Lipidomics, Microbiota, and Intestinal Clostridioides difficile Infection Outcome
Abstract
1. Introduction
2. Intestinal Microbiota and Its Impact on Maintaining Health and the Onset of Disease
3. The Relationship Between Lipidomics and Intestinal Microbiota
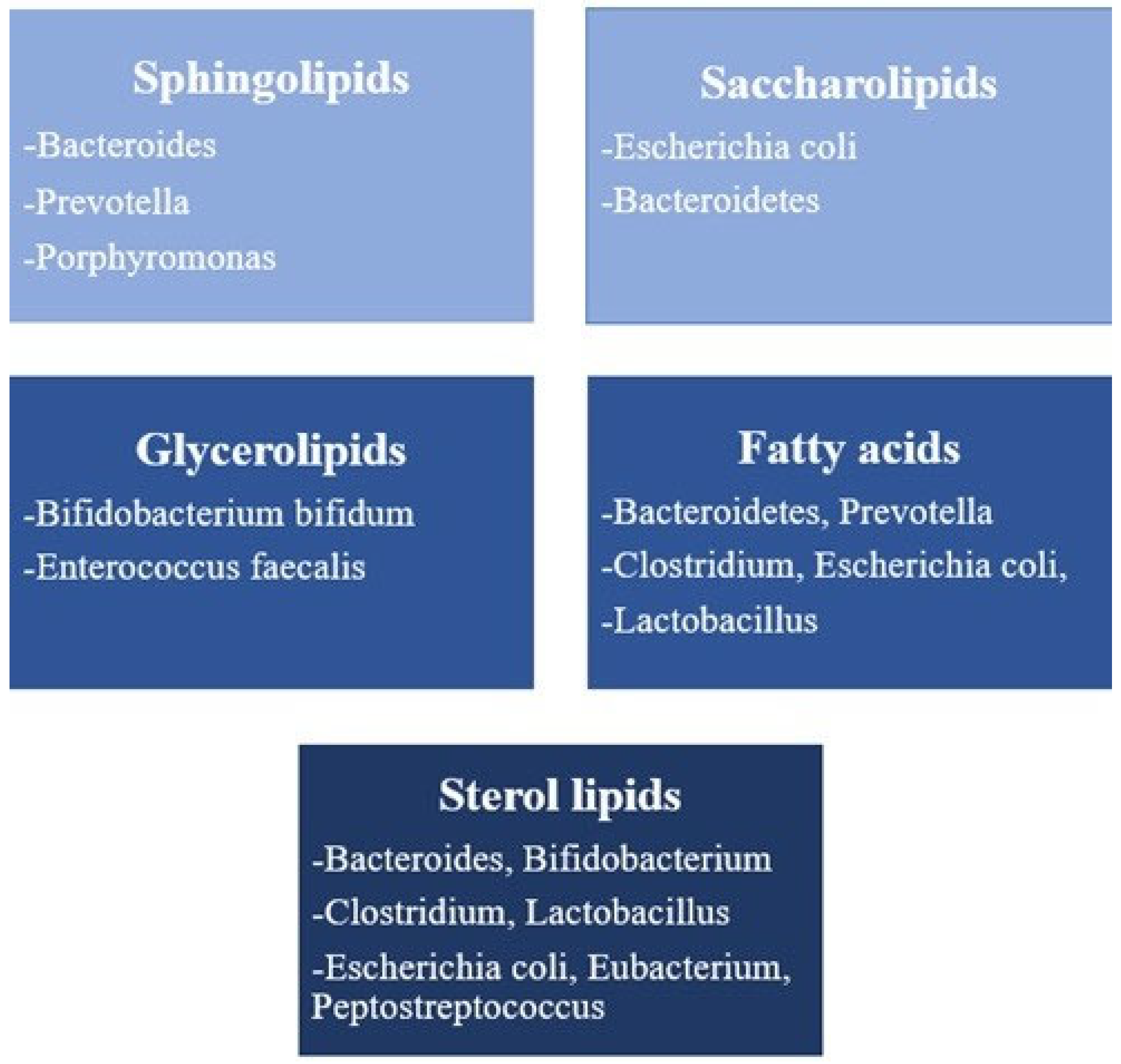
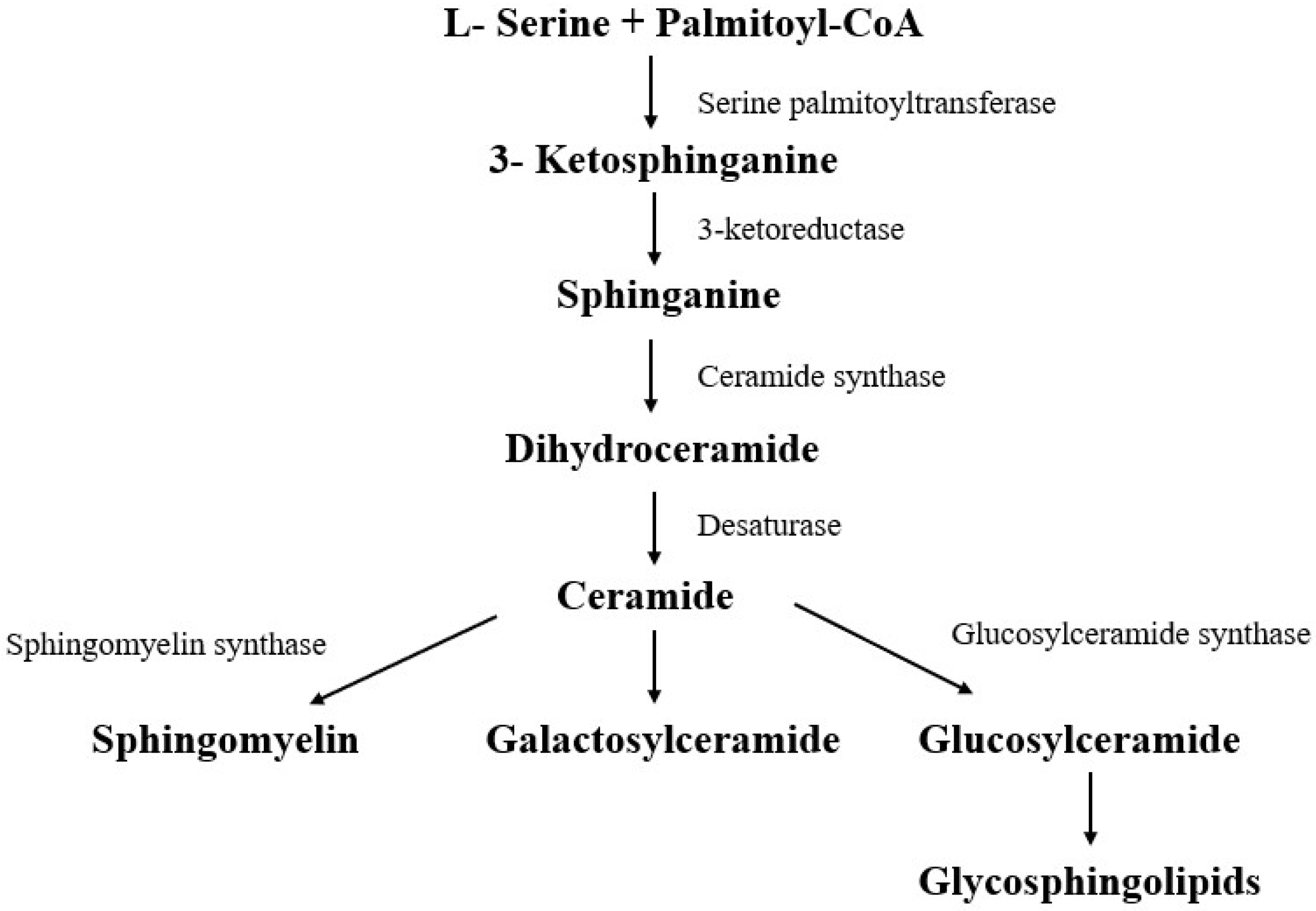
3.1. Sphingolipids
3.2. Saccharolipids
3.3. Fatty Acids
3.4. Glycerolipids
3.5. Sterol Lipids
4. Intestinal Microbiota and Its Role in Drug Biotransformation—Is There a Connection with Lipidomics?
5. Clostridioides difficile—Today’s Epidemiological Problem in Hospital Conditions
6. The Importance of Knowing Lipidomics and Microbiota for the Outcome of Clostridioides difficile Infection
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| C. difficile | Clostridioides difficile |
References
- Kishimoto, K.; Urade, R.; Ogawa, T.; Moriyama, T. Nondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: Suitable methods for “lipidome” analysis. Biochem. Biophys. Res. Commun. 2001, 281, 657–662. [Google Scholar] [CrossRef]
- Lindblom, G.; Orädd, G.; Rilfors, L.; Morein, S. Regulation of lipid composition in Acholeplasma laidlawii and Escherichia coli membranes: NMR studies of lipid lateral diffusion at different growth temperatures. Biochemistry 2002, 41, 11512–11515. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Kurreck, A.; Vandergrift, L.A.; Fuss, T.L.; Habbel, P.; Agar, N.Y.R.; Cheng, L.L. Prostate cancer diagnosis and characterization with mass spectrometry imaging. Prostate Cancer Prostatic Dis. 2018, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Smith, A.; Piga, I.; Galli, M.; Stella, M.; Denti, V.; Del Puppo, M.; Magni, F. Matrix-Assisted Laser Desorption/Ionisation Mass Spectrometry Imaging in the Study of Gastric Cancer: A Mini Review. Int. J. Mol. Sci. 2017, 18, 2588. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, L.; Yan, F.; Wang, X. Clinical lipidomics: A new way to diagnose human diseases. Clin. Transl. Med. 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, X.; Wang, X. Is the clinical lipidomics a potential goldmine? Cell Biol. Toxicol. 2018, 34, 421–423, Correction in Cell Biol. Toxicol. 2020, 36, 285. https://doi.org/10.1007/s10565-019-09490-8. [Google Scholar] [CrossRef]
- Lounila, J.; Ala-Korpela, M.; Jokisaari, J.; Savolainen, M.J.; Kesäniemi, Y.A. Effects of orientational order and particle size on the NMR line positions of lipoproteins. Phys. Rev. Lett. 1994, 72, 4049–4052. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.-R.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef]
- Giles, C.; Takechi, R.; Lam, V.; Dhaliwal, S.S.; Mamo, J.C.L. Contemporary lipidomic analytics: Opportunities and pitfalls. Prog. Lipid Res. 2018, 71, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Orešič, M. Optimizing the lipidomics workflow for clinical studies--practical considerations. Anal. Bioanal. Chem. 2015, 407, 4973–4993. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Avela, H.F.; Sirén, H. Advances in lipidomics. Clin. Chim. Acta 2020, 510, 123–141. [Google Scholar] [CrossRef]
- Kauhanen, D.; Sysi-Aho, M.; Koistinen, K.M.; Laaksonen, R.; Sinisalo, J.; Ekroos, K. Development and validation of a high-throughput LC-MS/MS assay for routine measurement of molecular ceramides. Anal. Bioanal. Chem. 2016, 408, 3475–3483. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
- Jayawardana, K.S.; Mundra, P.A.; Giles, C.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; et al. Changes in plasma lipids predict pravastatin efficacy in secondary prevention. JCI Insight 2019, 4, e128438. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Domenick, T.M.; Gill, E.L.; Vedam-Mai, V.; Yost, R.A. Mass Spectrometry-Based Cellular Metabolomics: Current Approaches, Applications, and Future Directions. Anal. Chem. 2021, 93, 546–566. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861, Correction in J. Lipid Res. 2010, 51, 1618. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed; Elsevier Science B.V.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Cohen, L.J.; Esterhazy, D.; Kim, S.H.; Lemetre, C.; Aguilar, R.R.; Gordon, E.A.; Pickard, A.J.; Cross, J.R.; Emiliano, A.B.; Han, S.M.; et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, 48–53, Correction in Nature 2018, 556, 135. https://doi.org/10.1038/nature25997. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148, Correction in Nature 2020, 579, E7. https://doi.org/10.1038/s41586-020-2030-5. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. The link among microbiota, epigenetics, and disease development. Environ. Sci. Pollut. Res. Int. 2021, 28, 28926–28964. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Radonjić, T.; Dukić, M.; Jovanović, I.; Zdravković, M.; Mandić, O.; Popadić, V.; Popović, M.; Nikolić, N.; Klašnja, S.; Divac, A.; et al. Aging of Liver in Its Different Diseases. Int. J. Mol. Sci. 2022, 23, 13085. [Google Scholar] [CrossRef]
- Guo, M.; Miao, M.; Wang, Y.; Duan, M.; Yang, F.; Chen, Y.; Yuan, W.; Zheng, H. Developmental differences in the intestinal microbiota of Chinese 1-year-old infants and 4-year-old children. Sci. Rep. 2020, 10, 19470. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Pellanda, P.; Ghosh, T.S.; O’Toole, P.W. Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr. Opin. Biotechnol. 2021, 70, 48–55. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. New York Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host-microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Dukić, M.; Radonjić, T.; Jovanović, I.; Zdravković, M.; Todorović, Z.; Kraišnik, N.; Aranđelović, B.; Mandić, O.; Popadić, V.; Nikolić, N.; et al. Alcohol, Inflammation, and Microbiota in Alcoholic Liver Disease. Int. J. Mol. Sci. 2023, 24, 3735. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Wlodarska, M.; Kostic, A.D.; Xavier, R.J. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 2015, 17, 577–591. [Google Scholar] [CrossRef]
- Branković, M.; Dukić, M.; Gmizić, T.; Popadić, V.; Nikolić, N.; Sekulić, A.; Brajković, M.; Đokić, J.; Mahmutović, E.; Lasica, R.; et al. New Therapeutic Approaches for the Treatment of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Increased Cardiovascular Risk. Diagnostics 2024, 14, 229. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Djurasevic, S.; Bojic, S.; Nikolic, B.; Dimkic, I.; Todorovic, Z.; Djordjevic, J.; Mitic-Culafic, D. Beneficial Effect of Virgin Coconut Oil on Alloxan-Induced Diabetes and Microbiota Composition in Rats. Plant Foods Hum. Nutr. 2018, 73, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Basso, P.J.; Fonseca, M.T.; Bonfá, G.; Alves, V.B.F.; Sales-Campos, H.; Nardini, V.; Cardoso, C.R.B. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz. J. Med. Biol. Res. 2014, 47, 727–737. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [PubMed]
- Bar, N.; Korem, T.; Weissbrod, O.; Zeevi, D.; Rothschild, D.; Leviatan, S.; Kosower, N.; Lotan-Pompan, M.; Weinberger, A.; Le Roy, C.I.; et al. A reference map of potential determinants for the human serum metabolome. Nature 2020, 588, 135–140. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Author Correction: Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Frangioudakis, G.; Garrard, J.; Raddatz, K.; Nadler, J.L.; Mitchell, T.W.; Schmitz-Peiffer, C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: Reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology 2010, 151, 4187–4196. [Google Scholar] [CrossRef]
- Nicholson, R.J.; Holland, W.L.; Summers, S.A. Ceramides and Acute Kidney Injury. Semin. Nephrol. 2022, 42, 151281. [Google Scholar] [CrossRef]
- Loft, L.M.I.; Moseholm, K.F.; Pedersen, K.K.W.; Jensen, M.K.; Koch, M.; Cronjé, H.T. Sphingomyelins and ceramides: Possible biomarkers for dementia? Curr. Opin. Lipidol. 2022, 33, 57–67. [Google Scholar] [CrossRef]
- Pilátová, M.B.; Solárová, Z.; Mezencev, R.; Solár, P. Ceramides and their roles in programmed cell death. Adv. Med. Sci. 2023, 68, 417–425. [Google Scholar] [CrossRef]
- An, D.; Oh, S.F.; Olszak, T.; Neves, J.F.; Avci, F.Y.; Erturk-Hasdemir, D.; Lu, X.; Zeissig, S.; Blumberg, R.S.; Kasper, D.L. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014, 156, 123–133. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Na, C.; Bielawski, J.; Hannun, Y.A.; Kasper, D.L. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4666–4671. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Le, H.H.; Johnson, E.L. Dietary sphinganine is selectively assimilated by members of the mammalian gut microbiome. J. Lipid Res. 2021, 62, 100034. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.; Kim, D.D.; Lee, S.; Lee, B.; Lee, Y.; Kim, S.; Kwon, S.; Noh, M.; Lee, M.-O.; et al. Bioactive Lipids and Their Derivatives in Biomedical Applications. Biomol. Ther. 2021, 29, 465–482. [Google Scholar] [CrossRef]
- Kostarnoy, A.V.; Gancheva, P.G.; Logunov, D.Y.; Verkhovskaya, L.V.; Bobrov, M.A.; Scheblyakov, D.V.; Tukhvatulin, A.I.; Filippova, N.E.; Naroditsky, B.S.; Gintsburg, A.L. Topical bacterial lipopolysaccharide application affects inflammatory response and promotes wound healing. J. Interferon Cytokine Res. 2013, 33, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, M.; Raetz, C.R. Membrane lipid biogenesis in Escherichia coli: Identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J. Biol. Chem. 1979, 254, 7837–7844. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Qureshi, N.; Mascagni, P.; Nashed, M.A.; Anderson, L.; Raetz, C.R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J. Biol. Chem. 1983, 258, 7379–7385. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853, Correction in Cell 2016, 165, 1551. https://doi.org/10.1016/j.cell.2016.05.056. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. (Eds.) Section 22.5—Acetyl Coenzyme A carboxylase plays a key role in controlling fatty acid metabolism. In Biochemistry, 5th ed.; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Ratnayake, W.M.; Galli, C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: A background review paper. Ann. Nutr. Metab. 2009, 55, 8–43. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.; Lanza, E.; Greenwald, P. Dietary fiber, vegetables, and colon cancer: Critical review and meta-analyses of the epidemiologic evidence. J. Natl. Cancer Inst. 1990, 82, 650–661. [Google Scholar] [CrossRef]
- Hague, A.; Elder, D.J.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and pan-PPAR partial agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 2013, 59, 214–224. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Brondz, I.; Olsen, I. Multivariate analyses of cellular fatty acids in Bacteroides, Prevotella, Porphyromonas, Wolinella, and Campylobacter spp. J. Clin. Microbiol. 1991, 29, 183–189. [Google Scholar] [CrossRef]
- Hari, S.B.; Grant, R.A.; Sauer, R.T. Structural and Functional Analysis of E. coli Cyclopropane Fatty Acid Synthase. Structure 2018, 26, 1251–1258.e3. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef]
- Hölzl, G.; Dörmann, P. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
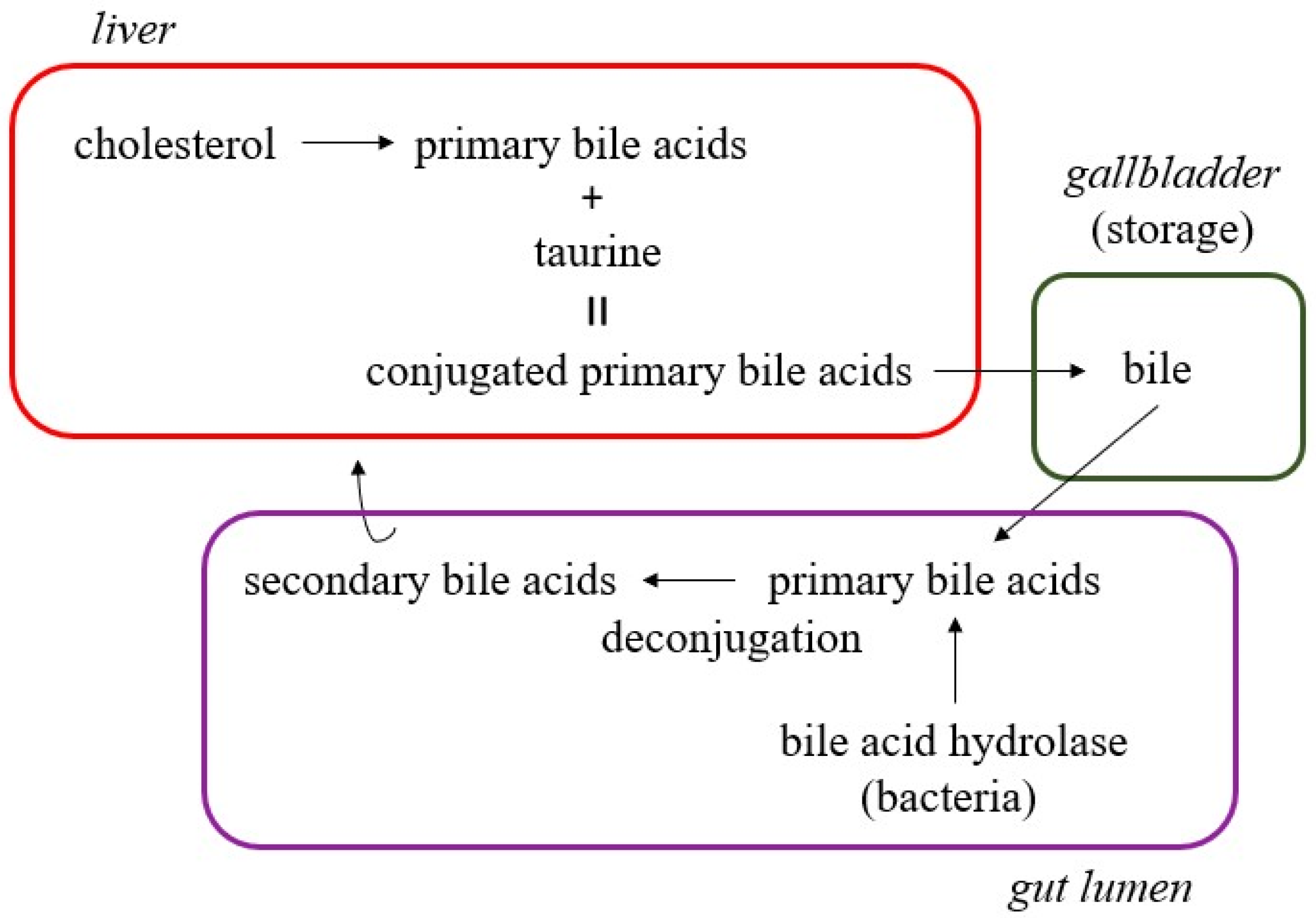
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Arcay, R.; Barceló-Nicolau, M.; Suárez, L.; Martín, L.; Reigada, R.; Höring, M.; Liebisch, G.; Garrido, C.; Cabot, G.; Vílchez, H.; et al. Gut microbiome and plasma lipidome analysis reveals a specific impact of Clostridioides difficile infection on intestinal bacterial communities and sterol metabolism. mBio 2024, 15, e0134724. [Google Scholar] [CrossRef]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. S1), s15–s20. [Google Scholar] [CrossRef]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef]
- Stiefel, U.; Tima, M.A.; Nerandzic, M.M. Metallo-β-lactamase-producing bacteroides species can shield other members of the gut microbiota from antibiotics. Antimicrob. Agents Chemother. 2015, 59, 650–653. [Google Scholar] [CrossRef]
- Luis, A.S.; Baslé, A.; Byrne, D.P.; Wright, G.S.A.; London, J.A.; Jin, C.; Karlsson, N.G.; Hansson, G.C.; Eyers, P.A.; Czjzek, M.; et al. Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota. Nat. Chem. Biol. 2022, 18, 841–849, Correction in Nat. Chem. Biol. 2022, 18, 1032. https://doi.org/10.1038/s41589-022-01132-1. [Google Scholar] [CrossRef]
- Wilkinson, E.M.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. Microbiota-drug interactions: Impact on metabolism and efficacy of therapeutics. Maturitas 2018, 112, 53–63. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Fiayyaz, F.; Rehman, K.; Sabir, S.; Rasool, M.H. Gut Microbiota and Metabolic Disorders: Advances in Therapeutic Interventions. Crit. Rev. Immunol. 2019, 39, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Zak-Gołąb, A.; Olszanecka-Glinianowicz, M.; Kocełak, P.; Chudek, J. Rola flory jelitowej w patogenezie otyłości [The role of gut microbiota in the pathogenesis of obesity]. Postep. Hig. Med. Dosw. 2014, 68, 84–90. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Liebisch, G.; Plagge, J.; Höring, M.; Seeliger, C.; Ecker, J. The effect of gut microbiota on the intestinal lipidome of mice. Int. J. Med. Microbiol. 2021, 311, 151488. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- LoGuidice, A.; Wallace, B.D.; Bendel, L.; Redinbo, M.R.; Boelsterli, U.A. Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J. Pharmacol. Exp. Ther. 2012, 341, 447–454. [Google Scholar] [CrossRef]
- Wu, Q.; Song, D.; Zhao, Y.; Verdegaal, A.A.; Turocy, T.; Duncan-Lowey, B.; Goodman, A.L.; Palm, N.W.; Crawford, J.M. Activity of GPCR-targeted drugs influenced by human gut microbiota metabolism. Nat. Chem. 2025, 17, 808–821. [Google Scholar] [CrossRef]
- Ali, Y.; Hamid, S.A.; Rashid, U. Biomedical Applications of Aromatic Azo Compounds. Mini Rev. Med. Chem. 2018, 18, 1548–1558. [Google Scholar] [CrossRef]
- Vonberg, R.P.; Kuijper, E.J.; Wilcox, M.H.; Barbut, F.; Tüll, P.; Gastmeier, P.; European C difficile-Infection Control Group; European Centre for Disease Prevention and Control (ECDC); van den Broek, P.J.; Colville, A.; et al. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol. Infect. 2008, 14 (Suppl. S5), 2–20. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Mizusawa, M. Laboratory Tests for the Diagnosis of Clostridium difficile. Clin. Colon. Rectal Surg. 2020, 33, 73–81. [Google Scholar] [CrossRef]
- Heinlen, L.; Ballard, J.D. Clostridium difficile infection. Am. J. Med. Sci. 2010, 340, 247–252. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Chang, T.W.; Gurwith, M.; Gorbach, S.L.; Onderdonk, A.B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 1978, 298, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile infection. Nat. Rev. Dis. Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile Infection. N. Engl. J. Med. 2015, 373, 287–288. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens, R.C., Jr.; Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Theriault, N.; Tillotson, G. The burden of CDI in the United States: A multifactorial challenge. BMC Infect. Dis. 2023, 23, 132. [Google Scholar] [CrossRef]
- Fazl Alizadeh, R.; Li, S.; Sullivan, B.; Manasa, M.; Ruhi-Williams, P.; Nahmias, J.; Carmichael, J.; Nguyen, N.T.; Stamos, M.J. Surgical Outcome in Laparoscopic Abdominal Surgical Operations with Clostridium difficile Infection. Am. Surg. 2022, 88, 2519–2524. [Google Scholar] [CrossRef]
- El Feghaly, R.E.; Stauber, J.L.; Deych, E.; Gonzalez, C.; Tarr, P.I.; Haslam, D.B. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin. Infect. Dis. 2013, 56, 1713–1721. [Google Scholar] [CrossRef]
- Borriello, S.P. Pathogenesis of Clostridium difficile infection. J. Antimic. Chemother. 1998, 41 (Suppl. C), 13–19. [Google Scholar] [CrossRef]
- Janoir, C. Virulence factors of Clostridium difficile and their role during infection. Anaerobe 2016, 37, 13–24. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Shin, J.H.; Chaves-Olarte, E.; Warren, C.A. Clostridium difficile Infection. Microbiol. Spectr. 2016, 4, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Hryckowian, A.J.; Pruss, K.M.; Sonnenburg, J.L. The emerging metabolic view of Clostridium difficile pathogenesis. Curr. Opin. Microbiol. 2017, 35, 42–47. [Google Scholar] [CrossRef] [PubMed]
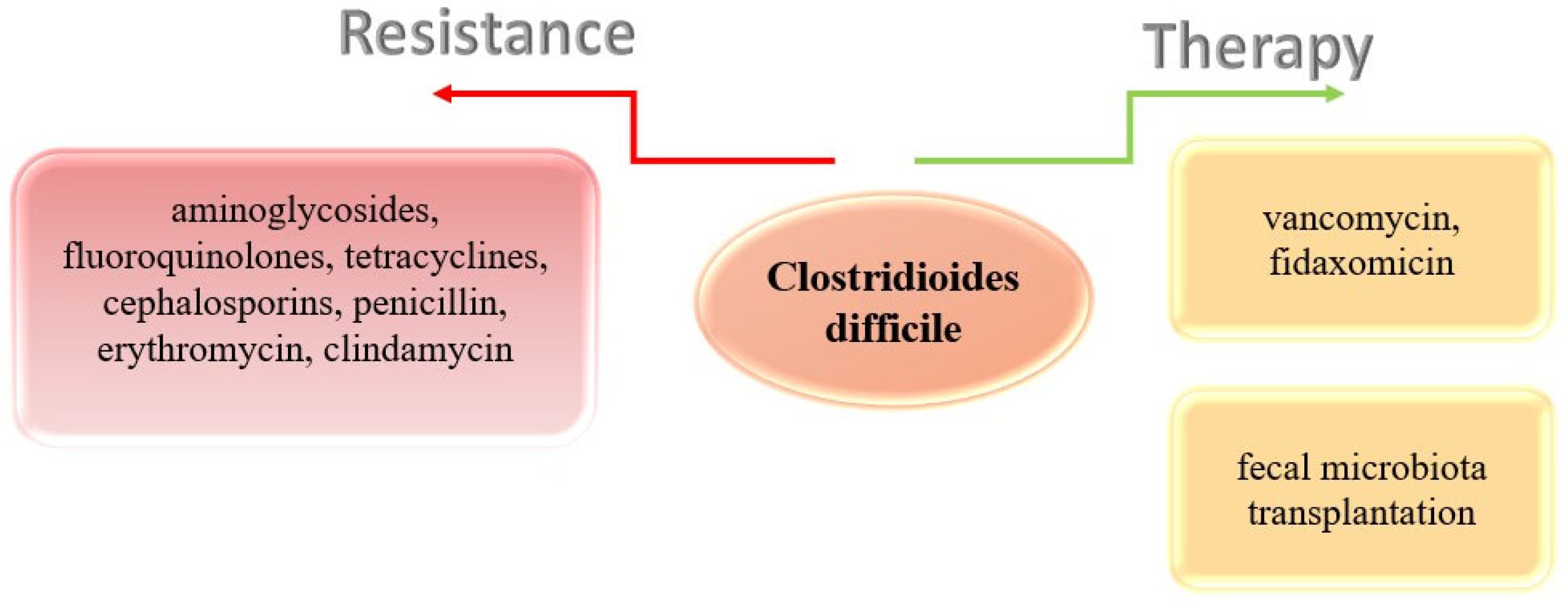
- Peng, Z.; Jin, D.; Kim, H.B.; Stratton, C.W.; Wu, B.; Tang, Y.-W.; Sun, X. Update on Antimicrobial Resistance in Clostridium difficile: Resistance Mechanisms and Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2017, 55, 1998–2008. [Google Scholar] [CrossRef]
- Spigaglia, P.; Mastrantonio, P.; Barbanti, F. Antibiotic Resistances of Clostridium difficile. Adv. Exp. Med. Biol. 2018, 1050, 137–159. [Google Scholar] [CrossRef]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef]
- Tkhawkho, L.; Nitzan, O.; Pastukh, N.; Brodsky, D.; Jackson, K.; Peretz, A. Antimicrobial susceptibility of Clostridium difficile isolates in Israel. J. Glob. Antimicrob. Resist. 2017, 10, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Banawas, S.S. Clostridium difficile Infections: A Global Overview of Drug Sensitivity and Resistance Mechanisms. Biomed. Res. Int. 2018, 2018, 8414257. [Google Scholar] [CrossRef]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014, 20 (Suppl. S2), 1–26. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, 755–757. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention Control. European Surveillance of Clostridioides (Clostridium) Difficile Infections Surveillance Protocol, 24th ed.; ECDC: Solna, Sweden, 2019; ISBN 978-92-9498-450-0. [Google Scholar] [CrossRef]
- Perumalsamy, S.; Riley, T.V. Molecular Epidemiology of Clostridioides difficile Infections in Children. J. Pediatric. Infect. Dis. Soc. 2021, 10 (Suppl. S3), S34–S40. [Google Scholar] [CrossRef]
- Lee, H.S.; Plechot, K.; Gohil, S.; Le, J. Clostridium difficile: Diagnosis and the Consequence of Over Diagnosis. Infect. Dis. Ther. 2021, 10, 687–697. [Google Scholar] [CrossRef]
- Minkoff, N.Z.; Aslam, S.; Medina, M.; Tanner-Smith, E.E.; Zackular, J.P.; Acra, S.; Nicholson, M.R.; Imdad, A. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile). Cochrane Database Syst. Rev. 2023, 4, CD013871. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; Imdad, A.; Altayar, O.; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on Fecal Microbiota-Based Therapies for Select Gastrointestinal Diseases. Gastroenterology 2024, 166, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.C.; Winston, J.A. Collaborative Metabolism: Gut Microbes Play a Key Role in Canine and Feline Bile Acid Metabolism. Vet. Sci. 2024, 11, 94. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Francis, M.B.; Ding, X.; McAllister, K.N.; Shrestha, R.; Sorg, J.A. Reexamining the Germination Phenotypes of Several Clostridium difficile Strains Suggests Another Role for the CspC Germinant Receptor. J. Bacteriol. 2015, 198, 777–786. [Google Scholar] [CrossRef]
- Foley, M.H.; Walker, M.E.; Stewart, A.K.; O’Flaherty, S.; Gentry, E.C.; Patel, S.; Beaty, V.V.; Allen, G.; Pan, M.; Simpson, J.B.; et al. Bile salt hydrolases shape the bile acid landscape and restrict Clostridioides difficile growth in the murine gut. Nat. Microbiol. 2023, 8, 611–628. [Google Scholar] [CrossRef]
- Thanissery, R.; Winston, J.A.; Theriot, C.M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 2017, 45, 86–100. [Google Scholar] [CrossRef]
- Kisthardt, S.C.; Thanissery, R.; Pike, C.M.; Foley, M.H.; Theriot, C.M. The microbial-derived bile acid lithocholate and its epimers inhibit Clostridioides difficile growth and pathogenicity while sparing members of the gut microbiota. J. Bacteriol. 2023, 205, e0018023. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Icho, S.; Utama, E.; Orrell, K.E.; Gómez-Biagi, R.F.; Theriot, C.M.; Kroh, H.K.; Rutherford, S.A.; Lacy, D.B.; Melnyk, R.A. Intestinal bile acids directly modulate the structure and function of C. difficile TcdB toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 6792–6800. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Dosa, P.I.; DeWinter, E.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Khoruts, A.; Sadowsky, M.J. Changes in Colonic Bile Acid Composition following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium difficile Germination and Growth. PLoS ONE 2016, 11, e0147210. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Theriot, C.M.; Rao, K.; Chang, Y.-M.; Freeman, A.E.; Kao, Y.J.; Young, V.B. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019, 15, e1007581. [Google Scholar] [CrossRef]
- Aguirre, A.M.; Adegbite, A.O.; Sorg, J.A. Clostridioides difficile bile salt hydrolase activity has substrate specificity and affects biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 94. [Google Scholar] [CrossRef]
- McMillan, A.S.; Foley, M.H.; Perkins, C.E.; Theriot, C.M. Loss of Bacteroides thetaiotaomicron bile acid-altering enzymes impacts bacterial fitness and the global metabolic transcriptome. Microbiol. Spectr. 2024, 12, e0357623. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Rud, T.; Rath, S.; Pieper, D.H.; Schlüter, D. Diversity of Bacteria Exhibiting Bile Acid-inducible 7α-dehydroxylation Genes in the Human Gut. Comput. Struct. Biotechnol. J. 2019, 17, 1016–1019. [Google Scholar] [CrossRef]
- Theriot, C.M.; Bowman, A.A.; Young, V.B. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere 2016, 1, e00045-15. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The role of bile acids in carcinogenesis. Cell Mol. Life Sci. 2022, 79, 243. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branković, M.; Kraišnik, M.; Zdravković, D.; Kraišnik, N.; Jelić, F.; Nikolić, N.; Đurašević, S.; Tosti, T.; Gmizić, T.; Todorović, Z. Lipidomics, Microbiota, and Intestinal Clostridioides difficile Infection Outcome. Int. J. Mol. Sci. 2025, 26, 8214. https://doi.org/10.3390/ijms26178214
Branković M, Kraišnik M, Zdravković D, Kraišnik N, Jelić F, Nikolić N, Đurašević S, Tosti T, Gmizić T, Todorović Z. Lipidomics, Microbiota, and Intestinal Clostridioides difficile Infection Outcome. International Journal of Molecular Sciences. 2025; 26(17):8214. https://doi.org/10.3390/ijms26178214
Chicago/Turabian StyleBranković, Marija, Marija Kraišnik, Dimitrije Zdravković, Nemanja Kraišnik, Filip Jelić, Novica Nikolić, Siniša Đurašević, Tomislav Tosti, Tijana Gmizić, and Zoran Todorović. 2025. "Lipidomics, Microbiota, and Intestinal Clostridioides difficile Infection Outcome" International Journal of Molecular Sciences 26, no. 17: 8214. https://doi.org/10.3390/ijms26178214
APA StyleBranković, M., Kraišnik, M., Zdravković, D., Kraišnik, N., Jelić, F., Nikolić, N., Đurašević, S., Tosti, T., Gmizić, T., & Todorović, Z. (2025). Lipidomics, Microbiota, and Intestinal Clostridioides difficile Infection Outcome. International Journal of Molecular Sciences, 26(17), 8214. https://doi.org/10.3390/ijms26178214










