Reactivity of Autologous Serum IgG to Gut Microbes in Pediatric Ulcerative Colitis
Abstract
1. Introduction
2. Results
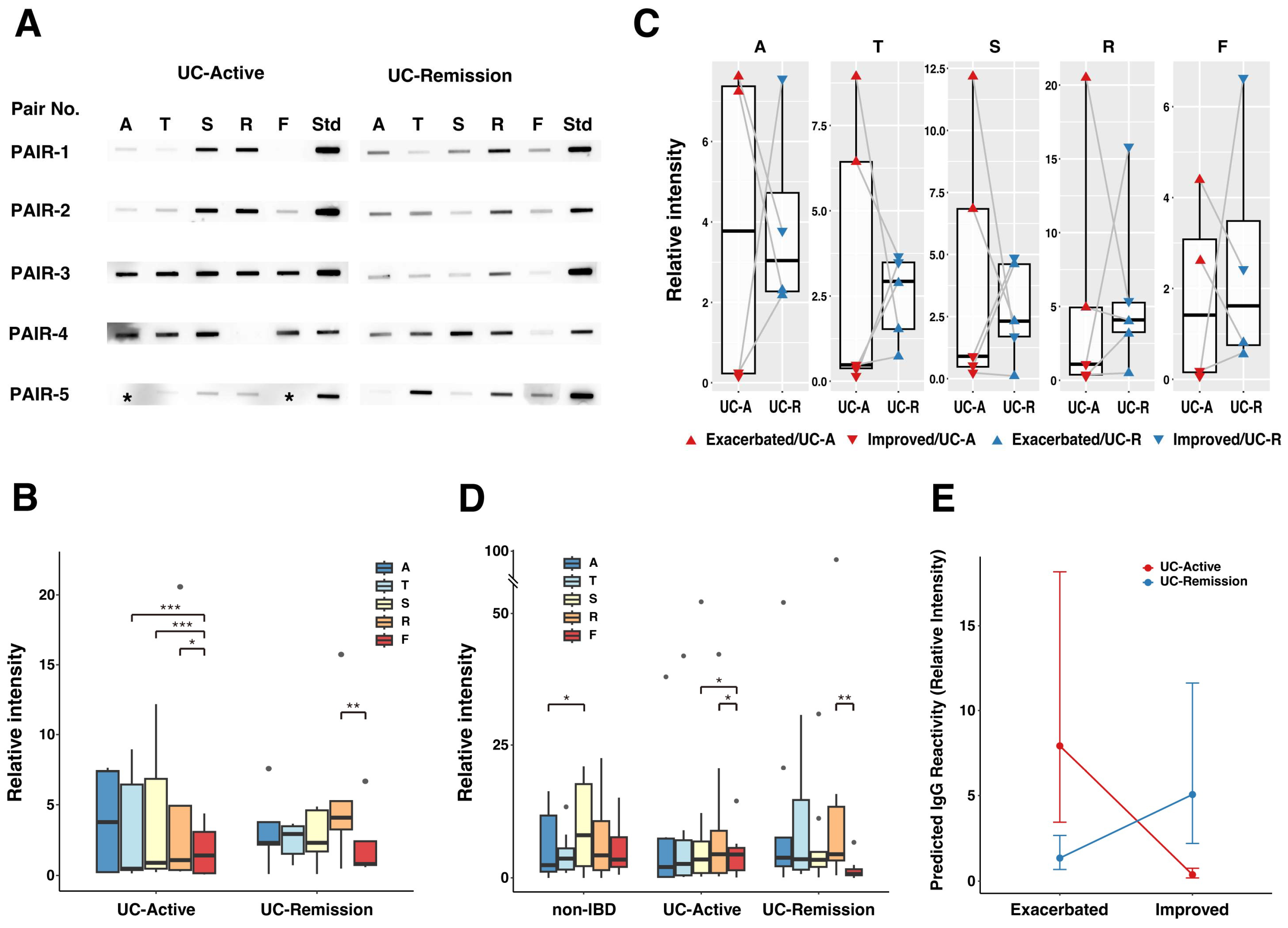
2.1. Reactivity of Serum IgG to Protein Extracts from CLFs or Feces from Pediatric Patients with UC
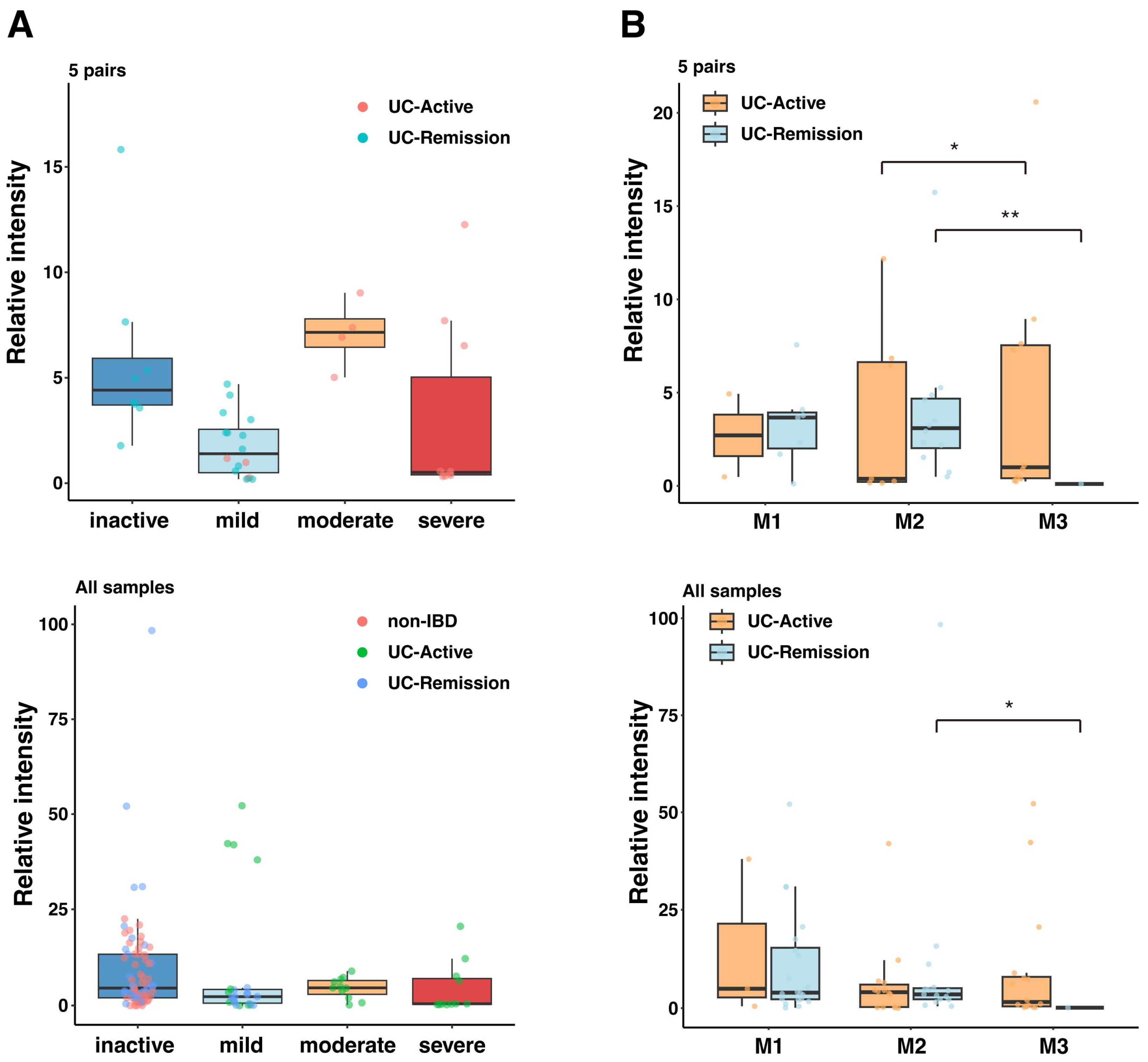
2.2. Association of Serum IgG Response with UC Disease Severity and Mucosal Inflammation
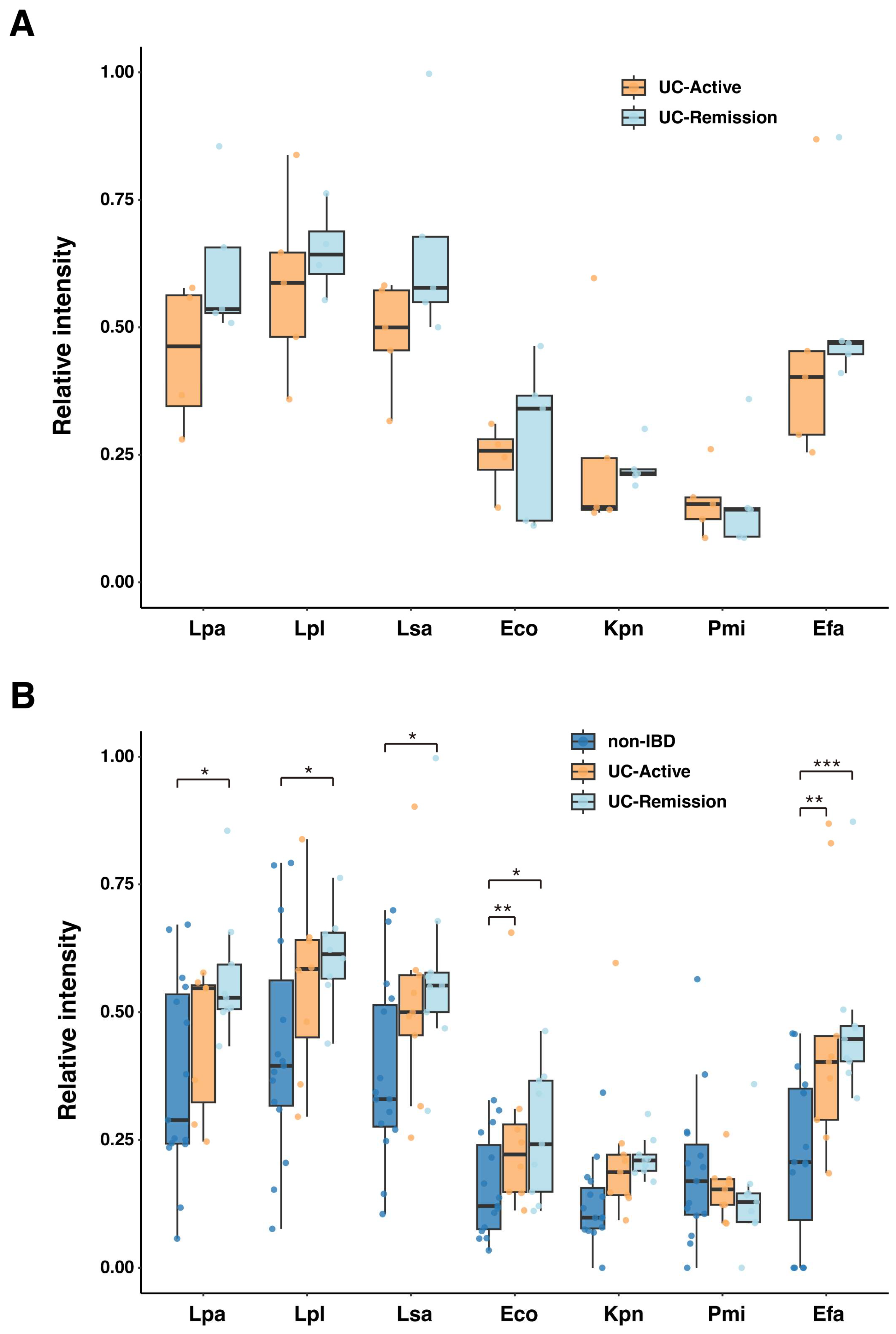
2.3. Serum IgG Reactivity to Specific Fecal Bacteria
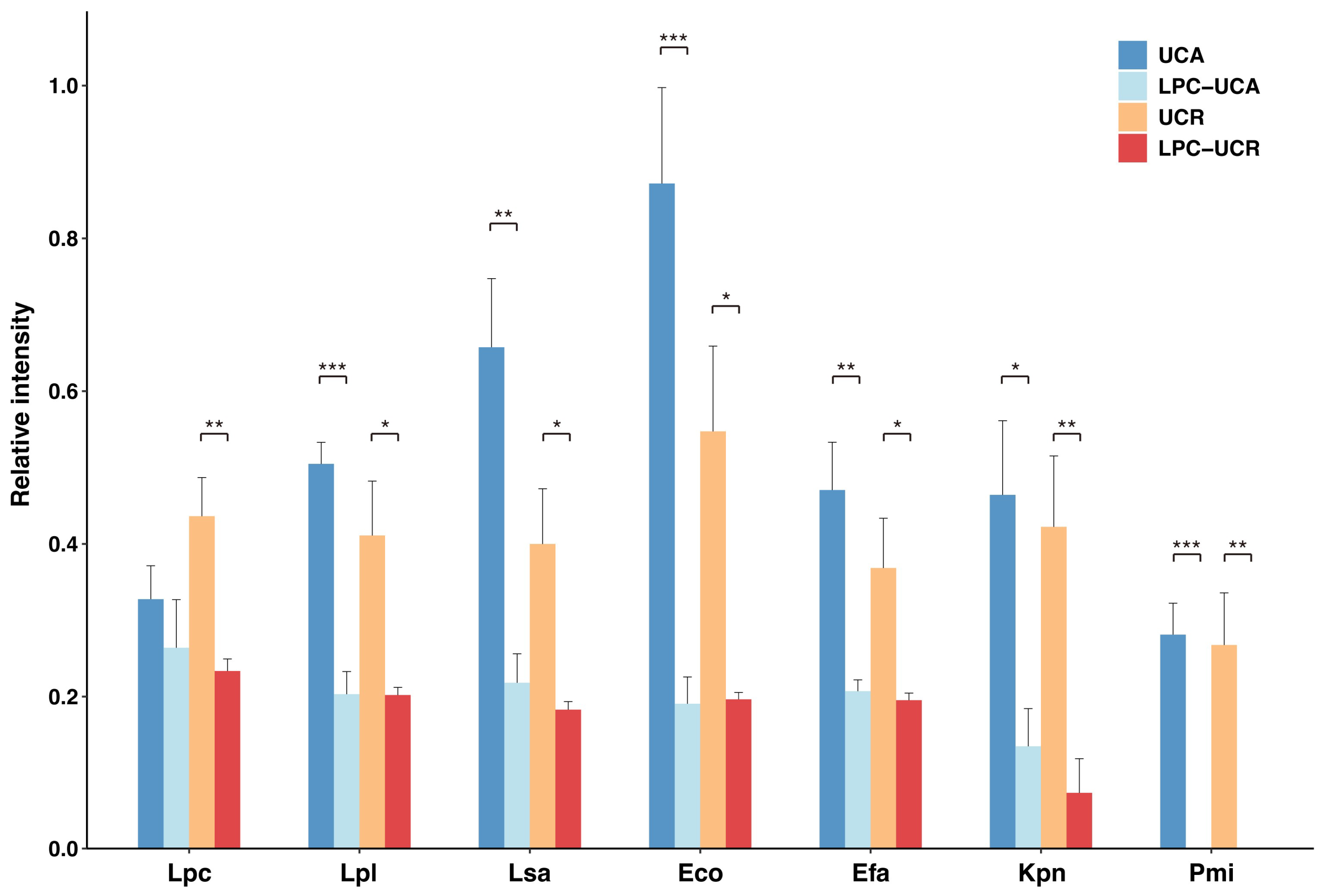
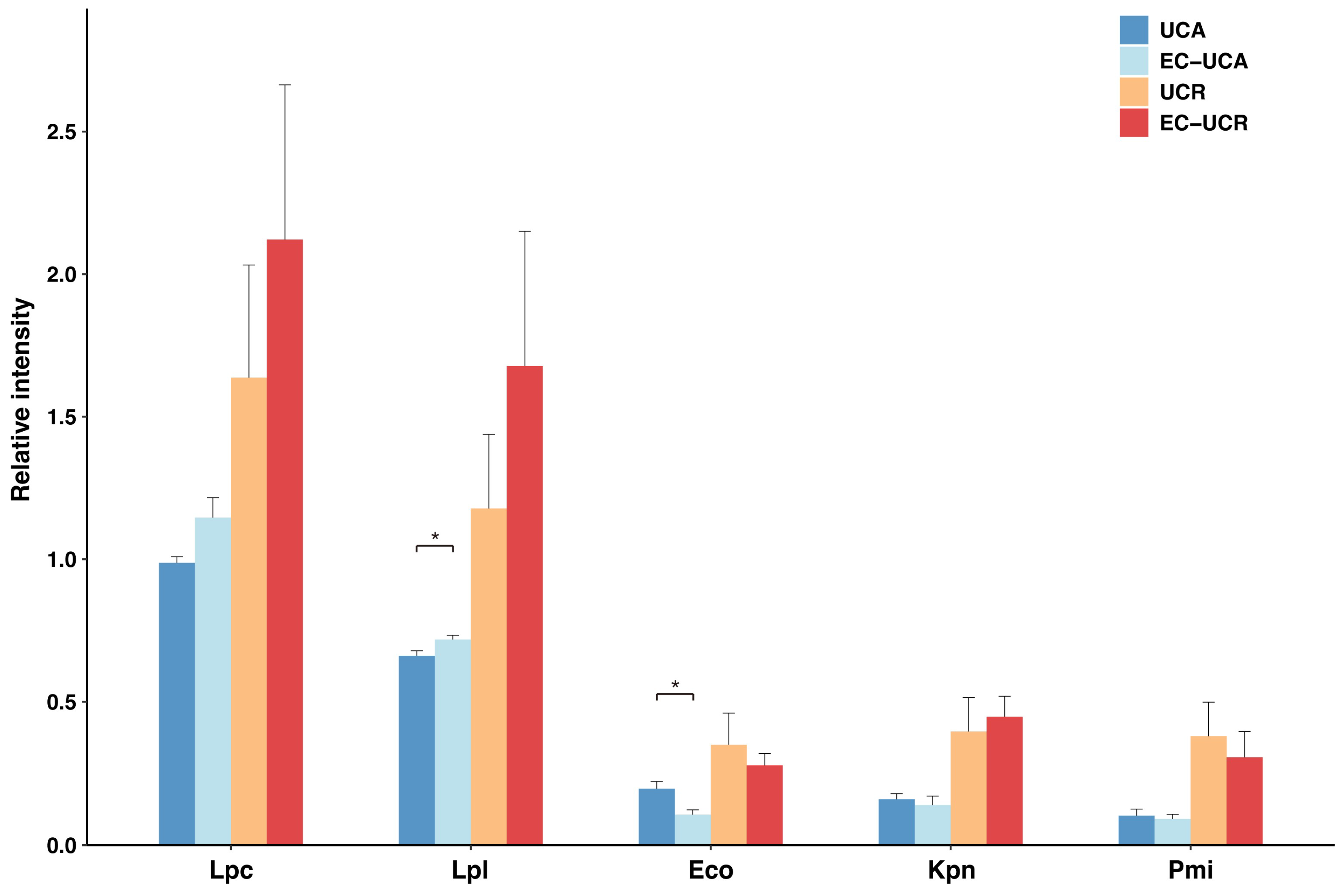
2.4. Serum IgG Reactivity to Selected Fecal Microbes After Absorption by L. paracasei or L. plantarum
2.5. Altered Serum IgG Reactivity to Gut Microbes After Absorption by E. coli
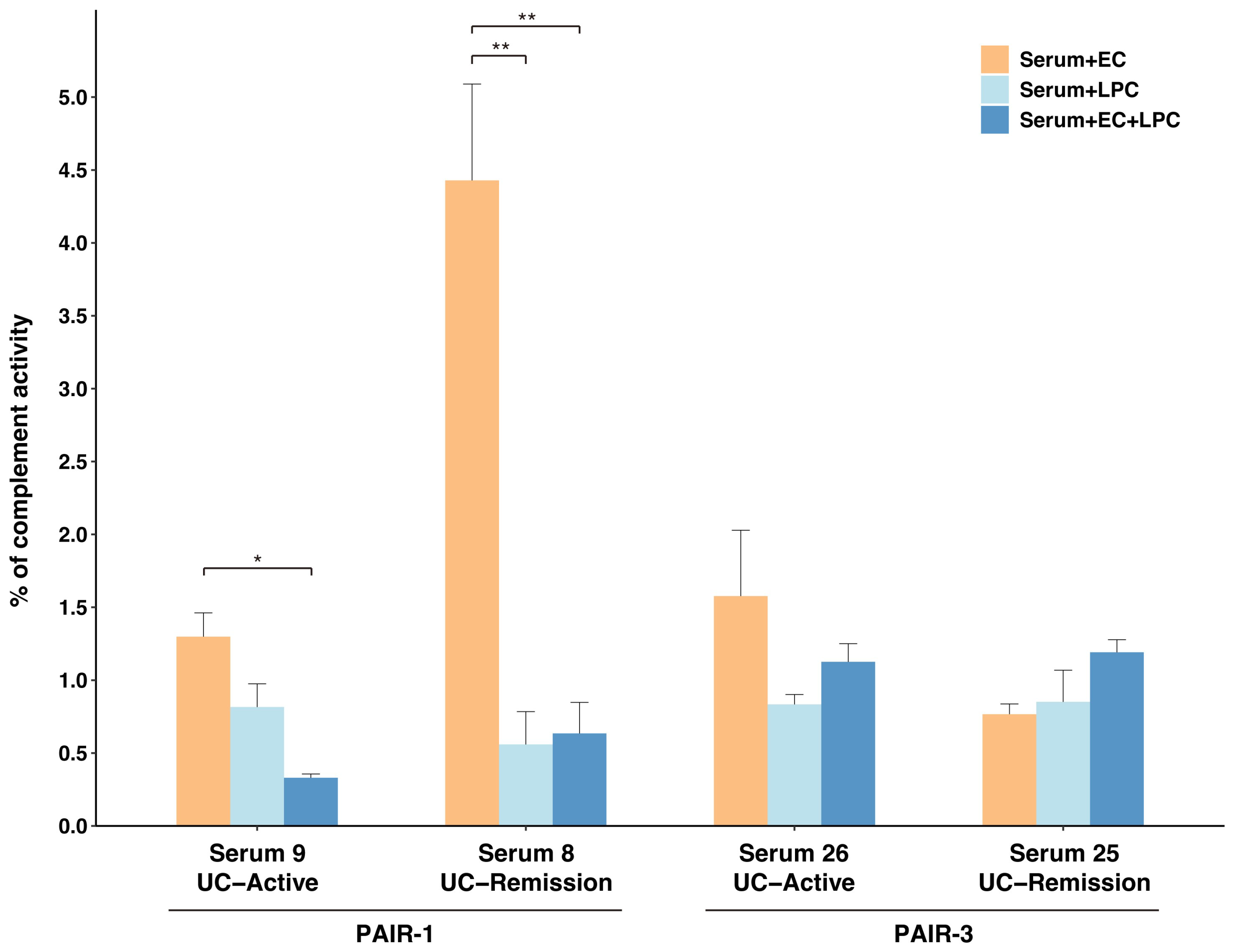
2.6. Complement Activation by Immune Complexes Composed of UC-Derived Serum IgG and L. paracasei and/or E. coli
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Purification of Serum IgG
4.3. Protein Extraction from CLFs and Feces
4.4. Isolation of Gut Microbes from Feces
4.5. Protein Extraction from Fecal Isolates from Pediatric Patients with UC
4.6. Serum IgG Reactivity to Protein Extracts from CLFs, Stools or Fecal Isolates
4.7. Preparation of L. paracasei-, L. plantarum- or E. coli-Absorbed Serum IgG
4.8. Complement Pathway Activation by Immune Complexes in IgG-Fecal Isolates
4.9. Statistical Analysis
4.9.1. Generalized Linear Mixed-Effects Models (GLMMs)
4.9.2. Other Comparisons
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| LM | Luminal microbes |
| MAM | Mucosa-associated microbes |
| IBDS | Inflammatory bowel diseases |
| OTUs | Operational taxonomic units |
| sIgA | Secretory IgA |
| FcRn | Neonatal Fc receptor |
| Ag | Antigen |
| CLFs | Colonoscopic lavage fluids |
| A | Ascending colon |
| T | Transverse colon |
| D | Descending colon |
| S | Sigmoid colon |
| R | Rectum |
| F | Feces |
| PUCAI | Pediatric UC activity index |
| Std | Standard |
| PFA | 4% Paraformaldehyde |
| PVDF | Polyvinylidene difluoride |
| UC-A | Ulcerative colitis active |
| UC-R | Ulcerative colitis remission |
| LSA | Ligilactobacillus salivarius |
| LPL | Lactiplantibacillus plantarum |
| LPC | Lactocaseibacilus paracasei |
| Eco | Escherichia coli |
| Kpn | Klebsiella pneumoniae |
| Pmi | Proteus mirabilis |
| Efa | Enterococcus faecalis |
| LPC-UCA | L. paracasei-absorbed Ulcerative colitis active serum |
| LPC-UCR | L. paracasei-absorbed Ulcerative colitis remission serum |
| LPL-UCA | L. plantarum-absorbed Ulcerative colitis active serum |
| LPL-UCR | L. plantarum-absorbed Ulcerative colitis remission serum |
| EC-UCA | E. coli-absorbed Ulcerative colitis active serum |
| EC-UCR | E. coli-absorbed Ulcerative colitis active serum |
| BHIS | Brain Heart Infusion Agar |
| PBS | Phosphate Buffered Saline |
References
- Bourgonje, A.R.; Roo-Brand, G.; Lisotto, P.; Sadaghian Sadabad, M.; Reitsema, R.D.; de Goffau, M.C.; Faber, K.N.; Dijkstra, G.; Harmsen, H.J.M. Patients with Inflammatory Bowel Disease Show IgG Immune Responses Towards Specific Intestinal Bacterial Genera. Front. Immunol. 2022, 13, 842911. [Google Scholar] [CrossRef]
- Armstrong, H.; Alipour, M.; Valcheva, R.; Bording-Jorgensen, M.; Jovel, J.; Zaidi, D.; Shah, P.; Lou, Y.; Ebeling, C.; Mason, A.L.; et al. Host Immunoglobulin G Selectively Identifies Pathobionts in Pediatric Inflammatory Bowel Diseases. Microbiome 2019, 7, 1. [Google Scholar] [CrossRef]
- Lin, R.; Chen, H.; Shu, W.; Sun, M.; Fang, L.; Shi, Y.; Pang, Z.; Wu, W.; Liu, Z. Clinical Significance of Soluble Immunoglobulins A and G and Their Coated Bacteria in Feces of Patients with Inflammatory Bowel Disease. J. Transl. Med. 2018, 16, 359. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Jiang, D.; Hoffman, S.N.; Guntaka, S.; Mays, J.L.; Wang, A.; Gomes, J.; Sorrentino, D. Impact of Diagnostic Delay and Associated Factors on Clinical Outcomes in a U.S. Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2017, 23, 1825–1831. [Google Scholar] [CrossRef]
- Sorrentino, D.; Nguyen, V.Q.; Chitnavis, M. V Capturing the Biologic Onset of Inflammatory Bowel Diseases: Impact on Translational and Clinical Science. Cells 2019, 8, 548. [Google Scholar] [CrossRef]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The Influence of Cytokines on the Complex Pathology of Ulcerative Colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Hughes, E.R.; Winter, M.G.; Duerkop, B.A.; Spiga, L.; de Carvalho, T.F.; Zhu, W.; Gillis, C.C.; Büttner, L.; Smoot, M.P.; Behrendt, C.L.; et al. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe 2017, 21, 208–219. [Google Scholar] [CrossRef]
- Yu, J.; Cheon, J.H. Microbial Modulation in Inflammatory Bowel Diseases. Immune Netw. 2022, 22, e44. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Liu, Z. Interplay of Intestinal Microbiota and Mucosal Immunity in Inflammatory Bowel Disease: A Relationship of Frenemies. Ther. Adv. Gastroenterol. 2020, 13, 1756284820935188. [Google Scholar] [CrossRef]
- Choden, T.; Cohen, N.A. The Gut Microbiome and the Immune System. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.; Corr, S.C. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients 2021, 13, 4259. [Google Scholar] [CrossRef]
- Thomazini, C.M.; Samegima, D.A.G.; Rodrigues, M.A.M.; Victoria, C.R.; Rodrigues, J. High Prevalence of Aggregative Adherent Escherichia Coli Strains in the Mucosa-Associated Microbiota of Patients with Inflammatory Bowel Diseases. Int. J. Med. Microbiol. 2011, 301, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Geng, J.; Tang, X.; Fan, H.; Xu, J.; Wen, X.; Ma, Z.; Shi, P. Spatial Heterogeneity and Co-Occurrence Patterns of Human Mucosal-Associated Intestinal Microbiota. ISME J. 2014, 8, 881–893. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.Y.; Oh, H.S.; Jee, S.R.; Park, S.J.; Lee, S.H.; Yoon, J.S.; Yu, S.J.; Yoon, I.C.; Lee, H.S. Comparison of Sampling Methods in Assessing the Microbiome from Patients with Ulcerative Colitis. BMC Gastroenterol. 2021, 21, 396. [Google Scholar] [CrossRef]
- Juge, N. Relationship between Mucosa-Associated Gut Microbiota and Human Diseases. Biochem. Soc. Trans. 2022, 50, 1225–1236. [Google Scholar] [CrossRef]
- Ohkusa, T.; Okayasu, I.; Ogihara, T.; Morita, K.; Ogawa, M.; Sato, N. Induction of Experimental Ulcerative Colitis by Fusobacterium Varium Isolated from Colonic Mucosa of Patients with Ulcerative Colitis. Gut 2003, 52, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Michaud, E.; Waeckel, L.; Gayet, R.; Goguyer-Deschaumes, R.; Chanut, B.; Jospin, F.; Bathany, K.; Monnoye, M.; Genet, C.; Prier, A.; et al. Alteration of Microbiota Antibody-mediated Immune Selection Contributes to Dysbiosis in Inflammatory Bowel Diseases. EMBO Mol. Med. 2022, 14, e15386. [Google Scholar] [CrossRef]
- Zhao, L.; Grimes, S.M.; Greer, S.U.; Kubit, M.; Lee, H.; Nadauld, L.D.; Ji, H.P. Characterization of the Consensus Mucosal Microbiome of Colorectal Cancer. NAR Cancer 2021, 3, zcab049. [Google Scholar] [CrossRef]
- Maccio-Maretto, L.; Piqueras, V.; Barrios, B.E.; Romagnoli, P.A.; Denning, T.L.; Correa, S.G. Luminal Bacteria Coated with IgA and IgG during Intestinal Inflammation as a New and Abundant Stimulus for Colonic Macrophages. Immunology 2022, 167, 64–76. [Google Scholar] [CrossRef]
- Eriksen, C.; Moll, J.M.; Myers, P.N.; Pinto, A.R.A.; Danneskiold-Samsøe, N.B.; Dehli, R.I.; Rosholm, L.B.; Dalgaard, M.D.; Penders, J.; Jonkers, D.M.; et al. IgG and IgM Cooperate in Coating of Intestinal Bacteria in IgA Deficiency. Nat. Commun. 2023, 14, 8124. [Google Scholar] [CrossRef]
- Yoshida, M.; Kobayashi, K.; Kuo, T.T.; Bry, L.; Glickman, J.N.; Claypool, S.M.; Kaser, A.; Nagaishi, T.; Higgins, D.E.; Mizoguchi, E.; et al. Neonatal Fc Receptor for IgG Regulates Mucosal Immune Responses to Luminal Bacteria. J. Clin. Investig. 2006, 116, 2142–2151. [Google Scholar] [CrossRef]
- Masu, Y.; Kanazawa, Y.; Kakuta, Y.; Shimoyama, Y. Immunoglobulin Subtype-Coated Bacteria Are Correlated with the Disease Activity of Inflammatory Bowel Disease. Sci. Rep. 2021, 11, 16672. [Google Scholar] [CrossRef]
- Sterlin, D.; Fadlallah, J.; Adams, O.; Fieschi, C.; Parizot, C.; Dorgham, K.; Rajkumar, A.; Autaa, G.; El-Kafsi, H.; Charuel, J.L.; et al. Human IgA Binds a Diverse Array of Commensal Bacteria. J. Exp. Med. 2020, 217, e20181635. [Google Scholar] [CrossRef] [PubMed]
- Benckert, J.; Schmolka, N.; Kreschel, C.; Zoller, M.J.; Sturm, A.; Wiedenmann, B.; Wardemann, H. The Majority of Intestinal IgA+ and IgG+ Plasmablasts in the Human Gut Are Antigen-Specific. J. Clin. Investig. 2011, 121, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, S.; Vivio, E.E.; Parkes, M.; Peterson, D.A.; Roberson, E.D.O.; Newberry, R.D.; Ciorba, M.A.; Hsieh, C.S. Dynamic Immunoglobulin Responses to Gut Bacteria during Inflammatory Bowel Disease. Gut Microbes 2020, 11, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis—An Evidence-Based Consensus Guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef]
- Yoshida, M.; Claypool, S.M.; Wagner, J.S.; Mizoguchi, E.; Mizoguchi, A.; Roopenian, D.C.; Lencer, W.I.; Blumberg, R.S. Human Neonatal Fc Receptor Mediates Transport of IgG into Luminal Secretions for Delivery of Antigens to Mucosal Dendritic Cells. Immunity 2004, 20, 769–783. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, D.; Yang, Z.; Li, R.; Zhang, X.; Gao, F.; Yu, L. The Role of Immunoglobulin Transport Receptor, Neonatal Fc Receptor in Mucosal Infection and Immunity and Therapeutic Intervention. Int. Immunopharmacol. 2024, 138, 112583. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Pouwels, S.D.; Funke, A.; Bos, N.A.; Dijkstra, G. Crohn’s Disease Patients Have More IgG-Binding Fecal Bacteria than Controls. Clin. Vaccine Immunol. 2012, 19, 515–521. [Google Scholar] [CrossRef]
- Zheng, L.; Duan, S.L.; Dai, Y.C.; Wu, S.C. Role of Adherent Invasive Escherichia Coli in Pathogenesis of Inflammatory Bowel Disease. World J. Clin. Cases 2022, 10, 11671–11689. [Google Scholar] [CrossRef]
- Tanaka, R.; Imai, J.; Tsugawa, H.; Eap, K.B.; Yazawa, M.; Kaneko, M.; Ohno, M.; Sugihara, K.; Kitamoto, S.; Nagao-Kitamoto, H.; et al. Adherent-Invasive E. coli—Induced Specific IgA Limits Pathobiont Localization to the Epithelial Niche in the Gut. Front. Microbiol. 2023, 14, 1031997. [Google Scholar] [CrossRef]
- Zhang, W.; An, E.K.; Kim, S.J.; Park, H.B.; Lee, P.C.W.; Jin, J.O. Escherichia Coli Adhesion Protein FimH Exacerbates Colitis via CD11b+CD103− Dendritic Cell Activation. Front. Immunol. 2023, 14, 1284770. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased Proportions of Bifidobacterium and the Lactobacillus Group and Loss of Butyrate-Producing Bacteria in Inflammatory Bowel Disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.J.C.; Wang, Q.W.; Hu, Y.Y.; He, J.M.; Ge, Q.W.; Qi, Y.D.; Chen, L.Y.; Zhang, Y.; Fan, L.N.; Lin, Y.F.; et al. Lactobacillus Johnsonii Alleviates Colitis by TLR1/2-STAT3 Mediated CD206+ MacrophagesIL-10 Activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef]
- Diebolder, C.A.; Beurskens, F.J.; Jong, R.N.; De Koning, R.I.; Strumane, K.; Lindorfer, M.A.; Voorhorst, M.; Ugurlar, D.; Heck, A.J.R.; Van De Winkel, J.G.J.; et al. Complement Is Activated by IgG Hexamers Assembled at the Cell Surface. Science 2014, 343, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Strasser, J.; De Jong, R.N.; Beurskens, F.J.; Wang, G.; Heck, A.J.R.; Schuurman, J.; Parren, P.W.H.I.; Hinterdorfer, P.; Preiner, J. Unraveling the Macromolecular Pathways of IgG Oligomerization and Complement Activation on Antigenic Surfaces. Nano Lett. 2019, 19, 4787–4796. [Google Scholar] [CrossRef]
- Cruz, A.R.; den Boer, M.A.; Strasser, J.; Zwarthoff, S.A.; Beurskens, F.J.; de Haas, C.J.C.; Aerts, P.C.; Wang, G.; de Jong, R.N.; Bagnoli, F.; et al. Staphylococcal Protein A Inhibits Complement Activation by Interfering with IgG Hexamer Formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2016772118. [Google Scholar] [CrossRef]
- Chen, X.; Gula, H.; Pius, T.; Ou, C.; Gomozkova, M.; Wang, L.-X.; Schneewind, O.; Missiakas, D. Immunoglobulin G Subclasses Confer Protection against Staphylococcus Aureus Bloodstream Dissemination through Distinct Mechanisms in Mouse Models. Proc. Natl. Acad. Sci. USA 2017, 120, 2017. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Anderco, P.; Birsan, S. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Biomedicines 2023, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The Risk of Inflammatory Bowel Disease Flares after Fecal Microbiota Transplantation: Systematic Review and Meta-Analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
| UC | Non-IBD | p-Value *** | |
|---|---|---|---|
| Number of patients | 13 | 15 | - |
| %Male | 46 | 60 | 0.729 (a) |
| Age | 5 to 17 | 4 to 16 | 0.139 (a) |
| %Antibiotics | 30.8 | 6.67 | 0.346 (a) |
| Number of serum samples | 18 | 15 | - |
| Disease activity | |||
| Active | 9 | - | - |
| Remission | 9 | - | - |
| Numbers of samples (total) | 83 | 62 | - |
| Ascending colon | 17 | 15 | - |
| Transverse colon | 17 | 11 | - |
| Sigmoid colon | 18 | 14 | - |
| Rectum | 17 | 13 | - |
| Feces | 14 | 9 | - |
| Matts grade | |||
| 1 | 23 | - | - |
| 2 | 29 | - | - |
| 3 | 17 | - | - |
| Blood analysis | |||
| White blood cell * | 8989 ± 4093 | 6889 ± 4220 | 0.0247 |
| Hemoglobin * | 12.1 ± 1.61 | 13.8 ± 1.24 | 0.0014 |
| Hematocrit * | 36.5 ± 4.56 | 39.3 ± 3.29 | 0.0858 |
| Platelet * | 32.3 ±10.4 | 25.4 ± 5.20 | 0.0399 |
| ESR * | 17.0 ± 15.3 | 7.54 ± 5.05 | 0.123 |
| Serological analysis | |||
| Albumin * | 3.97 ± 0.777 | 4.51 ± 0.352 | 0.0236 |
| C-reactive protein ** | 0.04 (0.01–4.6) | 0.01 (0.01–0.19) | 0.11 |
| Serum amyloid A ** | 14.8 (0.3–354) | 1.6 (0.2–6.3) | 0.0971 |
| IgG * | 1330 ± 364.5 | 1050 ± 237.8 | 0.0222 |
| IgM * | 125 ± 74.2 | 107 ± 81.6 | 0.336 |
| IgA * | 166 ± 44.3 | 141 ± 50.6 | 0.232 |
| %FOB | 60 | 16.7 | 0.0473 (a) |
| Fecal calprotectin ** | 1515 (47.3–14,400) | 33.4 (7.6–456) | 0.0002 |
| Predictor | Estimate (β) 4 | Std. Error (SE) | 95% CI | Ratio (exp(β)) 3 | p-Value |
|---|---|---|---|---|---|
| (Intercept) 1 | 2.07 | 0.42 | [1.24, 2.90] | — | <0.001 |
| Disease activity: Remission | −1.77 | 0.55 | [−2.84, −0.69] | 0.17 | 0.001 |
| Clinical Trend: Improve | −3.04 | 0.55 | [−4.12, −1.95] | 0.05 | <0.001 |
| Interaction Term 2 | 4.36 | 0.78 | [2.83, 5.88] | 77.9 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabassum, N.; Nakayama-Imaohji, H.; Munyeshyaka, E.; Tada, A.; Kondo, T.; Kondo, S.; Kusaka, T.; Kuwahara, T. Reactivity of Autologous Serum IgG to Gut Microbes in Pediatric Ulcerative Colitis. Int. J. Mol. Sci. 2025, 26, 8196. https://doi.org/10.3390/ijms26178196
Tabassum N, Nakayama-Imaohji H, Munyeshyaka E, Tada A, Kondo T, Kondo S, Kusaka T, Kuwahara T. Reactivity of Autologous Serum IgG to Gut Microbes in Pediatric Ulcerative Colitis. International Journal of Molecular Sciences. 2025; 26(17):8196. https://doi.org/10.3390/ijms26178196
Chicago/Turabian StyleTabassum, Nafisa, Haruyuki Nakayama-Imaohji, Emmanuel Munyeshyaka, Ayano Tada, Takeo Kondo, Sonoko Kondo, Takashi Kusaka, and Tomomi Kuwahara. 2025. "Reactivity of Autologous Serum IgG to Gut Microbes in Pediatric Ulcerative Colitis" International Journal of Molecular Sciences 26, no. 17: 8196. https://doi.org/10.3390/ijms26178196
APA StyleTabassum, N., Nakayama-Imaohji, H., Munyeshyaka, E., Tada, A., Kondo, T., Kondo, S., Kusaka, T., & Kuwahara, T. (2025). Reactivity of Autologous Serum IgG to Gut Microbes in Pediatric Ulcerative Colitis. International Journal of Molecular Sciences, 26(17), 8196. https://doi.org/10.3390/ijms26178196






