The Role of Selected Myokines in the Development of Cardiovascular Diseases, and Their Involvement in Developing Heart Failure in Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Role of Selected Myokines in Cardiovascular Diseases
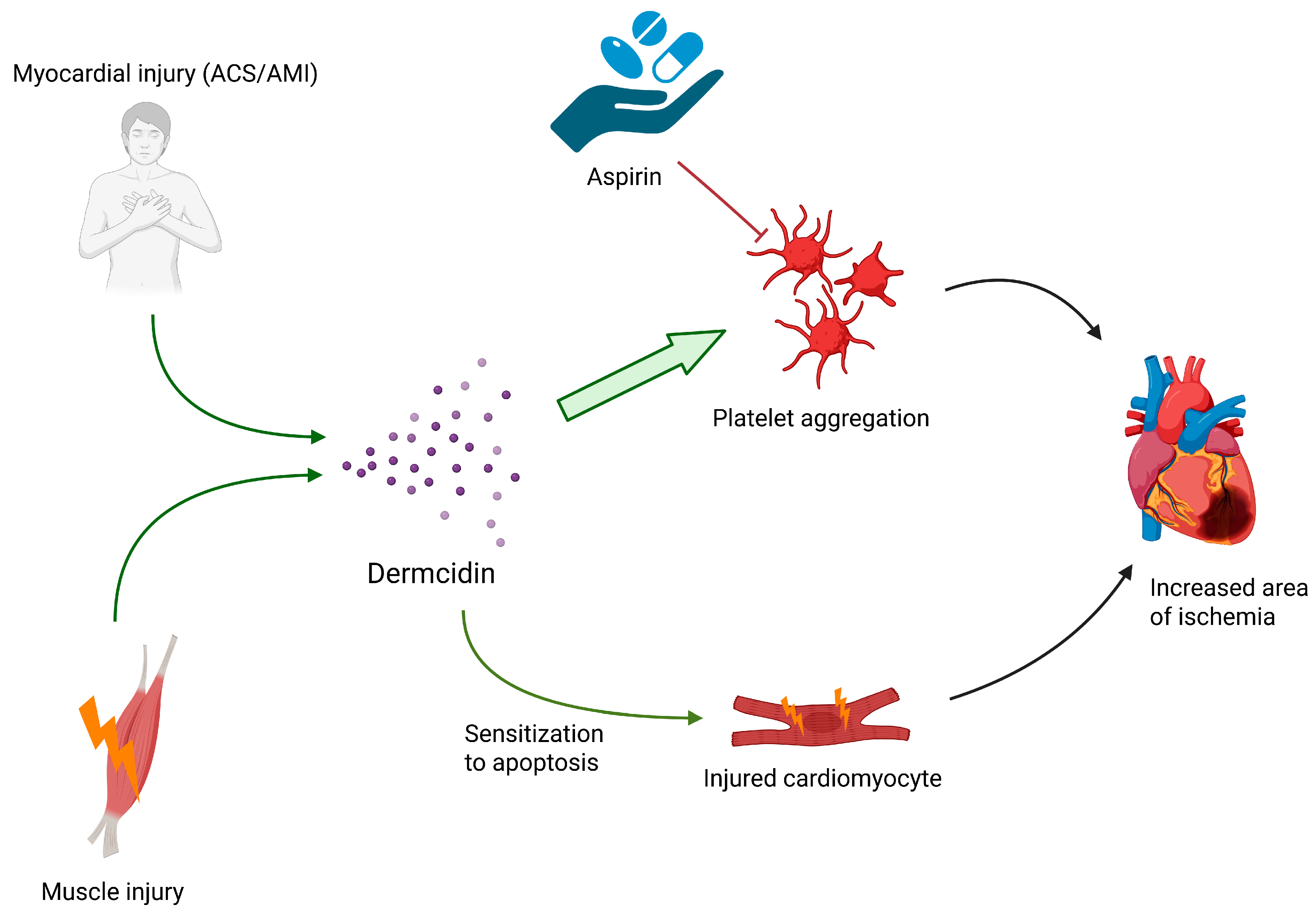
2.1. Dermcidin
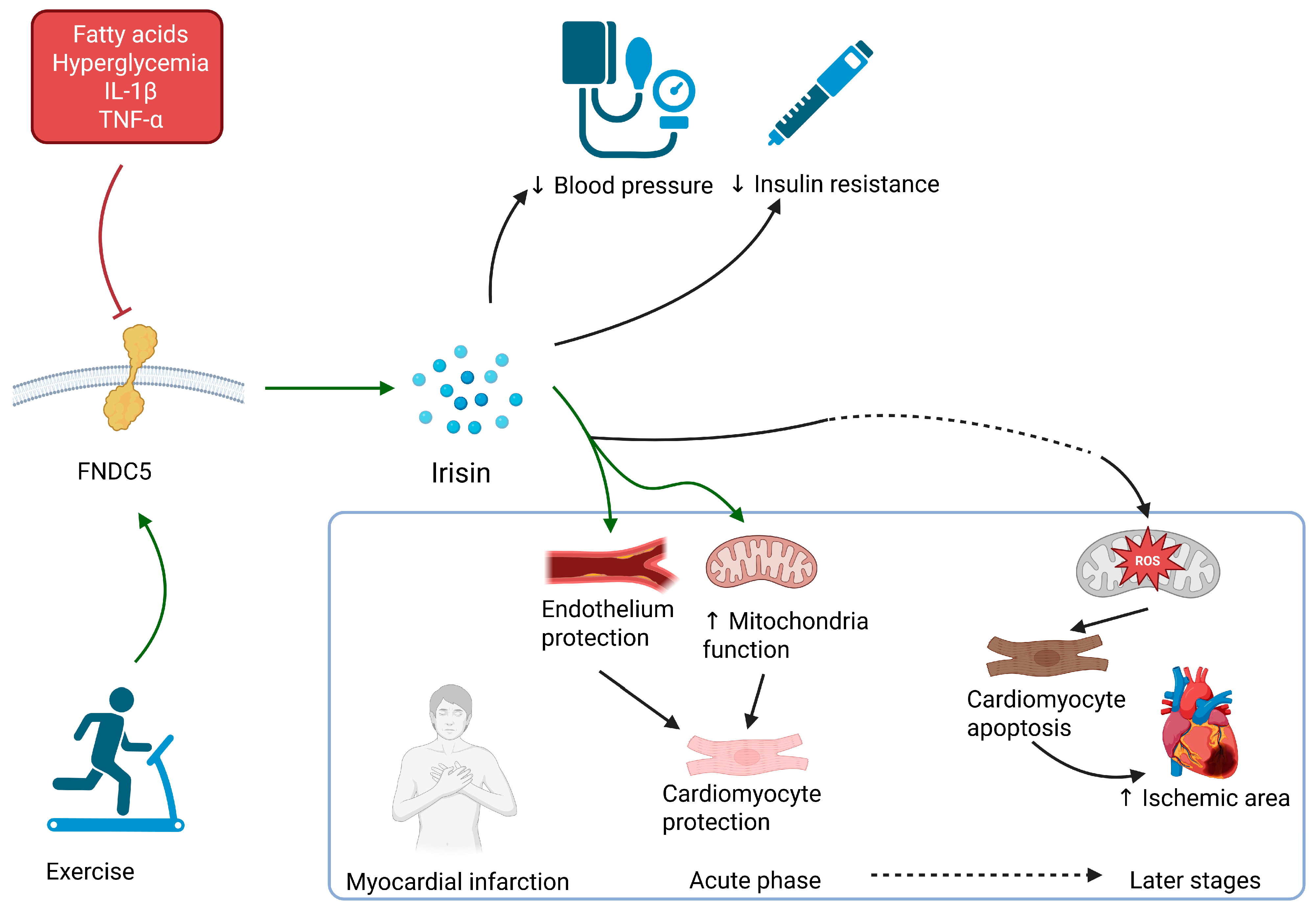
2.2. Irisin
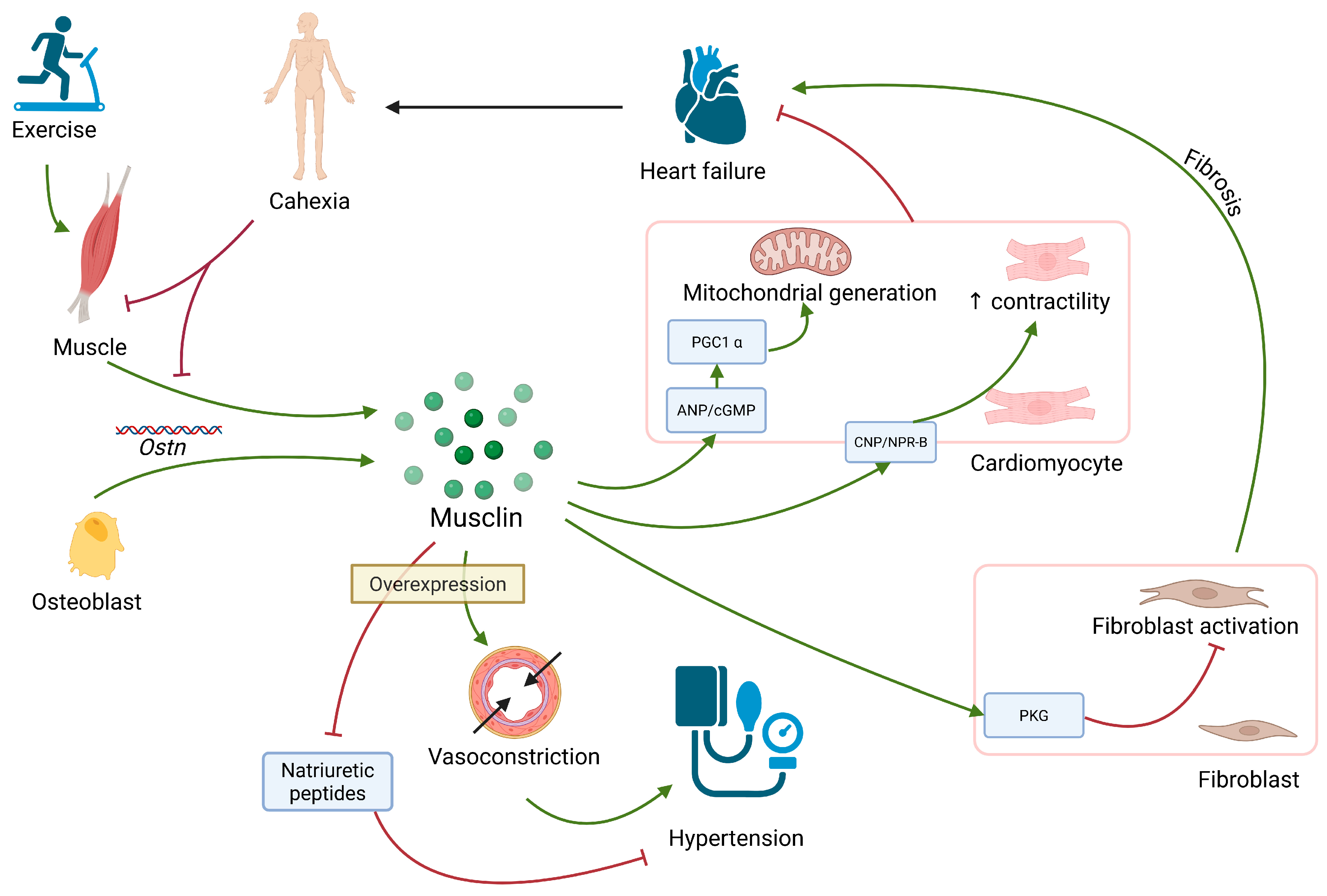
2.3. Musclin
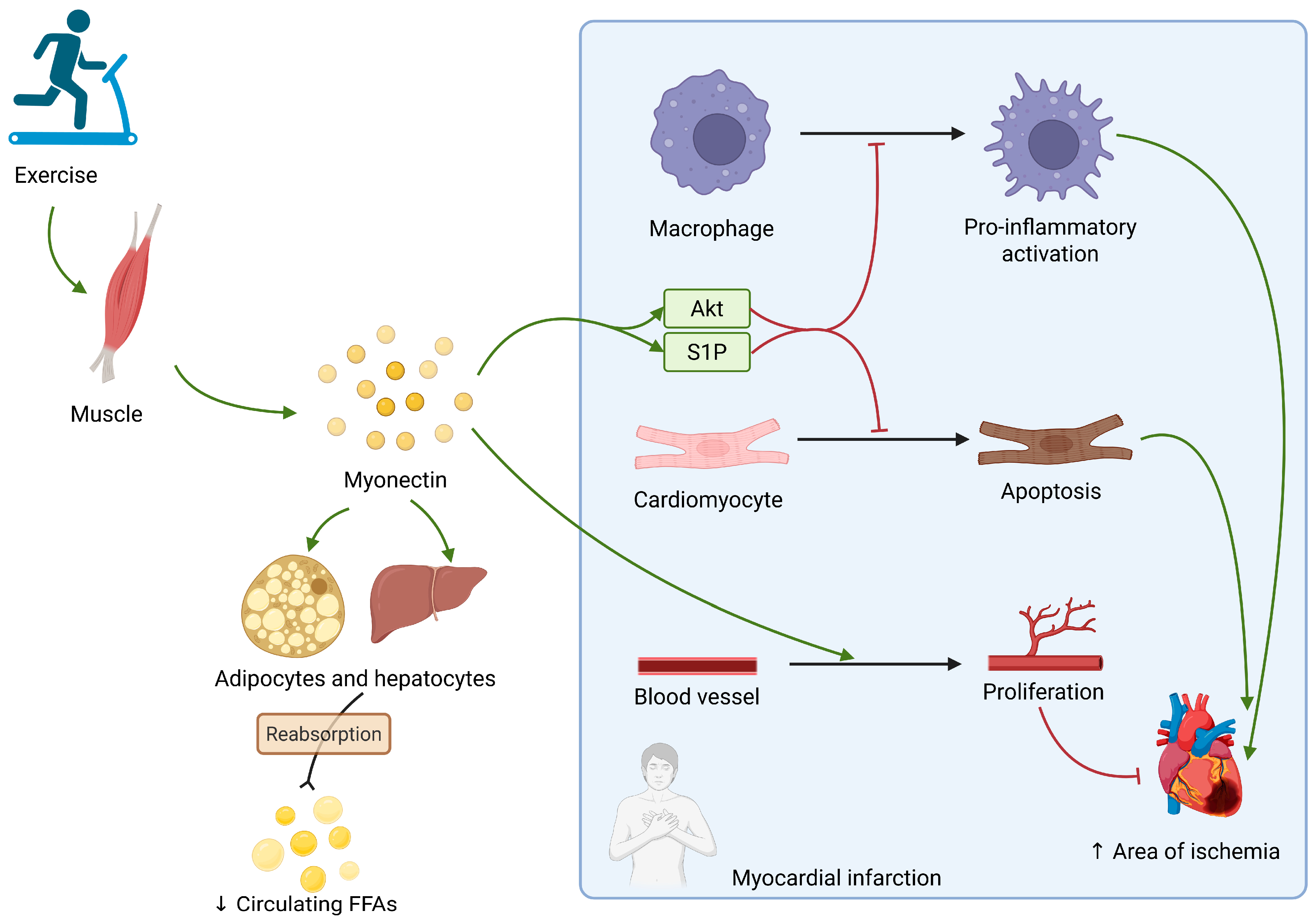
2.4. Myonectin
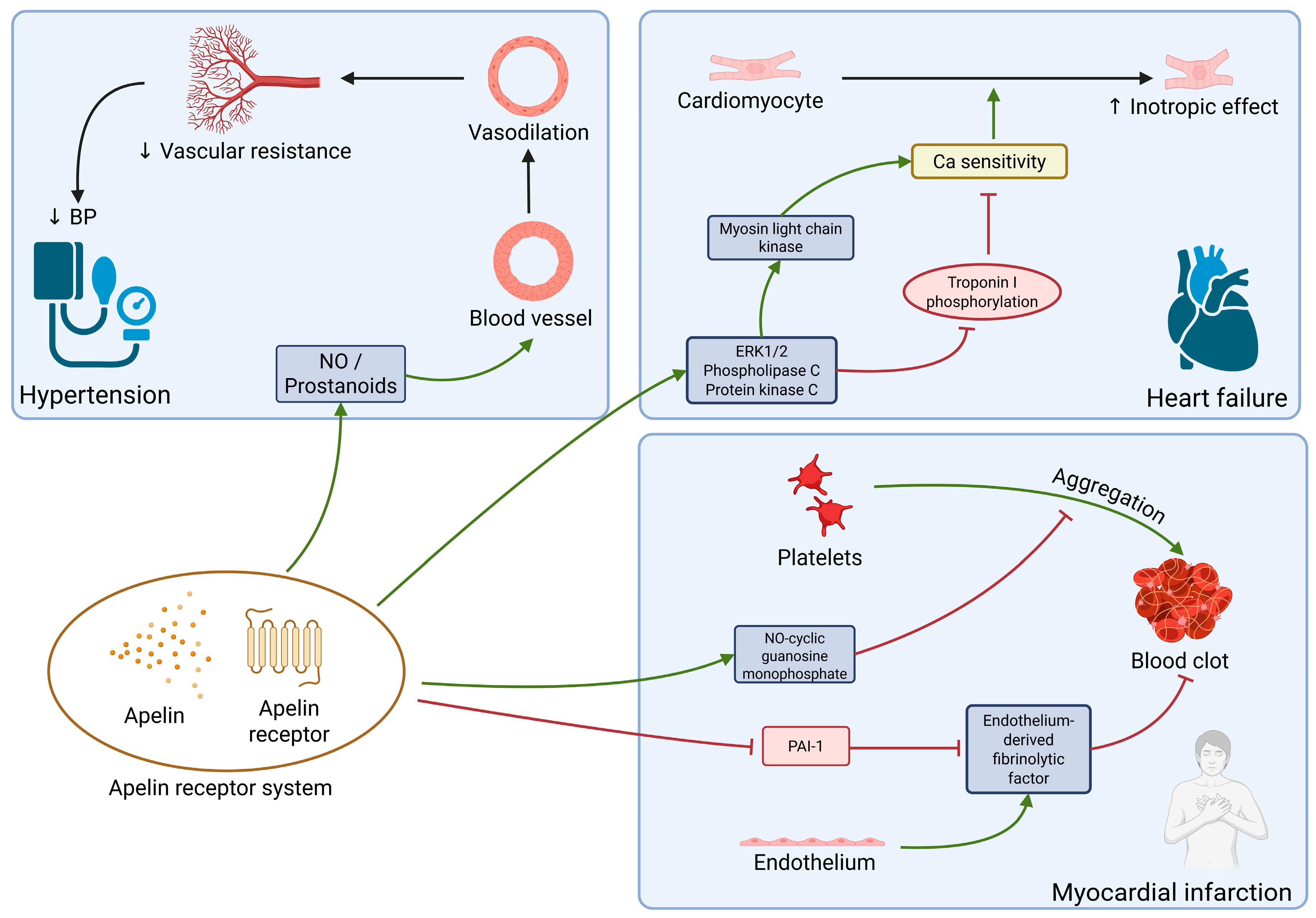
2.5. Apelin
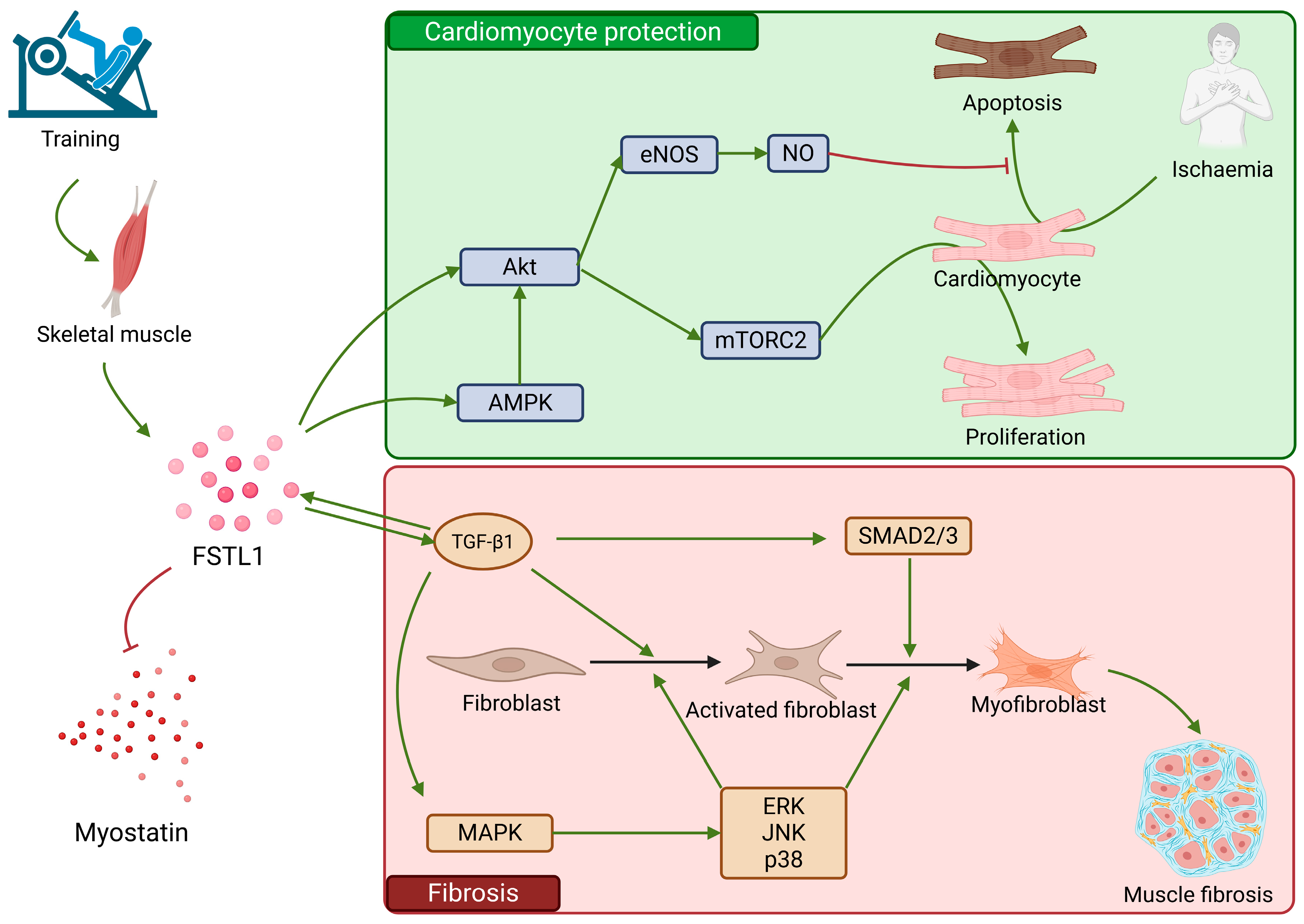
2.6. FSTL1
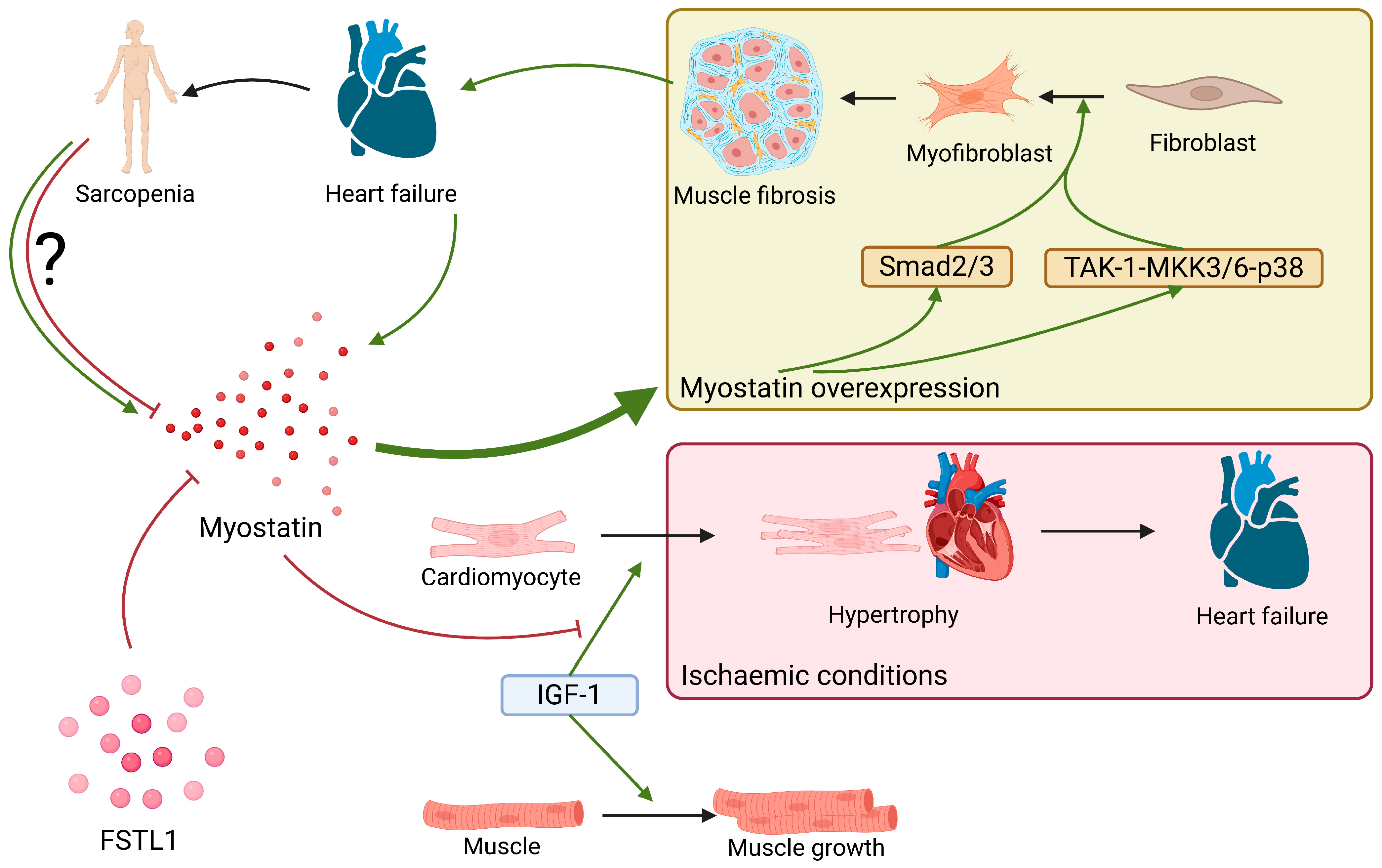
2.7. Myostatin
3. RA as a Risk Factor of Heart Failure and Sarcopenia
4. Materials and Methods
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609, Erratum in Endocr. Rev. 2021, 42, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Shimano, M.; Ouchi, N.; Walsh, K. Cardiokines: Recent progress in elucidating the cardiac secretome. Circulation 2012, 126, e327–e332. [Google Scholar] [CrossRef]
- Lin, J.-Z.; Ma, J.-D.; Yang, L.-J.; Zou, Y.-W.; Zhang, X.-P.; Pan, J.; Li, Q.-H.; Li, H.-G.; Yang, Z.-H.; Wu, T.; et al. Myokine myostatin is a novel predictor of one-year radiographic progression in patients with rheumatoid arthritis: A prospective cohort study. Front. Immunol. 2022, 13, 1005161. [Google Scholar] [CrossRef]
- Full Article: The Emerging Role of Irisin in Experimentally Induced Arthritis: A Recent Update Involving HMGB1/MCP1/Chitotriosidase I–Mediated Necroptosis. Available online: https://www.tandfonline.com/doi/full/10.1080/13510002.2022.2031516 (accessed on 9 July 2025).
- Gamal, R.M.; Mohamed, M.E.; Hammam, N.; El Fetoh, N.A.; Rashed, A.M.; Furst, D.E. Preliminary study of the association of serum irisin levels with poor sleep quality in rheumatoid arthritis patients. Sleep Med. 2020, 67, 71–76. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Maio, M.C.; Barbalho, S.M.; Guiguer, E.L.; Araújo, A.C.; de Alvares Goulart, R.; Flato, U.A.P.; Júnior, E.B.; Detregiachi, C.R.P.; Dos Santos Haber, J.F.; et al. Organokines in Rheumatoid Arthritis: A Critical Review. Int. J. Mol. Sci. 2022, 23, 6193. [Google Scholar] [CrossRef]
- Duan, J.; Zhu, B.; Wu, Y.; Chen, Z.; Yang, L. Myokines: An Available Biomarker to Evaluate Cardiac Functions? Cardiology 2019, 142, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell. Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.-C.; Ho, M.-Y.; Wen, M.-S.; Chen, C.-C.; Hsieh, M.-J.; Lin, C.-P.; Yeh, J.-K.; Tsai, M.-L.; Yang, C.-H.; Wu, V.C.-C.; et al. Serum irisin levels are associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Int. J. Cardiol. 2018, 261, 12–17. [Google Scholar] [CrossRef]
- Kohara, M.; Masuda, T.; Shiizaki, K.; Akimoto, T.; Watanabe, Y.; Honma, S.; Sekiguchi, C.; Miyazawa, Y.; Kusano, E.; Kanda, Y.; et al. Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLoS ONE 2017, 12, e0178971. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Luo, Y.; Chen, L.; Li, S.; Pan, Y.; Lei, X.; Wu, D.; Xu, D. Predictive value of serum myostatin for the severity and clinical outcome of heart failure. Eur. J. Intern. Med. 2019, 64, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-L.; Qiu, F.-H.; Dayarathna, T.K.; Wu, J.; Kuang, M.; Li, S.S.-C.; Peng, B.-G.; Nie, J. Identification of Dermcidin as a novel binding protein of Nck1 and characterization of its role in promoting cell migration. Biochim. Biophys. Acta 2011, 1812, 703–710. [Google Scholar] [CrossRef]
- Deans, D.A.C.; Wigmore, S.J.; Gilmour, H.; Tisdale, M.J.; Fearon, K.C.H.; Ross, J.A. Expression of the proteolysis-inducing factor core peptide mRNA is upregulated in both tumour and adjacent normal tissue in gastro-oesophageal malignancy. Br. J. Cancer 2006, 94, 731–736. [Google Scholar] [CrossRef]
- Stocki, P.; Wang, X.N.; Morris, N.J.; Dickinson, A.M. HSP70 natively and specifically associates with an N-terminal dermcidin-derived peptide that contains an HLA-A*03 antigenic epitope. J. Biol. Chem. 2011, 286, 12803–12811. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Skipworth, R.J.; Ross, J.A.; Fearon, K.C.; Baracos, V.E. The dermcidin gene in cancer: Role in cachexia, carcinogenesis and tumour cell survival. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 208–213. [Google Scholar] [CrossRef]
- Chang, W.C.; Huang, M.S.; Yang, C.J.; Wang, W.Y.; Lai, T.C.; Hsiao, M.; Chen, C.H. Dermcidin identification from exhaled air for lung cancer diagnosis. Eur. Respir. J. 2010, 35, 1182–1185. [Google Scholar] [CrossRef]
- Stewart, G.D.; Lowrie, A.G.; Riddick, A.C.P.; Fearon, K.C.H.; Habib, F.K.; Ross, J.A. Dermcidin expression confers a survival advantage in prostate cancer cells subjected to oxidative stress or hypoxia. Prostate 2007, 67, 1308–1317. [Google Scholar] [CrossRef]
- Esposito, G.; Schiattarella, G.G.; Perrino, C.; Cattaneo, F.; Pironti, G.; Franzone, A.; Gargiulo, G.; Magliulo, F.; Serino, F.; Carotenuto, G.; et al. Dermcidin: A skeletal muscle myokine modulating cardiomyocyte survival and infarct size after coronary artery ligation. Cardiovasc. Res. 2015, 107, 431–441. [Google Scholar] [CrossRef]
- Brevetti, G.; Piscione, F.; Schiano, V.; Galasso, G.; Scopacasa, F.; Chiariello, M. Concomitant coronary and peripheral arterial disease: Relationship between the inflammatory status of the affected limb and the severity of coronary artery disease. J. Vasc. Surg. 2009, 49, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Langer, R.D.; Fronek, A.; Feigelson, H.S.; Klauber, M.R.; McCann, T.J.; Browner, D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992, 326, 381–386. [Google Scholar] [CrossRef]
- Brevetti, G.; Piscione, F.; Cirillo, P.; Galasso, G.; Schiano, V.; Barbato, E.; Scopacasa, F.; Chiariello, M. In concomitant coronary and peripheral arterial disease, inflammation of the affected limbs predicts coronary artery endothelial dysfunction. Atherosclerosis 2008, 201, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bank, S.; Jana, P.; Maiti, S.; Guha, S.; Sinha, A.K. Dermcidin isoform-2 induced nullification of the effect of acetyl salicylic acid in platelet aggregation in acute myocardial infarction. Sci. Rep. 2014, 4, 5804. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, F.; Tang, Y.; Cai, L.; Zeng, C.; Yang, Y.; Yang, J. The Emerging Role of Irisin in Cardiovascular Diseases. J. Am. Heart Assoc. 2021, 10, e022453. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-Y.; Wang, C.-Y. Role of Irisin in Myocardial Infarction, Heart Failure, and Cardiac Hypertrophy. Cells 2021, 10, 2103. [Google Scholar] [CrossRef]
- Ferrer-Martínez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, İ.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Efe, T.H.; Açar, B.; Ertem, A.G.; Yayla, K.G.; Algül, E.; Yayla, Ç.; Ünal, S.; Bilgin, M.; Çimen, T.; Kirbaş, Ö.; et al. Serum Irisin Level Can Predict the Severity of Coronary Artery Disease in Patients with Stable Angina. Korean Circ. J. 2017, 47, 44–49. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, B.; Wang, X. Lower irisin levels in coronary artery disease: A meta-analysis. Minerva Endocrinol. 2020, 45, 61–69. [Google Scholar] [CrossRef]
- Khorasani, Z.M.; Bagheri, R.K.; Yaghoubi, M.A.; Chobkar, S.; Aghaee, M.A.; Abbaszadegan, M.R.; Sahebkar, A. The association between serum irisin levels and cardiovascular disease in diabetic patients. Diabetes Metab. Syndr. 2019, 13, 786–790. [Google Scholar] [CrossRef]
- Aronis, K.N.; Moreno, M.; Polyzos, S.A.; Moreno-Navarrete, J.M.; Ricart, W.; Delgado, E.; de la Hera, J.; Sahin-Efe, A.; Chamberland, J.P.; Berman, R.; et al. Circulating irisin levels and coronary heart disease: Association with future acute coronary syndrome and major adverse cardiovascular events. Int. J. Obes. 2015, 39, 156–161. [Google Scholar] [CrossRef]
- Bashar, S.M.; Samir El-Sherbeiny, S.M.; Boraie, M.Z. Correlation between the blood level of irisin and the severity of acute myocardial infarction in exercise-trained rats. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 59–71. [Google Scholar] [CrossRef]
- Abd El-Mottaleb, N.A.; Galal, H.M.; El Maghraby, K.M.; Gadallah, A.I. Serum irisin level in myocardial infarction patients with or without heart failure. Can. J. Physiol. Pharmacol. 2019, 97, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.T.; Zhang, S.; Dubielecka, P.M.; Du, J.; Yano, N.; Chin, Y.E.; Zhuang, S.; Qin, G.; Zhao, T.C. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell. Physiol. 2017, 232, 3775–3785. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Porter, G.A.; Hom, J.; Hoffman, D.; Quintanilla, R.; de Mesy Bentley, K.; Sheu, S.-S. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011, 31, 75–81. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 2012, 22, 98–105. [Google Scholar] [CrossRef]
- Oka, S.-I.; Sabry, A.D.; Cawley, K.M.; Warren, J.S. Multiple Levels of PGC-1α Dysregulation in Heart Failure. Front. Cardiovasc. Med. 2020, 7, 2. [Google Scholar] [CrossRef]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, A.K.; Cakmak, H.A.; Erturk, M.; Kalkan, K.E.; Uzun, F.; Tasbulak, O.; Diker, V.O.; Aydin, S.; Celik, A. Adropin and Irisin in Patients with Cardiac Cachexia. Arq. Bras. Cardiol. 2018, 111, 39–47. [Google Scholar] [CrossRef]
- Sobieszek, G.; Powrózek, T.; Mazurek, M.; Skwarek-Dziekanowska, A.; Małecka-Massalska, T. Electrical and Hormonal Biomarkers in Cachectic Elderly Women with Chronic Heart Failure. J. Clin. Med. 2020, 9, 1021. [Google Scholar] [CrossRef]
- Campbell, N.R.; Burgess, E.; Choi, B.C.; Taylor, G.; Wilson, E.; Cléroux, J.; Fodor, J.G.; Leiter, L.A.; Spence, D. Lifestyle modifications to prevent and control hypertension. 1. Methods and an overview of the Canadian recommendations. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ Can. Med. Assoc. J. 1999, 160, S1–S6. [Google Scholar]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Xie, Q.; Tang, C.-S.; Zhang, A.-H. Expressions of irisin and urotensin II and their relationships with blood pressure in patients with preeclampsia. Clin. Exp. Hypertens. 2017, 39, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.T.; Akkaya, N.; Erdamar, H.; Gok, S.; Kazanci, F.; Demircelik, B.; Cakmak, M.; Yigitoglu, R. The Effects of Valsartan and Amlodipine on the Levels of Irisin, Adropin, and Perilipin. Clin. Lab. 2015, 61, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-W.; Tsai, C.-C.; Chen, L.-J.; Niu, H.-S.; Chang, C.K.; Niu, C.-S. Characterization of musclin as a new target for treatment of hypertension. BioMed Res. Int. 2014, 2014, 354348. [Google Scholar] [CrossRef]
- Li, Y.-X.; Cheng, K.-C.; Asakawa, A.; Kato, I.; Sato, Y.; Amitani, H.; Kawamura, N.; Cheng, J.-T.; Inui, A. Role of musclin in the pathogenesis of hypertension in rat. PLoS ONE 2013, 8, e72004. [Google Scholar] [CrossRef]
- Nishizawa, H.; Matsuda, M.; Yamada, Y.; Kawai, K.; Suzuki, E.; Makishima, M.; Kitamura, T.; Shimomura, I. Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chem. 2004, 279, 19391–19395. [Google Scholar] [CrossRef]
- Kita, S.; Nishizawa, H.; Okuno, Y.; Tanaka, M.; Yasui, A.; Matsuda, M.; Yamada, Y.; Shimomura, I. Competitive binding of musclin to natriuretic peptide receptor 3 with atrial natriuretic peptide. J. Endocrinol. 2009, 201, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Abbey-Hosch, S.; Dickey, D.M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 2006, 27, 47–72. [Google Scholar] [CrossRef]
- Harris, M.P.; Zeng, S.; Zhu, Z.; Lira, V.A.; Yu, L.; Hodgson-Zingman, D.M.; Zingman, L.V. Myokine Musclin Is Critical for Exercise-Induced Cardiac Conditioning. Int. J. Mol. Sci. 2023, 24, 6525. [Google Scholar] [CrossRef] [PubMed]
- Szaroszyk, M.; Kattih, B.; Martin-Garrido, A.; Trogisch, F.A.; Dittrich, G.M.; Grund, A.; Abouissa, A.; Derlin, K.; Meier, M.; Holler, T.; et al. Skeletal muscle derived Musclin protects the heart during pathological overload. Nat. Commun. 2022, 13, 149. [Google Scholar] [CrossRef]
- Karstoft, K.; Pedersen, B.K. Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef]
- Ogura, Y.; Ouchi, N.; Ohashi, K.; Shibata, R.; Kataoka, Y.; Kambara, T.; Kito, T.; Maruyama, S.; Yuasa, D.; Matsuo, K.; et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation 2012, 126, 1728–1738. [Google Scholar] [CrossRef]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef]
- Seldin, M.M.; Lei, X.; Tan, S.Y.; Stanson, K.P.; Wei, Z.; Wong, G.W. Skeletal muscle-derived myonectin activates the mammalian target of rapamycin (mTOR) pathway to suppress autophagy in liver. J. Biol. Chem. 2013, 288, 36073–36082. [Google Scholar] [CrossRef]
- Fujio, Y.; Nguyen, T.; Wencker, D.; Kitsis, R.N.; Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 2000, 101, 660–667. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002, 277, 32124–32132. [Google Scholar] [CrossRef]
- Masutomi, T.; Ouchi, N.; Yamaguchi, S.; Ohashi, K.; Otaka, N.; Pu, Z.; Shimizu, Y.; Murohara, T.; Shibata, R. Myonectin stimulates endothelial angiogenic activity in vitro and in vivo. Nagoya J. Med. Sci. 2025, 87, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Wang, W.; Jin, H.-Y.; Chen, X.; Cheng, Y.-W.; Xu, Y.-L.; Song, B.; Penninger, J.M.; Oudit, G.Y.; Zhong, J.-C. Apelin Is a Negative Regulator of Angiotensin II-Mediated Adverse Myocardial Remodeling and Dysfunction. Hypertension 2017, 70, 1165–1175. [Google Scholar] [CrossRef]
- Zhong, J.-C.; Yu, X.-Y.; Huang, Y.; Yung, L.-M.; Lau, C.-W.; Lin, S.-G. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc. Res. 2007, 74, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Zhang, L.; Imai, Y.; Arab, S.; Chen, M.; Maekawa, Y.; Leschnik, M.; Leibbrandt, A.; Markovic, M.; Schwaighofer, J.; et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 2007, 101, e32–e42, Erratum in Circ. Res. 2008, 102, e36. [Google Scholar] [CrossRef] [PubMed]
- Japp, A.G.; Cruden, N.L.; Barnes, G.; van Gemeren, N.; Mathews, J.; Adamson, J.; Johnston, N.R.; Denvir, M.A.; Megson, I.L.; Flapan, A.D.; et al. Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure. Circulation 2010, 121, 1818–1827. [Google Scholar] [CrossRef]
- Schinzari, F.; Veneziani, A.; Mores, N.; Barini, A.; Di Daniele, N.; Cardillo, C.; Tesauro, M. Beneficial Effects of Apelin on Vascular Function in Patients With Central Obesity. Hypertension 2017, 69, 942–949. [Google Scholar] [CrossRef]
- Chapman, F.A.; Maguire, J.J.; Newby, D.E.; Davenport, A.P.; Dhaun, N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc. Res. 2023, 119, 2683–2696. [Google Scholar] [CrossRef]
- Charo, D.N.; Ho, M.; Fajardo, G.; Kawana, M.; Kundu, R.K.; Sheikh, A.Y.; Finsterbach, T.P.; Leeper, N.J.; Ernst, K.V.; Chen, M.M.; et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1904–H1913. [Google Scholar] [CrossRef]
- Freyer, L.; Hsu, C.-W.; Nowotschin, S.; Pauli, A.; Ishida, J.; Kuba, K.; Fukamizu, A.; Schier, A.F.; Hoodless, P.A.; Dickinson, M.E.; et al. Loss of Apela Peptide in Mice Causes Low Penetrance Embryonic Lethality and Defects in Early Mesodermal Derivatives. Cell Rep. 2017, 20, 2116–2130. [Google Scholar] [CrossRef]
- Zhong, J.-C.; Zhang, Z.-Z.; Wang, W.; McKinnie, S.M.K.; Vederas, J.C.; Oudit, G.Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1942–1950. [Google Scholar] [CrossRef]
- Ye, L.; Ding, F.; Zhang, L.; Shen, A.; Yao, H.; Deng, L.; Ding, Y. Serum apelin is associated with left ventricular hypertrophy in untreated hypertension patients. J. Transl. Med. 2015, 13, 290. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Amer, D.A.B.; Li, V.K.Y.; Goudie, E.B.; Johnston, N.R.; Sharma, S.; Neilson, I.; Webb, D.J.; Megson, I.L.; et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008, 52, 908–913. [Google Scholar] [CrossRef]
- Najafipour, H.; Vakili, A.; Shahouzehi, B.; Soltani Hekmat, A.; Masoomi, Y.; Yeganeh Hajahmadi, M.; Esmaeli-Mahani, S. Investigation of changes in apelin receptor mRNA and protein expression in the myocardium and aorta of rats with two-kidney, one-clip (2K1C) Goldblatt hypertension. J. Physiol. Biochem. 2015, 71, 165–175. [Google Scholar] [CrossRef]
- Marsault, E.; Llorens-Cortes, C.; Iturrioz, X.; Chun, H.J.; Lesur, O.; Oudit, G.Y.; Auger-Messier, M. The apelinergic system: A perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 2019, 1455, 12–33. [Google Scholar] [CrossRef]
- Maguire, J.J.; Kleinz, M.J.; Pitkin, S.L.; Davenport, A.P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease. Hypertension 2009, 54, 598–604. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Lampropoulos, S.; Kapelouzou, A.; Gkontopoulos, A.; Theofilogiannakos, E.K.; Fotiadis, G.; Kottas, G. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease—KOZANI STUDY. Transl. Res. J. Lab. Clin. Med. 2010, 155, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Khatib, A.-M.; Lopez, J.J.; Vatier, C.; Turpin, S.; Muscat, A.; Soulet, F.; Aries, A.; Jardin, I.; Bobe, R.; et al. Apelin: An antithrombotic factor that inhibits platelet function. Blood 2016, 127, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, K.; Hampton, J.; Khan, S.; Zadory, D.; Gleaves, L.; Vaughan, D.E.; Smith, L.H. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 2011, 29, 724–731. [Google Scholar] [CrossRef]
- Perjés, Á.; Skoumal, R.; Tenhunen, O.; Kónyi, A.; Simon, M.; Horváth, I.G.; Kerkelä, R.; Ruskoaho, H.; Szokodi, I. Apelin increases cardiac contractility via protein kinase Cε- and extracellular signal-regulated kinase-dependent mechanisms. PLoS ONE 2014, 9, e93473. [Google Scholar] [CrossRef] [PubMed]
- Perjés, Á.; Kilpiö, T.; Ulvila, J.; Magga, J.; Alakoski, T.; Szabó, Z.; Vainio, L.; Halmetoja, E.; Vuolteenaho, O.; Petäjä-Repo, U.; et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res. Cardiol. 2016, 111, 2. [Google Scholar] [CrossRef]
- Chen, M.M.; Ashley, E.A.; Deng, D.X.F.; Tsalenko, A.; Deng, A.; Tabibiazar, R.; Ben-Dor, A.; Fenster, B.; Yang, E.; King, J.Y.; et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003, 108, 1432–1439. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Hu, Z.; Li, L.; Yang, Y. Follistatin-like 1: A dual regulator that promotes cardiomyocyte proliferation and fibrosis. J. Cell. Physiol. 2020, 235, 5893–5902. [Google Scholar] [CrossRef]
- Mattiotti, A.; Prakash, S.; Barnett, P.; van den Hoff, M.J.B. Follistatin-like 1 in development and human diseases. Cell. Mol. Life Sci. 2018, 75, 2339–2354. [Google Scholar] [CrossRef]
- Görgens, S.W.; Raschke, S.; Holven, K.B.; Jensen, J.; Eckardt, K.; Eckel, J. Regulation of Follistatin-Like Protein 1 Expression and Secretion in Primary Human Skeletal Muscle Cells. Arch. Physiol. Biochem. 2013, 119, 75–80. Available online: https://www.tandfonline.com/doi/full/10.3109/13813455.2013.768270 (accessed on 9 July 2025).
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise Induces a Marked Increase in Plasma Follistatin: Evidence That Follistatin Is a Contraction-Induced Hepatokine. Endocrinology 2011, 152, 164–171. [Google Scholar] [CrossRef]
- Achttien, R.J.; Staal, J.B.; van der Voort, S.; Kemps, H.M.; Koers, H.; Jongert, M.W.A.; Hendriks, E.J.M.; Practice Recommendations Development Group. Exercise-based cardiac rehabilitation in patients with chronic heart failure: A Dutch practice guideline. Neth. Heart J. 2015, 23, 6–17. [Google Scholar] [CrossRef]
- Jin, Y.-K.; Li, X.-H.; Wang, W.; Liu, J.; Zhang, W.; Fang, Y.-S.; Zhang, Z.-F.; Dai, H.-P.; Ning, W.; Wang, C. Follistatin-Like 1 Promotes Bleomycin-Induced Pulmonary Fibrosis through the Transforming Growth Factor Beta 1/Mitogen-Activated Protein Kinase Signaling Pathway. Chin. Med. J. 2018, 131, 1917. [Google Scholar] [CrossRef]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cuñado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Asaumi, Y.; Ohashi, K.; Higuchi, A.; Sono-Romanelli, S.; Oshima, Y.; Walsh, K. DIP2A Functions as a FSTL1 Receptor *. J. Biol. Chem. 2010, 285, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Young, L.H. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol. Metab. 2015, 26, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef]
- Gao, F.; Gao, E.; Yue, T.-L.; Ohlstein, E.H.; Lopez, B.L.; Christopher, T.A.; Ma, X.-L. Nitric Oxide Mediates the Antiapoptotic Effect of Insulin in Myocardial Ischemia-Reperfusion. Circulation 2002, 105, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Sibinska, Z.; Tian, X.; Korfei, M.; Kojonazarov, B.; Kolb, J.S.; Klepetko, W.; Kosanovic, D.; Wygrecka, M.; Ghofrani, H.A.; Weissmann, N.; et al. Amplified canonical transforming growth factor-β signalling via heat shock protein 90 in pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1501941. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.-J.; et al. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Dong, Y.; Geng, Y.; Li, L.; Li, X.; Yan, X.; Fang, Y.; Li, X.; Dong, S.; Liu, X.; Li, X.; et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J. Exp. Med. 2015, 212, 235–252. [Google Scholar] [CrossRef]
- Maruyama, S.; Nakamura, K.; Papanicolaou, K.N.; Sano, S.; Shimizu, I.; Asaumi, Y.; van den Hoff, M.J.; Ouchi, N.; Recchia, F.A.; Walsh, K. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol. Med. 2016, 8, 949–966. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S.; Bunner, W.P.; Chang, Y.; Shi, R. Exercise induced Right Ventricular Fibrosis is Associated with Myocardial Damage and Inflammation. Korean Circ. J. 2018, 48, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Nikooie, R.; Samaneh, S. Exercise-induced lactate accumulation regulates intramuscular triglyceride metabolism via transforming growth factor-β1 mediated pathways. Mol. Cell. Endocrinol. 2016, 419, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.D.; Heineke, J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1973–H1982. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Biesemann, N.; Mendler, L.; Kostin, S.; Wietelmann, A.; Borchardt, T.; Braun, T. Myostatin induces interstitial fibrosis in the heart via TAK1 and p38. Cell Tissue Res. 2015, 361, 779–787. [Google Scholar] [CrossRef]
- Springer, J.; Springer, J.-I.; Anker, S.D. Muscle wasting and sarcopenia in heart failure and beyond: Update 2017. ESC Heart Fail. 2017, 4, 492–498. [Google Scholar] [CrossRef]
- Baán, J.A.; Varga, Z.V.; Leszek, P.; Kuśmierczyk, M.; Baranyai, T.; Dux, L.; Ferdinandy, P.; Braun, T.; Mendler, L. Myostatin and IGF-I signaling in end-stage human heart failure: A qRT-PCR study. J. Transl. Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Rossignol, P.; Masson, S.; Barlera, S.; Girerd, N.; Castelnovo, A.; Zannad, F.; Clemenza, F.; Tognoni, G.; Anand, I.S.; Cohn, J.N.; et al. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: Insights from the GISSI-HF and Val-HeFT trials. Eur. J. Heart Fail. 2015, 17, 424–433. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Bish, L.T.; Kamalakkannan, G.; Petrilli, C.M.; Oz, M.C.; Naka, Y.; Sweeney, H.L.; Maybaum, S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur. J. Heart Fail. 2010, 12, 444–453. [Google Scholar] [CrossRef]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Takada, S.; Homma, T.; Masaki, Y.; Abe, T.; Yokota, T.; Oba, K.; Okita, K.; et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int. J. Cardiol. 2016, 220, 483–487. [Google Scholar] [CrossRef]
- Bączek, J.; Charkiewicz, M.; Kasiukiewicz, A.; Witkowska, A.M.; Magnuszewski, Ł.; Bączek, M.; Wojszel, Z.B. Evaluation of Serum Myostatin Concentration in Chronic Heart Failure with Preserved and Impaired Left Ventricular Ejection Fraction. J. Clin. Med. 2024, 13, 1741. [Google Scholar] [CrossRef]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef]
- Lim, S.; McMahon, C.D.; Matthews, K.G.; Devlin, G.P.; Elston, M.S.; Conaglen, J.V. Absence of Myostatin Improves Cardiac Function Following Myocardial Infarction. Heart Lung Circ. 2018, 27, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Andonian, B.J.; Huffman, K.M. Skeletal muscle disease in rheumatoid arthritis: The center of cardiometabolic comorbidities? Curr. Opin. Rheumatol. 2020, 32, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Bag-Ozbek, A.; Giles, J.T. Inflammation, adiposity, and atherogenic dyslipidemia in rheumatoid arthritis: Is there a paradoxical relationship? Curr. Allergy Asthma Rep. 2015, 15, 497. [Google Scholar] [CrossRef]
- Giles, J.T.; Wasko, M.C.M.; Chung, C.P.; Szklo, M.; Blumenthal, R.S.; Kao, A.; Bokhari, S.; Zartoshti, A.; Stein, C.M.; Bathon, J.M. Exploring the Lipid Paradox Theory in Rheumatoid Arthritis: Associations of Low Circulating Low-Density Lipoprotein Concentration With Subclinical Coronary Atherosclerosis. Arthritis Rheumatol. 2019, 71, 1426–1436. [Google Scholar] [CrossRef]
- Kadier, K.; Dilixiati, D.; Zhang, X.; Li, H.; Kuang, L.; Huang, J.; Cai, X.; Ling, T.; Kong, F.; Liu, X. Rheumatoid arthritis increases the risk of heart failure: Results from the cross-sectional study in the US population and mendelian randomization analysis in the European population. Front. Immunol. 2024, 15, 1377432. [Google Scholar] [CrossRef]
- Mantel, Ä.; Holmqvist, M.; Andersson, D.C.; Lund, L.H.; Askling, J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1275–1285. [Google Scholar] [CrossRef]
- Miró, O.; Pedrol, E.; Casademont, J.; García-Carrasco, M.; Sanmartí, R.; Cebrián, M.; Grau, J.M. Muscle involvement in rheumatoid arthritis: Clinicopathological study of 21 symptomatic cases. Semin. Arthritis Rheum. 1996, 25, 421–428. [Google Scholar] [CrossRef]
- Halla, J.T.; Koopman, W.J.; Fallahi, S.; Oh, S.J.; Gay, R.E.; Schrohenloher, R.E. Rheumatoid myositis. Clinical and histologic features and possible pathogenesis. Arthritis Rheum. 1984, 27, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Touno, M.; Senda, M.; Nakago, K.; Yokoyama, Y.; Inoue, H. Muscle fiber changes of the vastus medialis in rheumatoid patients. Acta Med. Okayama 1996, 50, 157–164. [Google Scholar] [CrossRef]
- Beenakker, K.G.M.; Duijnisveld, B.J.; Van Der Linden, H.M.J.; Visser, C.P.J.; Westendorp, R.G.J.; Butler-Brown, G.; Nelissen, R.G.H.H.; Maier, A.B. Muscle characteristics in patients with chronic systemic inflammation. Muscle Nerve 2012, 46, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Boutrup, R.J.; Farup, J.; Vissing, K.; Kjaer, M.; Mikkelsen, U.R. Skeletal muscle stem cell characteristics and myonuclei content in patients with rheumatoid arthritis: A cross-sectional study. Rheumatol. Int. 2018, 38, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Mostoufi-Moab, S.; Long, J.; Zemel, B.; Ibrahim, S.; Taratuta, E.; Leonard, M.B. Intramuscular Fat Accumulation and Associations With Body Composition, Strength, and Physical Functioning in Patients With Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Ling, S.M.; Ferrucci, L.; Bartlett, S.J.; Andersen, R.E.; Towns, M.; Muller, D.; Fontaine, K.R.; Bathon, J.M. Abnormal body composition phenotypes in older rheumatoid arthritis patients: Association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008, 59, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.C.; Hizmetli, S.; Hayta, E.; Kaptanoğlu, E.; Erselcan, T.; Güler, E. Sarcopenia in women with rheumatoid arthritis. Eur. J. Rheumatol. 2015, 2, 57–61. [Google Scholar] [CrossRef]
- Baker, J.F.; Giles, J.T.; Weber, D.; Leonard, M.B.; Zemel, B.S.; Long, J.; Ibrahim, S.; Katz, P.P. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology 2017, 56, 981–988. [Google Scholar] [CrossRef]
- Khoja, S.S.; Patterson, C.G.; Goodpaster, B.H.; Delitto, A.; Piva, S.R. Skeletal muscle fat in individuals with rheumatoid arthritis compared to healthy adults. Exp. Gerontol. 2020, 129, 110768. [Google Scholar] [CrossRef]
- Baker, J.F.; Mostoufi-Moab, S.; Long, J.; Taratuta, E.; Leonard, M.B.; Zemel, B. Association of Low Muscle Density With Deteriorations in Muscle Strength and Physical Functioning in Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 355–363. [Google Scholar] [CrossRef]
- Yun, H.-W.; Kim, C.-J.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H.; Jung, J.-Y. The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 3458. [Google Scholar] [CrossRef]
| Myokine | Organ of Origin | Myocardial Injury | Heart Failure | Arterial Hypertension |
|---|---|---|---|---|
| Dermcidin | Sweat glands, skeletal muscle | Increased area of ischemia (induction of apoptosis of injured muscle cells) | - | - |
| Irisin | Skeletal muscle, adipose tissue, myocardium | Protection from apoptosis in early stages, induction of apoptosis in later stages | Downregulated in HFrEF, which may lead to cardiac hypertrophy | Lowering of blood pressure |
| Musclin | Skeletal muscle, osteoblasts, arteries | - | Protection from fibrosis. Improvement of cardiomyocyte function | Overexpression leads to induction of hypertension |
| Myonectin | Skeletal muscle, adipose tissue | Inhibition of cardiomyocyte apoptosis. Improvement of endothelial function | Downregulated in developing HF | - |
| Apelin | Brain, stomach, adipose tissue | Induction of antiplatelet effects. Improvement of endothelial function | Prevention of myocardium hypertrophy. Inotropic effect. Downregulation in HF leads to fibrosis | Antihypertensive mechanisms: diuresis induction, dilation of blood vessels |
| FSTL1 | Numerous tissues, including myocardium and endothelium | Protection from cardiomyocyte apoptosis, induction of myocyte proliferation | Involvement in profibrotic mechanisms | - |
| Myostatin | Myocardium, skeletal muscle, adipose tissue | Prevention of HF development in ischaemic conditions | Upregulated in HF, induces myocardial fibrosis | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuna, J.; Chmielewski, G.; Jaśkiewicz, Ł.; Knapik, M.; Krajewska-Włodarczyk, M. The Role of Selected Myokines in the Development of Cardiovascular Diseases, and Their Involvement in Developing Heart Failure in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2025, 26, 8194. https://doi.org/10.3390/ijms26178194
Kuna J, Chmielewski G, Jaśkiewicz Ł, Knapik M, Krajewska-Włodarczyk M. The Role of Selected Myokines in the Development of Cardiovascular Diseases, and Their Involvement in Developing Heart Failure in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2025; 26(17):8194. https://doi.org/10.3390/ijms26178194
Chicago/Turabian StyleKuna, Jakub, Grzegorz Chmielewski, Łukasz Jaśkiewicz, Michalina Knapik, and Magdalena Krajewska-Włodarczyk. 2025. "The Role of Selected Myokines in the Development of Cardiovascular Diseases, and Their Involvement in Developing Heart Failure in Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 26, no. 17: 8194. https://doi.org/10.3390/ijms26178194
APA StyleKuna, J., Chmielewski, G., Jaśkiewicz, Ł., Knapik, M., & Krajewska-Włodarczyk, M. (2025). The Role of Selected Myokines in the Development of Cardiovascular Diseases, and Their Involvement in Developing Heart Failure in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 26(17), 8194. https://doi.org/10.3390/ijms26178194







