Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications
Abstract
1. Introduction
2. PCOS, Autoimmune Diseases, and Related Autoantibodies—Current Evidence
2.1. Autoimmune Thyroiditis
2.2. Graves’ Disease
2.3. Type 1 Diabetes Mellitus
2.4. Antinuclear Antibody-Related Diseases
2.5. Beyond Organ-Specific Autoimmunity
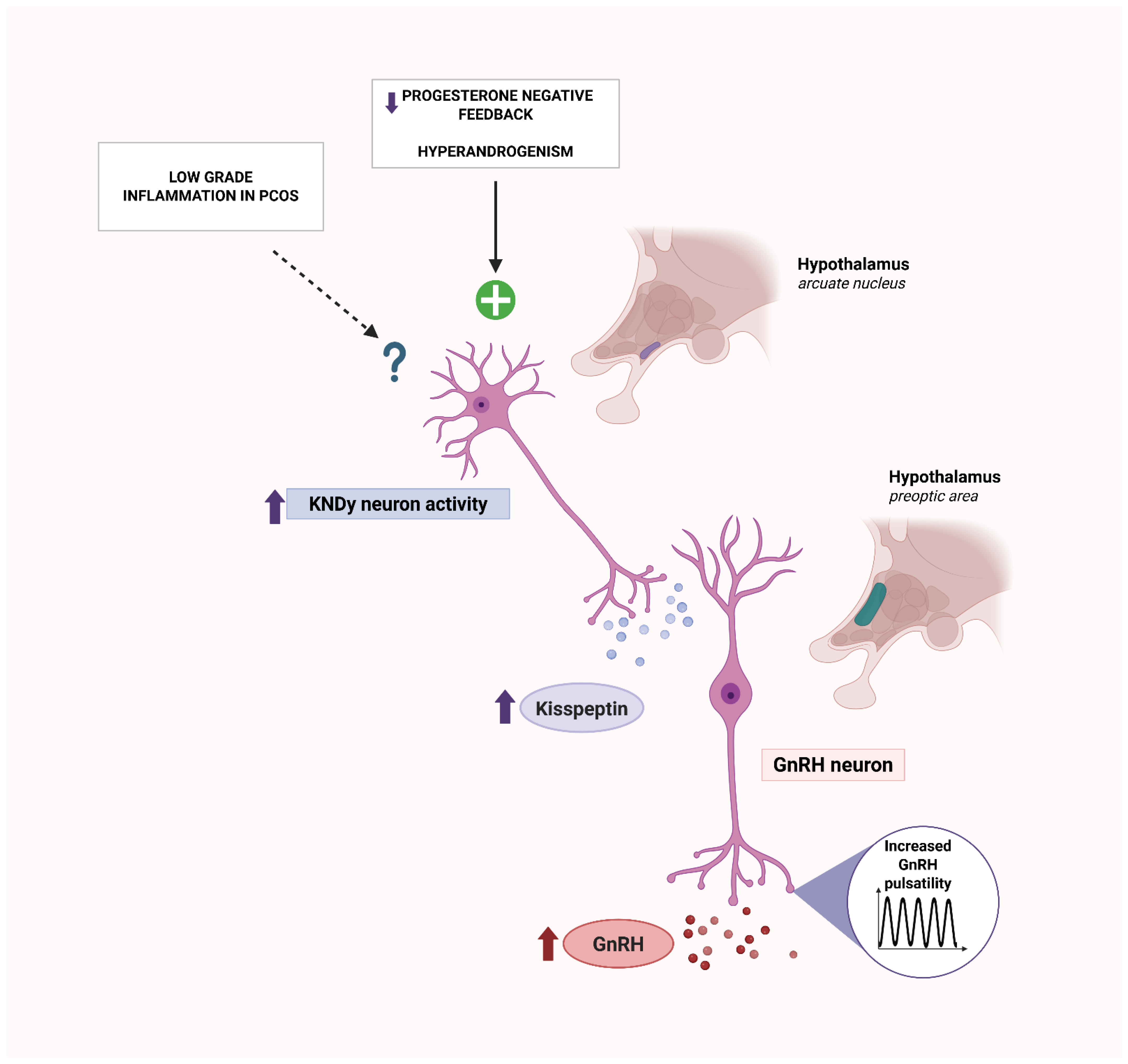
2.6. Autoantibodies Targeting Hypothalamic–Pituitary–Ovarian Axis
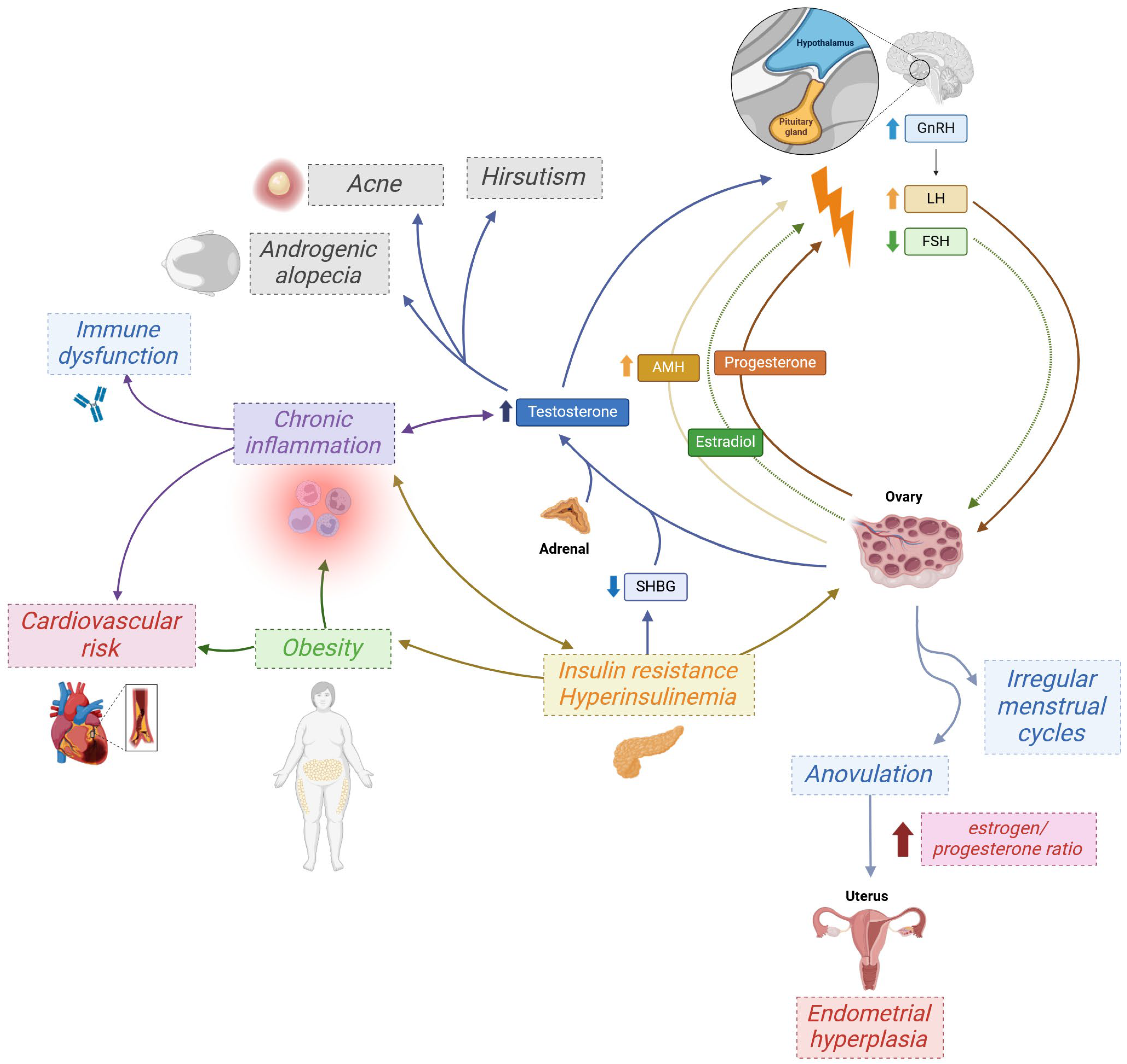
3. PCOS as a Chronic Low-Grade Inflammatory Disease
4. Autoantibodies as Markers of Inflammation
4.1. Mechanisms of Autoantibody Production: Insights from Rheumatoid Arthritis and Systemic Lupus Erythematosus
4.2. Increased B-Cell Activity in PCOS
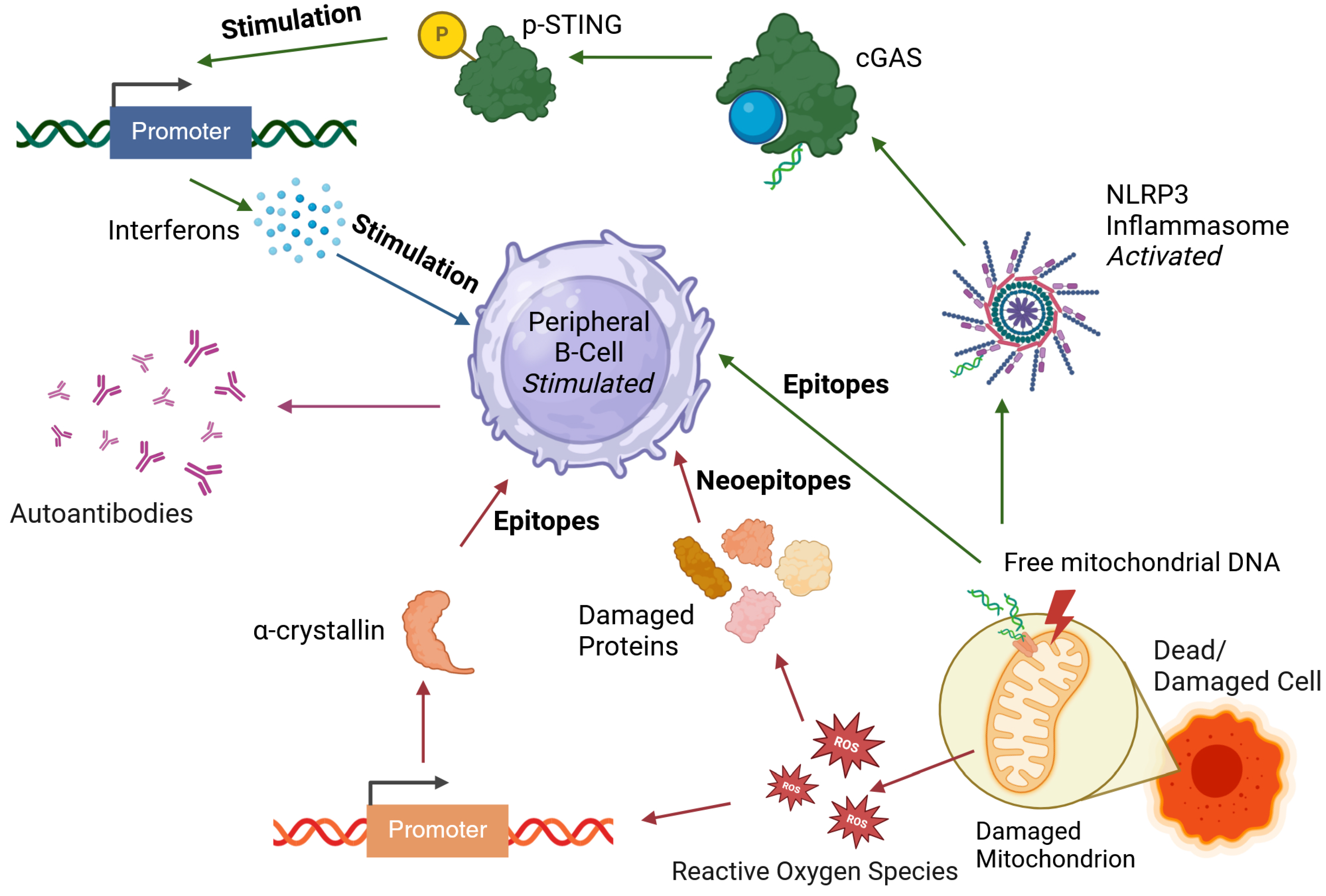
4.3. Oxidative Stress
4.4. Release of Nuclear and Mitochondrial Antigens
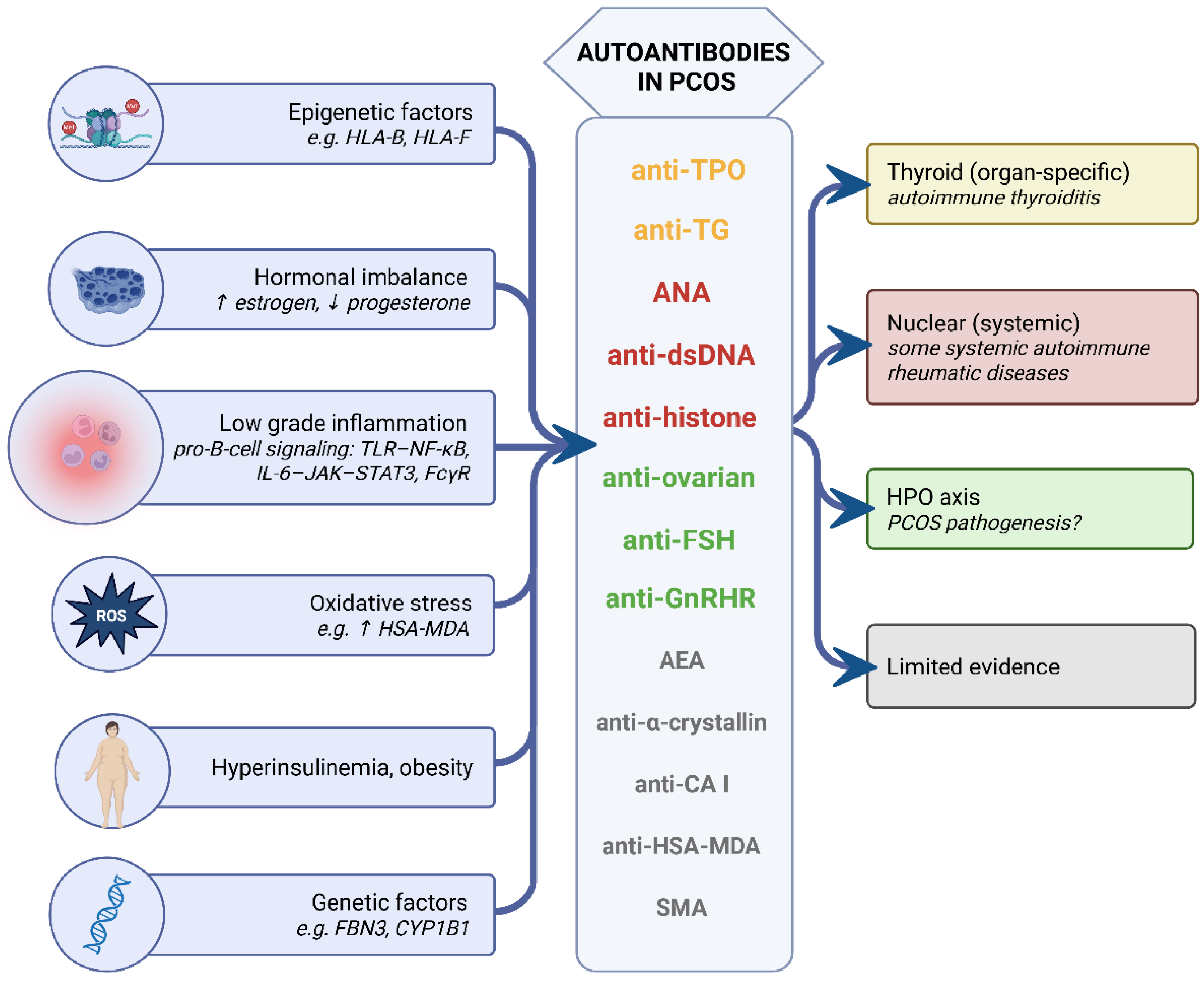
5. Drivers of Elevated Autoantibody Levels in PCOS
6. Conclusions
- Shared susceptibility: Genetic, epigenetic, and transcriptomic data converge on MHC/HLA-centered pathways, suggesting a permissive antigen-presentation background for autoimmunity in a subset of women with PCOS. Clinically, this endotype may justify targeted screening for thyroid autoimmunity (anti-TPO, anti-TG), while non-thyroid autoantibodies are insufficiently studied and require prospective validation.
- Inflammation and oxidative stress: The chronic low-grade inflammatory environment provides autoantigen supply (neoepitopes, nuclear antigens) and pro-B-cell signaling (TLR–NF-κB, IL-6–JAK–STAT3, complement, FcγR), plausibly facilitating seroconversion, especially in insulin-resistant or obese phenotypes. Anti-inflammatory or metabolic interventions should be investigated for their capacity to lower specific autoantibody titers, and the contribution of androgens to inflammatory regulation in PCOS should be clarified in interventional studies.
- Strongest functional signal: Anti-GnRHR antibodies show agonist-like activity, are blocked by cetrorelix, and induce PCOS-like features in vivo; these remain the most compelling pathogenic autoantibodies in PCOS. Prospective, bioassay-stratified studies should test whether targeted therapies benefit women with demonstrable anti-GnRHR activity and define practical selection criteria for such treatment.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | absorbance units |
| ACPA+ | anti-citrullinated protein antibody |
| AEAs | anti-endometrial antibodies |
| AGEs | advanced glycation end products |
| AIT | autoimmune thyroiditis |
| AMAs | antimitochondrial antibodies |
| AMH | anti-Müllerian hormone |
| ANAs | antinuclear antibodies |
| AOAs | antiovarian antibodies |
| anti-CA | anti-carbonic anhydrase antibodies |
| anti-dsDNA | anti-double-stranded DNA |
| anti-GAD65 | anti-glutamic acid decarboxylase 65 |
| anti-HSA-MDA | anti-malondialdehyde-modified human serum albumin |
| anti-IA2 | anti-insulinoma-associated antigen-2 |
| anti-SSA | anti-Sjögren’s-syndrome-related antigen A |
| anti-TG | anti-thyroglobulin |
| anti-TPO | anti-thyroid peroxidase |
| anti-β2GPI | anti-β2-glycoprotein I antibodies |
| ARAs | antireticulin antibodies |
| cGAS | cyclic GMP-AMP synthase |
| CI | confidence interval |
| CRP | C-reactive protein |
| ECL2 | second extracellular loop |
| ELSs | ectopic lymphoid structures |
| EWASs | epigenome-wide association studies |
| FSH | follicle-stimulating hormone |
| FSHR | follicle-stimulating hormone receptor |
| GD | Graves’ disease |
| GDM | gestational diabetes mellitus |
| GnRH | gonadotropin-releasing hormone |
| GNRHR | gonadotropin-releasing hormone receptor |
| GWASs | genome-wide association studies |
| HLA | human leukocyte antigen |
| HPO | hypothalamic–pituitary–ovarian |
| HR | hazard ratio |
| HSP | heat shock protein |
| HT | Hashimoto thyroiditis |
| IA2 | anti-insulinoma-associated antigen-2 |
| IgM | immunoglobulin M |
| IL-1 | interleukin-1 |
| IL-18 | interleukin-18 |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| INSR | insulin receptor |
| IU/mL | international units per milliliter |
| IVF | in vitro fertilization |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KNDy | kisspeptin/neurokinin B/dynorphin |
| LH | luteinizing hormone |
| LHR | luteinizing hormone receptor |
| LKMAs | liver/kidney microsomal antibodies |
| LN | lupus nephritis |
| MCP-1 | monocyte chemoattractant protein-1 |
| MCTD | mixed connective tissue disease |
| MHC | major histocompatibility complex |
| MIP-1α | macrophage inflammatory protein-1α |
| mtDNA | mitochondrial DNA |
| NF-κB | nuclear factor kappa B |
| OD | odds ratio |
| OR | odds ratio |
| PAD | peptidyl arginine deiminase |
| PCAs | parietal cell antibodies |
| PCOM | polycystic ovarian morphology |
| PCOS | polycystic ovary syndrome |
| PTX3 | pentraxin-3 |
| RA | rheumatoid arthritis |
| RAGE | receptor for advanced glycation end products |
| RF+ | rheumatoid factor |
| ROS | reactive oxygen species |
| RR | relative risk |
| SHPG | sex hormone-binding globulin |
| SjD | Sjögren’s disease |
| SLE | systemic lupus erythematosus |
| SMA | smooth muscle antibodies |
| STING | stimulation of interferon genes |
| T1D | type 1 diabetes |
| TGF-β | transforming growth factor β |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| TLR | Toll-like receptors |
| TMA | thyroid microsomal antibodies |
| TNF-α | tumor necrosis factor-α |
| Treg | regulatory T cell |
| TSH | thyroid-stimulating hormone |
| uNK | uterine natural killer |
| WBC | white blood cell count |
References
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Wekker, V.; van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C. Hormonal changes in PCOS. J. Endocrinol. 2024, 261, e230342. [Google Scholar] [CrossRef]
- Damone, A.L.; Joham, A.E.; Loxton, D.; Earnest, A.; Teede, H.J.; Moran, L.J. Depression, anxiety and perceived stress in women with and without PCOS: A community-based study. Psychol. Med. 2019, 49, 1510–1520. [Google Scholar] [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Myers, S.H.; Pintaudi, B.; D’Anna, R.; Morelli, M.; Unfer, V. Gestational diabetes mellitus and polycystic ovary syndrome, a position statement from EGOI-PCOS. Front. Endocrinol. 2025, 16, 1501110. [Google Scholar] [CrossRef] [PubMed]
- Alur-Gupta, S.; Boland, M.R.; Barnhart, K.T.; Sammel, M.D.; Dokras, A. Postpartum complications increased in women with polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2021, 224, 280-e1. [Google Scholar] [CrossRef]
- Palomba, S.; Colombo, C.; Busnelli, A.; Caserta, D.; Vitale, G. Polycystic ovary syndrome and thyroid disorder: A comprehensive narrative review of the literature. Front. Endocrinol. 2023, 14, 1251866. [Google Scholar] [CrossRef]
- Sharmeen, S.; Nomani, H.; Taub, E.; Carlson, H.; Yao, Q. Polycystic ovary syndrome: Epidemiologic assessment of prevalence of systemic rheumatic and autoimmune diseases. Clin. Rheumatol. 2021, 40, 4837–4843. [Google Scholar] [CrossRef]
- Busiah, K.; Colmenares, A.; Bidet, M.; Tubiana-Rufi, N.; Levy-Marchal, C.; Delcroix, C.; Jacquin, P.; Martin, D.; Benadjaoud, L.; Jacqz-Aigrain, E.; et al. High Prevalence of Polycystic Ovary Syndrome in Type 1 Diabetes Mellitus Adolescents: Is There a Difference Depending on the NIH and Rotterdam Criteria? Horm. Res. Paediatr. 2017, 87, 333–341. [Google Scholar] [CrossRef]
- Bayona, A.; Martinez-Vaello, V.; Zamora, J.; Nattero-Chavez, L.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Prevalence of PCOS and related hyperandrogenic traits in premenopausal women with type 1 diabetes: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 501–517. [Google Scholar] [CrossRef]
- Buteva-Hristova, I.; Lazarov, V.; Lozanov, V.; Gateva, A.; Bechev, B.; Kavaldzieva, K.; Mladenov, N.; Trifonova, N.; Dimitrova-Dikanarova, D.; Kamenov, Z. Serum anti-α-crystallin antibodies in women with endocrine disorders. Biotechnol. Biotechnol. Equip. 2017, 31, 574–580. [Google Scholar] [CrossRef]
- Hepsen, S.; Karakose, M.; Cakal, E.; Oztekin, S.; Unsal, I.; Akhanli, P.; Ucan, B.; Ozbek, M. The assessment of thyroid autoantibody levels in euthyroid patients with polycystic ovary syndrome. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 215–219. [Google Scholar] [CrossRef]
- Ott, J.; Aust, S.; Kurz, C.; Nouri, K.; Wirth, S.; Huber, J.C.; Mayerhofer, K. Elevated antithyroid peroxidase antibodies indicating Hashimoto’s thyroiditis are associated with the treatment response in infertile women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 2895–2897. [Google Scholar] [CrossRef]
- Rashid, A.; Bhat, J.A.; Ganie, M.A.; Wani, I.A.; Bhat, M.H.; Shah, Z.A.; Masoodi, S.R.; Marwaha, R.K. Evaluation of serum anti-nuclear antibody among women with PCOS: A hospital based single center cross sectional study. Gynecol. Endocrinol. 2018, 34, 965–969. [Google Scholar] [CrossRef]
- Mobeen, H.; Afzal, N.; Kashif, M. Polycystic Ovary Syndrome May Be an Autoimmune Disorder. Scientifica 2016, 2016, 4071735. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Roldan-Martin, M.B. Type 1 Diabetes and Polycystic Ovary Syndrome: Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Zhou, S.; Fei, W.; Yang, Y.; Que, H. Correlation between Hashimoto’s thyroiditis and polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1025267. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Li, X. The relationship between thyroiditis and polycystic ovary syndrome: A meta-analysis. Int. J. Clin. Exp. Med. 2013, 6, 880–889. [Google Scholar] [PubMed]
- Bahreiny, S.S.; Ahangarpour, A.; Amraei, M.; Mansouri, Z.; Pirsadeghi, A.; Kazemzadeh, R.; Javidan, M.; Karamali, N.; Bastani, M.N.; Dabbagh, M.R. Autoimmune thyroid disorders and polycystic ovary syndrome: Tracing links through systematic review and meta-analysis. J. Reprod. Immunol. 2024, 163, 104215. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, C.C.; Hsieh, M.C.; Ho, C.W.; Hsu, S.P.; Yip, H.T.; Kao, C.H. Graves’ disease could increase polycystic ovary syndrome and comorbidities in Taiwan. Curr. Med. Res. Opin. 2020, 36, 1063–1067. [Google Scholar] [CrossRef]
- Merlino, L.A.; Cerhan, J.R.; Criswell, L.A.; Mikuls, T.R.; Saag, K.G. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin. Arthritis Rheum. 2003, 33, 72–82. [Google Scholar] [CrossRef]
- McCoy, S.S.; Hetzel, S.; VanWormer, J.J.; Bartels, C.M. Sex hormones, body mass index, and related comorbidities associated with developing Sjogren’s disease: A nested case-control study. Clin. Rheumatol. 2022, 41, 3065–3074. [Google Scholar] [CrossRef]
- Lee, T.H.; Wu, C.H.; Chen, M.L.; Yip, H.T.; Lee, C.I.; Lee, M.S.; Wei, J.C. Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study. J. Clin. Med. 2020, 9, 1947. [Google Scholar] [CrossRef]
- Nanah, R.; Jansson-Knodell, C.; Chatterjee, A.; Nanah, R.; Nanah, M.H.; Almasri, J.; Ford, A.; Hamid, O.; Telbany, A.; Rubio-Tapia, A. Women’s Health Disorders in a Coeliac Disease Population After Diagnosis—A Nationwide Cohort Analysis. Aliment. Pharmacol. Ther. 2025, 61, 1603–1611. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Hennig, E.E. Genetic Susceptibility to Joint Occurrence of Polycystic Ovary Syndrome and Hashimoto’s Thyroiditis: How Far Is Our Understanding? Front. Immunol. 2021, 12, 606620. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Suchta, K.; Kulecka, M.; Kluska, A.; Piatkowska, M.; Dabrowski, M.J.; Jankowska, K.; Grymowicz, M.; Smolarczyk, R.; Hennig, E.E. Exome sequencing to explore the possibility of predicting genetic susceptibility to the joint occurrence of polycystic ovary syndrome and Hashimoto’s thyroiditis. Front. Immunol. 2023, 14, 1193293. [Google Scholar] [CrossRef]
- Monteleone, P.; Parrini, D.; Faviana, P.; Carletti, E.; Casarosa, E.; Uccelli, A.; Cela, V.; Genazzani, A.R.; Artini, P.G. Female infertility related to thyroid autoimmunity: The ovarian follicle hypothesis. Am. J. Reprod. Immunol. 2011, 66, 108–114. [Google Scholar] [CrossRef]
- Kelkar, R.L.; Meherji, P.K.; Kadam, S.S.; Gupta, S.K.; Nandedkar, T.D. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J. Reprod. Immunol. 2005, 66, 53–67. [Google Scholar] [CrossRef]
- Arduc, A.; Aycicek Dogan, B.; Bilmez, S.; Imga Nasiroglu, N.; Tuna, M.M.; Isik, S.; Berker, D.; Guler, S. High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: Does the imbalance between estradiol and progesterone play a role? Endocr. Res. 2015, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Skrzynska, K.; Zachurzok, A.; Tomaszewski, R.; Gawlik, A.; Malecka-Tendera, E. The prevalence of autoimmune thyroiditis in adolescent girls with polycystic ovary syndrome. Ginekol. Pol. 2022, 93, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.; Goerges, J.; Keck, C.; Muller-Wieland, D.; Diederich, S.; Janssen, O.E. Impact of Autoimmune Thyroiditis on Reproductive and Metabolic Parameters in Patients with Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2018, 126, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, S.C.; Gunes, M. Investigation of the Relationship Between Autoimmune and Nodular Goiter in Patients with Euthyroid Polycystic Ovary Syndrome and Their Phenotypes. Horm. Metab. Res. 2022, 54, 396–406. [Google Scholar] [CrossRef]
- Janssen, O.E.; Mehlmauer, N.; Hahn, S.; Offner, A.H.; Gartner, R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 2004, 150, 363–369. [Google Scholar] [CrossRef]
- Kim, J.J.; Yoon, J.W.; Kim, M.J.; Kim, S.M.; Hwang, K.R.; Choi, Y.M. Thyroid autoimmunity markers in women with polycystic ovary syndrome and controls. Hum. Fertil. 2022, 25, 128–134. [Google Scholar] [CrossRef]
- Adamska, A.; Lebkowska, A.; Krentowska, A.; Hryniewicka, J.; Adamski, M.; Lesniewska, M.; Polak, A.M.; Kowalska, I. Ovarian Reserve and Serum Concentration of Thyroid Peroxidase Antibodies in Euthyroid Women With Different Polycystic Ovary Syndrome Phenotypes. Front. Endocrinol. 2020, 11, 440. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Akpang, N.; Zaborowska, L.; Ludwin, A. Prevalence and Levels of Thyroid Autoantibodies in Polycystic Ovary Syndrome-Impact of TSH- and BMI-Matched Comparisons: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 7525. [Google Scholar] [CrossRef]
- Chapman, J.C.; Min, S.H.; Freeh, S.M.; Michael, S.D. The estrogen-injected female mouse: New insight into the etiology of PCOS. Reprod. Biol. Endocrinol. 2009, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Bayona, A.; Nattero-Chavez, L.; Luque-Ramirez, M. Type 1 diabetes mellitus and polycystic ovary syndrome. Nat. Rev. Endocrinol. 2021, 17, 701–702. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. The link between polycystic ovary syndrome and both Type 1 and Type 2 diabetes mellitus: What do we know today? Womens Health 2012, 8, 147–154. [Google Scholar] [CrossRef]
- Tan, Q. Deciphering the DNA Methylome of Polycystic Ovary Syndrome. Mol. Diagn. Ther. 2020, 24, 245–250. [Google Scholar] [CrossRef]
- Li, S.; Zhu, D.; Duan, H.; Tan, Q. The epigenomics of polycystic ovarian syndrome: From pathogenesis to clinical manifestations. Gynecol. Endocrinol. 2016, 32, 942–946. [Google Scholar] [CrossRef]
- Li, S.; Zhu, D.; Duan, H.; Ren, A.; Glintborg, D.; Andersen, M.; Skov, V.; Thomassen, M.; Kruse, T.; Tan, Q. Differential DNA methylation patterns of polycystic ovarian syndrome in whole blood of Chinese women. Oncotarget 2017, 8, 20656–20666. [Google Scholar] [CrossRef]
- Wang, X.X.; Wei, J.Z.; Jiao, J.; Jiang, S.Y.; Yu, D.H.; Li, D. Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget 2014, 5, 6603–6610. [Google Scholar] [CrossRef]
- Perez-Bravo, F.; Carrasco, E.; Echiburu, B.; Maliqueo, M.; Diaz, J.; Sir-Petermann, T. Serological markers of autoimmunity in pregnant women with polycystic ovary syndrome: A pilot study. Gynecol. Endocrinol. 2010, 26, 889–893. [Google Scholar] [CrossRef]
- Bossuyt, X.; De Langhe, E.; Borghi, M.O.; Meroni, P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 715–726. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.Q.; Zou, J.W.; Li, M.; Pan, C.C.; Si, Y.Q. Association between serum antinuclear antibody and rheumatoid arthritis. Front. Immunol. 2024, 15, 1358114. [Google Scholar] [CrossRef] [PubMed]
- Hefler-Frischmuth, K.; Walch, K.; Huebl, W.; Baumuehlner, K.; Tempfer, C.; Hefler, L. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil. Steril. 2010, 93, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Samsami Dehaghani, A.; Karimaghaei, N.; Parsanezhad, M.E.; Malekzadeh, M.; Mehrazmay, M.; Erfani, N. Anti-Nuclear Antibodies in Patients with Polycystic Ovary Syndrome before and after Laparoscopic Electrocauterization. Iran. J. Med. Sci. 2013, 38, 187–190. [Google Scholar] [PubMed]
- Hamedi, B.; Sarvestani, E.K.; Khalili, A.; Fard, M.B.; Alborzi, S.; Davoodi, S.; Nazarinia, M.A. Evaluation of ANA-related serologic autoantibodies in polycystic ovary syndrome. Immunol. Endocr. Metab. Agents Med. Chem. (Former. Curr. Med. Chem.-Immunol. Endocr. Metab. Agents) 2014, 14, 21–25. [Google Scholar] [CrossRef]
- Reimand, K.; Talja, I.; Metskula, K.; Kadastik, U.; Matt, K.; Uibo, R. Autoantibody studies of female patients with reproductive failure. J. Reprod. Immunol. 2001, 51, 167–176. [Google Scholar] [CrossRef]
- Haller, K.; Sarapik, A.; Talja, I.; Salumets, A.; Uibo, R. Controlled ovarian hyperstimulation changes the prevalence of serum autoantibodies in in vitro fertilization patients. Am. J. Reprod. Immunol. 2006, 56, 364–370. [Google Scholar] [CrossRef]
- Palacio, J.R.; Iborra, A.; Ulcova-Gallova, Z.; Badia, R.; Martinez, P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin. Exp. Immunol. 2006, 144, 217–222. [Google Scholar] [CrossRef]
- Novais Jde, S.; Benetti-Pinto, C.L.; Garmes, H.M.; Jales, R.M.; Juliato, C.R. Polycystic ovary syndrome and chronic autoimmune thyroiditis. Gynecol. Endocrinol. 2015, 31, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Makled, A.K.; Fathi, H.M.; Gomaa, M.F.; Bakr, R.M. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Middle East. Fertil. Soc. J. 2015, 20, 86–90. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Kadhim, E.J.; Abbas, N.S.; Younis, S.R.; Fawzi, H.A. Serological markers of autoimmunity in women with polycystic ovary syndrome. Int. J. Res. Pharm. Sci. 2019, 10, 1746–1750. [Google Scholar] [CrossRef]
- Petrikova, J.; Lazurova, I.; Dravecka, I.; Vrbikova, J.; Kozakova, D.; Figurova, J.; Vaczy, Z.; Rosocha, J. The prevalence of non organ specific and thyroid autoimmunity in patients with polycystic ovary syndrome. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 302–306. [Google Scholar] [CrossRef]
- Menteşe, A.; Güven, S.; Sumer, A.; Turan, I.; Demir, S.; Karahan, S.; Alver, A. Serum anti-carbonic anhydrase I and II antibodies and polycystic ovary syndrome. Turk. J. Biochem.-Turk. Biyokim. Derg. 2013, 38, 43. [Google Scholar] [CrossRef]
- Weedin, E.A.; Burks, H.R.; Yu, X.; Li, H.L.; Aston, C.E.; Kem, D.C.; Craig, L.B. Elevated activity levels of activating autoantibodies to the GnRH receptor in patients with polycystic ovary syndrome. F S Rep. 2020, 1, 299–304. [Google Scholar] [CrossRef]
- Sattler, L.M.; Schniewind, H.A.; Minich, W.B.; Haudum, C.W.; Niklowitz, P.; Munzker, J.; Kovacs, G.L.; Reinehr, T.; Obermayer-Pietsch, B.; Schomburg, L. Natural autoantibodies to the gonadotropin-releasing hormone receptor in polycystic ovarian syndrome. PLoS ONE 2021, 16, e0249639. [Google Scholar] [CrossRef]
- Kem, D.C.; Li, H.; Yu, X.; Weedin, E.; Reynolds, A.C.; Forsythe, E.; Beel, M.; Fischer, H.; Hines, B.; Guo, Y.; et al. The Role of GnRH Receptor Autoantibodies in Polycystic Ovary Syndrome. J. Endocr. Soc. 2020, 4, bvaa078. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Deng, J.; Fischer, H.; Weedin, E.A.; Burks, H.R.; Craig, L.B.; Yu, X. Increased testosterone and proinflammatory cytokines in patients with polycystic ovary syndrome correlate with elevated GnRH receptor autoantibody activity assessed by a fluorescence resonance energy transfer-based bioassay. Endocrine 2021, 74, 163–171. [Google Scholar] [CrossRef]
- Haller, K.; Mathieu, C.; Rull, K.; Matt, K.; Bene, M.C.; Uibo, R. IgG, IgA and IgM antibodies against FSH: Serological markers of pathogenic autoimmunity or of normal immunoregulation? Am. J. Reprod. Immunol. 2005, 54, 262–269. [Google Scholar] [CrossRef]
- Schniewind, H.A.; Sattler, L.M.; Haudum, C.W.; Munzker, J.; Minich, W.B.; Obermayer-Pietsch, B.; Schomburg, L. Autoimmunity to the Follicle-Stimulating Hormone Receptor (FSHR) and Luteinizing Hormone Receptor (LHR) in Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2021, 22, 13667. [Google Scholar] [CrossRef]
- Shoukry, M.M.; Amer, H.A.; El-Kabarity, R.H.; Wahba, N.S. The role of anti-ovarian autoantibodies in Polycystic Ovary Syndrome. Egypt. J. Hosp. Med. 2020, 81, 1326–1329. [Google Scholar] [CrossRef]
- Akpang, N.; Kwiatkowski, J.; Zaborowska, L.; Ludwin, A. Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis? Int. J. Mol. Sci. 2025, 26, 4121. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Salih, B.; Othman, B. Relation of Anti FSH Antibodies and Polycystic Ovarian Syndrome in Women. Indian J. Public Health Res. Dev. 2020, 11, 1954. [Google Scholar] [CrossRef]
- Fénichel, P.; Gobert, B.; Carré, Y.; Barbarino-Monnier, P.; Hiéronimus, S. Polycystic o vary syndrome in autoimmune disease. Lancet 1999, 353, 2210. [Google Scholar] [CrossRef]
- Luborsky, J.; Shatavi, S.; Adamczyk, P.; Chiong, C.; Llanes, B.; Lafniztzegger, J.; Soltes, B.; McGovern, P.; Santoro, N. Polycystic ovary syndrome and ovarian autoimmunity—Assessment of ovarian antibodies by EIA. J. Reprod. Immunol. 1999, 42, 79–84. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Deng, J.; Gali, H.; Weedin, E.A.; Burks, H.R.; Craig, L.B.; Yu, X. GnRH receptor-activating autoantibodies in polycystic ovary syndrome: Identification of functional epitopes and development of epitope mimetic inhibitors. Endocrine 2022, 75, 959–963. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Zhang, G.; Deng, J.; Fischer, H.; Craig, L.B.; Yu, X.; Kem, D.C. Gonadotrophin-releasing hormone receptor autoantibodies induce polycystic ovary syndrome-like features in a rat model. Exp. Physiol. 2021, 106, 902–912. [Google Scholar] [CrossRef]
- Davies, T.F.; Andersen, S.; Latif, R.; Nagayama, Y.; Barbesino, G.; Brito, M.; Eckstein, A.K.; Stagnaro-Green, A.; Kahaly, G.J. Graves’ disease. Nat. Rev. Dis. Primers 2020, 6, 52. [Google Scholar] [CrossRef]
- McCartney, C.R.; Campbell, R.E.; Marshall, J.C.; Moenter, S.M. The role of gonadotropin-releasing hormone neurons in polycystic ovary syndrome. J. Neuroendocrinol. 2022, 34, e13093. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Lim, S.S.; Davies, M.J.; Norman, R.J.; Moran, L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef]
- Keskin Kurt, R.; Okyay, A.G.; Hakverdi, A.U.; Gungoren, A.; Dolapcioglu, K.S.; Karateke, A.; Dogan, M.O. The effect of obesity on inflammatory markers in patients with PCOS: A BMI-matched case-control study. Arch. Gynecol. Obstet. 2014, 290, 315–319. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; Mueller, A.; Sailer, M.; Gonzalez Santos, F.; Rizo, J.M.; Egea, F.M. Chronic Inflammation in PCOS: The Potential Benefits of Specialized Pro-Resolving Lipid Mediators (SPMs) in the Improvement of the Resolutive Response. Int. J. Mol. Sci. 2020, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; de Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef]
- de Medeiros, S.F.; de Medeiros, M.A.S.; Santos, N.S.; Barbosa, B.B.; Yamamoto, M.M.W. Combined Oral Contraceptive Effects on Low-Grade Chronic Inflammatory Mediators in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Inflam. 2018, 2018, 9591509. [Google Scholar] [CrossRef]
- Goodman, M.P. Are all estrogens created equal? A review of oral vs. transdermal therapy. J. Womens Health 2012, 21, 161–169. [Google Scholar] [CrossRef]
- Bahceci, M.; Tuzcu, A.; Canoruc, N.; Tuzun, Y.; Kidir, V.; Aslan, C. Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance. Horm. Res. 2004, 62, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Winnett, C.; Lu, G.; Grogan, T.R.; Abbott, D.H.; Naik, R.; Chazenbalk, G.D. Randomized clinical trial: Effect of low-dose flutamide on abdominal adipogenic function in normal-weight women with polycystic ovary syndrome. Fertil. Steril. 2023, 119, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Yasui, T.; Matsui, S.; Kato, T.; Tsuchiya, N.; Yuzurihara, M.; Kase, Y.; Irahara, M. Circulating levels of monocyte chemoattractant protein-1 and interleukin-7 in women who have undergone bilateral salpingo-oophorectomy. J. Inflamm. Res. 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Yang, Q.; Jia, S.; Tao, J.; Zhang, J.; Fan, Z. Multiple effects of kisspeptin on neuroendocrine, reproduction, and metabolism in polycystic ovary syndrome. J. Neuroendocrinol. 2025, 37, e13482. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Kinsey-Jones, J.S.; Cheng, Y.; Knox, A.M.; Lin, Y.; Petrou, N.A.; Roseweir, A.; Lightman, S.L.; Milligan, S.R.; Millar, R.P.; et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 2009, 4, e8334. [Google Scholar] [CrossRef]
- Decourt, C.; Watanabe, Y.; Evans, M.C.; Inglis, M.A.; Fisher, L.C.; Jasoni, C.L.; Campbell, R.E.; Anderson, G.M. Deletion of Androgen Receptors From Kisspeptin Neurons Prevents PCOS Features in a Letrozole Mouse Model. Endocrinology 2023, 164, bqad077. [Google Scholar] [CrossRef]
- Okada, H.; Kanasaki, H.; Tumurbaatar, T.; Tumurgan, Z.; Oride, A.; Kyo, S. Hyperandrogenism induces proportional changes in the expression of Kiss-1, Tac2, and DynA in hypothalamic KNDy neurons. Reprod. Biol. Endocrinol. 2022, 20, 91. [Google Scholar] [CrossRef]
- Moore, A.M. Neuroendocrine mechanisms responsible for elevated gonadotrophin-releasing hormone and luteinising hormone pulses in polycystic ovary syndrome. J. Neuroendocrinol. 2025, 37, e70028. [Google Scholar] [CrossRef]
- Liu, J.; Qu, T.; Li, Z.; Yu, L.; Zhang, S.; Yuan, D.; Wu, H. Serum kisspeptin levels in polycystic ovary syndrome: A meta-analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2157–2165. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Ornat, L.; Lopez-Baena, M.T.; Santabarbara, J.; Saviron-Cornudella, R.; Perez-Roncero, G.R. Circulating kisspeptin and anti-mullerian hormone levels, and insulin resistance in women with polycystic ovary syndrome: A systematic review, meta-analysis, and meta-regression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 85–98. [Google Scholar] [CrossRef]
- Makowski, K.N.; Kreisman, M.J.; McCosh, R.B.; Raad, A.A.; Breen, K.M. Peripheral interleukin-1beta inhibits arcuate kiss1 cells and LH pulses in female mice. J. Endocrinol. 2020, 246, 149–160. [Google Scholar] [CrossRef]
- Castellano, J.M.; Bentsen, A.H.; Romero, M.; Pineda, R.; Ruiz-Pino, F.; Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Pinilla, L.; Mikkelsen, J.D.; Tena-Sempere, M. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E54–E61. [Google Scholar] [CrossRef]
- Renwick, A.N.; Whitlock, B.K.; Nestor, C.C.; Daniel, J.A.; Strickland, L.; Lear, A.S.; Adkins, M.; Griffin, C.; Esteller-Vico, A. Chronic inflammation decreases arcuate kisspeptin expression in male sheep. Domest. Anim. Endocrinol. 2024, 89, 106868. [Google Scholar] [CrossRef]
- Luedde, M.; Spehlmann, M.E.; Hippe, H.J.; Loosen, S.H.; Roy, S.; Vargas Cardenas, D.; Vucur, M.; Frey, N.; Koch, A.; Luedde, T.; et al. Serum levels of kisspeptin are elevated in critically ill patients. PLoS ONE 2018, 13, e0206064. [Google Scholar] [CrossRef]
- Akad, M.; Socolov, R.; Covali, R.; Stan, C.D.; Crauciuc, E.; Popovici, D.; Stan, C.I.; Akad, F.; Socolov, D. Kisspeptin Serum Levels in Patients with Endometriosis, New Research Pathways Regarding Female Infertility. Maedica 2022, 17, 557–560. [Google Scholar] [CrossRef]
- Garcia-Ortega, J.; Pinto, F.M.; Fernandez-Sanchez, M.; Prados, N.; Cejudo-Roman, A.; Almeida, T.A.; Hernandez, M.; Romero, M.; Tena-Sempere, M.; Candenas, L. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum. Reprod. 2014, 29, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Mondal, P.; Wolfe, A.; Alonso, L.C.; Stamateris, R.; Ong, B.W.; Lim, O.C.; Yang, K.S.; Radovick, S.; Novaira, H.J.; et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014, 19, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Cockwell, H.; Wilkinson, D.A.; Bouzayen, R.; Imran, S.A.; Brown, R.; Wilkinson, M. KISS1 expression in human female adipose tissue. Arch. Gynecol. Obstet. 2013, 287, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S. Targeting KNDy neurons to control GnRH pulses. Curr. Opin. Pharmacol. 2022, 67, 102316. [Google Scholar] [CrossRef]
- Katulski, K.; Podfigurna, A.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 2018, 61, 149–157. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef]
- Norman, R.J.; Brannstrom, M. Cytokines in the ovary: Pathophysiology and potential for pharmacological intervention. Pharmacol. Ther. 1996, 69, 219–236. [Google Scholar] [CrossRef]
- Ben-Rafael, Z.; Orvieto, R. Cytokines-involvement in reproduction. Fertil. Steril. 1992, 58, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, M.; Wang, R.; Jiang, J.; Hu, Y.; Wang, W.; Wang, Y.; Li, H. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: Evidence from a large national cohort study. Lipids Health Dis. 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Robertson, A.K.; Soderberg-Naucler, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Sahin, F.K.; Sahin, S.B.; Balik, G.; Ural, U.M.; Tekin, Y.B.; Cure, M.C.; Senturk, S.; Yuce, S.; Cure, E. Does low pentraxin-3 levels associate with polycystic ovary syndrome and obesity? Int. J. Clin. Exp. Med. 2014, 7, 3512–3519. [Google Scholar]
- Chodkowski, A.; Nabrdalik, K.; Kwiendacz, H.; Gumprecht, J. Association of pentraxin 3 with atherosclerotic cardiovascular diseases—General knowledge in 2018. Clin. Diabetol. 2018, 7, 203–206. [Google Scholar] [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M. Immunological determinants of implantation success. Int. J. Dev. Biol. 2014, 58, 205–217. [Google Scholar] [CrossRef]
- Dosiou, C.; Giudice, L.C. Natural killer cells in pregnancy and recurrent pregnancy loss: Endocrine and immunologic perspectives. Endocr. Rev. 2005, 26, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum. Reprod. Update 2021, 27, 584–618. [Google Scholar] [CrossRef]
- Salama, S.A.; Kamel, M.W.; Diaz-Arrastia, C.R.; Xu, X.; Veenstra, T.D.; Salih, S.; Botting, S.K.; Kumar, R. Effect of tumor necrosis factor-alpha on estrogen metabolism and endometrial cells: Potential physiological and pathological relevance. J. Clin. Endocrinol. Metab. 2009, 94, 285–293. [Google Scholar] [CrossRef]
- Schmidt, J.; Weijdegard, B.; Mikkelsen, A.L.; Lindenberg, S.; Nilsson, L.; Brannstrom, M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol. Hum. Reprod. 2014, 20, 49–58. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, H.; Ding, H.; Xu, T.; Liu, X.; Huang, Z.; Wu, H.; Ge, H. Hyperandrogenism drives ovarian inflammation and pyroptosis: A possible pathogenesis of PCOS follicular dysplasia. Int. Immunopharmacol. 2023, 125, 111141. [Google Scholar] [CrossRef]
- Adams, J.; Liu, Z.; Ren, Y.A.; Wun, W.S.; Zhou, W.; Kenigsberg, S.; Librach, C.; Valdes, C.; Gibbons, W.; Richards, J. Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women With Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3459–3468. [Google Scholar] [CrossRef]
- Shen, H.; Liang, Z.; Zheng, S.; Li, X. Pathway and network-based analysis of genome-wide association studies and RT-PCR validation in polycystic ovary syndrome. Int. J. Mol. Med. 2017, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Bai, F.; Deng, Y.; Zhang, X.; Wang, L. Investigating the Role of Inflammatory Response in Polycystic Ovary Syndrome Using Integrated RNA-Seq Analysis. J. Inflamm. Res. 2024, 17, 4701–4719. [Google Scholar] [CrossRef]
- Su, N.J.; Ma, J.; Feng, D.F.; Zhou, S.; Li, Z.T.; Zhou, W.P.; Deng, H.; Liang, J.Y.; Yang, X.H.; Zhang, Y.M.; et al. The peripheral blood transcriptome identifies dysregulation of inflammatory response genes in polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, Q.; Liu, S.; Cheng, L.; Li, B.; Cheng, D. Integrated multi-omics analysis reveals complement component 3 as a central driver of immune dysregulation in polycystic ovary syndrome. Fron. Endocrinol. 2025, 16, 1523488. [Google Scholar] [CrossRef] [PubMed]
- DeMarshall, C.; Goldwaser, E.L.; Sarkar, A.; Godsey, G.A.; Acharya, N.K.; Thayasivam, U.; Belinka, B.A.; Nagele, R.G. Autoantibodies as diagnostic biomarkers for the detection and subtyping of multiple sclerosis. J. Neuroimmunol. 2017, 309, 51–57. [Google Scholar] [CrossRef]
- Musa, R.; Rout, P.; Qurie, A. Lupus Nephritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mohan, C.; Assassi, S. Biomarkers in rheumatic diseases: How can they facilitate diagnosis and assessment of disease activity? BMJ 2015, 351, h5079. [Google Scholar] [CrossRef]
- Lu, D.R.; McDavid, A.N.; Kongpachith, S.; Lingampalli, N.; Glanville, J.; Ju, C.H.; Gottardo, R.; Robinson, W.H. T Cell-Dependent Affinity Maturation and Innate Immune Pathways Differentially Drive Autoreactive B Cell Responses in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1732–1744. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yamanaka, H.; Ishiguro, N.; Miyasaka, N.; Mukai, M.; Matsubara, T.; Uchida, S.; Akama, H.; Kupper, H.; Arora, V.; et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: The HOPEFUL 1 study. Ann. Rheum. Dis. 2014, 73, 536–543. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Arnaud, L.; Chasset, F.; Martin, T. Immunopathogenesis of systemic lupus erythematosus: An update. Autoimmun. Rev. 2024, 23, 103648. [Google Scholar] [CrossRef]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J. Clin. Investig. 2015, 125, 2194–2202. [Google Scholar] [CrossRef]
- Xiao, N.; He, K.; Gong, F.; Xie, Q.; Peng, J.; Su, X.; Lu, Y.; Xia, X.; Lin, G.; Cheng, L. Altered subsets and activities of B lymphocytes in polycystic ovary syndrome. J. Allergy Clin. Immunol. 2019, 143, 1943–1945.e4. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, X.; Xiao, N.; Yan, Y.; Liu, X.; Xie, Q.; Su, X.; Chen, M.; Peng, J.; Wang, S.; et al. The therapeutic effect of anti-CD19 antibody on DHEA-induced PCOS mice. Int. Immunopharmacol. 2024, 130, 111711. [Google Scholar] [CrossRef]
- Petrikova, J.; Lazurova, I. Ovarian failure and polycystic ovary syndrome. Autoimmun. Rev. 2012, 11, A471–A478. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Smithson, G.; Couse, J.F.; Lubahn, D.B.; Korach, K.S.; Kincade, P.W. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J. Immunol. 1998, 161, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.M.; Cleary, J.; Dagtas, A.S.; Moussai, D.; Diamond, B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Investig. 2002, 109, 1625–1633. [Google Scholar] [CrossRef]
- Shabbir, S.; Khurram, E.; Moorthi, V.S.; Eissa, Y.T.H.; Kamal, M.A.; Butler, A.E. The interplay between androgens and the immune response in polycystic ovary syndrome. J. Transl. Med. 2023, 21, 259. [Google Scholar] [CrossRef]
- Gao, Q.; Ren, H.; Chen, M.; Niu, Z.; Tao, H.; Jia, Y.; Zhang, J.; Li, W. Long non-coding RNAs regulate effects of beta-crystallin B2 on mouse ovary development. Mol. Med. Rep. 2016, 14, 4223–4231. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao Ch, M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta 2015, 1854, 291–319. [Google Scholar] [CrossRef]
- Maggi, E.; Bellazzi, R.; Falaschi, F.; Frattoni, A.; Perani, G.; Finardi, G.; Gazo, A.; Nai, M.; Romanini, D.; Bellomo, G. Enhanced LDL oxidation in uremic patients: An additional mechanism for accelerated atherosclerosis? Kidney Int. 1994, 45, 876–883. [Google Scholar] [CrossRef]
- Maggi, E.; Marchesi, E.; Ravetta, V.; Martignoni, A.; Finardi, G.; Bellomo, G. Presence of autoantibodies against oxidatively modified low-density lipoprotein in essential hypertension: A biochemical signature of an enhanced in vivo low-density lipoprotein oxidation. J. Hypertens. 1995, 13, 129–138. [Google Scholar] [CrossRef]
- Bellomo, G.; Maggi, E.; Poli, M.; Agosta, F.G.; Bollati, P.; Finardi, G. Autoantibodies against oxidatively modified low-density lipoproteins in NIDDM. Diabetes 1995, 44, 60–66. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
| Associated Autoimmune Disease | Study | Year | Type of Study | Co-Occurrence Frequency/Risk |
|---|---|---|---|---|
| Type 1 diabetes mellitus | Bayona et al. [18] | 2022 | systematic review and meta-analysis | The overall prevalence of PCOS among women with T1D was estimated at 26% (95% CI: 19–34%). |
| Escobar-Morreale et al. [24] | 2016 | systematic review and meta-analysis | The prevalence of PCOS in women with type 1 diabetes was 24% (95% CI: 15–34). | |
| Autoimmune thyroiditis | Hu et al. [25] | 2022 | systematic review and meta-analysis | The mean prevalence of HT in PCOS patients was 25.24%. PCOS patients had a higher risk of developing HT under a random-effects model (OR = 2.28, 95% Cl: 1.61–3.22). |
| Du et al. [26] | 2013 | systematic review and meta-analysis | The prevalence of AIT in PCOS patients was higher than that in control groups, with the effect size calculated as OR = 4.81, 95% CI: 2.88–8.04. | |
| Bahreiny et al. [27] | 2024 | systematic review and meta-analysis | A considerable association was detected between PCOS and the presence of AIT (OR = 2.38, 95% CI: 1.63–3.49). | |
| Graves’ disease | Chen et al. [28] | 2020 | retrospective cohort study | Women with GD could be at risk of developing PCOS. The adjusted HR of PCOS for patients with GD relative to patients without GD was 1.47, 95% CI: 1.09–1.98. |
| Rheumatoid arthritis | Merlino et al. [29] | 2003 | prospective cohort study | The development of RA in elderly women showed association with PCOS (RR = 2.58, 95% CI: 1.06–6.30). |
| Sjögren’s disease | McCoy et al. [30] | 2022 | retrospective case–control study | PCOS, including ovarian cysts and hirsutism, was associated with greater RR for SjD (RR = 1.65, 95% CI: 1.28–2.12). |
| Psoriasis | Lee et al. [31] | 2020 | retrospective population-based cohort study | The risk of psoriasis was higher in the PCOS group by an HR of 2.07 (95% CI: 1.25–3.43) compared with the control group. |
| Celiac disease | Nanah et al. [32] | 2025 | retrospective observational analysis | Women with celiac disease had higher odds of later women’s health conditions including PCOS (3.3% vs. 1.0%; OR = 3.2, 95% CI: 2.94–3.68). |
| Systemic sclerosis | Sharmeen et al. [16] | 2021 | retrospective study | Systemic sclerosis was significantly more frequent in the PCOS patients than the non-PCOS (0.40% vs. 0.0%, p = 0.0369). |
| Undifferentiated connective tissue disease | Sharmeen et al. [16] | 2021 | retrospective study | Undifferentiated connective tissue disease was significantly more frequent in the PCOS patients than the non-PCOS (0.53% vs. 0.0%, p = 0.0123). |
| Autoantibody | Study | Year | Results (PCOS vs. Controls) | Conclusions |
|---|---|---|---|---|
| anti-TPO | Arduc et al. [37] | 2015 | 26.7% vs. 6.6% p = 0.002 2.8 (0.2–600) vs. 1.5 (0.2–95) IU/mL p = 0.012 | A higher concentration of anti-TPO along with an increased occurrence of anti-TPO positivity was identified in women with PCOS in comparison to those in the non-PCOS control group. Interestingly, Pearson correlation analysis revealed a positive association between anti-TPO levels and estradiol, the estradiol/progesterone ratio, and TSH levels. |
| Hepşen et al. [20] | 2018 | 37.9% vs. 11.1% p < 0.001 52 (0.2–1300) vs. 10 (10–1000) IU/mL p < 0.001 | Anti-TPO levels and anti-TPO positivity prevalence were significantly higher in euthyroid PCOS patients in comparison with controls. | |
| Janssen et al. [41] | 2004 | 26.9% vs. 8.3% p < 0.001 123 ± 328 vs. 10 ± 18 IU/mL p < 0.001 | Both anti-TPO levels and the prevalence of anti-TPO positivity were significantly elevated in PCOS patients compared to the control group. Although LH and FSH levels did not differ individually, the LH-to-FSH ratio was higher in antibody-positive patients. | |
| Kim et al. [42] | 2019 | 4.8% vs. 7.6% p = 0.88 | Neither anti-TPO positivity nor thyroid parenchymal changes suggestive of thyroiditis were more prevalent in women with PCOS than in controls. | |
| Adamska et al. [43] | 2020 | 22.0% vs. 23.9% p = 0.07 | The frequency of positive serum anti-TPO did not differ between women with PCOS and controls or among phenotypes A, B, and C. Interestingly, women presenting phenotype D were characterized by the lowest frequency of occurrence of positive anti-TPO. | |
| anti-TG | Arduc et al. [37] | 2015 | 16.2% vs. 5.0% p = 0.039 17.5 (0.9–1098) vs. 10.8 (0.9–239) IU/mL p = 0.014 | The study demonstrated a higher prevalence of anti-TG levels in PCOS patients. |
| Hepşen et al. [20] | 2018 | 15.3% vs. 5.1% p = 0.013 26 (0.9–524) vs. 20 (10–308) IU/mL p < 0.001 | Anti-TG antibody levels were determined to be significantly higher in the euthyroid PCOS group. Anti-TG antibody positivity prevalence of euthyroid PCOS patients was significantly higher as compared to controls too. | |
| Janssen et al. [41] | 2004 | 26.9% vs. 8.3% p < 0.001 113 ± 312 vs. 4 ± 17 IU/mL p < 0.001 | Anti-TG levels and the rate of anti-TG positivity were notably higher in PCOS patients compared to the control group. While LH and FSH levels were similar individually, the LH-to-FSH ratio was increased in patients with positive antibody results. | |
| Novais et al. [62] | 2014 | 9.2% vs. 7.7% p = 0.7527 | There was no difference between the two groups with respect to the presence of anti-TG antibodies. | |
| ANAs | Rashid et al. [22] | 2018 | 18.4% vs. 2.29% p < 0.01 | Serum ANA positivity was significantly more prevalent in women with PCOS than in controls. It correlated with clinical signs of hyperandrogenism and plasma glucose but showed no significant link to other hormonal parameters. |
| Makled et al. [63] | 2015 | 36% vs. 6% p < 0.001 9.0 ± 6.1 vs. 5.4 ± 2.3 IU/mL p < 0.001 | Mean serum ANA levels were significantly higher in women with PCOS than in controls and showed a notable association with TSH levels. | |
| Ibrahim et al. [64] | 2019 | 8.0 ± 2.7 vs. 5.1 ± 2.6 IU/mL p < 0.001 | Serum ANA levels were significantly higher in PCOS than in controls and were strongly correlated with FSH and LH. | |
| Petrikova et al. [65] | 2015 | 0.66% vs. 2.7% p = 0.250 | There were no significant differences in the prevalence of ANA between PCOS and controls. | |
| anti-dsDNA | Hefler-Frischmuth et al. [56] | 2010 | 4.6 ± 3.8 vs. 3.8 ± 1.6 IU/mL p = 0.02 | Serum levels of anti-dsDNA were significantly higher in women with PCOS. |
| Makled et al. [63] | 2015 | 28% vs. 2% p < 0.001 56.3 ± 25.7 vs. 26.0 ± 10.8 IU/mL p < 0.001 | Mean serum anti-dsDNA levels and prevalence of serum anti-dsDNA positivity were significantly higher in women with PCOS than in controls. | |
| Ibrahim et al. [64] | 2019 | 54.2 ± 20.3 vs. 24.0 ± 15.0 IU/mL p < 0.001 | Serum anti-dsDNA levels were significantly higher in PCOS compared to control women. | |
| Hamedi et al. [58] | 2014 | 42.5 ± 38 vs. 35.4 ± 39 IU/mL p = 0.23 | Serologic markers of autoimmunity, anti-dsDNA, were not elevated in PCOS patients. | |
| anti-histone | Hefler-Frischmuth et al. [56] | 2010 | 7.8 ± 7.7 vs. 5.5 ± 6.1 IU/mL p = 0.02 | Serum levels of anti-histone antibodies were significantly higher in women with PCOS. |
| AEAs | Palacio et al. [61] | 2006 | mean AEA level (exact values not provided) p < 0.01 | Women with PCOS had a significantly higher mean AEA level compared to the control group. |
| anti-α-crystallin | Buteva-Hristova et al. [19] | 2017 | 25.4% vs. 0% p = 0.029 0.6031 (0.36–1.26) vs. 0.4979 (0.42–0.6) OD (492 nm) p = 0.021 | In the PCOS group, the levels of anti-α-crystallin antibodies were significantly higher than in the control group. Moreover, the proportion of positive sera in this group was considerably greater compared to the control group. |
| anti-CA I | Menteşe et al. [66] | 2013 | 26% vs. 5.3% p < 0.05 0.311 ± 0.180 vs. 0.190 ± 0.098 A (480 nm) p < 0.0001 | Women with PCOS had significantly higher mean anti-CA I antibody levels, while anti-CA II levels showed no significant difference from controls. All patients positive for anti-CA II were also positive for anti-CA I. |
| anti-CA II | 4% vs. 0% p > 0.05 0.332 ± 0.174 vs. 0.333 ± 0.107 A (480 nm) p > 0.05 | |||
| anti-HSA-MDA | Palacio et al. [61] | 2006 | 0.09 ± 0.03 vs. 0.041 ± 0.03 A (620 nm) p < 0.05 | Patients with PCOS had a significantly higher mean serum anti-HSA-MDA level compared to the control group. |
| SMAs | Reimand et al. [59] | 2001 | 15% vs. 5.1% p < 0.005 | SMAs were significantly more frequent in PCOS than in the control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, J.; Akpang, N.; Ziemkiewicz, Z.; Zaborowska, L.; Ludwin, A. Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 8192. https://doi.org/10.3390/ijms26178192
Kwiatkowski J, Akpang N, Ziemkiewicz Z, Zaborowska L, Ludwin A. Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2025; 26(17):8192. https://doi.org/10.3390/ijms26178192
Chicago/Turabian StyleKwiatkowski, Jakub, Nicole Akpang, Zofia Ziemkiewicz, Lucja Zaborowska, and Artur Ludwin. 2025. "Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications" International Journal of Molecular Sciences 26, no. 17: 8192. https://doi.org/10.3390/ijms26178192
APA StyleKwiatkowski, J., Akpang, N., Ziemkiewicz, Z., Zaborowska, L., & Ludwin, A. (2025). Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 26(17), 8192. https://doi.org/10.3390/ijms26178192






