Ultrastructural Insight into Rift Valley Fever Virus Pathogenesis in Different Human Cell Types
Abstract
1. Introduction
2. Results
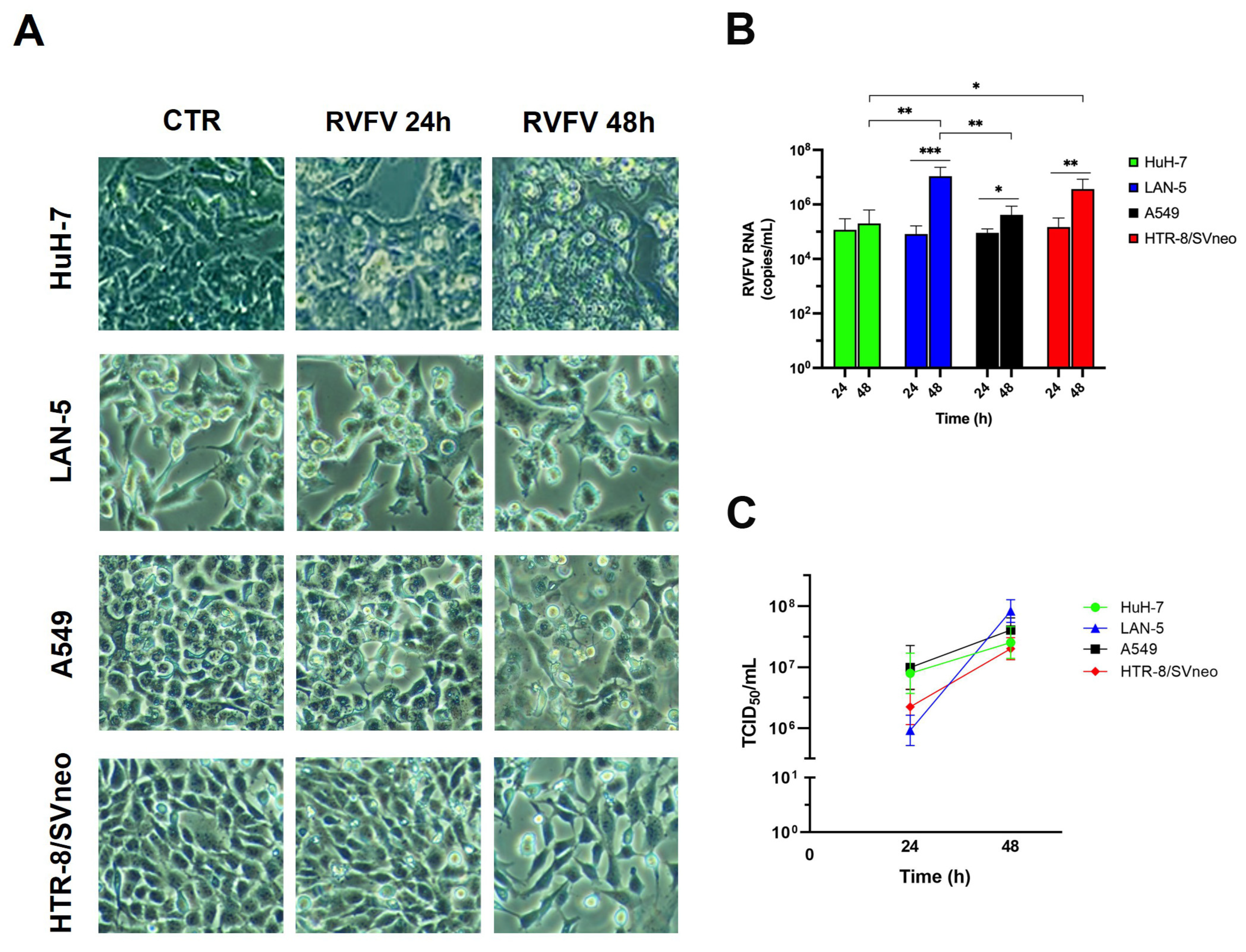
2.1. RVFV Is Able to Infect Human HuH-7, LAN5, A549, and HTR-8/SVneo Cell Lines and to Establish a Productive Infection
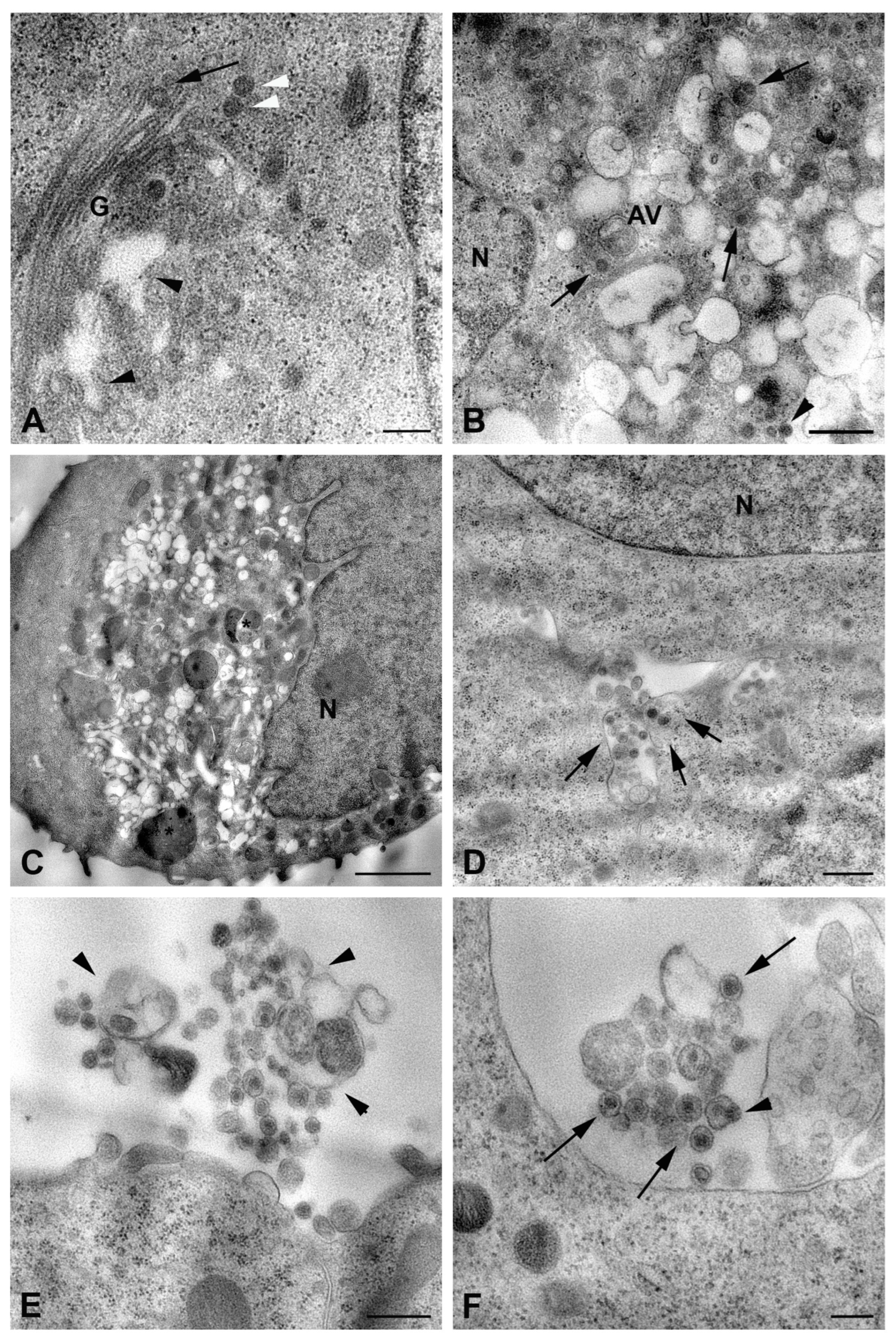
2.2. Ultrastructural Analysis of RVFV Infection
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Viral Production
4.3. Viral Infection
4.4. TCID50 Assay
4.5. Viral Quantification by Real-Time RT-PCR
4.6. Transmission Electron Microscopy
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RVFV | Rift Valley Fever Virus |
| TEM | Transmission Electron Microscopy |
| NCPV | National Collection of Pathogenic Viruses |
| MOI | Multiplicity of Infection |
| TCID50 | Tissue Culture Infectious Dose 50 |
| p.i. | Post Infection |
| BSL-3 | Biosafety Level 3 |
| CPE | Cytopathic Effect |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| BUNV | Bunyamwera Virus |
| EVs | Extracellular Membrane Vesicles |
| ZIKV | Zika Virus |
| ROS | Reactive Oxygen Species |
| MAVS | Mitochondrial Antiviral Signaling Protein |
| DAMPs | Damage-Associated Molecular Patterns |
| PE | Preeclampsia |
| FGR | Fetal Growth Restriction |
References
- Sun, M.H.; Ji, Y.F.; Li, G.H.; Shao, J.W.; Chen, R.X.; Gong, H.Y.; Chen, S.Y.; Chen, J.M. Highly Adaptive Phenuiviridae with Biomedical Importance in Multiple Fields. J. Med. Virol. 2022, 94, 2388–2401. [Google Scholar] [CrossRef] [PubMed]
- Javelle, E.; Lesueur, A.; De Santi, V.P.; De Laval, F.; Lefebvre, T.; Holweck, G.; Durand, G.A.; Leparc-Goffart, I.; Texier, G.; Simon, F. The Challenging Management of Rift Valley Fever in Humans: Literature Review of the Clinical Disease and Algorithm Proposal. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 4. [Google Scholar] [CrossRef]
- Gibson, S.; Noronha, L.E.; Tubbs, H.; Cohnstaedt, L.W.; Wilson, W.C.; Mire, C.; Mitzel, D.; Anyamba, A.; Rostal, M.; Linthicum, K.J. The Increasing Threat of Rift Valley Fever Virus Globalization: Strategic Guidance for Protection and Preparation. J. Med. Entomol. 2023, 60, 1197–1213. [Google Scholar] [CrossRef]
- Ebogo-Belobo, J.T.; Kenmoe, S.; Abanda, N.N.; Bowo-Ngandji, A.; Mbaga, D.S.; Magoudjou-Pekam, J.N.; Kame-Ngasse, G.I.; Tchatchouang, S.; Menkem, E.Z.; Okobalemba, E.A.; et al. Contemporary Epidemiological Data of Rift Valley Fever Virus in Humans, Mosquitoes and Other Animal Species in Africa: A Systematic Review and Meta-analysis. Vet. Med. Sci. 2023, 9, 2309. [Google Scholar] [CrossRef]
- Lapa, D.; Pauciullo, S.; Ricci, I.; Garbuglia, A.R.; Maggi, F.; Scicluna, M.T.; Tofani, S. Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics. Biomedicines 2024, 12, 540. [Google Scholar] [CrossRef]
- Odendaal, L.; Clift, S.J.; Fosgate, G.T.; Davis, A.S. Ovine Fetal and Placental Lesions and Cellular Tropism in Natural Rift Valley Fever Virus Infections. Vet. Pathol. 2020, 57, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Wang, Z.; Yang, W.; Li, E.; Xia, X.; Yan, F.; Chiu, S. Emerging and Reemerging Infectious Diseases: Global Trends and New Strategies for Their Prevention and Control. Signal Transduct. Target. Ther. 2024, 9, 223. [Google Scholar] [CrossRef]
- Baudin, M.; Jumaa, A.M.; Jomma, H.J.E.; Karsany, M.S.; Bucht, G.; Näslund, J.; Ahlm, C.; Evander, M.; Mohamed, N. Association of Rift Valley Fever Virus Infection with Miscarriage in Sudanese Women: A Cross-Sectional Study. Lancet Glob. Health 2016, 4, e864–e871. [Google Scholar] [CrossRef]
- McMillen, C.M.; Hartman, A.L. Rift Valley Fever: A Threat to Pregnant Women Hiding in Plain Sight? J. Virol. 2021, 95, e01394-19. [Google Scholar] [CrossRef]
- Freiberg, A.N.; Sherman, M.B.; Morais, M.C.; Holbrook, M.R.; Watowich, S.J. Three-Dimensional Organization of Rift Valley Fever Virus Revealed by Cryoelectron Tomography. J. Virol. 2008, 82, 10341–10348. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Plegge, T.; Pöhlmann, S. The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release. Viruses 2016, 8, 202. [Google Scholar] [CrossRef]
- Weber, F.; Bouloy, M.; Lozach, P.Y. An Introduction to Rift Valley Fever Virus. Methods Mol. Biol. 2024, 2824, 1–14. [Google Scholar] [CrossRef]
- Smith, D.R.; Bird, B.H.; Lewis, B.; Johnston, S.C.; McCarthy, S.; Keeney, A.; Botto, M.; Donnelly, G.; Shamblin, J.; Albariño, C.G.; et al. Development of a Novel Nonhuman Primate Model for Rift Valley Fever. J. Virol. 2012, 86, 2109. [Google Scholar] [CrossRef]
- Hartman, A. Rift Valley Fever. Clin. Lab. Med. 2017, 37, 285–301. [Google Scholar] [CrossRef]
- Odendaal, L.; Clift, S.J.; Fosgate, G.T.; Davis, A.S. Lesions and Cellular Tropism of Natural Rift Valley Fever Virus Infection in Adult Sheep. Vet. Pathol. 2019, 56, 61–77. [Google Scholar] [CrossRef]
- Brown, J.L.; Dominik, J.W.; Morrissey, R.L. Respiratory Infectivity of a Recently Isolated Egyptian Strain of Rift Valley Fever Virus. Infect. Immun. 1981, 33, 848–853. [Google Scholar] [CrossRef]
- Reed, C.; Lin, K.; Wilhelmsen, C.; Friedrich, B.; Nalca, A.; Keeney, A.; Donnelly, G.; Shamblin, J.; Hensley, L.E.; Olinger, G.; et al. Aerosol Exposure to Rift Valley Fever Virus Causes Earlier and More Severe Neuropathology in the Murine Model, Which Has Important Implications for Therapeutic Development. PLoS Negl. Trop. Dis. 2013, 7, e2156. [Google Scholar] [CrossRef] [PubMed]
- Gwon, Y.D.; Mahani, S.A.N.; Nagaev, I.; Mincheva-Nilsson, L.; Evander, M. Rift Valley Fever Virus Propagates in Human Villous Trophoblast Cell Lines and Induces Cytokine Mrna Responses Known to Provoke Miscarriage. Viruses 2021, 13, 2265. [Google Scholar] [CrossRef] [PubMed]
- Oymans, J.; Wichgers Schreur, P.J.; van Keulen, L.; Kant, J.; Kortekaas, J. Rift Valley Fever Virus Targets the Maternal-Foetal Interface in Ovine and Human Placentas. PLoS Negl. Trop. Dis. 2020, 14, 1–18. [Google Scholar] [CrossRef]
- Anywaine, Z.; Lule, S.A.; Hansen, C.; Warimwe, G.; Elliott, A. Clinical Manifestations of Rift Valley Fever in Humans: Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010233. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Steele, K.E.; Honko, A.; Shamblin, J.; Hensley, L.E.; Smith, D.R. Ultrastructural Study of Rift Valley Fever Virus in the Mouse Model. Virology 2012, 431, 58–70. [Google Scholar] [CrossRef]
- Bamia, A.; Marcato, V.; Boissière, M.; Mansuroglu, Z.; Tamietti, C.; Romani, M.; Simon, D.; Tian, G.; Niedergang, F.; Panthier, J.-J.; et al. The NSs Protein Encoded by the Virulent Strain of Rift Valley Fever Virus Targets the Expression of Abl2 and the Actin Cytoskeleton of the Host, Affecting Cell Mobility, Cell Shape, and Cell-Cell Adhesion. J. Virol. 2020, 95, e01768-20. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; López-Montero, N.; Elliott, R.M.; Fernández, J.J.; Risco, C. The Unique Architecture of Bunyamwera Virus Factories around the Golgi Complex. Cell. Microbiol. 2008, 10, 2012–2028. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Ahmed, I.; Urmi, T.J. The Pathogenicity and Risk Evaluation of Rift Valley Virus to Cause Mysterious “Disease X”: An Update on Recent Evidences. Ann. Med. Surg. 2024, 86, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.S.; Simpson, D.I.H.; Stamford, S.; Abdel Wahab, K.S.E. Rift Valley Fever Virus: Some Ultrastructural Observations on Material from the Outbreak in Egypt 1977. J. Gen. Virol. 1979, 42, 329–337. [Google Scholar] [CrossRef]
- Smith, D.R.; Steele, K.E.; Shamblin, J.; Honko, A.; Johnson, J.; Reed, C.; Kennedy, M.; Chapman, J.L.; Hensley, L.E. The Pathogenesis of Rift Valley Fever Virus in the Mouse Model. Virology 2010, 407, 256–267. [Google Scholar] [CrossRef]
- Walter, C.T.; Barr, J.N. Recent Advances in the Molecular and Cellular Biology of Bunyaviruses. J. Gen. Virol. 2011, 92, 2467–2484. [Google Scholar] [CrossRef]
- Carnec, X.; Ermonval, M.; Kreher, F.; Flamand, M.; Bouloy, M. Role of the Cytosolic Tails of Rift Valley Fever Virus Envelope Glycoproteins in Viral Morphogenesis. Virology 2014, 448, 1–14. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kordbacheh, R.; Sin, J. Extracellular Vesicles: A Novel Mode of Viral Propagation Exploited by Enveloped and Non-Enveloped Viruses. Microorganisms 2024, 12, 274. [Google Scholar] [CrossRef]
- Fu, Y.; Zi, R.; Xiong, S. Infection by Exosome-Carried Coxsackievirus B3 Induces Immune Escape Resulting in an Aggravated Pathogenesis. Microbes Infect. 2023, 25, 105148. [Google Scholar] [CrossRef]
- Segredo-Otero, E.; Sanjuán, R. The Effect of Genetic Complementation on the Fitness and Diversity of Viruses Spreading as Collective Infectious Units. Virus Res. 2019, 267, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes Mediate Zika Virus Transmission through SMPD3 Neutral Sphingomyelinase in Cortical Neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Kutchy, N.A.; Peeples, E.S.; Sil, S.; Liao, K.; Chivero, E.T.; Hu, G.; Buch, S. Extracellular Vesicles in Viral Infections of the Nervous System. Viruses 2020, 12, 700. [Google Scholar] [CrossRef]
- Dasgupta, A. Targeting TFIIH to Inhibit Host Cell Transcription by Rift Valley Fever Virus. Mol. Cell 2004, 13, 456–458. [Google Scholar] [CrossRef]
- Léger, P.; Nachman, E.; Richter, K.; Tamietti, C.; Koch, J.; Burk, R.; Kummer, S.; Xin, Q.; Stanifer, M.; Bouloy, M.; et al. NSs Amyloid Formation Is Associated with the Virulence of Rift Valley Fever Virus in Mice. Nat. Commun. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Peng, K.; Lozach, P.Y. Rift Valley Fever Virus: A New Avenue of Research on the Biological Functions of Amyloids? Future Virol. 2021, 16, 677–689. [Google Scholar] [CrossRef]
- Léger, P.; Lara, E.; Jagla, B.; Sismeiro, O.; Mansuroglu, Z.; Coppée, J.Y.; Bonnefoy, E.; Bouloy, M. Dicer-2- and Piwi-Mediated RNA Interference in Rift Valley Fever Virus-Infected Mosquito Cells. J. Virol. 2013, 87, 1631–1648. [Google Scholar] [CrossRef]
- Duan, X.; Liu, R.; Lan, W.; Liu, S. The Essential Role of Mitochondrial Dynamics in Viral Infections. Int. J. Mol. Sci. 2025, 26, 1955. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Yao, C.H.; Wang, R.; Wang, Y.; Kung, C.P.; Weber, J.D.; Patti, G.J. Mitochondrial Fusion Supports Increased Oxidative Phosphorylation during Cell Proliferation. Elife 2019, 8, e41351. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Huang, X.; Shi, Y.; Jiang, Y.; Han, Y.; Zhang, Y. Mitochondrial Dysfunction in Pregnancy Loss: A Review. Mol. Cell. Biochem. 2024, 480, 2749–2764. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapa, D.; Romeo, M.A.; Duca, L.; Castelli, C.; Specchiarello, E.; Maggi, F.; Falasca, L. Ultrastructural Insight into Rift Valley Fever Virus Pathogenesis in Different Human Cell Types. Int. J. Mol. Sci. 2025, 26, 8183. https://doi.org/10.3390/ijms26178183
Lapa D, Romeo MA, Duca L, Castelli C, Specchiarello E, Maggi F, Falasca L. Ultrastructural Insight into Rift Valley Fever Virus Pathogenesis in Different Human Cell Types. International Journal of Molecular Sciences. 2025; 26(17):8183. https://doi.org/10.3390/ijms26178183
Chicago/Turabian StyleLapa, Daniele, Maria Anele Romeo, Leonardo Duca, Carlotta Castelli, Eliana Specchiarello, Fabrizio Maggi, and Laura Falasca. 2025. "Ultrastructural Insight into Rift Valley Fever Virus Pathogenesis in Different Human Cell Types" International Journal of Molecular Sciences 26, no. 17: 8183. https://doi.org/10.3390/ijms26178183
APA StyleLapa, D., Romeo, M. A., Duca, L., Castelli, C., Specchiarello, E., Maggi, F., & Falasca, L. (2025). Ultrastructural Insight into Rift Valley Fever Virus Pathogenesis in Different Human Cell Types. International Journal of Molecular Sciences, 26(17), 8183. https://doi.org/10.3390/ijms26178183




