Novel Insights into the Molecular Mechanisms of Chicken Breast Muscle Development by Integrating Non-Coding RNA and mRNA Profiles
Abstract
1. Introduction
2. Results
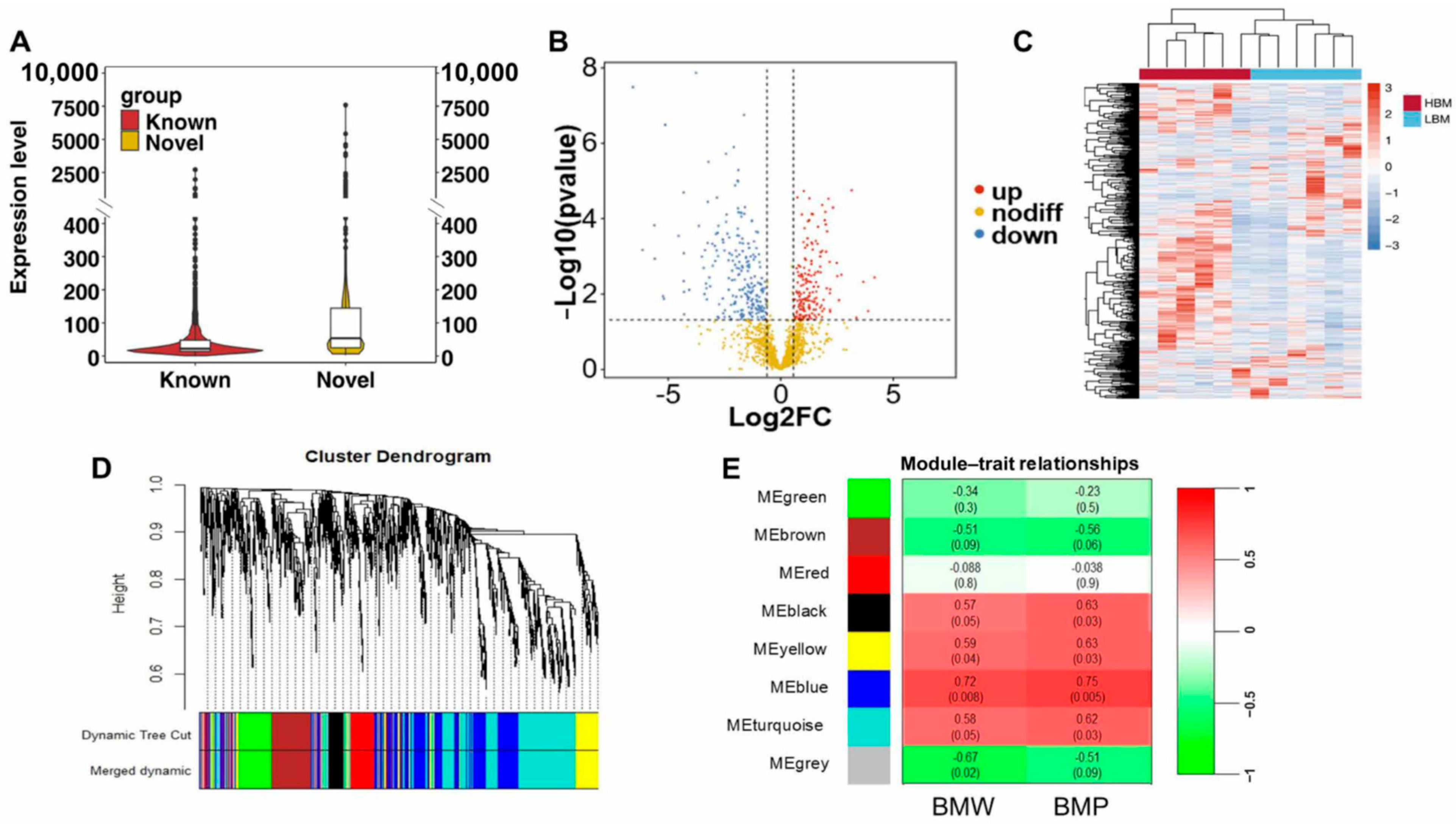
2.1. LncRNA/circRNA Profiling of Breast Muscle in Chicken
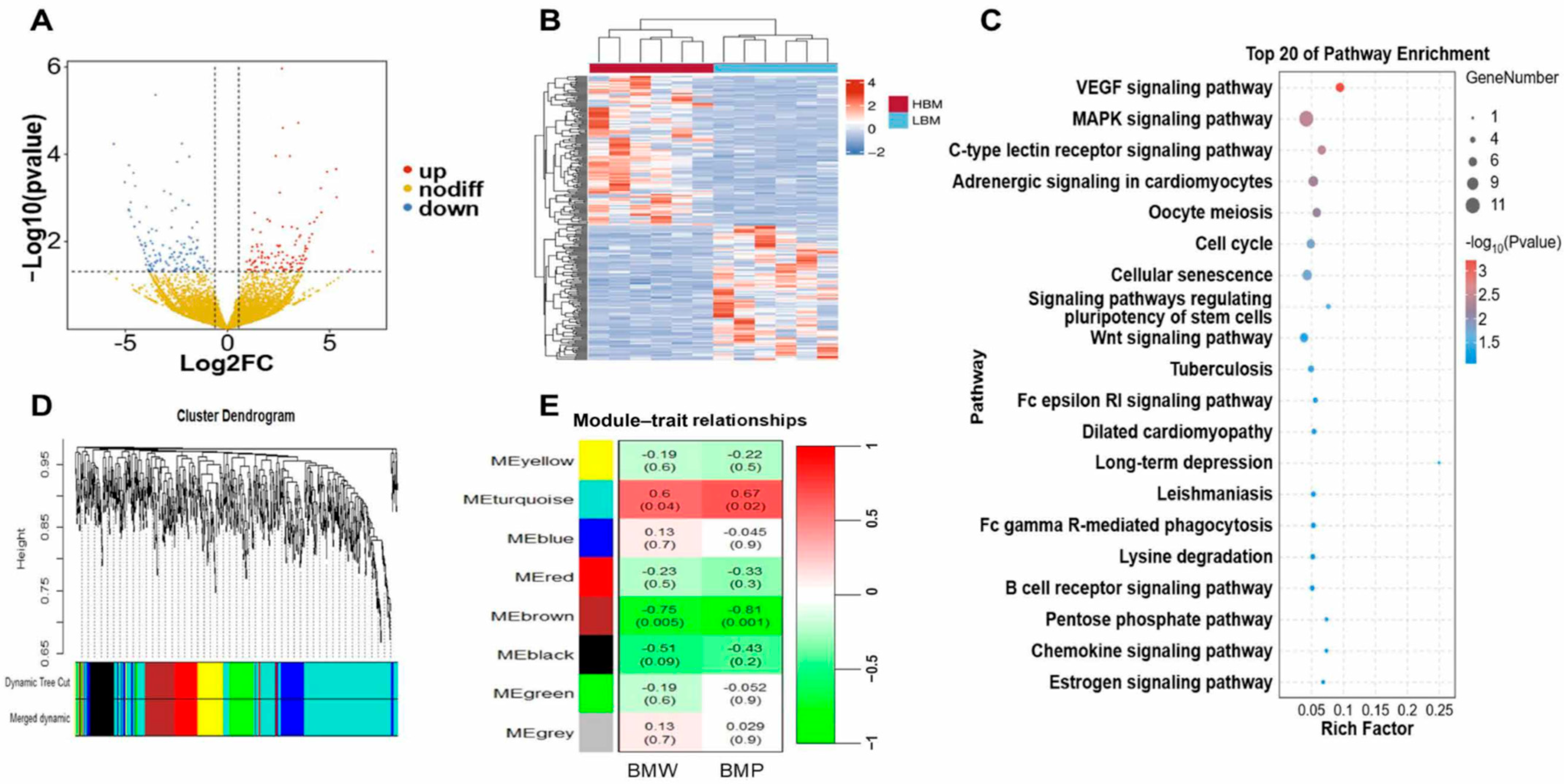
2.2. Identification of Candidate lncRNAs Affecting Breast Muscle Development
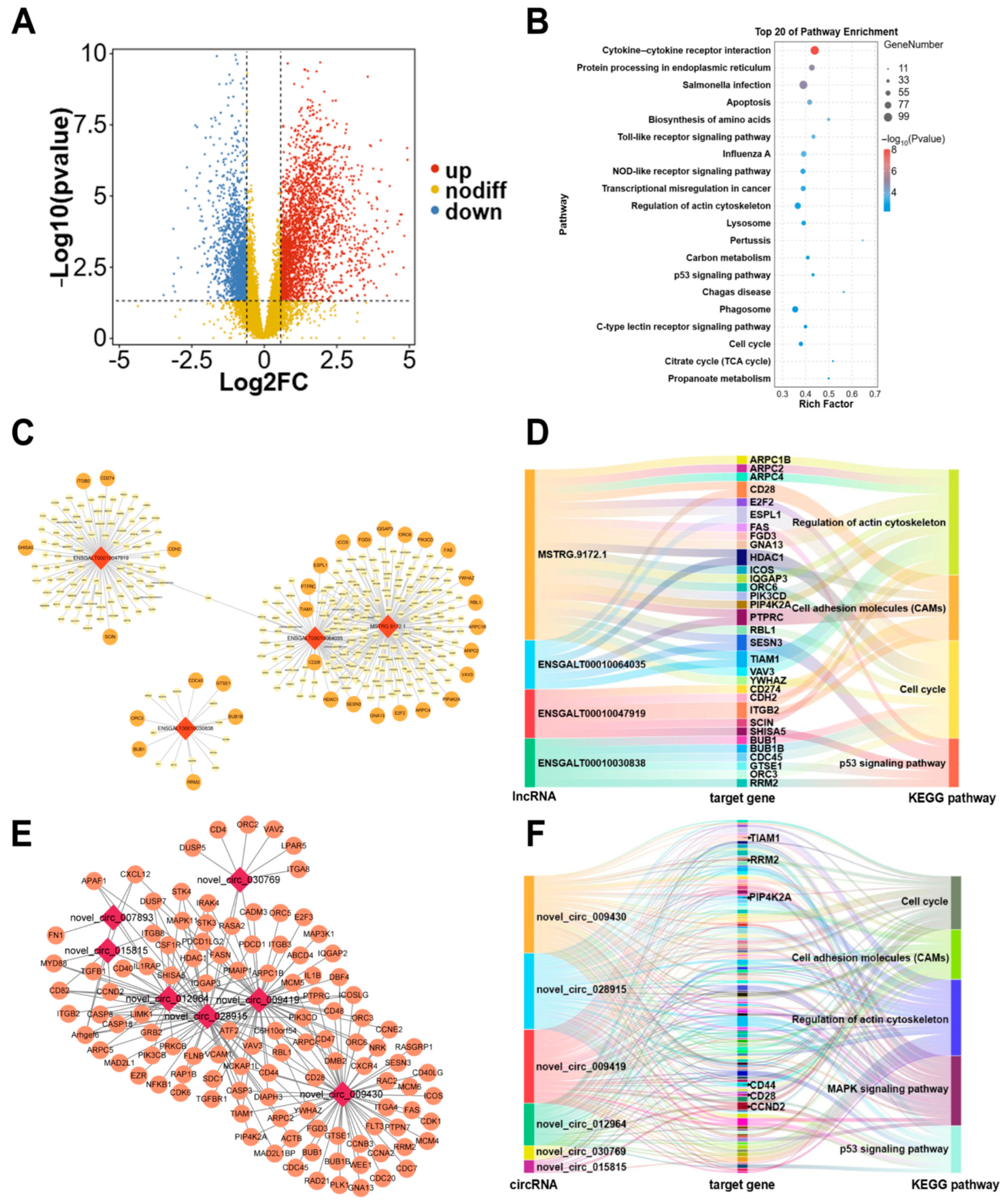
2.3. Screening for Candidate circRNAs Affecting Breast Muscle Development
2.4. Identification of Hub Genes and Construction of lncRNA/circRNA-mRNA Networks
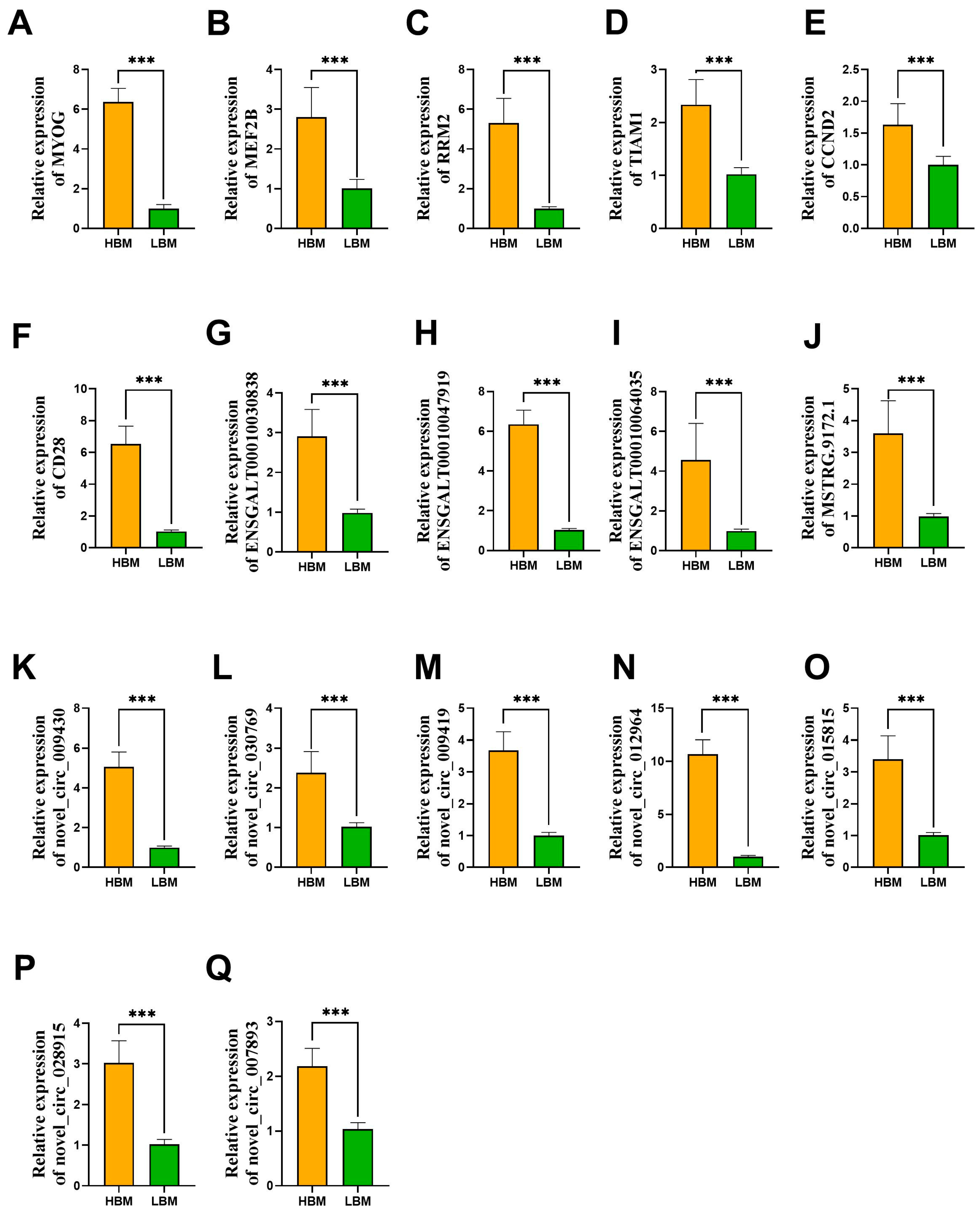
2.5. Validation of Candidate lncRNAs/circRNAs and mRNAs
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Total RNA Isolation and Detection
4.3. Library Preparation and Sequencing of Non-Coding RNAs
4.4. Quality Control, rRNA Mapping and Removal, and Genome Alignment
4.5. Prediction and Quantification of Novel lncRNAs
4.6. Identification and Quantification of circRNAs
4.7. Detection of Differentially Expressed Genes/Non-Coding RNAs
4.8. Weighted Gene Co-Expression Network Analysis
4.9. Functional Enrichment Analysis
4.10. Construction of the CeRNA Network for lncRNA/circRNA–mRNA Pairs
4.11. Validation of Candidate lncRNAs, circRNAs, and mRNAs
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The formation of skeletal muscle: From somite to limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, K.; Zhang, J.; Ling, X.; Zhang, X.; Zhang, L.; Li, P.; Wei, Q.; Zhang, T.; Wang, X.; et al. Identification of crucial circRNAs in skeletal muscle during chicken embryonic development. BMC Genom. 2022, 23, 330. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, J.W.; Zhang, R.P.; Chen, X.; Yu, H.Y.; Jin, H.B.; Li, L.; Han, C.C.; Xu, F.; Kang, B.; et al. In ovo feeding of IGF-1 to ducks influences neonatal skeletal muscle hypertrophy and muscle mass growth upon satellite cell activation. J. Cell Physiol. 2012, 227, 1465–1475. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes. Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef]
- Chen, R.; Lei, S.; Jiang, T.; Zeng, J.; Zhou, S.; She, Y. Roles of lncRNAs and circRNAs in regulating skeletal muscle development. Acta Physiol. 2020, 228, e13356. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ouyang, H.; Zheng, M.; Cai, B.; Han, P.; Abdalla, B.A.; Nie, Q.; Zhang, X. Integrated Analysis of Long Non-coding RNAs (LncRNAs) and mRNA Expression Profiles Reveals the Potential Role of LncRNAs in Skeletal Muscle Development of the Chicken. Front. Physiol. 2017, 7, 687. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, M.; Yuan, R.; Zhou, Z.; Zhang, J.; Kong, S.; Lin, D.; Lian, L.; Li, J.; Zhang, X.; et al. MYH1G-AS is a chromatin-associated lncRNA that regulates skeletal muscle development in chicken. Cell Mol. Biol. Lett. 2024, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Tang, H.; Sha, Z.; Chen, R.; Chen, L.; Yu, Y.; Rowe, G.C.; Das, S.; Xiao, J. Inhibition of lncRNA MAAT Controls Multiple Types of Muscle Atrophy by cis- and trans-Regulatory Actions. Mol. Ther. 2020, 29, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jia, X.; Xu, J. The new function of circRNA: Translation. Clin. Transl. Oncol. 2020, 22, 2162–2169. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Yin, H.; Shen, X.; Zhao, J.; Cao, X.; He, H.; Han, S.; Chen, Y.; Cui, C.; Wei, Y.; Wang, Y.; et al. Circular RNA CircFAM188B Encodes a Protein That Regulates Proliferation and Differentiation of Chicken Skeletal Muscle Satellite Cells. Front. Cell Dev. Biol. 2020, 8, 522588. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, M.; Zhang, B.; Zhang, J.; Shi, Y.; Ma, T.; Sun, Y. CircGRB14 Inhibits Proliferation and Promotes Apoptosis of Granulosa Cells in Chicken Follicle Selection Through Sponging miR-12264-3p and miR-6660-3p. Int. J. Mol. Sci. 2025, 26, 2214. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Wu, W.; Zou, J. Studies on the Role of circRNAs in Osteoarthritis. Biomed. Res. Int. 2021, 2021, 8231414. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Yan, S.; Pei, Y.; Li, J.; Tang, Z.; Yang, Y. Recent Progress on Circular RNAs in the Development of Skeletal Muscle and Adipose Tissues of Farm Animals. Biomolecules 2023, 13, 314. [Google Scholar] [CrossRef]
- Wei, Y.; Tian, Y.; Li, X.; Amevor, F.K.; Shen, X.; Zhao, J.; Zhao, X.; Zhang, X.; Huang, W.; Hu, J.; et al. Circular RNA circFNDC3AL Upregulates BCL9 Expression to Promote Chicken Skeletal Muscle Satellite Cells Proliferation and Differentiation by Binding to miR-204. Front. Cell Dev. Biol. 2021, 9, 736749. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, J.; Guo, L.; Byers, M.S.; Wang, Z.; Chen, X.; Xu, H.; Nie, Q. Circular RNA circHIPK3 Promotes the Proliferation and Differentiation of Chicken Myoblast Cells by Sponging miR-30a-3p. Cells 2019, 8, 177. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, X.; Du, Y.; Zhao, J.; Ge, C. Regulation of Non-Coding RNA in the Growth and Development of Skeletal Muscle in Domestic Chickens. Genes 2022, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, R.; Li, W.; Zheng, M.; Zhu, D.; Liu, D.; Feng, F.; Li, Q.; Liu, L.; Wen, J.; et al. Assessment the effect of genomic selection and detection of selective signature in broilers. Poult. Sci. 2022, 101, 101856. [Google Scholar] [CrossRef]
- Tan, X.; Liu, R.; Zhao, D.; He, Z.; Li, W.; Zheng, M.; Li, Q.; Wang, Q.; Liu, D.; Feng, F.; et al. Large-scale genomic and transcriptomic analyses elucidate the genetic basis of high meat yield in chickens. J. Adv. Res. 2024, 55, 1–16. [Google Scholar] [CrossRef]
- Hubert, S.; Athrey, G. Transcriptomic signals of mitochondrial dysfunction and OXPHOS dynamics in fast-growth chicken. PeerJ 2022, 10, e13364. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Selamat, J.; Jambari, N.N.; Sukor, R.; Murugesu, S.; Khatib, A. Muscle and Serum Metabolomics for Different Chicken Breeds under Commercial Conditions by GC-MS. Foods 2021, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Alessandroni, L.; Sagratini, G.; Gagaoua, M. Integrated Chemometrics and Data-Independent Acquisition Proteomics for the Discovery of Meat Authenticity Biomarkers: A Study on Early Post-Mortem Pectoralis major Muscle Proteomes of Ross 308 and Ranger Classic Chicken Produced by Organic versus Antibiotic-Free Farming Systems. J. Agric. Food Chem. 2024, 72, 20153–20170. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, M.; Zhang, J.; Wang, Z.; Kong, S.; Zhou, Z.; Lian, L.; Zhang, J.; Li, J.; Wang, Y.; et al. LncEDCH1 improves mitochondrial function to reduce muscle atrophy by interacting with SERCA2. Mol. Ther.–Nucleic Acids 2021, 27, 319–334. [Google Scholar] [CrossRef]
- Greco, S.; Cardinali, B.; Falcone, G.; Martelli, F. Circular RNAs in Muscle Function and Disease. Int. J. Mol. Sci. 2018, 19, 3454. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Jawasreh, K.; Al Athamneh, S.; Al-Zghoul, M.B.; Al Amareen, A.; AlSukhni, I.; Aad, P. Evaluation of growth performance and muscle marker genes expression in four different broiler strains in Jordan. Ital. J. Anim. Sci. 2019, 18, 766–776. [Google Scholar] [CrossRef]
- Tan, X.; He, Z.; Fahey, A.G.; Zhao, G.; Liu, R.; Wen, J. Research progress and applications of genome-wide association study in farm animals. Anim. Res. One Health 2023, 1, 56–77. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Ye, H.; Tu, W. Weighted gene co-expression network analysis in biomedicine research. Sheng Wu Gong Cheng Xue Bao 2017, 33, 1791–1801. [Google Scholar] [CrossRef]
- Becsky, D.; Gyulai-Nagy, S.; Balind, A.; Horvath, P.; Dux, L.; Keller-Pinter, A. Myoblast Migration and Directional Persistence Affected by Syndecan-4-Mediated Tiam-1 Expression and Distribution. Int. J. Mol. Sci. 2020, 21, 823. [Google Scholar] [CrossRef]
- Li, M.; Zhang, N.; Zhang, W.; Hei, W.; Cai, C.; Yang, Y.; Lu, C.; Gao, P.; Guo, X.; Cao, G.; et al. Comprehensive analysis of differentially expressed circRNAs and ceRNA regulatory network in porcine skeletal muscle. BMC Genom. 2021, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, H.; Deng, C.; Lin, Q.; Yu, P.; Chen, S.; Ruan, J. RRM2 Improves Cardiomyocyte Proliferation after Myocardial Ischemia Reperfusion Injury through the Hippo-YAP Pathway. Dis. Markers 2021, 2021, 5089872. [Google Scholar] [CrossRef]
- Chen, G.; Luo, Y.; Warncke, K.; Sun, Y.; Yu, D.S.; Fu, H.; Behera, M.; Ramalingam, S.S.; Doetsch, P.W.; Duong, D.M.; et al. Acetylation regulates ribonucleotide reductase activity and cancer cell growth. Nat. Commun. 2019, 10, 3213. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Y.; Niu, Y.; Wang, Y.; Liu, Y.; Ji, H.; Han, R.; Tian, Y.; Liu, X.; Kang, X.; et al. RRM2 promotes the proliferation of chicken myoblasts, inhibits their differentiation and muscle regeneration. Poult. Sci. 2024, 103, 103407. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.; Yu, S.; Wang, W.; Liu, G.; Jiang, X.; Sun, D. Circular RNAs regulate parental gene expression: A new direction for molecular oncology research. Front. Oncol. 2022, 12, 947775. [Google Scholar] [CrossRef]
- Keren, A.; Tamir, Y.; Bengal, E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol. Cell Endocrinol. 2006, 252, 224–230. [Google Scholar] [CrossRef]
- Xie, S.J.; Li, J.H.; Chen, H.F.; Tan, Y.Y.; Liu, S.R.; Zhang, Y.; Xu, H.; Yang, J.H.; Liu, S.; Zheng, L.L.; et al. Inhibition of the JNK/MAPK signaling pathway by myogenesis-associated miRNAs is required for skeletal muscle development. Cell Death Differ. 2018, 25, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, Z.; Deng, K.; Li, J.; Liu, Z.; Huang, X.; Wang, F.; Fan, Y. circUSP13 facilitates the fast-to-slow myofiber shift via the MAPK/ERK signaling pathway in goat skeletal muscles. J. Cell Physiol. 2024, 239, e31226. [Google Scholar] [CrossRef]
- Kegley, K.M.; Gephart, J.; Warren, G.L.; Pavlath, G.K. Altered primary myogenesis in NFATC3(-/-) mice leads to decreased muscle size in the adult. Dev. Biol. 2001, 232, 115–126. [Google Scholar] [CrossRef]
- Boyer, J.G.; Prasad, V.; Song, T.; Lee, D.; Fu, X.; Grimes, K.M.; Sargent, M.A.; Sadayappan, S.; Molkentin, J.D. ERK1/2 signaling induces skeletal muscle slow fiber-type switching and reduces muscular dystrophy disease severity. JCI Insight 2019, 5, e127356. [Google Scholar] [CrossRef]
- Alexander, M.S.; Rozkalne, A.; Colletta, A.; Spinazzola, J.M.; Johnson, S.; Rahimov, F.; Meng, H.; Lawlor, M.W.; Estrella, E.; Kunkel, L.M.; et al. CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell 2016, 19, 800–807. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Li, X.; Li, W.; Bai, Z. Gene identification and functional analysis of a D-type cyclin (CCND2) in freshwater pearl mussel (Hyriopsis cumingii). Mol. Biol. Rep. 2022, 49, 6601–6611. [Google Scholar] [CrossRef]
- Khanjyan, M.V.; Yang, J.; Kayali, R.; Caldwell, T.; Bertoni, C. A high-content, high-throughput siRNA screen identifies cyclin D2 as a potent regulator of muscle progenitor cell fusion and a target to enhance muscle regeneration. Hum. Mol. Genet. 2013, 22, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Apponi, L.H.; Long, K.K.; Pavlath, G.K. Chemokine expression and control of muscle cell migration during myogenesis. J. Cell Sci. 2010, 123, 3052–3060. [Google Scholar] [CrossRef]
- Yang, Z.J.; Broz, D.K.; Noderer, W.L.; Ferreira, J.P.; Overton, K.W.; Spencer, S.L.; Meyer, T.; Tapscott, S.J.; Attardi, L.D.; Wang, C.L. p53 suppresses muscle differentiation at the myogenin step in response to genotoxic stress. Cell Death Differ. 2015, 22, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Liu, Y.; Shan, Y.; Ji, G.; Zhang, M.; Tu, Y.; Zou, J.; Chen, X.; Geng, Z.; Shu, J. Analysis of potential regulatory LncRNAs and CircRNAs in the oxidative myofiber and glycolytic myofiber of chickens. Sci. Rep. 2021, 11, 20861. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 54. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 2018, 7, 1338. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Shumate, A.; Wong, B.; Pertea, G.; Pertea, M. Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Comput. Biol. 2022, 18, e1009730. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Wucher, V.; Legeai, F.; Hédan, B.; Rizk, G.; Lagoutte, L.; Leeb, T.; Jagannathan, V.; Cadieu, E.; David, A.; Lohi, H.; et al. FEELnc: A tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res. 2017, 45, e57. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, J.; Zhao, F. Accurate quantification of circular RNAs identifies extensive circular isoform switching events. Nat. Commun. 2020, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Dong, J.; Li, J.; Huang, M.; Wang, D.; Tan, X. Novel Insights into the Molecular Mechanisms of Chicken Breast Muscle Development by Integrating Non-Coding RNA and mRNA Profiles. Int. J. Mol. Sci. 2025, 26, 8181. https://doi.org/10.3390/ijms26178181
Jin Y, Dong J, Li J, Huang M, Wang D, Tan X. Novel Insights into the Molecular Mechanisms of Chicken Breast Muscle Development by Integrating Non-Coding RNA and mRNA Profiles. International Journal of Molecular Sciences. 2025; 26(17):8181. https://doi.org/10.3390/ijms26178181
Chicago/Turabian StyleJin, Yuting, Jie Dong, Jiahua Li, Minjie Huang, Deqian Wang, and Xiaodong Tan. 2025. "Novel Insights into the Molecular Mechanisms of Chicken Breast Muscle Development by Integrating Non-Coding RNA and mRNA Profiles" International Journal of Molecular Sciences 26, no. 17: 8181. https://doi.org/10.3390/ijms26178181
APA StyleJin, Y., Dong, J., Li, J., Huang, M., Wang, D., & Tan, X. (2025). Novel Insights into the Molecular Mechanisms of Chicken Breast Muscle Development by Integrating Non-Coding RNA and mRNA Profiles. International Journal of Molecular Sciences, 26(17), 8181. https://doi.org/10.3390/ijms26178181





