Physical Exercise as a Therapeutic Approach for Patients Living with Type 2 Diabetes: Does the Explanation Reside in Exerkines?—A Review
Abstract
1. Introduction
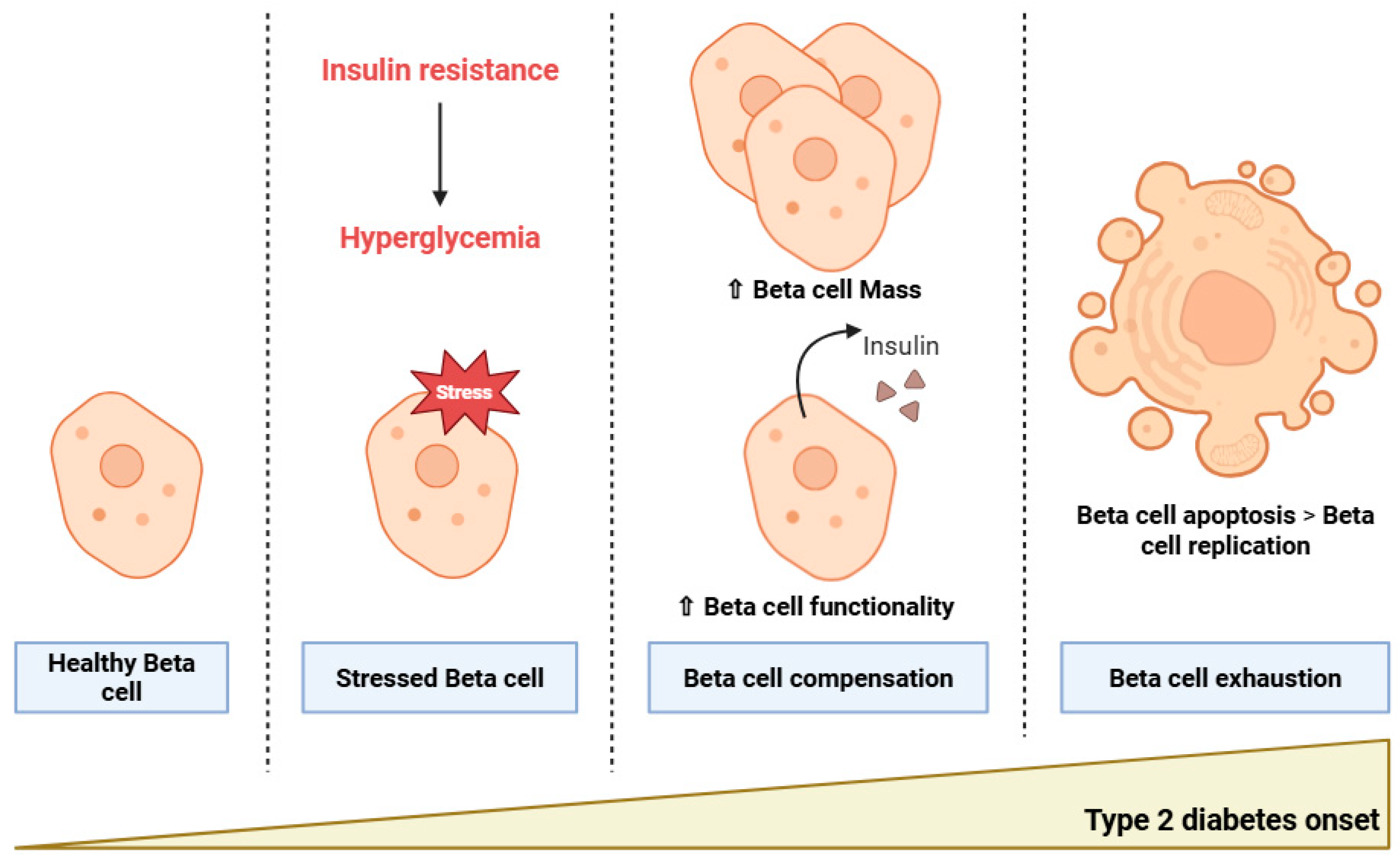
2. Type 2 Diabetes Pathophysiology: From Insulin Resistance to Beta Cell Dysfunction
2.1. Insulin Resistance
2.2. Beta Cell Dysfunction
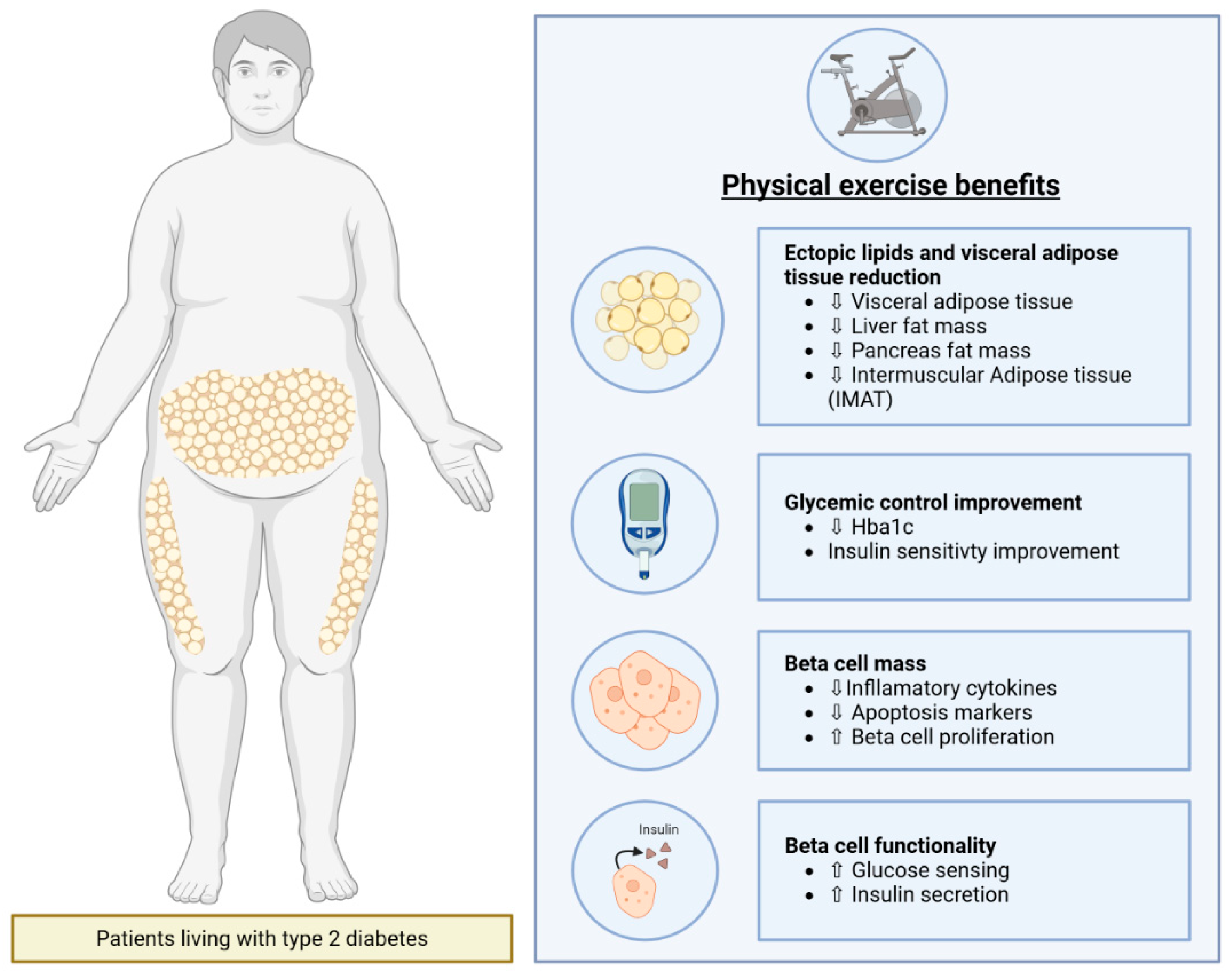
3. Beneficial Impact of Physical Exercise on Type 2 Diabetes
3.1. Physical Exercise Improves Glycemic Control in Patients Living with Type 2 Diabetes
3.2. Physical Exercise Improves Insulin Sensitivity in Patients Living with Type 2 Diabetes
3.3. Physical Exercise Decreases Ectopic Lipids and Visceral Adipose Tissue in Patients Living with Type 2 Diabetes
3.4. Physical Exercise Enhances Beta Cell Mass in Patients Living with T2D
3.5. Physical Exercise Enhances Beta Cell Functionality in Patients Living with Type 2 Diabetes
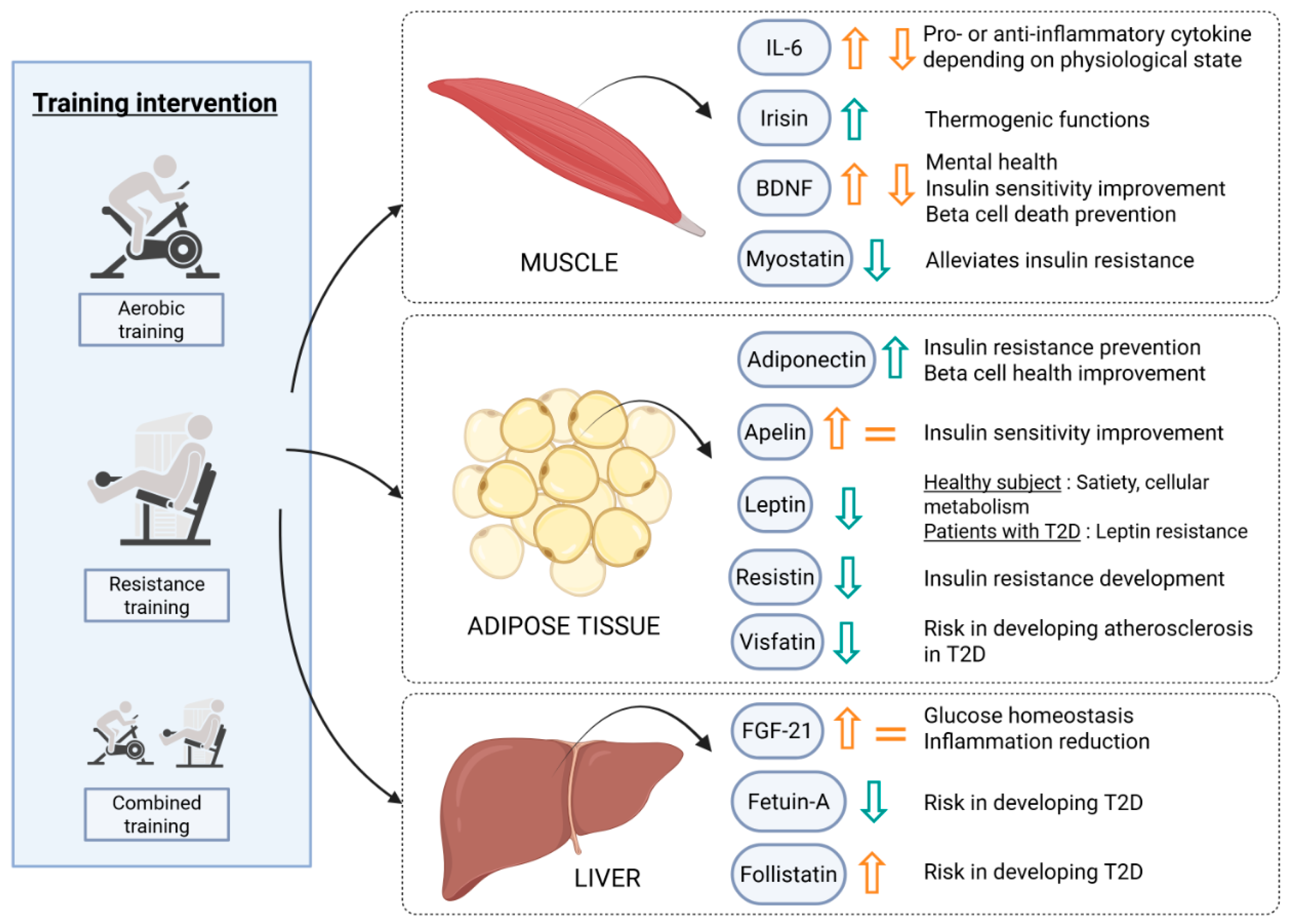
4. Physical Exercise’s Beneficial Impact Could Rely on Endocrine Secretions
4.1. Impact of Physical Exercise on Exerkine Secretion in Patients with Type 2 Diabetes
4.1.1. Skeletal Muscle Secretions
4.1.2. Adipose Tissue Secretions
4.1.3. Liver Secretions
| Exerkines | Studies | Patients | Type of Intervention | Physical Exercise Impact |
|---|---|---|---|---|
| Muscle Secretions | ||||
| IL-6 | [74]: MA RCT | T2D (w or w/o obesity, CAD, overweight) | Aex, RT, CT | ↘ |
| [75]: MA RCT | T2D | Aex, HIIT, RT, CT | ↘ | |
| Irisin | [82]: RCT | T2D (men) | CT (Aex + RT or RT + Aex) | ↗ |
| [84]: SR | MS | NA | ↗ | |
| [83]: SR RCT | T2D | Aex, RT, HIT, MIT | 4 ↗ and 1 = | |
| BDNF | [88]: SR RCT | T2D (4 humans and 7 animals) | Aex (humans) | 5 ↗; 4 ↘ and 2 = |
| Myostatin | [92] | IR (men) | Aex | ↘ |
| [93]: RCT | T2D (men) | Aex (HIIT vs. MICT) | ↘ | |
| [94]: RCT | T2D (elderly men) | RT | ↘ | |
| Adipose Tissue Secretion | ||||
| Adiponectin | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↗ |
| [97]: RCT | T2D + MS | Aex, CT | ↗ | |
| [98] | IGT, T2D | Aex | ↗ | |
| Apelin | [108]: RCT | T2D + overweight | AEX | ↗ |
| [75]: MA RCT | T2D | Aex, RT, CT, HIIT | = | |
| Leptin | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↘ |
| [113]: MA RCT | T2D and pre-diabetes | Aex, RT, CT | ↘ | |
| Resistin | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↘ |
| [114]: RCT | T2D + overweight/obese | Aex | ↘ | |
| [115]: RCT | T2D (PM women) | Aex + diet intervention | = | |
| [116] | NGT, IGT, T2D | Cardiovascular exercise + caloric restriction | = | |
| Visfatin | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↘ |
| Liver Secretions | ||||
| FGF-21 | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↗ |
| [120] | T2D (men) + overweight/obese | Aex | ↗ | |
| [82]: RCT | T2D (men) | CT (Aex + RT or RT + Aex) | = | |
| Fetuin-A | [75]: MA RCT | T2D | Aex, RT, CT, HIIT | ↘ |
| [125]: MA RCT | T2D, obese, NAFLD, CVD | Aex, CT | obese ↘ but T2D = | |
| Follistatin | [82]: RCT | T2D (men) | CT (Aex + RT or RT + Aex) | ↗ |
4.2. Impact of Exercise-Induced Secretions on Pancreatic Islet Health
4.2.1. Impact of Post-Exercise Plasma on Pancreatic Islet Health
4.2.2. Impact of Exerkines on Human Pancreatic Islet Health
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAT | Brown Adipose Tissue |
| BDNF | Brain-derived Neurotrophic Factor |
| BMI | Body Mass Index |
| CVD | Cardiovascular Disease |
| DI | Disposition Index |
| FGF-21 | Fibroblast Growth Factor 21 |
| GDR | Glucose Disposal Rate |
| HIIT | High Intensity Interval Training |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IMAT | Intermuscular Adipose Tissue |
| IGT | Impaired Glucose Tolerance |
| ISI | Insulin Sensitivity Index |
| MICT | Moderate Intensity Continuous Training |
| NAFLD | Non-alcoholic fatty liver disease |
| NGT | Normal Glucose Tolerance |
| T2D | Type 2 Diabetes |
| TNF-α | Tumor Necrosis Factor α |
References
- Strati, M.; Moustaki, M.; Psaltopoulou, T.; Vryonidou, A.; Paschou, S.A. Early Onset Type 2 Diabetes Mellitus: An Update. Endocrine 2024, 85, 965–978. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Watanabe, R.M.; Stram, D.O.; Buchanan, T.A.; Xiang, A.H. High Calorie Intake Is Associated with Worsening Insulin Resistance and β-Cell Function in Hispanic Women After Gestational Diabetes Mellitus. Diabetes Care 2014, 37, 3294–3300. [Google Scholar] [CrossRef]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical Exercise as Therapy for Type 2 Diabetes Mellitus. Diabetes Metab. Res. Rev. 2014, 30 (Suppl. S1), 13–23. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint Position Statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Q.; Pan, B.; Li, R.; Li, Y.; He, J.; Qin, T.; Cao, L.; Zhang, N.; Cao, C.; et al. Exercise Modalities for Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Randomized Trials. Diabetes Metab. Res. Rev. 2023, 39, e3591. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Hepler, C.; Vishvanath, L.; Gupta, R.K. Sorting out Adipocyte Precursors and Their Role in Physiology and Disease. Genes Dev. 2017, 31, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose Tissue Heterogeneity: Implication of Depot Differences in Adipose Tissue for Obesity Complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wysham, C.; Shubrook, J. Beta-Cell Failure in Type 2 Diabetes: Mechanisms, Markers, and Clinical Implications. Postgrad. Med. 2020, 132, 676–686. [Google Scholar] [CrossRef]
- Mezza, T.; Cinti, F.; Cefalo, C.M.A.; Pontecorvi, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Fate in Human Insulin Resistance and Type 2 Diabetes: A Perspective on Islet Plasticity. Diabetes 2019, 68, 1121–1129. [Google Scholar] [CrossRef]
- Prentki, M. Islet Beta Cell Failure in Type 2 Diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-Cell Deficit and Increased Beta-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Yuan, X.; Dai, X.; Liu, L.; Hsue, C.; Miller, J.D.; Fang, Z.; Li, J.; Feng, J.; Huang, Y.; Liu, C.; et al. Comparing the Effects of 6 Months Aerobic Exercise and Resistance Training on Metabolic Control and β-Cell Function in Chinese Patients with Prediabetes: A Multicenter Randomized Controlled Trial. J. Diabetes 2020, 12, 25–37. [Google Scholar] [CrossRef]
- Miranda-Tueros, M.; Ramirez-Peña, J.; Cabanillas-Lazo, M.; Paz-Ibarra, J.L.; Pinedo-Torres, I. Effects of aerobic exercise on components of the metabolic syndrome in older adults with type 2 diabetes mellitus: Systematic review and meta-analysis. Rev. Peru. Med. Exp. Salud Publica 2024, 41, 146–155. [Google Scholar] [CrossRef]
- Wrench, E.; Rattley, K.; Lambert, J.E.; Killick, R.; Hayes, L.D.; Lauder, R.M.; Gaffney, C.J. There Is No Dose-Response Relationship between the Amount of Exercise and Improvement in HbA1c in Interventions over 12 Weeks in Patients with Type 2 Diabetes: A Meta-Analysis and Meta-Regression. Acta Diabetol. 2022, 59, 1399–1415. [Google Scholar] [CrossRef]
- Jansson, A.K.; Chan, L.X.; Lubans, D.R.; Duncan, M.J.; Plotnikoff, R.C. Effect of Resistance Training on HbA1c in Adults with Type 2 Diabetes Mellitus and the Moderating Effect of Changes in Muscular Strength: A Systematic Review and Meta-Analysis. BMJ Open Diabetes Res. Care 2022, 10, e002595. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.P.; Cureau, F.V.; Iorra, F.d.Q.; Bottino, L.G.; R C Monteiro, L.E.; Leivas, G.; Umpierre, D.; Schaan, B.D. Effects of Exercise Training and Physical Activity Advice on HbA1c in People with Type 2 Diabetes: A Network Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2025, 221, 112027. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Batrakoulis, A.; Wan Ghazali, W.S.; Mohamed, M.; Aldayel, A.; Alhussain, M.H.; Afolabi, H.A.; Wada, Y.; Gülü, M.; Elkholi, S.; et al. Effects of Combined Aerobic and Resistance Training on Glycemic Control, Blood Pressure, Inflammation, Cardiorespiratory Fitness and Quality of Life in Patients with Type 2 Diabetes and Overweight/Obesity: A Systematic Review and Meta-Analysis. PeerJ 2024, 12, e17525. [Google Scholar] [CrossRef]
- Amin, U.; Adnan, Q.-U.-A.; Ahmad, T.; Farooqui, S.I. Exploring the Synergistic Effects of Concurrent Exercise for Managing Type-II Diabetes Mellitus: A Meta-Analysis. J. Coll. Physicians Surg. Pak. 2024, 34, 1355–1363. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Wu, Q.; Han, R.; Cheng, D.; Wu, L.; Guo, J.; Yu, X.; Ge, W.; Ni, J.; et al. Exercise-Induced Improvement of Glycemic Fluctuation and Its Relationship with Fat and Muscle Distribution in Type 2 Diabetes. J. Diabetes 2024, 16, e13549. [Google Scholar] [CrossRef]
- Valenti, V.E.; Chagas, A.D.S.; Chedraui, P.; de Souza, I.S.; Porto, A.A.; Sorpreso, I.C.E.; Soares Júnior, J.M.; Zangirolami-Raimundo, J.; Garner, D.M.; Raimundo, R.D. Effect of Combined Aerobic Exercise and Resistance Training on Postmenopausal Women with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2025, 41, 2450338. [Google Scholar] [CrossRef]
- Petersen, M.H.; de Almeida, M.E.; Wentorf, E.K.; Jensen, K.; Ørtenblad, N.; Højlund, K. High-Intensity Interval Training Combining Rowing and Cycling Efficiently Improves Insulin Sensitivity, Body Composition and VO2 max in Men with Obesity and Type 2 Diabetes. Front. Endocrinol. 2022, 13, 1032235. [Google Scholar] [CrossRef] [PubMed]
- Christ-Roberts, C.Y.; Pratipanawatr, T.; Pratipanawatr, W.; Berria, R.; Belfort, R.; Kashyap, S.; Mandarino, L.J. Exercise Training Increases Glycogen Synthase Activity and GLUT4 Expression but Not Insulin Signaling in Overweight Nondiabetic and Type 2 Diabetic Subjects. Metabolism 2004, 53, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Legaard, G.E.; Lyngbæk, M.P.P.; Almdal, T.P.; Karstoft, K.; Bennetsen, S.L.; Feineis, C.S.; Nielsen, N.S.; Durrer, C.G.; Liebetrau, B.; Nystrup, U.; et al. Effects of Different Doses of Exercise and Diet-Induced Weight Loss on Beta-Cell Function in Type 2 Diabetes (DOSE-EX): A Randomized Clinical Trial. Nat. Metab. 2023, 5, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Way, K.L.; Hackett, D.A.; Baker, M.K.; Johnson, N.A. The Effect of Regular Exercise on Insulin Sensitivity in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. J. 2016, 40, 253. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Amaravadi, S.K.; Maiya, G.A.; K, V.; Shastry, B.A. Effectiveness of Structured Exercise Program on Insulin Resistance and Quality of Life in Type 2 Diabetes Mellitus-A Randomized Controlled Trial. PLoS ONE 2024, 19, e0302831. [Google Scholar] [CrossRef]
- Gregory, J.M.; Muldowney, J.A.; Engelhardt, B.G.; Tyree, R.; Marks-Shulman, P.; Silver, H.J.; Donahue, E.P.; Edgerton, D.S.; Winnick, J.J. Aerobic Exercise Training Improves Hepatic and Muscle Insulin Sensitivity, but Reduces Splanchnic Glucose Uptake in Obese Humans with Type 2 Diabetes. Nutr. Diabetes 2019, 9, 25. [Google Scholar] [CrossRef]
- Hussey, S.E.; McGee, S.L.; Garnham, A.; Wentworth, J.M.; Jeukendrup, A.E.; Hargreaves, M. Exercise Training Increases Adipose Tissue GLUT4 Expression in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2011, 13, 959–962. [Google Scholar] [CrossRef]
- O’Gorman, D.J.; Karlsson, H.K.R.; McQuaid, S.; Yousif, O.; Rahman, Y.; Gasparro, D.; Glund, S.; Chibalin, A.V.; Zierath, J.R.; Nolan, J.J. Exercise Training Increases Insulin-Stimulated Glucose Disposal and GLUT4 (SLC2A4) Protein Content in Patients with Type 2 Diabetes. Diabetologia 2006, 49, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Sabag, A.; Way, K.L.; Keating, S.E.; Sultana, R.N.; O’Connor, H.T.; Baker, M.K.; Chuter, V.H.; George, J.; Johnson, N.A. Exercise and Ectopic Fat in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Metab. 2017, 43, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High Intensity Intermittent Exercise Improves Cardiac Structure and Function and Reduces Liver Fat in Patients with Type 2 Diabetes: A Randomised Controlled Trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- Sabag, A.; Way, K.L.; Sultana, R.N.; Keating, S.E.; Gerofi, J.A.; Chuter, V.H.; Byrne, N.M.; Baker, M.K.; George, J.; Caterson, I.D.; et al. The Effect of a Novel Low-Volume Aerobic Exercise Intervention on Liver Fat in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020, 43, 2371–2378. [Google Scholar] [CrossRef]
- Al Ozairi, E.; Alsaeed, D.; Al Roudhan, D.; Jalali, M.; Mashankar, A.; Taliping, D.; Abdulla, A.; Gill, J.M.R.; Sattar, N.; Welsh, P.; et al. The Effect of Home-Based Resistance Exercise Training in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Metab. Res. Rev. 2023, 39, e3677. [Google Scholar] [CrossRef]
- Freer, C.L.; George, E.S.; Tan, S.-Y.; Abbott, G.; Dunstan, D.W.; Daly, R.M. Effect of Progressive Resistance Training with Weight Loss Compared with Weight Loss Alone on the Fatty Liver Index in Older Adults with Type 2 Diabetes: Secondary Analysis of a 12-Month Randomized Controlled Trial. BMJ Open Diab. Res. Care 2022, 10, e002950. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Butler, A.E.; Butler, P.C. Pancreatic Fat Content and Beta-Cell Function in Men with and without Type 2 Diabetes: Response to Tushuizen et al. Diabetes Care 2008, 31, e38, author reply e39. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Q.; Miller, J.D.; Zuo, P.; Yuan, X.; Feng, J.; Liu, C.; Bao, S.; Lou, Q. Aerobic Training Reduces Pancreatic Fat Content and Improves β-Cell Function: A Randomized Controlled Trial Using IDEAL-IQ Magnetic Resonance Imaging. Diabetes Metab. Res. Rev. 2022, 38, e3516. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, M.A.; Motiani, K.K.; Mari, A.; Saunavaara, V.; Eskelinen, J.-J.; Virtanen, K.A.; Koivumäki, M.; Löyttyniemi, E.; Nuutila, P.; Kalliokoski, K.K.; et al. Exercise Training Decreases Pancreatic Fat Content and Improves Beta Cell Function Regardless of Baseline Glucose Tolerance: A Randomised Controlled Trial. Diabetologia 2018, 61, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Sachs, S.; Zarini, S.; Kahn, D.E.; Harrison, K.A.; Perreault, L.; Phang, T.; Newsom, S.A.; Strauss, A.; Kerege, A.; Schoen, J.A.; et al. Intermuscular Adipose Tissue Directly Modulates Skeletal Muscle Insulin Sensitivity in Humans. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E866–E879. [Google Scholar] [CrossRef]
- Granados, A.; Gebremariam, A.; Gidding, S.S.; Terry, J.G.; Carr, J.J.; Steffen, L.M.; Jacobs, D.R.; Lee, J.M. Association of Abdominal Muscle Composition with Prediabetes and Diabetes: The CARDIA Study. Diabetes Obes. Metab. 2019, 21, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, F.; Shabani, M.; Quinaglia A C Silva, T.; Bluemke, D.A.; Budoff, M.; Barr, R.G.; Allison, M.A.; Bertoni, A.G.; Post, W.S.; Lima, J.A.C.; et al. Adipose Tissue Biomarkers and Type 2 Diabetes Incidence in Normoglycemic Participants in the MESArthritis Ancillary Study: A Cohort Study. PLoS Med. 2021, 18, e1003700. [Google Scholar] [CrossRef]
- Tang, F.; Wang, W.; Wang, Y.; Lee, Y.; Lou, Q. Moderate Resistance Training Reduces Intermuscular Adipose Tissue and Risk Factors of Atherosclerotic Cardiovascular Disease for Elderly Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2024, 26, 3418–3428. [Google Scholar] [CrossRef]
- Gallagher, D.; Heshka, S.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, F.X.; Patricio, J.; Mancino, J.; Clark, J.M. MRI Ancillary Study Group of Look AHEAD Research Group Changes in Adipose Tissue Depots and Metabolic Markers Following a 1-Year Diet and Exercise Intervention in Overweight and Obese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 3325–3332. [Google Scholar] [CrossRef]
- Dhokte, S.; Czaja, K. Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes. Nutrients 2024, 16, 1015. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Bahrami Kerchi, A.; Behzadnejad, N.; Belyani, S.; Rosenkranz, S.K.; Bagheri, R.; Dutheil, F. The Effects of Exercise Interventions on Ectopic and Subcutaneous Fat in Patients with Type 2 Diabetes Mellitus: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Clin. Med. 2024, 13, 5005. [Google Scholar] [CrossRef]
- Paula, F.M.M.; Leite, N.C.; Borck, P.C.; Freitas-Dias, R.; Cnop, M.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Marchetti, P.; Boschero, A.C.; Zoppi, C.C.; et al. Exercise Training Protects Human and Rodent β Cells against Endoplasmic Reticulum Stress and Apoptosis. FASEB J. 2018, 32, 1524–1536. [Google Scholar] [CrossRef]
- Coomans de Brachène, A.; Scoubeau, C.; Musuaya, A.E.; Costa-Junior, J.M.; Castela, A.; Carpentier, J.; Faoro, V.; Klass, M.; Cnop, M.; Eizirik, D.L. Exercise as a Non-Pharmacological Intervention to Protect Pancreatic Beta Cells in Individuals with Type 1 and Type 2 Diabetes. Diabetologia 2023, 66, 450–460. [Google Scholar] [CrossRef]
- Carvalho, V.H.C.; Wang, Q.; Xu, X.; Liu, L.; Jiang, W.; Wang, X.; Wang, J.; Li, W.; Chen, J.; Li, T.; et al. Long-Term Exercise Preserves Pancreatic Islet Structure and β-Cell Mass through Attenuation of Islet Inflammation and Fibrosis. FASEB J. 2023, 37, e22822. [Google Scholar] [CrossRef]
- Király, M.A.; Bates, H.E.; Yue, J.T.Y.; Goche-Montes, D.; Fediuc, S.; Park, E.; Matthews, S.G.; Vranic, M.; Riddell, M.C. Attenuation of Type 2 Diabetes Mellitus in the Male Zucker Diabetic Fatty Rat: The Effects of Stress and Non-Volitional Exercise. Metabolism 2007, 56, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Gallo, L.A.; Wlodek, M.E.; Siebel, A.L.; Wadley, G.D.; McConell, G.K. Short-Term Exercise Training Early in Life Restores Deficits in Pancreatic β-Cell Mass Associated with Growth Restriction in Adult Male Rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E931–E940. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and Exercise May Enhance Beta-Cell Function and Mass via Insulin Receptor Substrate 2 Induction in Ovariectomized Diabetic Rats. Endocrinology 2005, 146, 4786–4794. [Google Scholar] [CrossRef]
- Coskun, O.; Ocakci, A.; Bayraktaroglu, T.; Kanter, M. Exercise Training Prevents and Protects Streptozotocin-Induced Oxidative Stress and Beta-Cell Damage in Rat Pancreas. Tohoku J. Exp. Med. 2004, 203, 145–154. [Google Scholar] [CrossRef]
- Curran, M.; Drayson, M.T.; Andrews, R.C.; Zoppi, C.; Barlow, J.P.; Solomon, T.P.J.; Narendran, P. The Benefits of Physical Exercise for the Health of the Pancreatic β-Cell: A Review of the Evidence. Exp. Physiol. 2020, 105, 579–589. [Google Scholar] [CrossRef]
- Vakilian, M.; Tahamtani, Y.; Ghaedi, K. A Review on Insulin Trafficking and Exocytosis. Gene 2019, 706, 52–61. [Google Scholar] [CrossRef]
- Király, M.A.; Bates, H.E.; Kaniuk, N.A.; Yue, J.T.Y.; Brumell, J.H.; Matthews, S.G.; Riddell, M.C.; Vranic, M. Swim Training Prevents Hyperglycemia in ZDF Rats: Mechanisms Involved in the Partial Maintenance of Beta-Cell Function. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E271–E283. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Hong, S.M.; Jun, D.W.; Park, S. Exercise and Dexamethasone Oppositely Modulate Beta-Cell Function and Survival via Independent Pathways in 90% Pancreatectomized Rats. J. Endocrinol. 2006, 190, 471–482. [Google Scholar] [CrossRef]
- Saisho, Y. Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 744. [Google Scholar] [CrossRef]
- Di Murro, E.; Di Giuseppe, G.; Soldovieri, L.; Moffa, S.; Improta, I.; Capece, U.; Nista, E.C.; Cinti, F.; Ciccarelli, G.; Brunetti, M.; et al. Physical Activity and Type 2 Diabetes: In Search of a Personalized Approach to Improving β-Cell Function. Nutrients 2023, 15, 4202. [Google Scholar] [CrossRef]
- Johansen, M.Y.; Karstoft, K.; MacDonald, C.S.; Hansen, K.B.; Ellingsgaard, H.; Hartmann, B.; Wewer Albrechtsen, N.J.; Vaag, A.A.; Holst, J.J.; Pedersen, B.K.; et al. Effects of an Intensive Lifestyle Intervention on the Underlying Mechanisms of Improved Glycaemic Control in Individuals with Type 2 Diabetes: A Secondary Analysis of a Randomised Clinical Trial. Diabetologia 2020, 63, 2410–2422. [Google Scholar] [CrossRef]
- Zhang, H.; Simpson, L.K.; Carbone, N.P.; Hirshman, M.F.; Nigro, P.; Vamvini, M.; Goodyear, L.J.; Middelbeek, R.J.W. Moderate-Intensity Endurance Training Improves Late Phase β-Cell Function in Adults with Type 2 Diabetes. iScience 2023, 26, 107226. [Google Scholar] [CrossRef] [PubMed]
- Ingersen, A.; Schmücker, M.; Alexandersen, C.; Graungaard, B.; Thorngreen, T.; Borch, J.; Holst, J.J.; Helge, J.W.; Dela, F. Effects of Aerobic Training and Semaglutide Treatment on Pancreatic β-Cell Secretory Function in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and Metabolic Health: Beyond Skeletal Muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, G.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Antonopoulos, C.; Tachmatzidis, D.; Didangelos, T.; Lambadiari, V.; Kadoglou, N.P.E. The Anti-Inflammatory Effects of Aerobic Exercise Training in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Cytokine 2023, 164, 156157. [Google Scholar] [CrossRef]
- Zhou, N.; Gong, L.; Zhang, E.; Wang, X. Exploring Exercise-Driven Exerkines: Unraveling the Regulation of Metabolism and Inflammation. PeerJ 2024, 12, e17267. [Google Scholar] [CrossRef]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-Induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529 Pt 1, 237–242. [Google Scholar] [CrossRef]
- Pedersen, B.K. IL-6 Signalling in Exercise and Disease. Biochem. Soc. Trans. 2007, 35, 1295–1297. [Google Scholar] [CrossRef]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 Increases Insulin-Stimulated Glucose Disposal in Humans and Glucose Uptake and Fatty Acid Oxidation in Vitro via AMP-Activated Protein Kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-Inflammatory Effects of Exercise: Role in Diabetes and Cardiovascular Disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Wang, C.; He, H. Effects of Exercise on Inflammatory Cytokines in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Oxid. Med. Cell. Longev. 2020, 2020, 6660557. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise Training-Induced Changes in Exerkine Concentrations May Be Relevant to the Metabolic Control of Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Sport Health Sci. 2023, 12, 147–157. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lin, S. Irisin: A Bridge between Exercise and Neurological Diseases. Heliyon 2022, 8, e12352. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhao, X.; Zhang, D.-Q.; Wang, R.; Feng, Y. Lower Levels of Irisin in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 175, 108788. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Song, R.; Zhao, X.; Yang, C.; Feng, Y. Lower Circulating Irisin Levels in Type 2 Diabetes Mellitus Patients with Chronic Complications: A Meta-Analysis. Heliyon 2023, 9, e21859. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-L.; Jiang, W.-X.; Lv, Z.-T. Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2016, 48, 644–652. [Google Scholar] [CrossRef]
- Motahari Rad, M.; Bijeh, N.; Attarzadeh Hosseini, S.R.; Raouf Saeb, A. The Effect of Two Concurrent Exercise Modalities on Serum Concentrations of FGF21, Irisin, Follistatin, and Myostatin in Men with Type 2 Diabetes Mellitus. Arch. Physiol. Biochem. 2023, 129, 424–433. [Google Scholar] [CrossRef]
- Vecchiato, M.; Zanardo, E.; Battista, F.; Quinto, G.; Bergia, C.; Palermi, S.; Duregon, F.; Ermolao, A.; Neunhaeuserer, D. The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review. J. Clin. Med. 2022, 12, 62. [Google Scholar] [CrossRef]
- Villamil-Parra, W.; Moscoso-Loaiza, L. Effects of Physical Exercise on Irisin and BDNF Concentrations, and Their Relationship with Cardiometabolic and Mental Health of Individuals with Metabolic Syndrome: A Systematic Review. Exp. Gerontol. 2024, 198, 112640. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Malek, L.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef]
- Jamali, A.; Shahrbanian, S.; Morteza Tayebi, S. The Effects of Exercise Training on the Brain-Derived Neurotrophic Factor (BDNF) in the Patients with Type 2 Diabetes: A Systematic Review of the Randomized Controlled Trials. J. Diabetes Metab. Disord. 2020, 19, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Myostatin: A Potential Therapeutic Target for Metabolic Syndrome. Front. Endocrinol. 2023, 14, 1181913. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Pourteymour, S.; Görgens, S.W.; Langleite, T.M.; Lee, S.; Holen, T.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A.; et al. Myostatin in Relation to Physical Activity and Dysglycaemia and Its Effect on Energy Metabolism in Human Skeletal Muscle Cells. Acta Physiol. 2016, 217, 45–60. [Google Scholar] [CrossRef]
- Brandt, C.; Nielsen, A.R.; Fischer, C.P.; Hansen, J.; Pedersen, B.K.; Plomgaard, P. Plasma and Muscle Myostatin in Relation to Type 2 Diabetes. PLoS ONE 2012, 7, e37236. [Google Scholar] [CrossRef]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin Decreases with Aerobic Exercise and Associates with Insulin Resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef]
- Riahy, S. The Effects of 12 Weeks of High-Intensity Interval Training and Moderate-Intensity Continuous Training on FGF21, Irisin, and Myostatin in Men with Type 2 Diabetes Mellitus. Growth Factors 2024, 42, 24–35. [Google Scholar] [CrossRef]
- Shabkhiz, F.; Khalafi, M.; Rosenkranz, S.; Karimi, P.; Moghadami, K. Resistance Training Attenuates Circulating FGF-21 and Myostatin and Improves Insulin Resistance in Elderly Men with and without Type 2 Diabetes Mellitus: A Randomised Controlled Clinical Trial. Eur. J. Sport Sci. 2021, 21, 636–645. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, X.; You, L.; Li, F.; Lian, H.; Wang, J.; Mao, N.; Ren, M.; Li, Y.; Wang, C.; et al. Association between Adiponectin and Newly Diagnosed Type 2 Diabetes in Population with the Clustering of Obesity, Dyslipidaemia and Hypertension: A Cross-Sectional Study. BMJ Open 2023, 13, e060377. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Matsushita, Y.; Nakagawa, T.; Hayashi, T.; Noda, M.; Mizoue, T. Circulating Adiponectin Levels and Risk of Type 2 Diabetes in the Japanese. Nutr. Diabetes 2014, 4, e130. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A.; et al. Anti-Inflammatory Effect of Exercise Training in Subjects with Type 2 Diabetes and the Metabolic Syndrome Is Dependent on Exercise Modalities and Independent of Weight Loss. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Bullen, J.W.; Lee, J.H.; Kralisch, S.; Fasshauer, M.; Klöting, N.; Niebauer, J.; Schön, M.R.; Williams, C.J.; Mantzoros, C.S. Circulating Adiponectin and Expression of Adiponectin Receptors in Human Skeletal Muscle: Associations with Metabolic Parameters and Insulin Resistance and Regulation by Physical Training. J. Clin. Endocrinol. Metab. 2006, 91, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Dong, L.Q. Adiponectin Signaling and Function in Insulin Target Tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Wijesekara, N.; Krishnamurthy, M.; Bhattacharjee, A.; Suhail, A.; Sweeney, G.; Wheeler, M.B. Adiponectin-Induced ERK and Akt Phosphorylation Protects against Pancreatic Beta Cell Apoptosis and Increases Insulin Gene Expression and Secretion. J. Biol. Chem. 2010, 285, 33623–33631. [Google Scholar] [CrossRef]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Magliulo, L.; Bondi, D.; Pini, N.; Marramiero, L.; Di Filippo, E.S. The Wonder Exerkines-Novel Insights: A Critical State-of-the-Art Review. Mol. Cell. Biochem. 2022, 477, 105–113. [Google Scholar] [CrossRef]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a Newly Identified Adipokine Up-Regulated by Insulin and Obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, C.; Li, X.; Ren, G.; Fan, X.; Ren, F.; Zhang, N.; Sun, J.; Yang, J. Low Plasma Apelin in Newly Diagnosed Type 2 Diabetes in Chinese People. Diabetes Care 2009, 32, e150. [Google Scholar] [CrossRef] [PubMed]
- Noori-Zadeh, A.; Bakhtiyari, S.; Khanjari, S.; Haghani, K.; Darabi, S. Elevated Blood Apelin Levels in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2019, 148, 43–53. [Google Scholar] [CrossRef]
- Mund, C.; Kellellu, C.K.; Rattan, R.; Mahapatra, S.; Lamare, A.A.; Jena, S. Study of Serum Apelin and Insulin Resistance in Type 2 Diabetes Mellitus Patients With or Without Obesity. Cureus 2023, 15, e43401. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Vrabas, I.S.; Kapelouzou, A.; Lampropoulos, S.; Sailer, N.; Kostakis, A.; Liapis, C.D.; Angelopoulou, N. The Impact of Aerobic Exercise Training on Novel Adipokines, Apelin and Ghrelin, in Patients with Type 2 Diabetes. Med. Sci. Monit. 2012, 18, CR290–CR295. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Polonio-González, M.L.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R. Implications of Resistin in Type 2 Diabetes Mellitus and Coronary Artery Disease: Impairing Insulin Function and Inducing pro-Inflammatory Cytokines. J. Cell. Physiol. 2019, 234, 21758–21769. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, D.-M.; Lin, K.-C.; Shin, S.-J.; Lee, Y.-J. Visfatin in Overweight/Obesity, Type 2 Diabetes Mellitus, Insulin Resistance, Metabolic Syndrome and Cardiovascular Diseases: A Meta-Analysis and Systemic Review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kärberg, K.; Forbes, A.; Lember, M. Visfatin and Subclinical Atherosclerosis in Type 2 Diabetes: Impact of Cardiovascular Drugs. Medicina 2023, 59, 1324. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Perrea, D.; Iliadis, F.; Angelopoulou, N.; Liapis, C.; Alevizos, M. Exercise Reduces Resistin and Inflammatory Cytokines in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 719–721. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Fernhall, B.; Carhart, R.; Weinstock, R.S.; Baynard, T.; Figueroa, A.; Kanaley, J.A. Effects of Diet and/or Exercise on the Adipocytokine and Inflammatory Cytokine Levels of Postmenopausal Women with Type 2 Diabetes. Metabolism 2005, 54, 866–875. [Google Scholar] [CrossRef]
- Monzillo, L.U.; Hamdy, O.; Horton, E.S.; Ledbury, S.; Mullooly, C.; Jarema, C.; Porter, S.; Ovalle, K.; Moussa, A.; Mantzoros, C.S. Effect of Lifestyle Modification on Adipokine Levels in Obese Subjects with Insulin Resistance. Obes. Res. 2003, 11, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Park, S.H.; Marquez, J.; Kwak, H.-B.; Kim, T.N.; Bae, J.H.; Koh, J.-H.; Han, J. Hepatokines as a Molecular Transducer of Exercise. J. Clin. Med. 2021, 10, 385. [Google Scholar] [CrossRef]
- Post, A.; Dam, W.A.; Sokooti, S.; Groothof, D.; Gloerich, J.; van Gool, A.J.; Kremer, D.; Gansevoort, R.T.; van den Born, J.; Kema, I.P.; et al. Circulating FGF21 Concentration, Fasting Plasma Glucose, and the Risk of Type 2 Diabetes: Results from the PREVEND Study. J. Clin. Endocrinol. Metab. 2023, 108, 1387–1393. [Google Scholar] [CrossRef]
- Chavez, A.O.; Molina-Carrion, M.; Abdul-Ghani, M.A.; Folli, F.; Defronzo, R.A.; Tripathy, D. Circulating Fibroblast Growth Factor-21 Is Elevated in Impaired Glucose Tolerance and Type 2 Diabetes and Correlates with Muscle and Hepatic Insulin Resistance. Diabetes Care 2009, 32, 1542–1546. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Pedersen, A.J.T.; Kristensen, J.M.; Handberg, A.; Wojtaszewski, J.F.P.; Højlund, K. Intact Regulation of Muscle Expression and Circulating Levels of Myokines in Response to Exercise in Patients with Type 2 Diabetes. Physiol. Rep. 2018, 6, e13723. [Google Scholar] [CrossRef]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Weigert, C.; Hoene, M.; Plomgaard, P. Hepatokines-a Novel Group of Exercise Factors. Pflug. Arch 2019, 471, 383–396. [Google Scholar] [CrossRef]
- Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Joost, H.-G.; Häring, H.-U.; Schulze, M.B. Plasma Fetuin-A Levels and the Risk of Type 2 Diabetes. Diabetes 2008, 57, 2762–2767. [Google Scholar] [CrossRef]
- Wang, Y.; Koh, W.P.; Jensen, M.K.; Yuan, J.M.; Pan, A. Plasma Fetuin-A Levels and Risk of Type 2 Diabetes Mellitus in A Chinese Population: A Nested Case-Control Study. Diabetes Metab. J. 2019, 43, 474–486. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; García-Hermoso, A.; Hackney, A.C.; Izquierdo, M. Effects of Exercise Training on Fetuin-a in Obese, Type 2 Diabetes and Cardiovascular Disease in Adults and Elderly: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2019, 18, 23. [Google Scholar] [CrossRef]
- Lee, S.; Norheim, F.; Gulseth, H.L.; Langleite, T.M.; Kolnes, K.J.; Tangen, D.S.; Stadheim, H.K.; Gilfillan, G.D.; Holen, T.; Birkeland, K.I.; et al. Interaction between Plasma Fetuin-A and Free Fatty Acids Predicts Changes in Insulin Sensitivity in Response to Long-Term Exercise. Physiol. Rep. 2017, 5, e13183. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Borné, Y.; Gao, R.; López Rodriguez, M.; Roell, W.C.; Wilson, J.M.; Regmi, A.; Luan, C.; Aly, D.M.; Peter, A.; et al. Elevated Circulating Follistatin Associates with an Increased Risk of Type 2 Diabetes. Nat. Commun. 2021, 12, 6486. [Google Scholar] [CrossRef]
- Ennequin, G.; Sirvent, P.; Whitham, M. Role of Exercise-Induced Hepatokines in Metabolic Disorders. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E11–E24. [Google Scholar] [CrossRef]
- Mizgier, M.L.; Fernández-Verdejo, R.; Cherfan, J.; Pinget, M.; Bouzakri, K.; Galgani, J.E. Insights on the Role of Putative Muscle-Derived Factors on Pancreatic Beta Cell Function. Front. Physiol. 2019, 10, 1024. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin Are Type II Muscle Specific Myokines Protecting Pancreatic Beta-Cells against Proinflammatory Cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef]
- Jasmine, M.R.; Nanda, N.; Sahoo, J.; Velkumary, S.; Pal, G.K. Increased Osteoprotegerin Level Is Associated with Impaired Cardiovagal Modulation in Type-2 Diabetic Patients Treated with Oral Antidiabetic Drugs. BMC Cardiovasc. Disord. 2020, 20, 453. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.M.; Fay, M.M.; Akiyama, Y.; Anderson, P.J.; Ivanov, P. RNA Biology of Angiogenin: Current State and Perspectives. RNA Biol. 2017, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.; Subramanian, V. The Cellular Uptake of Angiogenin, an Angiogenic and Neurotrophic Factor Is through Multiple Pathways and Largely Dynamin Independent. PLoS ONE 2018, 13, e0193302. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef]
- Siebert, J.; Reiwer-Gostomska, M.; Babińska, Z.; Myśliwska, J.; Myśliwski, A.; Skopińska-Rózewska, E.; Sommer, E.; Skopiński, P. Low Serum Angiogenin Concentrations in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 3086–3087. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.L.; Liu, S.; Liu, J.-J.; M, Y.; Zheng, H.; Chan, C.; Ang, K.; Subramaniam, T.; Sum, C.F.; Lim, S.C. Association of Plasma Angiogenin with Risk of Major Cardiovascular Events in Type 2 Diabetes. Cardiovasc. Diabetol. 2024, 23, 70. [Google Scholar] [CrossRef]
- Siebert, J.; Reiwer-Gostomska, M.; Mysliwska, J.; Marek, N.; Raczynska, K.; Glasner, L. Glycemic Control Influences Serum Angiogenin Concentrations in Patients with Type 2 Diabetes. Diabetes Care 2010, 33, 1829–1830. [Google Scholar] [CrossRef]
- Esteghamati, A.; Aflatoonian, M.; Rad, M.V.; Mazaheri, T.; Mousavizadeh, M.; Nakhjavani, M.; Noshad, S. Association of Osteoprotegerin with Peripheral Artery Disease in Patients with Type 2 Diabetes. Arch Cardiovasc. Dis. 2015, 108, 412–419. [Google Scholar] [CrossRef]
- Knudsen, S.T.; Foss, C.H.; Poulsen, P.L.; Andersen, N.H.; Mogensen, C.E.; Rasmussen, L.M. Increased Plasma Concentrations of Osteoprotegerin in Type 2 Diabetic Patients with Microvascular Complications. Eur. J. Endocrinol. 2003, 149, 39–42. [Google Scholar] [CrossRef]
- Terekeci, H.M.; Senol, M.G.; Top, C.; Sahan, B.; Celik, S.; Sayan, O.; Kucukardali, Y.; Ipcioglu, O.; Cagiltay, E.; Oktenli, C.; et al. Plasma Osteoprotegerin Concentrations in Type 2 Diabetic Patients and Its Association with Neuropathy. Exp. Clin. Endocrinol. Diabetes 2009, 117, 119–123. [Google Scholar] [CrossRef]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The Myokine Decorin Is Regulated by Contraction and Involved in Muscle Hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Jeyagaran, A.; Zbinden, A.; Lu, C.-E.; Marzi, J.; Kuhlburger, L.; Nahnsen, S.; Layland, S.L.; Duffy, G.; Schenke-Layland, K. Decorin Improves Human Pancreatic β-Cell Function and Regulates ECM Expression in Vitro. Matrix Biol. 2023, 115, 160–183. [Google Scholar] [CrossRef]
- Langlois, A.; Cherfan, J.; Meugnier, E.; Rida, A.; Arous, C.; Peronet, C.; Hamdard, H.; Zarrouki, B.; Wehrle-Haller, B.; Pinget, M.; et al. DECORIN, a Triceps-Derived Myokine, Protects Sorted β-Cells and Human Islets against Chronic Inflammation Associated with Type 2 Diabetes. Acta Physiol. 2025, 241, e14267. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin Interacting Network: A Comprehensive Analysis of Decorin-Binding Partners and Their Versatile Functions. Matrix Biol. 2016, 55, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Svärd, J.; Røst, T.H.; Sommervoll, C.E.N.; Haugen, C.; Gudbrandsen, O.A.; Mellgren, A.E.; Rødahl, E.; Fernø, J.; Dankel, S.N.; Sagen, J.V.; et al. Absence of the Proteoglycan Decorin Reduces Glucose Tolerance in Overfed Male Mice. Sci. Rep. 2019, 9, 4614. [Google Scholar] [CrossRef]
- Chen, F.; Lai, J.; Zhu, Y.; He, M.; Hou, H.; Wang, J.; Chen, C.; Wang, D.W.; Tang, J. Cardioprotective Effect of Decorin in Type 2 Diabetes. Front. Endocrinol. 2020, 11, 479258. [Google Scholar] [CrossRef]
- Bolton, K.; Segal, D.; McMillan, J.; Jowett, J.; Heilbronn, L.; Abberton, K.; Zimmet, P.; Chisholm, D.; Collier, G.; Walder, K. Decorin Is a Secreted Protein Associated with Obesity and Type 2 Diabetes. Int. J. Obes. 2008, 32, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Stiernborg, M.; Fogdell-Hahn, A.; Månsson, K.; Furmark, T.; Berglind, D.; Melas, P.A.; Forsell, Y.; Lavebratt, C. Physical Exercise Is Associated with a Reduction in Plasma Levels of Fractalkine, TGF-Β1, Eotaxin-1 and IL-6 in Younger Adults with Mobility Disability. PLoS ONE 2022, 17, e0263173. [Google Scholar] [CrossRef]
- Lee, Y.S.; Morinaga, H.; Kim, J.J.; Lagakos, W.; Taylor, S.; Keshwani, M.; Perkins, G.; Dong, H.; Kayali, A.G.; Sweet, I.R.; et al. The Fractalkine/CX3CR1 System Regulates β Cell Function and Insulin Secretion. Cell 2013, 153, 413–425. [Google Scholar] [CrossRef]
- Rutti, S.; Arous, C.; Schvartz, D.; Timper, K.; Sanchez, J.-C.; Dermitzakis, E.; Donath, M.Y.; Halban, P.A.; Bouzakri, K. Fractalkine (CX3CL1), a New Factor Protecting β-Cells against TNFα. Mol. Metab. 2014, 3, 731–741. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Campia, U.; Cardillo, C. Increased Fractalkine and Vascular Dysfunction in Obesity and in Type 2 Diabetes. Effects of Oral Antidiabetic Treatment. Vascul. Pharmacol. 2020, 128–129, 106676. [Google Scholar] [CrossRef]
- Rodriguez, C.; Chocarro, L.; Echaide, M.; Ausin, K.; Escors, D.; Kochan, G. Fractalkine in Health and Disease. Int. J. Mol. Sci. 2024, 25, 8007. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Hinkle, C.C.; Ferguson, J.F.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine Is a Novel Human Adipochemokine Associated with Type 2 Diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Arefanian, H.; Al-Roub, A.A.; Ali, S.; Wilson, A.; Al-Hubail, A.; Al-Beloushi, S.; Al-Zanki, S.; Ahmad, R. Increased Circulatory Levels of Fractalkine (CX3CL1) Are Associated with Inflammatory Chemokines and Cytokines in Individuals with Type-2 Diabetes. J. Diabetes Metab. Disord. 2017, 16, 15. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Novelli, G.; Calcaterra, G.; Casciani, F.; Pecorelli, S.; Mehta, J.L. “Exerkines”: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines 2024, 12, 1975. [Google Scholar] [CrossRef]
- Jin, Y.; Wan, K.; Liu, C.; Cheng, W.; Wang, R. Mechanisms of Exercise Intervention in Type 2 Diabetes: A Bibliometric and Visualization Analysis Based on CiteSpace. Front. Endocrinol. 2024, 15, 1401342. [Google Scholar] [CrossRef]
- Tripathi, P.; Kadam, N.; Vyawahare, A.; Kuppusamy, M.; Vijayakumar, V. Long-Term Remission of Type 2 Diabetes through Intense Lifestyle Modification Program—A Case Series. J. Fam. Med. Prim. Care 2023, 12, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, D.; Sultan, A.; Bouzakri, K. Physical Exercise as a Therapeutic Approach for Patients Living with Type 2 Diabetes: Does the Explanation Reside in Exerkines?—A Review. Int. J. Mol. Sci. 2025, 26, 8182. https://doi.org/10.3390/ijms26178182
Bernard D, Sultan A, Bouzakri K. Physical Exercise as a Therapeutic Approach for Patients Living with Type 2 Diabetes: Does the Explanation Reside in Exerkines?—A Review. International Journal of Molecular Sciences. 2025; 26(17):8182. https://doi.org/10.3390/ijms26178182
Chicago/Turabian StyleBernard, Daphné, Ariane Sultan, and Karim Bouzakri. 2025. "Physical Exercise as a Therapeutic Approach for Patients Living with Type 2 Diabetes: Does the Explanation Reside in Exerkines?—A Review" International Journal of Molecular Sciences 26, no. 17: 8182. https://doi.org/10.3390/ijms26178182
APA StyleBernard, D., Sultan, A., & Bouzakri, K. (2025). Physical Exercise as a Therapeutic Approach for Patients Living with Type 2 Diabetes: Does the Explanation Reside in Exerkines?—A Review. International Journal of Molecular Sciences, 26(17), 8182. https://doi.org/10.3390/ijms26178182







