Genome-Wide Association Study of Osteoporosis Risk in Korean Pre-Menopausal Women: The Korean Genome and Epidemiology Study
Abstract
1. Introduction
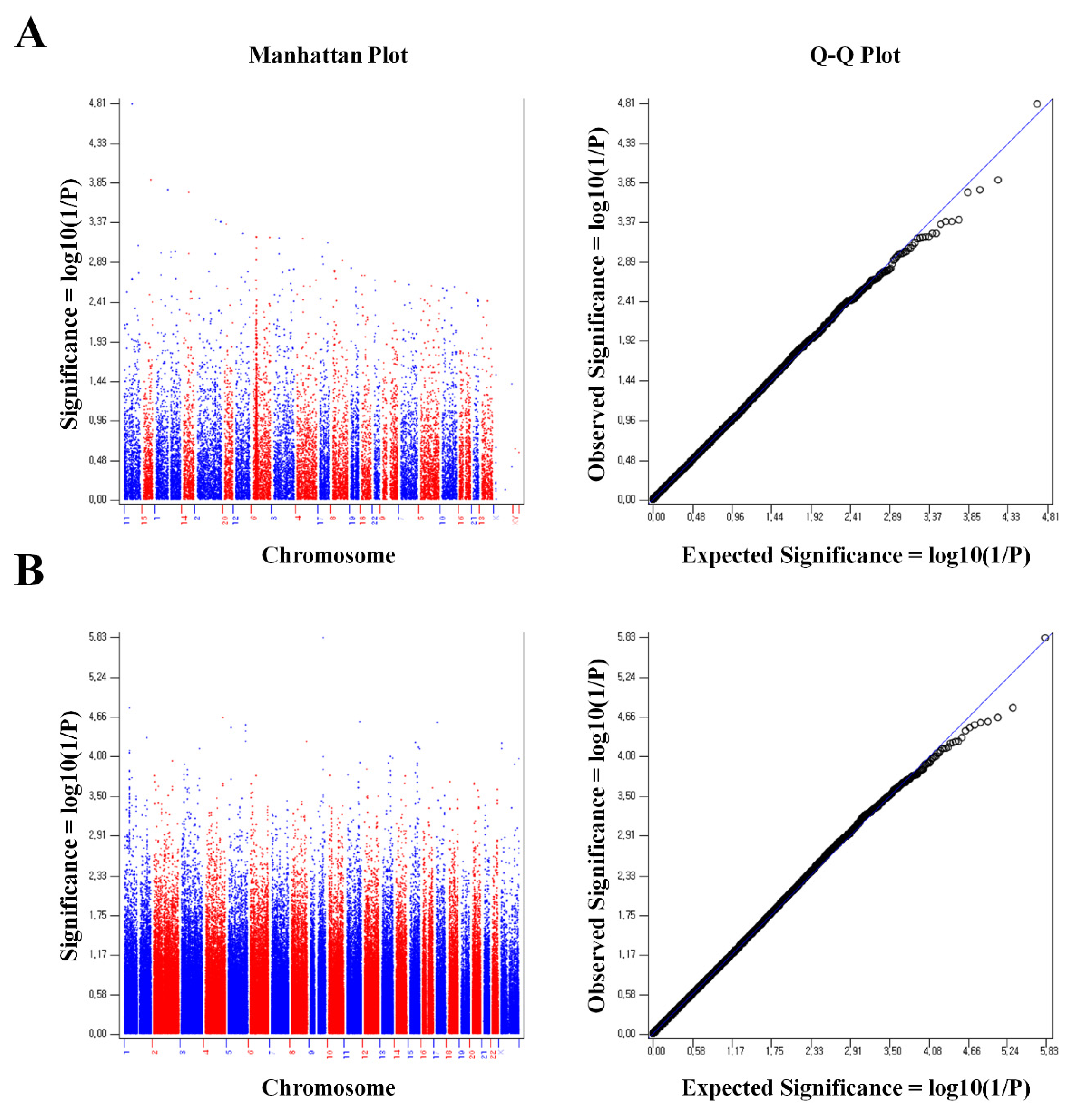
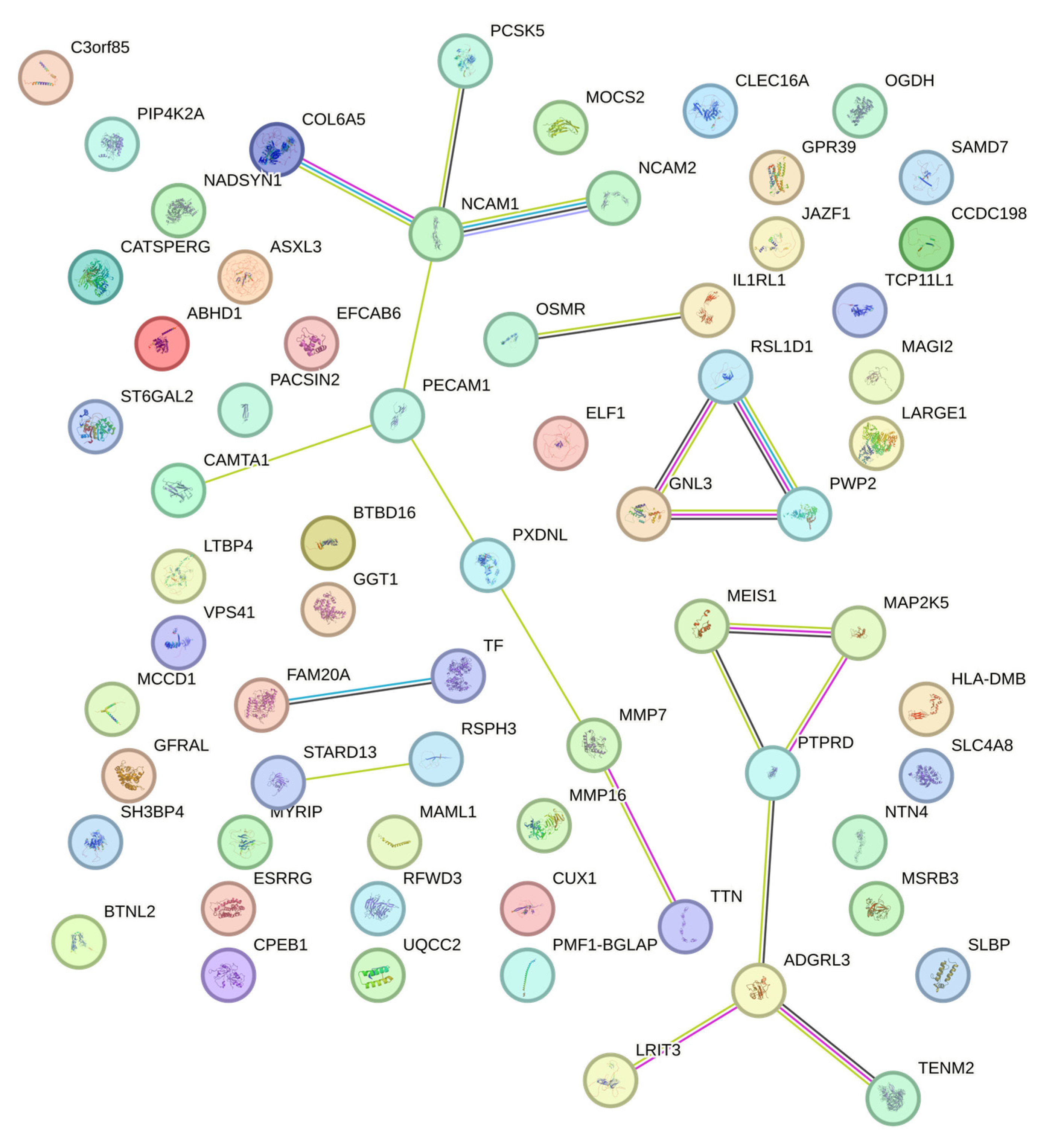
2. Results
3. Discussion
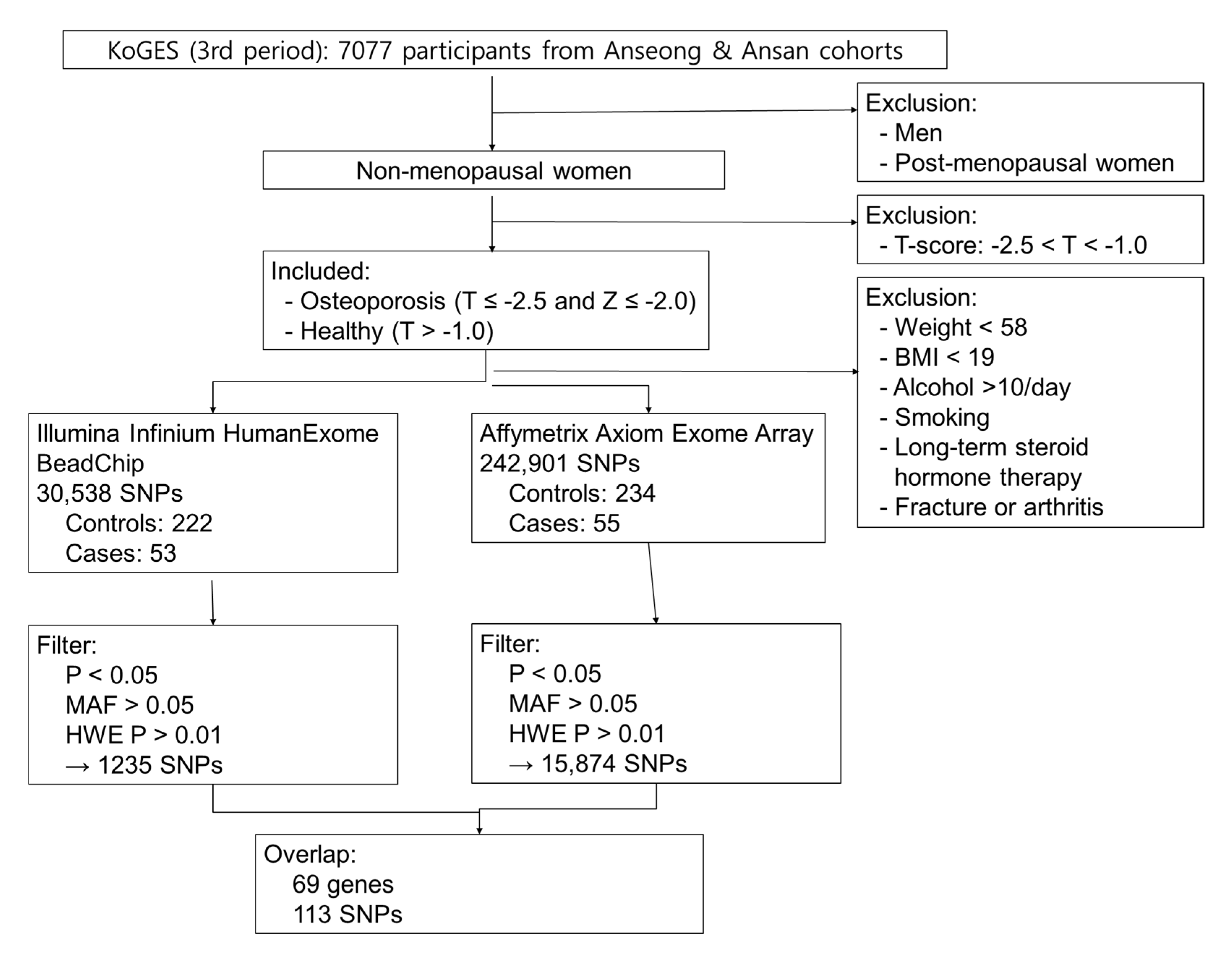
4. Materials and Methods
4.1. Study Subjects
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adachi, J.D.; Ioannidis, G.; Pickard, L.; Berger, C.; Prior, J.C.; Joseph, L.; Hanley, D.A.; Olszynski, W.P.; Murray, T.M.; Anastassiades, T.; et al. The association between osteoporotic fractures and health-related quality of life as measured by the Health Utilities Index in the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos. Int. 2003, 14, 895–904. [Google Scholar] [CrossRef]
- Smith, R. Osteoporosis: Cause and management. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 329–332. [Google Scholar] [CrossRef]
- McGuigan, F.E.; Murray, L.; Gallagher, A.; Davey-Smith, G.; Neville, C.E.; Van’t Hof, R.; Boreham, C.; Ralston, S.H. Genetic and environmental determinants of peak bone mass in young men and women. J. Bone Miner. Res. 2002, 17, 1273–1279. [Google Scholar] [CrossRef]
- Chen, K.Y.; Wang, C.H.; Lin, T.Y.; Chang, C.Y.; Liu, C.L.; Hsiao, Y.C.; Hung, C.C.; Wang, N.C. Monitoring early developed low bone mineral density in HIV-infected patients by intact parathyroid hormone and circulating fibroblast growth factor 23. J. Microbiol. Immunol. Infect. 2019, 52, 693–699. [Google Scholar] [CrossRef]
- Sato, K. Graves’ disease and bone metabolism. Nihon. Rinsho. 2006, 64, 2317–2322. [Google Scholar]
- Seo, S.; Chun, S.; Newell, M.A.; Yun, M. Association between alcohol consumption and Korean young women’s bone health: A cross sectional study from the 2008 to 2011 Korea National Health and Nutrition Examination Survey. BMJ Open 2015, 5, e007914. [Google Scholar] [CrossRef] [PubMed]
- Briot, K. Bone and glucocorticoids. Ann. Endocrinol. 2018, 79, 115–118. [Google Scholar] [CrossRef]
- Jouanny, P.; Guillemin, F.; Kuntz, C.; Jeandel, C.; Pourel, J. Environmental and genetic factors affecting bone mass. Similarity of bone density among members of healthy families. Arthritis Rheum. 1995, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, D.; Shen, G.; Cui, Y.; Lv, Q.; Wei, F. Association of VDR and OPG gene polymorphism with osteoporosis risk in Chinese postmenopausal women. Climacteric 2019, 22, 208–212. [Google Scholar] [CrossRef]
- Mondockova, V.; Adamkovicova, M.; Lukacova, M.; Grosskopf, B.; Babosova, R.; Galbavy, D.; Martiniakova, M.; Omelka, R. The estrogen receptor 1 gene affects bone mineral density and osteoporosis treatment efficiency in Slovak postmenopausal women. BMC Med. Genet. 2018, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Wolski, H.; Drews, K.; Bogacz, A.; Kaminski, A.; Barlik, M.; Bartkowiak-Wieczorek, J.; Klejewski, A.; Ozarowski, M.; Majchrzycki, M.; Seremak-Mrozikiewicz, A. The RANKL/RANK/OPG signal trail: Significance of genetic polymorphisms in the etiology of postmenopausal osteoporosis. Ginekol. Pol. 2016, 87, 347–352. [Google Scholar] [CrossRef]
- Igo, R.P., Jr.; Cooke Bailey, J.N.; Romm, J.; Haines, J.L.; Wiggs, J.L. Quality Control for the Illumina HumanExome BeadChip. Curr. Protoc. Hum. Genet. 2016, 90, 2.14.1–2.14.16. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi-Man, O.; Woehrmann, M.H.; Webster, T.A.; Gollub, J.; Bivol, A.; Keeble, S.M.; Aull, K.H.; Mittal, A.; Roter, A.H.; Wong, B.A.; et al. Novel genotyping algorithms for rare variants significantly improve the accuracy of Applied Biosystems Axiom array genotyping calls: Retrospective evaluation of UK Biobank array data. PLoS ONE 2022, 17, e0277680. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Choi, H.J.; Kim, M.J.; Kim, J.T.; Yu, S.H.; Koo, B.K.; Cho, H.Y.; Cho, S.W.; Kim, S.W.; Park, Y.J.; et al. Prevalence and risk factors of osteoporosis in Korea: A community-based cohort study with lumbar spine and hip bone mineral density. Bone 2010, 47, 378–387. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Higashiyama, A.; Kubota, Y.; Sugiyama, D.; Nishida, Y.; Hirata, T.; Kadota, A.; Nishimura, K.; Imano, H.; Miyamatsu, N.; et al. Underweight Young Women Without Later Weight Gain Are at High Risk for Osteopenia After Midlife: The KOBE Study. J. Epidemiol. 2016, 26, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef]
- Khosla, S.; Atkinson, E.J.; Melton, L.J., 3rd; Riggs, B.L. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: A population-based study. J. Clin. Endocrinol. Metab. 1997, 82, 1522–1527. [Google Scholar]
- Ebeling, P.R.; Sandgren, M.E.; DiMagno, E.P.; Lane, A.W.; DeLuca, H.F.; Riggs, B.L. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: Relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J. Clin. Endocrinol. Metab. 1992, 75, 176–182. [Google Scholar]
- Lips, P.; Wiersinga, A.; van Ginkel, F.C.; Jongen, M.J.; Netelenbos, J.C.; Hackeng, W.H.; Delmas, P.D.; van der Vijgh, W.J. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J. Clin. Endocrinol. Metab. 1988, 67, 644–650. [Google Scholar] [CrossRef]
- Holick, M.F. Perspective on the impact of weightlessness on calcium and bone metabolism. Bone 1998, 22 (Suppl. 5), 105S–111S. [Google Scholar] [CrossRef]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.M.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone 2008, 43, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Haugh, M.G.; O’Brien, F.J.; McNamara, L.M. Estrogen withdrawal from osteoblasts and osteocytes causes increased mineralization and apoptosis. Horm. Metab. Res. 2014, 46, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Elfassihi, L.; Giroux, S.; Bureau, A.; Laflamme, N.; Cole, D.E.; Rousseau, F. Association with replication between estrogen-related receptor gamma (ESRRgamma) polymorphisms and bone phenotypes in women of European ancestry. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 901–911. [Google Scholar] [CrossRef]
- Cardelli, M.; Aubin, J.E. ERRgamma is not required for skeletal development but is a RUNX2-dependent negative regulator of postnatal bone formation in male mice. PLoS ONE 2014, 9, e109592. [Google Scholar] [CrossRef]
- Enoki, Y.; Sato, T.; Kokabu, S.; Hayashi, N.; Iwata, T.; Yamato, M.; Usui, M.; Matsumoto, M.; Tomoda, T.; Ariyoshi, W.; et al. Netrin-4 Promotes Differentiation and Migration of Osteoblasts. Vivo 2017, 31, 793–799. [Google Scholar]
- Enoki, Y.; Sato, T.; Tanaka, S.; Iwata, T.; Usui, M.; Takeda, S.; Kokabu, S.; Matsumoto, M.; Okubo, M.; Nakashima, K.; et al. Netrin-4 derived from murine vascular endothelial cells inhibits osteoclast differentiation in vitro and prevents bone loss in vivo. FEBS Lett. 2014, 588, 2262–2269. [Google Scholar] [CrossRef]
- D’Amelio, P.; Cristofaro, M.A.; Tamone, C.; Morra, E.; Di Bella, S.; Isaia, G.; Grimaldi, A.; Gennero, L.; Gariboldi, A.; Ponzetto, A.; et al. Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone 2008, 43, 1010–1015. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, T.H.; Kim, H.J.; Park, E.K.; Yang, E.K.; Kim, S.Y. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp. Mol. Med. 2010, 42, 376–385. [Google Scholar] [CrossRef]
- Swanberg, M.; McGuigan, F.E.; Ivaska, K.K.; Gerdhem, P.; Akesson, K. Polymorphisms in the inflammatory genes CIITA, CLEC16A and IFNG influence BMD, bone loss and fracture in elderly women. PLoS ONE 2012, 7, e47964. [Google Scholar] [CrossRef]
- Wu, Y.; Tworkoski, K.; Michaud, M.; Madri, J.A. Bone marrow monocyte PECAM-1 deficiency elicits increased osteoclastogenesis resulting in trabecular bone loss. J. Immunol. 2009, 182, 2672–2679. [Google Scholar] [CrossRef]
- Fang, J.; Hall, B.K. N-CAM is not required for initiation of secondary chondrogenesis: The role of N-CAM in skeletal condensation and differentiation. Int. J. Dev. Biol. 1999, 43, 335–342. [Google Scholar]
- Hiramatsu, K.; Asaba, Y.; Takeshita, S.; Nimura, Y.; Tatsumi, S.; Katagiri, N.; Niida, S.; Nakajima, T.; Tanaka, S.; Ito, M.; et al. Overexpression of gamma-glutamyltransferase in transgenic mice accelerates bone resorption and causes osteoporosis. Endocrinology 2007, 148, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Into, T.; Suzuki, K.; Miyauchi, M.; Takata, T.; Shibayama, K.; Niida, S. gamma-Glutamyltranspeptidase is an endogenous activator of Toll-like receptor 4-mediated osteoclastogenesis. Sci. Rep. 2016, 6, 35930. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pandey, A.K.; Mulligan, M.K.; Williams, E.G.; Mozhui, K.; Li, Z.; Jovaisaite, V.; Quarles, L.D.; Xiao, Z.; Huang, J.; et al. Joint mouse-human phenome-wide association to test gene function and disease risk. Nat. Commun. 2016, 7, 10464. [Google Scholar] [CrossRef]
- Chen, R.; Liao, X.; Chen, F.; Wang, B.; Huang, J.; Jian, G.; Huang, Z.; Yin, G.; Liu, H.; Jin, D. Circulating microRNAs, miR-10b-5p, miR-328-3p, miR-100 and let-7, are associated with osteoblast differentiation in osteoporosis. Int. J. Clin. Exp. Pathol. 2018, 11, 1383–1390. [Google Scholar]
- Tang, F.; Zhang, R.; He, Y.; Zou, M.; Guo, L.; Xi, T. MicroRNA-125b induces metastasis by targeting STARD13 in MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE 2012, 7, e35435. [Google Scholar] [CrossRef]
- Foucan, L.; Velayoudom-Cephise, F.L.; Larifla, L.; Armand, C.; Deloumeaux, J.; Fagour, C.; Plumasseau, J.; Portlis, M.L.; Liu, L.; Bonnet, F.; et al. Polymorphisms in GC and NADSYN1 Genes are associated with vitamin D status and metabolic profile in Non-diabetic adults. BMC Endocr. Disord. 2013, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Jiang, S.; Muyiduli, X.; Wang, S.; Mo, M.; Li, M.; Wang, Z.; Yu, Y. Vitamin D pathway gene polymorphisms influenced vitamin D level among pregnant women. Clin. Nutr. 2018, 37 Pt A, 2230–2237. [Google Scholar] [CrossRef]
- Simmons, K.M.; Beaudin, S.G.; Narvaez, C.J.; Welsh, J. Gene Signatures of 1,25-Dihydroxyvitamin D3 Exposure in Normal and Transformed Mammary Cells. J. Cell. Biochem. 2015, 116, 1693–1711. [Google Scholar] [CrossRef]
- Sheng, L.; Anderson, P.H.; Turner, A.G.; Pishas, K.I.; Dhatrak, D.J.; Gill, P.G.; Morris, H.A.; Callen, D.F. Identification of vitamin D(3) target genes in human breast cancer tissue. J. Steroid Biochem. Mol. Biol. 2016, 164, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Reginster, J.Y.; Bruyere, O. Association Between the Decline in Muscle Health and the Decline in Bone Health in Older Individuals from the SarcoPhAge Cohort. Calcif. Tissue Int. 2019, 104, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef]
- Popov, D.V.; Makhnovskii, P.A.; Kurochkina, N.S.; Lysenko, E.A.; Vepkhvadze, T.F.; Vinogradova, O.L. Intensity-dependent gene expression after aerobic exercise in endurance-trained skeletal muscle. Biol. Sport. 2018, 35, 277–289. [Google Scholar] [CrossRef]
- Negro-Vilar, A. Selective androgen receptor modulators (SARMs): A novel approach to androgen therapy for the new millennium. J. Clin. Endocrinol. Metab. 1999, 84, 3459–3462. [Google Scholar] [CrossRef]
- Niki, T.; Takahashi-Niki, K.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. DJBP: A novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. 2003, 1, 247–261. [Google Scholar]
- Fishilevich, S.; Zimmerman, S.; Kohn, A.; Iny Stein, T.; Olender, T.; Kolker, E.; Safran, M.; Lancet, D. Genic insights from integrated human proteomics in GeneCards. Database 2016, 2016, baw030. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, B.; Wang, Z.P.; He, J.W.; Fu, W.Z.; Fan, Y.B.; Zhang, Z.L. Whole-Exome Sequencing Identifies Novel Recurrent Somatic Mutations in Sporadic Parathyroid Adenomas. Endocrinology 2018, 159, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Quartier, A.; Chatrousse, L.; Redin, C.; Keime, C.; Haumesser, N.; Maglott-Roth, A.; Brino, L.; Le Gras, S.; Benchoua, A.; Mandel, J.L.; et al. Genes and Pathways Regulated by Androgens in Human Neural Cells, Potential Candidates for the Male Excess in Autism Spectrum Disorder. Biol. Psychiatry 2018, 84, 239–252. [Google Scholar] [CrossRef]
- Giguere, V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. 2002, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Esseghir, S.; Kennedy, A.; Seedhar, P.; Nerurkar, A.; Poulsom, R.; Reis-Filho, J.S.; Isacke, C.M. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3164–3173. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.N.; Ketabi, Z.; Rosenstierne, M.W.; Palle, C.; Boesen, H.C.; Norrild, B. Expression of CPEB, GAPDH and U6snRNA in cervical and ovarian tissue during cancer development. APMIS 2009, 117, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sousa Martins, J.P.; Liu, X.; Oke, A.; Arora, R.; Franciosi, F.; Viville, S.; Laird, D.J.; Fung, J.C.; Conti, M. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J. Cell Sci. 2016, 129, 1271–1282. [Google Scholar] [CrossRef]
- Hyon, C.; Mansour-Hendili, L.; Chantot-Bastaraud, S.; Donadille, B.; Kerlan, V.; Dode, C.; Jonard, S.; Delemer, B.; Gompel, A.; Reznik, Y.; et al. Deletion of CPEB1 Gene: A Rare but Recurrent Cause of Premature Ovarian Insufficiency. J. Clin. Endocrinol. Metab. 2016, 101, 2099–2104. [Google Scholar] [CrossRef]
- Zhang, J.; Lai, Z.; Shi, L.; Tian, Y.; Luo, A.; Xu, Z.; Ma, X.; Wang, S. Repeated superovulation increases the risk of osteoporosis and cardiovascular diseases by accelerating ovarian aging in mice. Aging 2018, 10, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Liu, Y.; Li, J.; Zhang, Y.; Wang, H.; Zhang, Y.; Jia, B. Identification of Potential Biomarkers Associated with Spermatogenesis in Azoospermia. Clin. Lab. 2024, 70, 2151–2160. [Google Scholar] [CrossRef]
- Vondracek, S.F.; Hansen, L.B.; McDermott, M.T. Osteoporosis risk in premenopausal women. Pharmacotherapy 2009, 29, 305–317. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Wang, S.; Dvorkin, D.; Da, Y. SNPEVG: A graphical tool for GWAS graphing with mouse clicks. BMC Bioinform. 2012, 13, 319. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat. Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 247) | Osteoporosis (n = 57) | p Value | |
|---|---|---|---|
| Age (years) | 47.08 ± 2.57 | 47.54 ± 2.46 | 0.218 |
| Weight (kg) | 63.94 ± 5.13 | 66.62 ± 7.98 | 0.018 |
| BMI (kg/m2) | 25.71 ± 2.35 | 27.23 ± 3.22 | 0.001 |
| Alcohol consumption (g/day) | 1.06 ± 2.03 | 0.66 ± 1.35 | 0.072 |
| Calcium consumption (mg/day) | 435.88 ± 196.07 | 485.01 ± 205.45 | 0.092 |
| Medical history of fracture | none | none | |
| Medical history of arthritis | none | none | |
| Smoking | none | none | |
| Long-term steroid | none | none | |
| Hormone therapy | none | none | |
| DR-SOS (m/s) | 4269.38 ± 123.94 | 4107.46 ± 152.21 | 0.000 * |
| DR-T (m/s) | 0.8 ± 0.99 | −0.45 ± 1.19 | 0.000 * |
| DR-Z (m/s) | 0.92 ± 1.02 | −0.29 ± 1.22 | 0.000 * |
| MT-SOS (m/s) | 3959.12 ± 65.93 | 3608.74 ± 90.86 | 0.000 * |
| MT-T (m/s) | 0.001 ± 0.63 | −3.4 ± 0.89 | 0.000 * |
| MT-Z (m/s) | 0.2 ± 0.63 | −3.2 ± 0.92 | 0.000 * |
| SNP | Chromosome | Position | Reference Allele | Alternate Allele | Gene | Amino Acid Change | PolyPhen-2 | SIFT | PROVEAN | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Prediction | Score | Prediction | Score | Prediction | |||||||
| rs1799852 | 3 | 133475722 | C | T | TF | p.Leu247= | - | - | 0.333 | tolerated | 0.00 | neutral |
| rs11917356 | 3 | 130110550 | A | G | COL6A5 | p.Asp982Val | 0.093 | benign | 0.717 | tolerated | −1.79 | neutral |
| rs2276360 | 11 | 71169547 | G | C | NADSYN1 | p.Val74Leu | 0.000 | benign | 1.000 | tolerated | 2.56 | neutral |
| rs1128431 | 15 | 82456227 | T | C | EFTUD1 | p.Ile617Val | 0.791 | possibly damaging | 0.010 | deleterious | −1.00 | neutral |
| rs7232237 | 18 | 31324934 | A | G | ASXL3 | p.Met1708Val | 0.000 | benign | 0.668 | tolerated | −0.84 | neutral |
| rs2282632 | 18 | 31320229 | A | G | ASXL3 | p.Asn954Ser | 0.003 | benign | 0.744 | tolerated | −0.73 | neutral |
| SNP | Gene | Chromosome | Position | p Value (Exome) | p Value (Affymetrix) |
|---|---|---|---|---|---|
| rs783540 | CPEB1 | 15 | 83254708 | 0.000 | 0.000 |
| rs3731646 | SH3BP4 | 2 | 235950002 | 0.000 | 0.003 |
| rs10506525 | MSRB3 | 12 | 65783378 | 0.001 | 0.003 |
| rs2110871 | MAGI2 | 7 | 78080548 | 0.002 | 0.002 |
| rs2172802 | LPHN3 | 4 | 62453209 | 0.003 | 0.001 |
| rs6895902 | MAML1 | 5 | 179201847 | 0.004 | 0.001 |
| rs2020945 | PWP2 | 21 | 45528919 | 0.004 | 0.003 |
| rs3756987 | RSPH3 | 6 | 159398700 | 0.004 | 0.010 |
| rs2286550 | CATSPERG | 19 | 38861362 | 0.004 | 0.008 |
| rs4729759 | CUX1 | 7 | 101536886 | 0.005 | 0.004 |
| rs10513680 | SAMD7 | 3 | 169644710 | 0.005 | 0.000 |
| rs1052053 | PMF1-BFLAP | 1 | 156202173 | 0.005 | 0.008 |
| rs2764020 | STARD13 | 13 | 34234642 | 0.006 | 0.003 |
| rs7088318 | PIP4K2A | 10 | 22852948 | 0.007 | 0.001 |
| rs151719 | HLA-DMB | 6 | 32903900 | 0.007 | 0.005 |
| rs2302234 | FAM20A | 17 | 66538239 | 0.007 | 0.008 |
| rs16990991 | EFCAB6 | 22 | 44167684 | 0.008 | 0.003 |
| rs12757165 | ESRRG | 1 | 216716537 | 0.009 | 0.003 |
| SNP | Chr. | Position | Gene | FC | p Value (Exome) | p Value (Affy) | Possible Mechanism in Osteoporosis | Function | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| rs12757165 | 1 | 216716537 | ESRRG | INT | 0.009 | 0.003 | Bone mineral density | Determination of bone density | [24] |
| rs1799852 | 3 | 133475722 | TF | SYN | 0.029 | 0.005 | Osteoclastogenesis | Bone mineral density | [28] |
| rs1436109 | 11 | 112991618 | NCAM1 | INT | 0.012 | 0.001 | Osteogenesis | Osteogenesis | [32] |
| rs4341610 | 12 | 96149288 | NTN4 | INT | 0.029 | 0.027 | To promote osteoblasts and inhibit osteoclast | [26,27] | |
| rs6498142 | 16 | 11081249 | CLECL16A | INT | 0.046 | 0.030 | Bone mineral density | [30] | |
| rs11917356 | 3 | 130110550 | COL6A5 | MIS | 0.014 | 0.005 | Variation in bone mineral density | [35] | |
| rs2812 | 17 | 62401118 | PECAM1 | 3′ UTR | 0.016 | 0.027 | Negative regulator of Osteoclastogenesis | [31] | |
| rs4820599 | 22 | 24990213 | GGT1 | INT | 0.003 | 0.041 | Osteoclastogenesis | [33] | |
| rs2764020 | 13 | 34234642 | STARD13 | INT | 0.006 | 0.003 | Target of miR-125, which is up-regulated in Osteoporosis | [36] | |
| rs2276360 | 11 | 71169547 | NADSYN1 | MIS | 0.038 | 0.027 | Vitamin D | Vitamin D status and metabolic profile | [38,39] |
| rs1128431 | 15 | 82456227 | EFTUD1 | MIS | 0.025 | 0.032 | Target gene for vitamin D | [36,40] | |
| rs12757165 | 1 | 216716537 | ESRRG | INT | 0.009 | 0.003 | Skeletal muscle | Skeletal muscle exercise | [44] |
| rs11090122 | 22 | 43308475 | PACSIN2 | INT | 0.045 | 0.028 | Skeletal muscle exercise | [44] | |
| rs12757165 | 1 | 216716537 | ESRRG | INT | 0.009 | 0.003 | Reproductive system | Estrogen pathways | [24,50] |
| rs16990991 | 22 | 44167684 | EFCAB6 | INT | 0.008 | 0.003 | Regulation of androgen receptor | [46] | |
| rs4341610 | 12 | 96149288 | NTN4 | INT | 0.029 | 0.027 | Prognosis of ER-positive breast cancer | [51] | |
| rs783540 | 15 | 83254708 | CPEB1 | INT | 0.000 | 0.000 | Oocyte maturation | [53,54] | |
| rs7232237 | 18 | 31324934 | ASXL3 | MIS | 0.015 | 0.011 | Androgen pathway | [49] | |
| rs2282632 | 18 | 31320229 | ASXL3 | MIS | 0.019 | 0.038 | Androgen pathway | [49] | |
| rs1128431 | 15 | 82456227 | EFTUD1 | MIS | 0.025 | 0.032 | Breast cancer | [41] | |
| rs2286550 | 19 | 38861362 | CATSPERG | MIS | 0.004 | 0.008 | Spermatogenesis | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.K.; Hong, S.-J.; Kim, G.; Ban, J.Y.; Kang, S.W. Genome-Wide Association Study of Osteoporosis Risk in Korean Pre-Menopausal Women: The Korean Genome and Epidemiology Study. Int. J. Mol. Sci. 2025, 26, 8177. https://doi.org/10.3390/ijms26178177
Kim SK, Hong S-J, Kim G, Ban JY, Kang SW. Genome-Wide Association Study of Osteoporosis Risk in Korean Pre-Menopausal Women: The Korean Genome and Epidemiology Study. International Journal of Molecular Sciences. 2025; 26(17):8177. https://doi.org/10.3390/ijms26178177
Chicago/Turabian StyleKim, Su Kang, Seoung-Jin Hong, Gyutae Kim, Ju Yeon Ban, and Sang Wook Kang. 2025. "Genome-Wide Association Study of Osteoporosis Risk in Korean Pre-Menopausal Women: The Korean Genome and Epidemiology Study" International Journal of Molecular Sciences 26, no. 17: 8177. https://doi.org/10.3390/ijms26178177
APA StyleKim, S. K., Hong, S.-J., Kim, G., Ban, J. Y., & Kang, S. W. (2025). Genome-Wide Association Study of Osteoporosis Risk in Korean Pre-Menopausal Women: The Korean Genome and Epidemiology Study. International Journal of Molecular Sciences, 26(17), 8177. https://doi.org/10.3390/ijms26178177





