Early Diagnosis and Follow-Up of a Novel Homozygous Mutation in SOST Gene in a Child with Recurrent Facial Palsy: A Case Report and Review of the Literature
Abstract
1. Introduction
2. Results
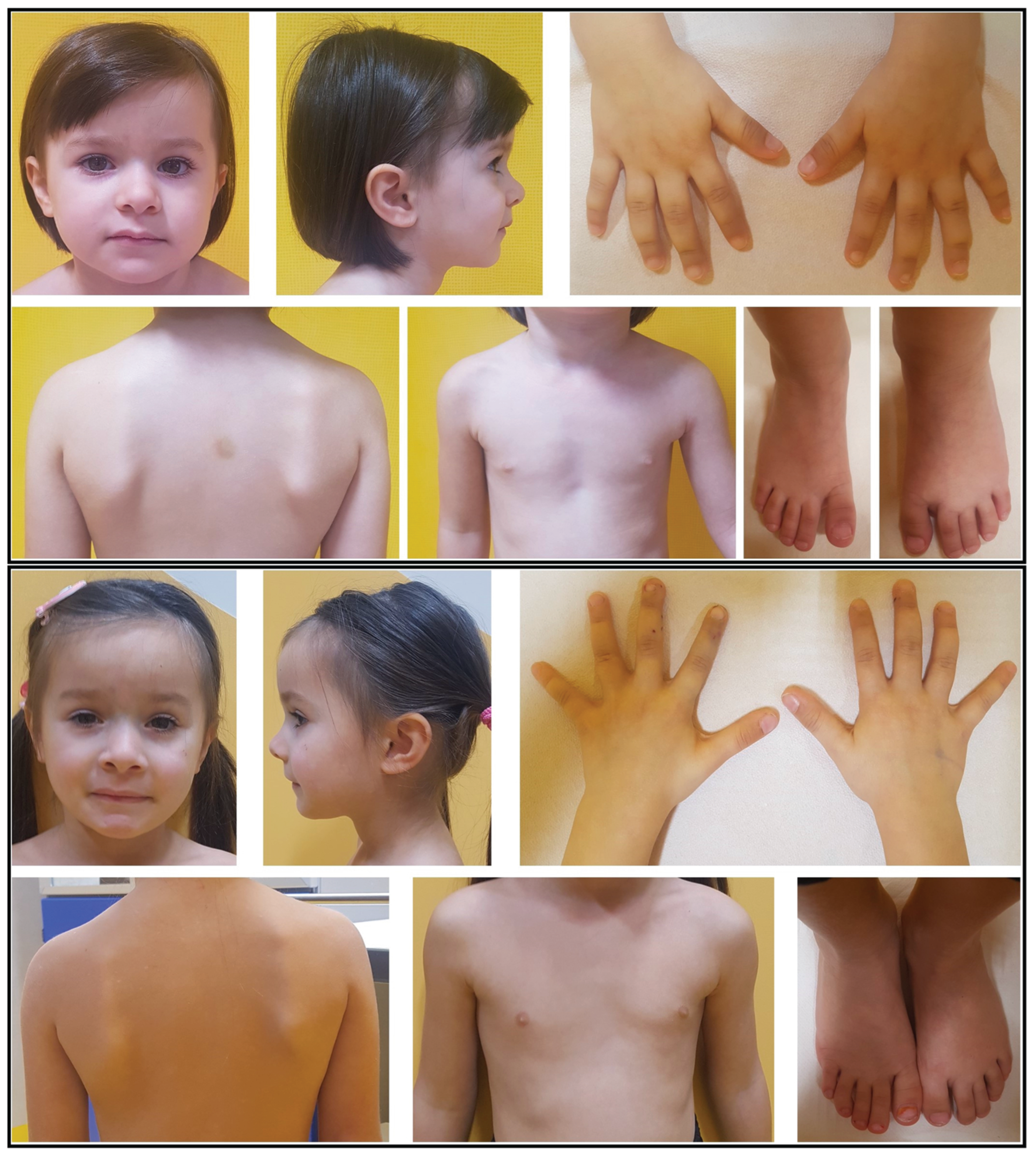
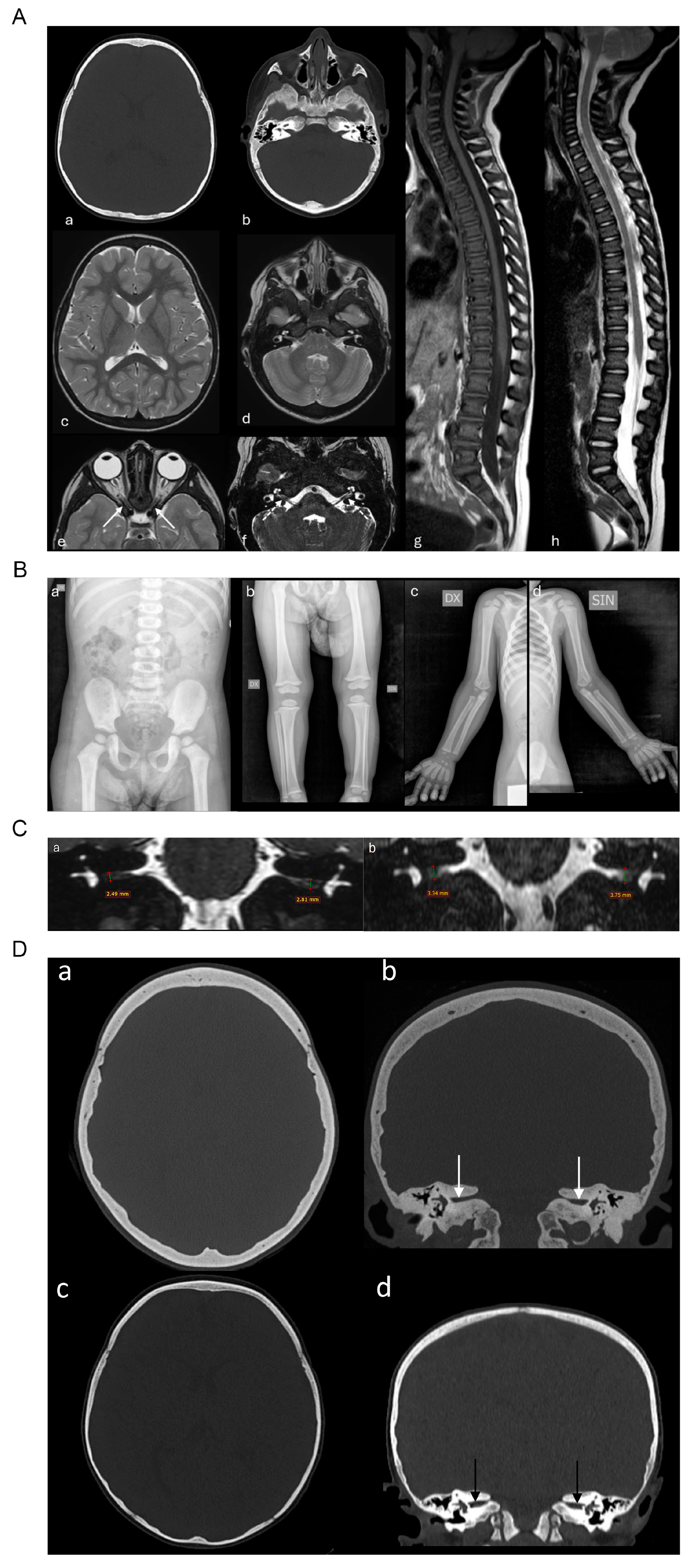
2.1. Case Report
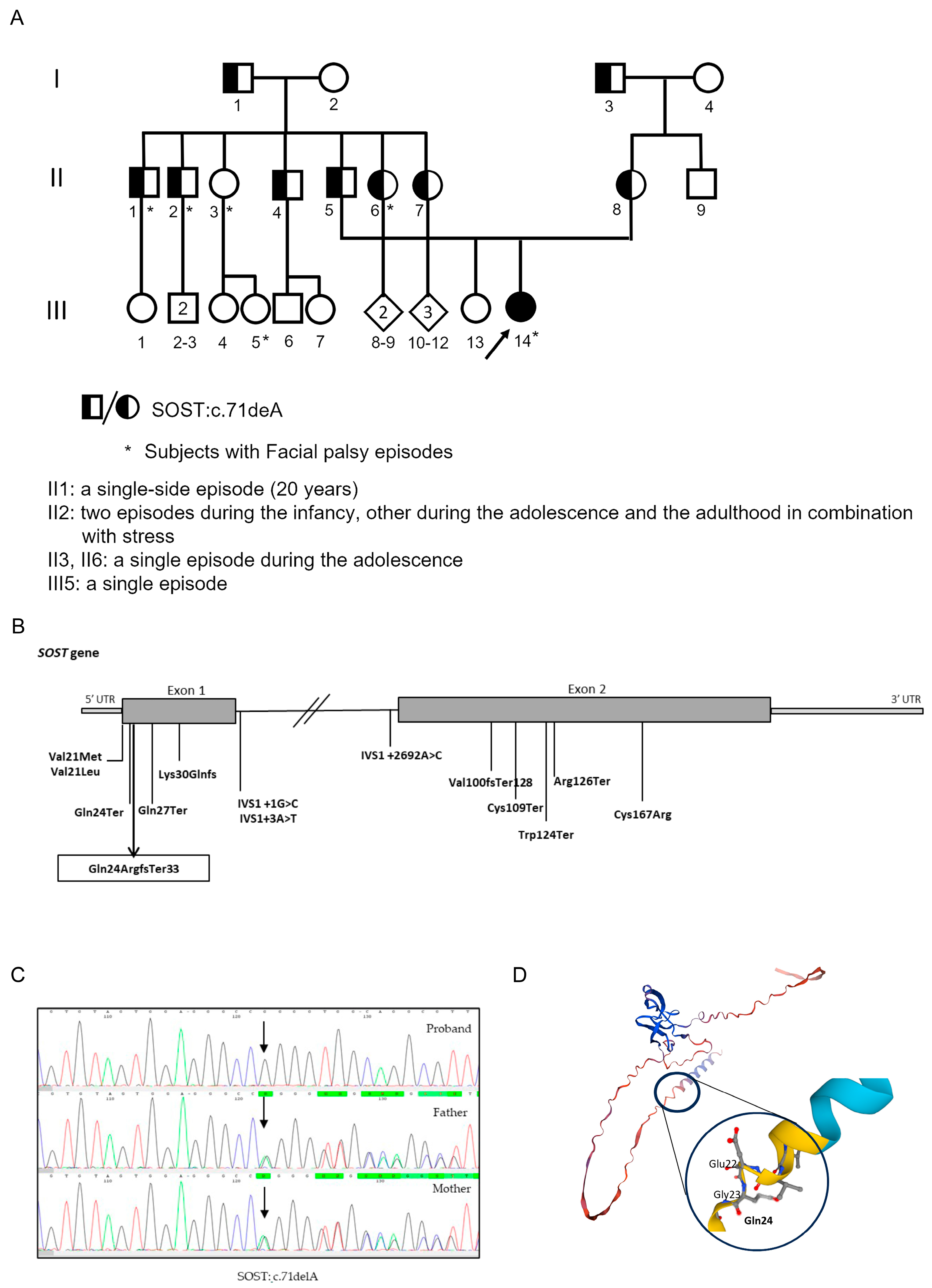
2.2. Genetic Analysis
3. Discussion
4. Materials and Methods
4.1. Patients’ Enrollment
4.2. DNA Extraction, NGS Sequencing, and Bioinformatics Analysis
4.3. Sanger Sequencing Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebastian, A.; Loots, G.G. Transcriptional Control of Sost in Bone. Bone 2017, 96, 76–84. [Google Scholar] [CrossRef]
- Sharifi, M.; Ereifej, L.; Lewiecki, E.M. Sclerostin and Skeletal Health. Rev. Endocr. Metab. Disord. 2015, 16, 149–156. [Google Scholar] [CrossRef]
- He, W.; Chen, C.; Pan, C.; Zhang, M.; Yu, X.; Wang, D.; Hu, S. Sclerosteosis Caused by a Novel Nonsense Mutation of SOST in a Consanguineous Family. Clin. Genet. 2016, 89, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.; Van Lierop, A.; Papapoulos, S. SOST-Related Sclerosing Bone Dysplasias. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Balemans, W. Increased Bone Density in Sclerosteosis Is Due to the Deficiency of a Novel Secreted Protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Brunkow, M.E.; Gardner, J.C.; Van Ness, J.; Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.; et al. Bone Dysplasia Sclerosteosis Results from Loss of the SOST Gene Product, a Novel Cystine Knot-Containing Protein. Am. J. Hum. Genet. 2001, 68, 577–589. [Google Scholar] [CrossRef]
- Itin, P.H.; Keserü, B.; Hauser, V. Syndactyly/Brachyphalangy and Nail Dysplasias as Marker Lesions for Sclerosteosis. Dermatology 2001, 202, 259–260. [Google Scholar] [CrossRef]
- Janssens, K. Molecular Genetics of Too Much Bone. Hum. Mol. Genet. 2002, 11, 2385–2393. [Google Scholar] [CrossRef][Green Version]
- Sebastian, A.; Loots, G.G. Genetics of Sost/SOST in Sclerosteosis and van Buchem Disease Animal Models. Metabolism 2018, 80, 38–47. [Google Scholar] [CrossRef]
- Loots, G.G.; Kneissel, M.; Keller, H.; Baptist, M.; Chang, J.; Collette, N.M.; Ovcharenko, D.; Plajzer-Frick, I.; Rubin, E.M. Genomic Deletion of a Long-Range Bone Enhancer Misregulates Sclerostin in Van Buchem Disease. Genome Res. 2005, 15, 928–935. [Google Scholar] [CrossRef]
- Balemans, W. Identification of a 52 Kb Deletion Downstream of the SOST Gene in Patients with van Buchem Disease. J. Med. Genet. 2002, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Bieganski, T.; Sohn, Y.B.; Kozlowski, K.; Semënov, M.; Okamoto, N.; Kim, C.H.; Ko, A.-R.; Ahn, G.H.; Choi, Y.-L.; et al. Identification of Signal Peptide Domain SOST Mutations in Autosomal Dominant Craniodiaphyseal Dysplasia. Hum. Genet. 2011, 129, 497–502. [Google Scholar] [CrossRef]
- Bieganski, T.; Baranska, D.; Miastkowska, I.; Kobielski, A.; Gorska-Chrzastek, M.; Kozlowski, K. A Boy with Severe Craniodiaphyseal Dysplasia and Apparently Normal Mother. Am. J. Med. Genet. Part A 2007, 143A, 2435–2443. [Google Scholar] [CrossRef]
- Jenke, A.C.; Stoek, L.-M.; Zilbauer, M.; Wirth, S.; Borusiak, P. Facial Palsy: Etiology, Outcome and Management in Children. Eur. J. Paediatr. Neurol. 2011, 15, 209–213. [Google Scholar] [CrossRef]
- Karalok, Z.S.; Taskin, B.D.; Ozturk, Z.; Gurkas, E.; Koc, T.B.; Guven, A. Childhood Peripheral Facial Palsy. Childs Nerv. Syst. 2018, 34, 911–917. [Google Scholar] [CrossRef]
- Rowhani-Rahbar, A.; Baxter, R.; Rasgon, B.; Ray, P.; Black, S.; Klein, J.O.; Klein, N.P. Epidemiologic and Clinical Features of Bell’s Palsy among Children in Northern California. Neuroepidemiology 2012, 38, 252–258. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Çubukçu, D.; Yılmaz, T.S.; Akıncı, G.; Özcan, M.; Güzel, O. Peripheral Facial Palsy in Children. J. Child. Neurol. 2014, 29, 1473–1478. [Google Scholar] [CrossRef]
- Wolfovitz, A.; Yehudai, N.; Luntz, M. Prognostic Factors for Facial Nerve Palsy in a Pediatric Population: A Retrospective Study and Review. Laryngoscope 2017, 127, 1175–1180. [Google Scholar] [CrossRef]
- Chen, W.-X.; Wong, V. Prognosis of Bell’s Palsy in Children—Analysis of 29 Cases. Brain Dev. 2005, 27, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Pitts, D.B.; Adour, K.K.; Hilsinger, R.L. Recurrent Bell’s Palsy: Analysis of 140 Patients. Laryngoscope 1988, 98, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.S.; Katz, K.A.; Hazen, A. Surgical Management of Facial Nerve Paralysis in the Pediatric Population. J. Pediatr. Surg. 2011, 46, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Drack, F.D.; Weissert, M. Outcome of Peripheral Facial Palsy in Children—A Catamnestic Study. Eur. J. Paediatr. Neurol. 2013, 17, 185–191. [Google Scholar] [CrossRef]
- Evans, A.K.; Licameli, G.; Brietzke, S.; Whittemore, K.; Kenna, M. Pediatric Facial Nerve Paralysis: Patients, Management and Outcomes. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1521–1528. [Google Scholar] [CrossRef]
- Yagi, H.; Takagi, M.; Hasegawa, Y.; Kayserili, H.; Nishimura, G. Sclerosteosis (Craniotubular Hyperostosis-Syndactyly) with Complex Hyperphalangy of the Index Finger. Pediatr. Radiol. 2015, 45, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Fayez, A.; Aglan, M.; Esmaiel, N.; El Zanaty, T.; Abdel Kader, M.; El Ruby, M. A Novel Loss-of-Sclerostin Function Mutation in a First Egyptian Family with Sclerosteosis. BioMed Res. Int. 2015, 2015, 517815. [Google Scholar] [CrossRef] [PubMed]
- Belkhribchia, M.R.; Collet, C.; Laplanche, J.-L.; Hassani, R. Novel SOST Gene Mutation in a Sclerosteosis Patient from Morocco: A Case Report. Eur. J. Med. Genet. 2014, 57, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Magno De Andrade, E.; Beer-Furlan, A.; Duarte, K.; Fonoff, E.; Teixeira, M. Management of Trigeminal Neuralgia in Sclerosteosis. Surg. Neurol. Int. 2013, 4, 455. [Google Scholar] [CrossRef]
- Tholpady, S.; Dodd, Z.H.; Havlik, R.J.; Fulkerson, D.H. Cranial Reconstruction for Treatment of Intracranial Hypertension from Sclerosteosis: Case-Based Update. World Neurosurg. 2014, 81, e1–e442. [Google Scholar] [CrossRef]
- Piters, E.; Culha, C.; Moester, M.; Van Bezooijen, R.; Adriaensen, D.; Mueller, T.; Weidauer, S.; Jennes, K.; de Freitas, F.; Löwik, C.; et al. First Missense Mutation in the SOST Gene Causing Sclerosteosis by Loss of Sclerostin Function. Hum. Mutat. 2010, 31, E1526–E1543. [Google Scholar] [CrossRef]
- Van Egmond, M.E.; Dikkers, F.G.; Boot, A.M.; Van Lierop, A.H.J.M.; Papapoulos, S.E.; Brouwer, O.F. A Rare Cause of Facial Nerve Palsy in Children: Hyperostosis Corticalis Generalisata (Van Buchem Disease). Three New Pediatric Cases and a Literature Review. Eur. J. Paediatr. Neurol. 2012, 16, 740–743. [Google Scholar] [CrossRef]
- Dixon, J.M.; Cull, R.E.; Gamble, P. Two Cases of Van Buchem’s Disease. J. Neurol. Neurosurg. Psychiatry 1982, 45, 913–918. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquaviva, F.; Bruno, G.; Palladino, F.; Rubino, A.; Russo, C.; Pandolfi, M.; Covelli, E.M.; Evangelista, E.; De Falco, L.; Tirozzi, A.; et al. Early Diagnosis and Follow-Up of a Novel Homozygous Mutation in SOST Gene in a Child with Recurrent Facial Palsy: A Case Report and Review of the Literature. Int. J. Mol. Sci. 2025, 26, 8175. https://doi.org/10.3390/ijms26178175
Acquaviva F, Bruno G, Palladino F, Rubino A, Russo C, Pandolfi M, Covelli EM, Evangelista E, De Falco L, Tirozzi A, et al. Early Diagnosis and Follow-Up of a Novel Homozygous Mutation in SOST Gene in a Child with Recurrent Facial Palsy: A Case Report and Review of the Literature. International Journal of Molecular Sciences. 2025; 26(17):8175. https://doi.org/10.3390/ijms26178175
Chicago/Turabian StyleAcquaviva, Fabio, Giorgia Bruno, Federica Palladino, Alfonso Rubino, Carmela Russo, Maria Pandolfi, Eugenio Maria Covelli, Eloisa Evangelista, Luigia De Falco, Alfonsina Tirozzi, and et al. 2025. "Early Diagnosis and Follow-Up of a Novel Homozygous Mutation in SOST Gene in a Child with Recurrent Facial Palsy: A Case Report and Review of the Literature" International Journal of Molecular Sciences 26, no. 17: 8175. https://doi.org/10.3390/ijms26178175
APA StyleAcquaviva, F., Bruno, G., Palladino, F., Rubino, A., Russo, C., Pandolfi, M., Covelli, E. M., Evangelista, E., De Falco, L., Tirozzi, A., De Brasi, D., & Varone, A. (2025). Early Diagnosis and Follow-Up of a Novel Homozygous Mutation in SOST Gene in a Child with Recurrent Facial Palsy: A Case Report and Review of the Literature. International Journal of Molecular Sciences, 26(17), 8175. https://doi.org/10.3390/ijms26178175





