Effects of Methotrexate and Tofacitinib on Mitochondrial Function and Oxidative Stress in Human Synovial Cells In Vitro
Abstract
1. Introduction
2. Results
2.1. Cell Viability (MTT—Test)
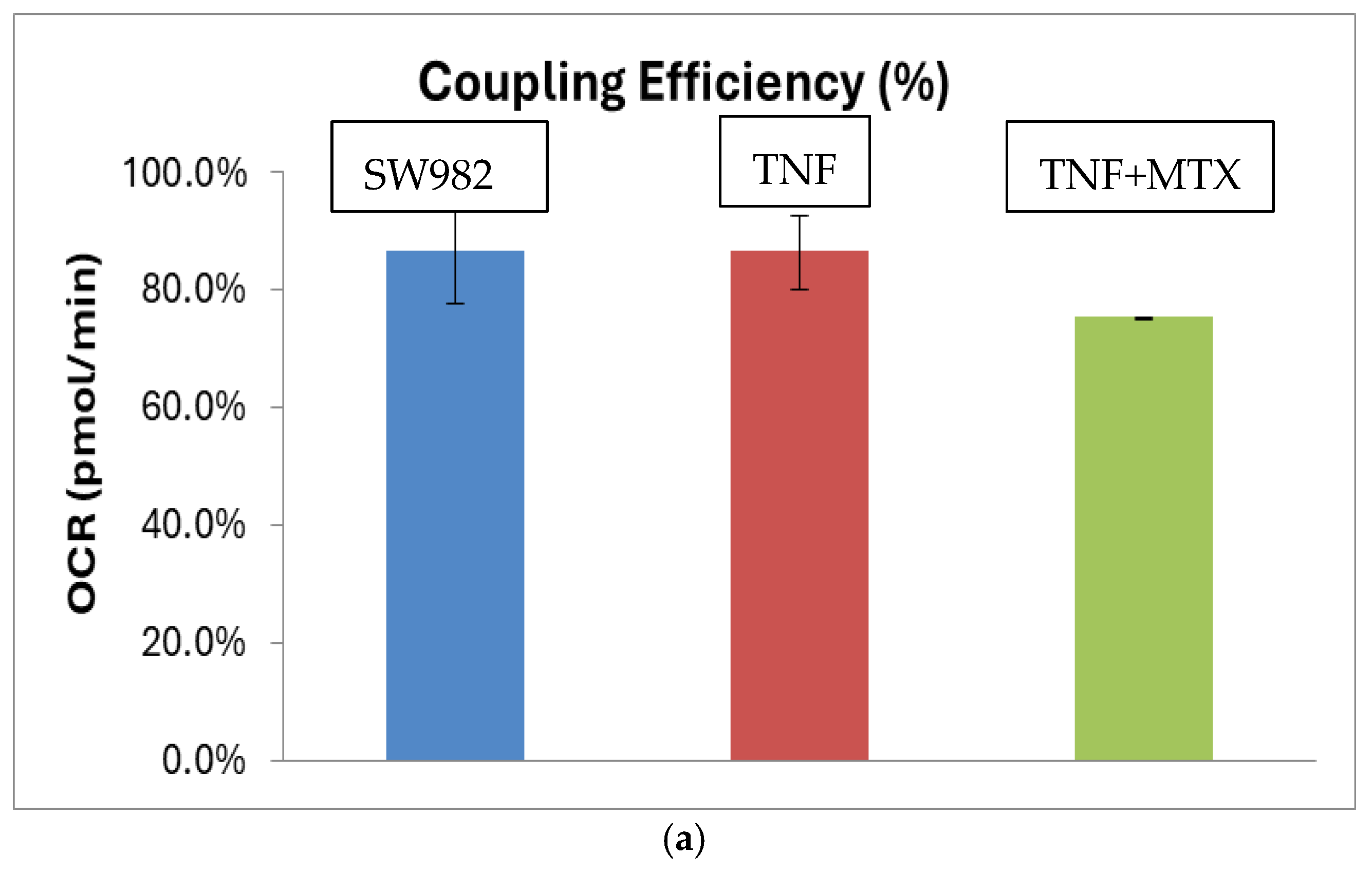
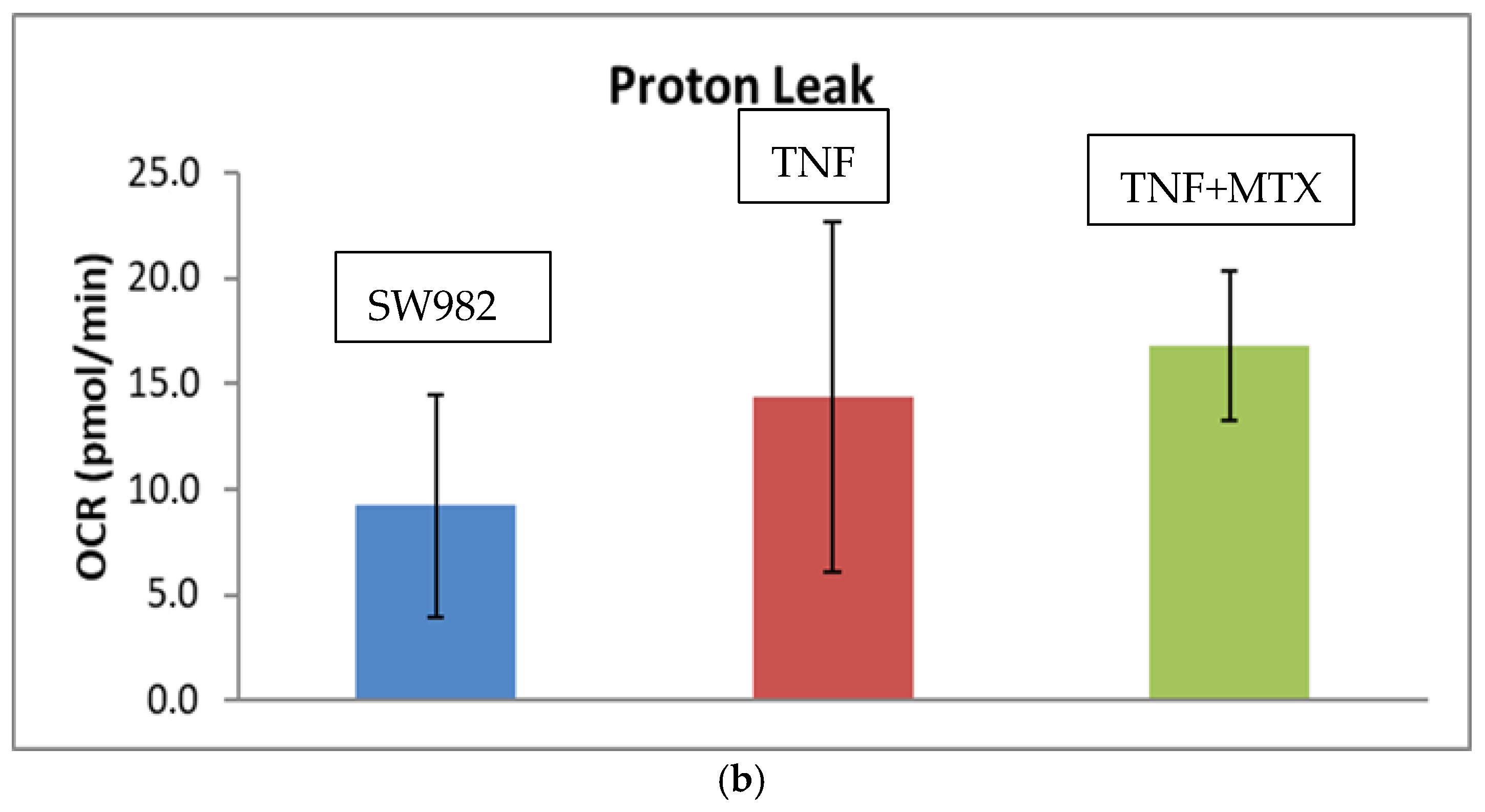
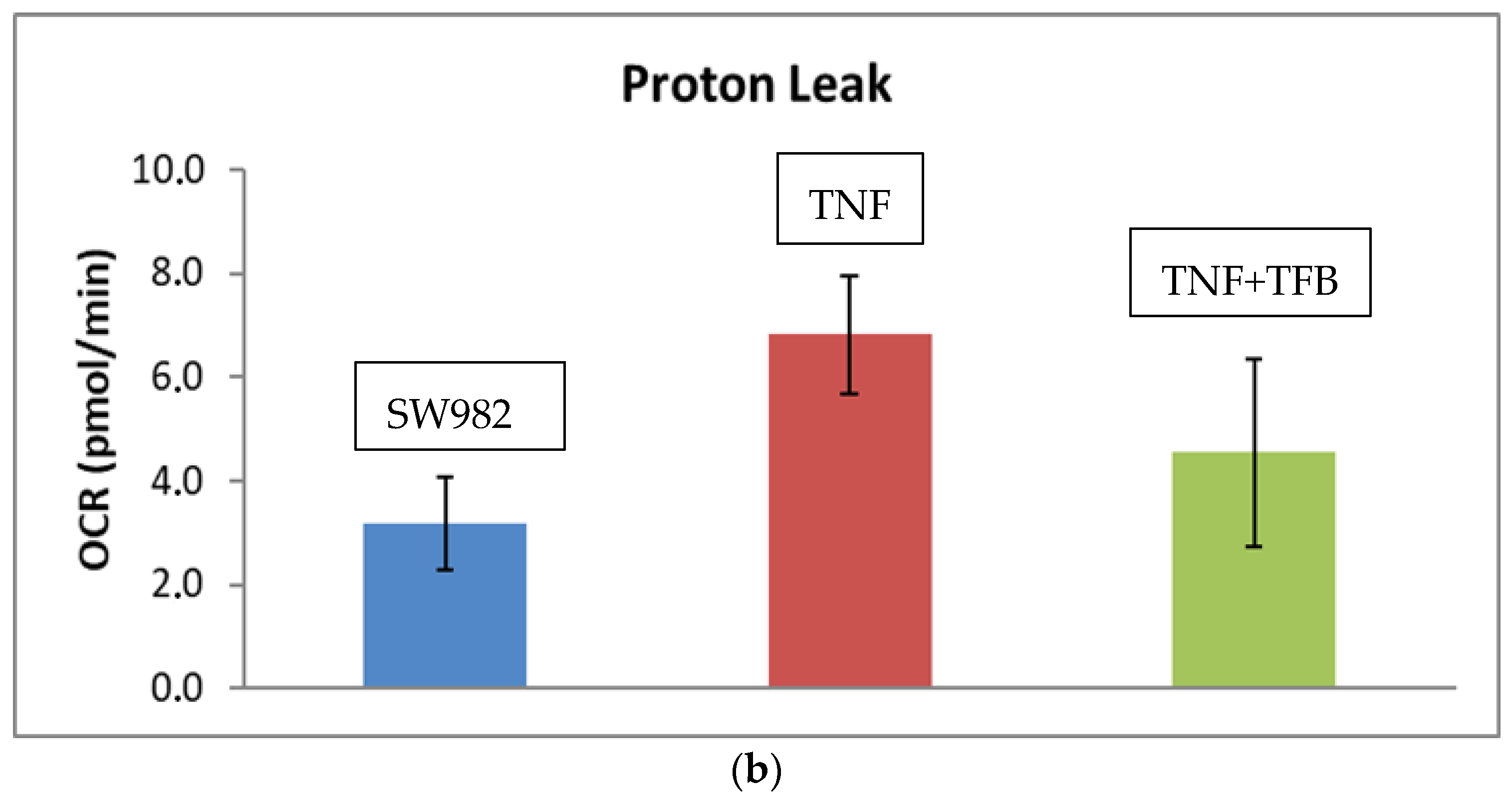
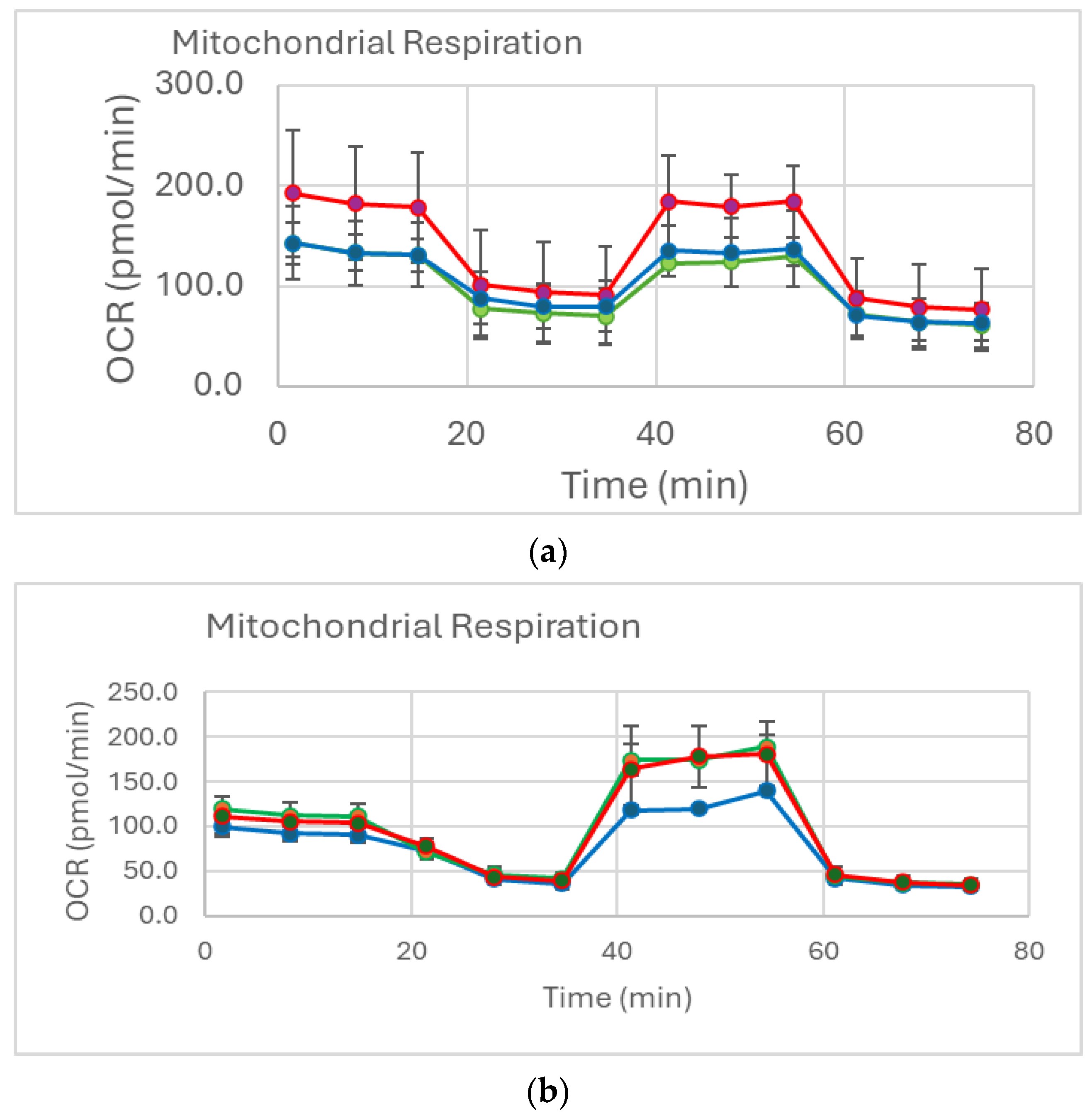
2.2. Mitochondrial Function (Mito Stress Test)
2.3. Oxidative Stress (Chromatographic Analysis for 8-IsoPGF2alpha Detection)
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Treatments with MTX and TFB
4.4. Detection of Mitochondrial Function
4.5. High-Performance Liquid Chromatography with Mass Detection for 8-IsoPGF2alpha
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef]
- Damerau, A.; Gaber, T. Modeling Rheumatoid Arthritis In Vitro: From Experimental Feasibility to Physiological Proximity. Int. J. Mol. Sci. 2020, 21, 7916. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038, Erratum in Lancet 2016, 388, 1984. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Hong, F.; Yang, S. Mitochondrial Dysfunction in Rheumatoid Arthritis. Biomolecules 2022, 12, 1216. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Fernández-Rodríguez, J.A.; Blanco, F.J. Mitochondrial Dysfunction and Oxidative Stress in Rheumatoid Arthritis. Antioxidants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Jávor, P.; Mácsai, A.; Butt, E.; Baráth, B.; Jász, D.K.; Horváth, T.; Baráth, B.; Csonka, Á.; Török, L.; Varga, E.; et al. Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently. Int. J. Mol. Sci. 2022, 23, 7553. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020, 328, 19–27. [Google Scholar] [CrossRef]
- Kostova, T.; Mihaylova, V.; Karalilova, R.; Batalov, Z.; Kazakova, M.; Batalov, A. Mitochondrial dysfunction and biological therapy: A new look at rheumatoid arthritis. Rheumatology 2022, 30, 51–65. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Seriolo, B.; Straub, R.H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benucci, M.; Bernardini, P.; Coccia, C.; De Luca, R.; Levani, J.; Economou, A.; Damiani, A.; Russo, E.; Amedei, A.; Guiducci, S.; et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun. Rev. 2023, 22, 103276. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in the development of rheumatoid arthritis. Immunol. Rev. 2020, 294, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, J.; Neuhoff, M.T.; Hoyler, T.; Noir, E.; Tessier, C.; Sarret, S.; Thorsen, T.N.; Littlewood-Evans, A.; Zhang, J.; Hasan, M.; et al. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 2021, 37, 109977. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.S.; Wu, J. Methotrexate and Pralatrexate. Dermatol. Clin. 2015, 33, 747–755. [Google Scholar] [CrossRef]
- Lopes de Melo, J.M.; Laursen, J.C.; Søndergaard-Heinrich, N.; Bull Rasmussen, I.K.; Hansen, C.S.; Frimodt-Møller, M.; Rossing, P.; Størling, J. Increased mitochondrial proton leak and glycolysis in peripheral blood mononuclear cells in type-1-diabetes. Heliyon 2022, 8, e12304. [Google Scholar] [CrossRef]
- Yamamoto, N.; Oliveira, M.B.; Campello, A.d.P.; Lopes, L.C.; Klüppel, M.L. Methotrexate: Studies on the cellular metabolism. I. Effect on mitochondrial oxygen uptake and oxidative phosphorylation. Cell Biochem. Funct. 1988, 6, 61–66. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Tanaka, Y. JAK inhibitors for rheumatoid arthritis. Expert Opin. Investig. Drugs 2023, 32, 333–344. [Google Scholar] [CrossRef]
- Andraos, R.; Qian, Z.; Bonenfant, D.; Rubert, J.; Vangrevelinghe, E.; Scheufler, C.; Marque, F.; Régnier, C.H.; De Pover, A.; Ryckelynck, H.; et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov. 2012, 2, 512–523. [Google Scholar] [CrossRef]
- Mihaylova, V.; Kazakova, M.; Batalov, Z.; Karalilova, R.; Batalov, A.; Sarafian, V. JAK inhibitors improve ATP production and mitochondrial function in rheumatoid arthritis: A pilot study. Rheumatol. Int. 2024, 44, 57–65. [Google Scholar] [CrossRef]
- van’t Erve, T.J.; Lih, F.B.; Kadiiska, M.B.; Deterding, L.J.; Eling, T.E.; Mason, R.P. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med. 2015, 83, 245–251. [Google Scholar] [CrossRef]
- Hursitoğlu, O.; Kurutas, E.B. Serum Levels of 8-Iso-Prostaglandin F2α and Raftlin in Patients with Generalized Anxiety Disorder. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2023, 21, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, L.; Văduva, C.C.; Caragea, D.C.; Novac, M.B.; Manasia, M.; Siloși, I.; Manolea, M.M.; Boldeanu, M.V.; Dijmărescu, A.L. Association between Serum 8-Iso-Prostaglandin F2α as an Oxidative Stress Marker and Immunological Markers in a Cohort of Preeclampsia Patients. Life 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, Y.; Liu, B.; Jia, X.; Wang, R.; Lu, Q. Associations of plasma 8-iso-prostaglandin F2α levels with fasting blood glucose (FBG) and intra-abdominal fat (IAF) area in various Glycometabolism populations. BMC Endocr. Disord. 2021, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yokoyama, T.; Akatsu, H.; Tukiyama, T.; Tokiwa, T. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. Vitr. Cell Dev Biol Anim. 2003, 39, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Dowty, M.E.; Lin, T.H.; Jesson, M.I.; Hegen, M.; Martin, D.A.; Katkade, V.; Menon, S.; Telliez, J.B. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol. Res. Perspect. 2019, 7, e00537. [Google Scholar] [CrossRef]
- Herman, R.; Veng-Pedersen, P.; Hoffman, J.; Koehnke, R.; Furst, D.E. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J. Pharm. Sci. 1989, 78, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tomova, Z.; Tomov, D.; Vlahova, A.; Chaova-Gizdakova, V.; Yoanidu, L.; Svinarov, D. Development and validation of an LC-MS/MS method for determination of 8-iso-prostaglandin F2 Alpha in human saliva. J. Med. Biochem. 2022, 41, 466–473. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, V.; Tomov, D.; Karalilova, R.; Batalov, Z.; Batalov, A.; Sarafian, V.; Kazakova, M. Effects of Methotrexate and Tofacitinib on Mitochondrial Function and Oxidative Stress in Human Synovial Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 8173. https://doi.org/10.3390/ijms26178173
Mihaylova V, Tomov D, Karalilova R, Batalov Z, Batalov A, Sarafian V, Kazakova M. Effects of Methotrexate and Tofacitinib on Mitochondrial Function and Oxidative Stress in Human Synovial Cells In Vitro. International Journal of Molecular Sciences. 2025; 26(17):8173. https://doi.org/10.3390/ijms26178173
Chicago/Turabian StyleMihaylova, Valentina, Desislav Tomov, Rositsa Karalilova, Zguro Batalov, Anastas Batalov, Victoria Sarafian, and Maria Kazakova. 2025. "Effects of Methotrexate and Tofacitinib on Mitochondrial Function and Oxidative Stress in Human Synovial Cells In Vitro" International Journal of Molecular Sciences 26, no. 17: 8173. https://doi.org/10.3390/ijms26178173
APA StyleMihaylova, V., Tomov, D., Karalilova, R., Batalov, Z., Batalov, A., Sarafian, V., & Kazakova, M. (2025). Effects of Methotrexate and Tofacitinib on Mitochondrial Function and Oxidative Stress in Human Synovial Cells In Vitro. International Journal of Molecular Sciences, 26(17), 8173. https://doi.org/10.3390/ijms26178173





