TGFBI Facilitates Myogenesis and Limits Fibrosis in Mouse Skeletal Muscle Regeneration
Abstract
1. Introduction
2. Results
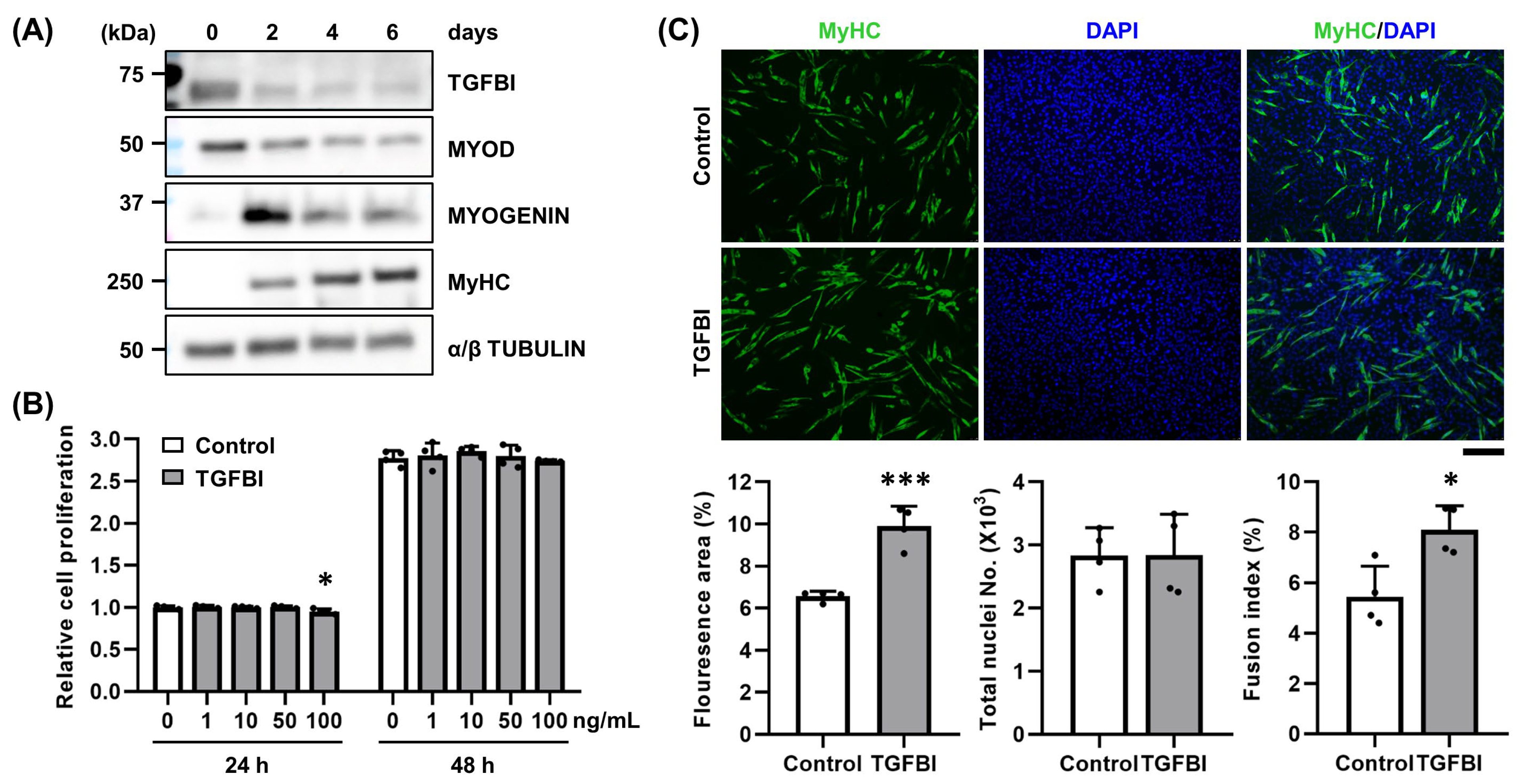
2.1. TGFBI Enhances Differentiation and Fusion of C2C12 Cells
2.2. TGFBI Rescues SV-Induced Cell Damage in C2C12 Cells
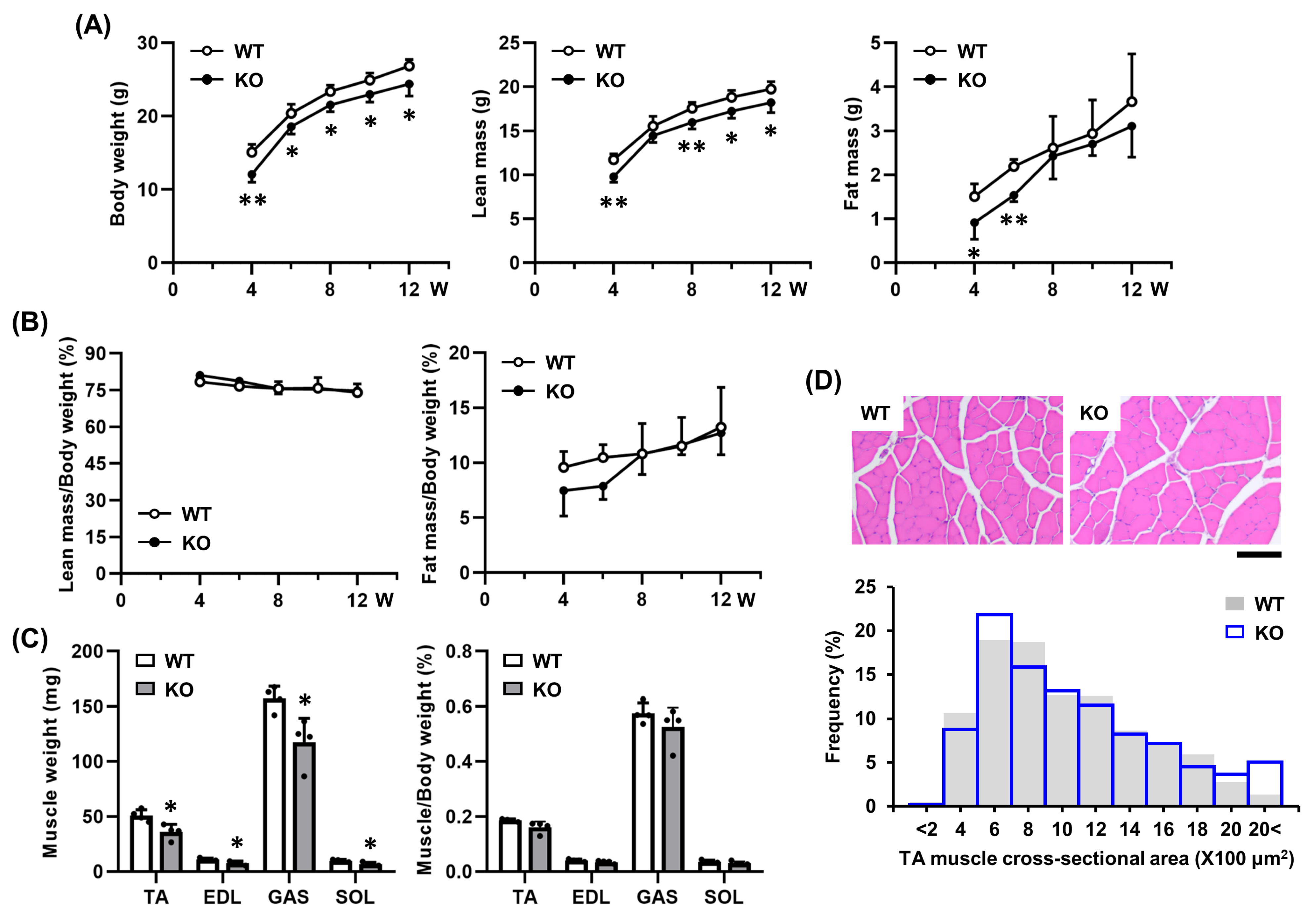
2.3. Tgfbi KO Mice Exhibit No Morphological Abnormalities in Skeletal Muscle Under Physiological Conditions
2.4. TGFBI Is Induced During Early Muscle Regeneration
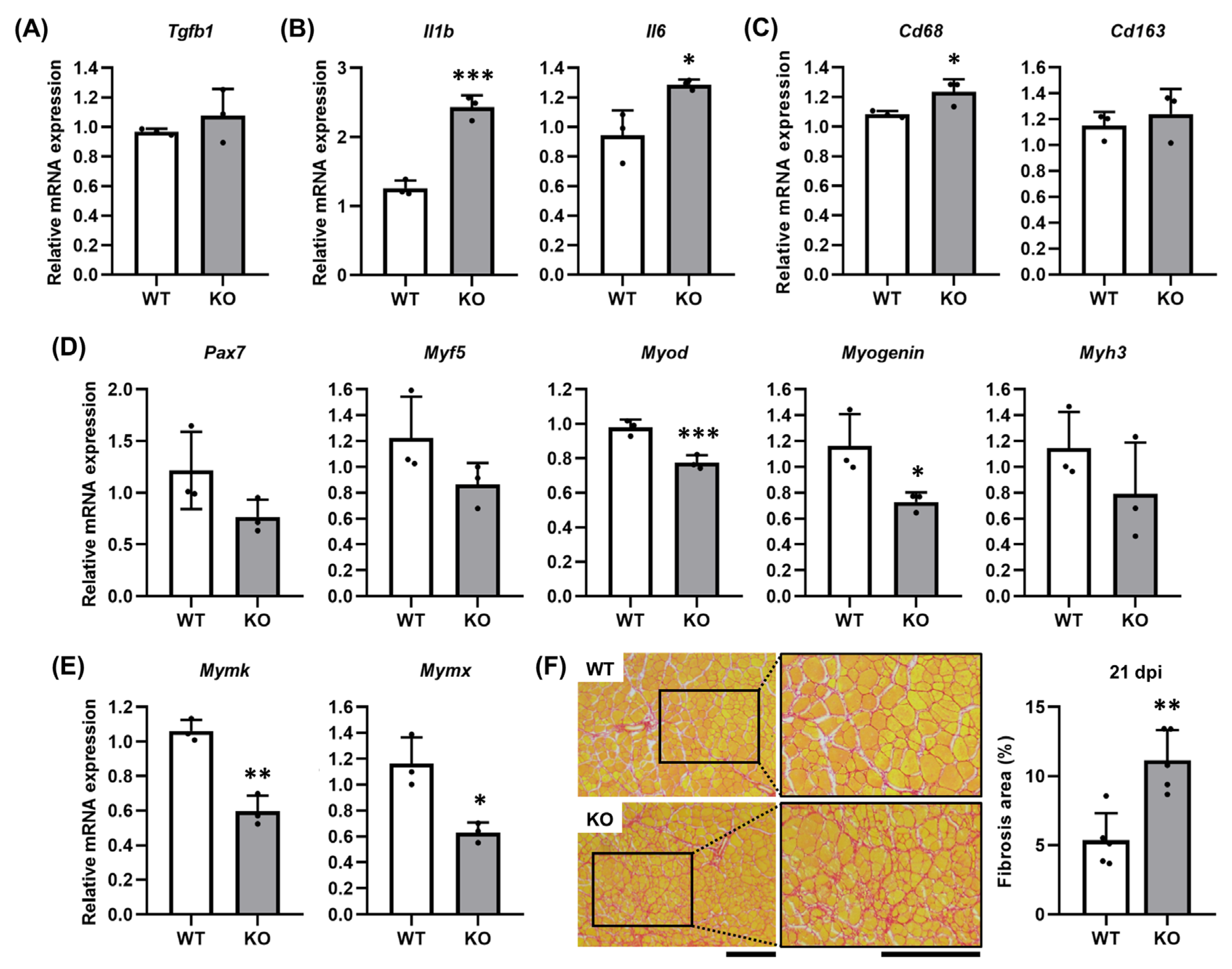
2.5. TGFBI Suppresses Fibrosis by Modulating Inflammatory Responses During Muscle Regeneration
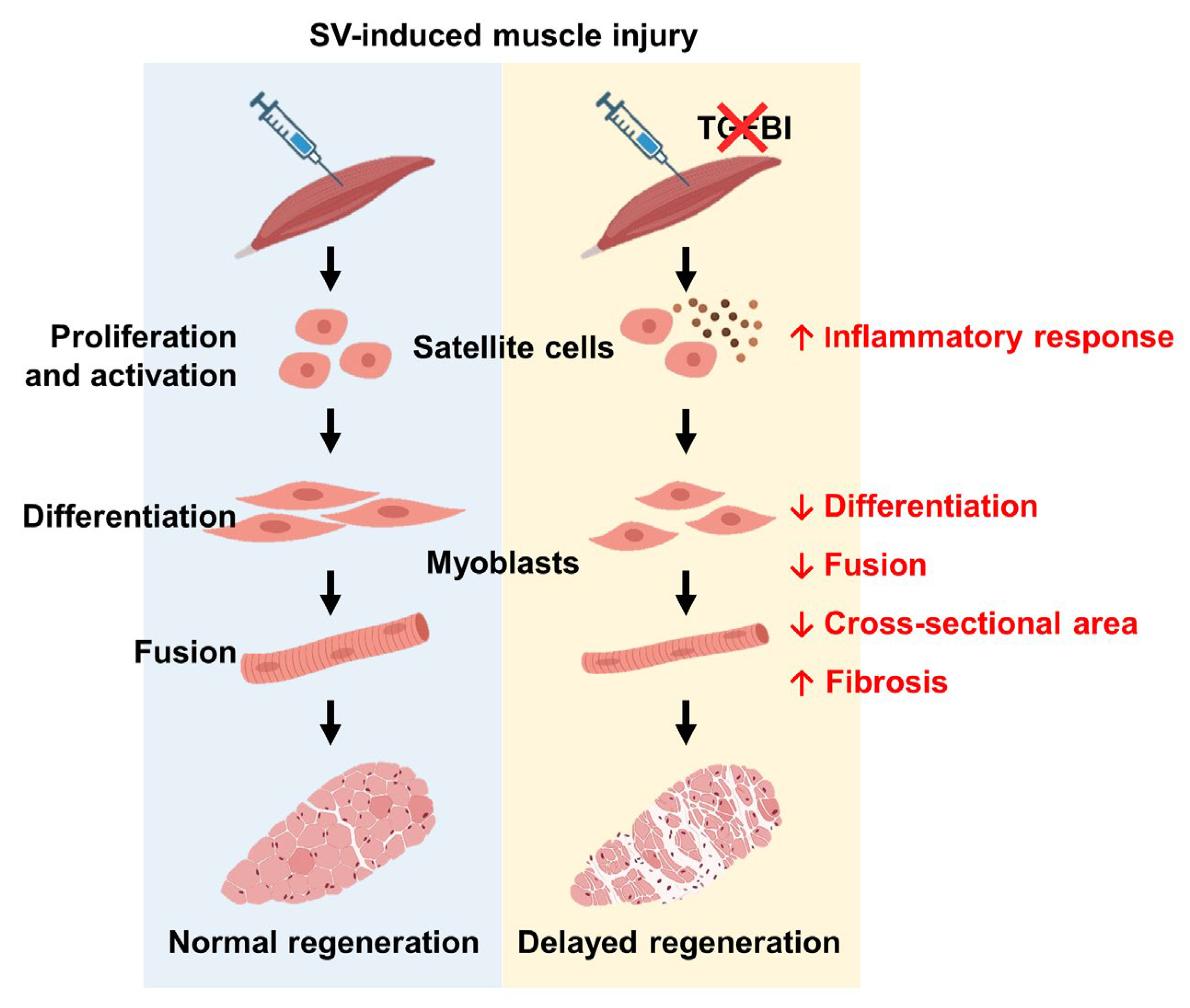
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture, MTT Assay, and SV-Induced Cell Damage
4.3. Western Blotting
4.4. Immunocytochemistry
4.5. Quantitative Real-Time PCR
4.6. Body Composition Analysis
4.7. Snake Venom-Induced Muscle Injury
4.8. Histological and Immunohistochemical Analyses
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BW | Body weight |
| dpi | Days post-injection |
| ECM | Extracellular matrix |
| EDL | Extensor digitorum longus |
| GAS | Gastrocnemius |
| H&E | Hematoxylin and eosin |
| Il1b | Interleukin 1 beta |
| Il6 | Interleukin 6 |
| KO | Knockout |
| MyHC | Myosin heavy chain |
| Mymk | Myomaker |
| Mymx | Myomixer |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| SOL | Soleus |
| SV | Snake venom |
| TA | Tibialis anterior |
| Tgfb1 | Transforming growth factor beta 1 |
| TGFBI | Transforming growth factor β-induced |
| qRT-PCR | Quantitative real-time PCR |
| WT | Wild-type |
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thépenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative study of injury models for studying muscle regeneration in mice. PLoS ONE 2016, 11, e014719. [Google Scholar] [CrossRef]
- Sanchez-Castro, E.E.; Pajuelo-Reyes, C.; Tejedo, R.; Soria-Juan, B.; Tapia-Limonchi, R.; Andreu, E.; Hitos, A.B.; Martin, F.; Cahuana, G.M.; Guerra-Duarte, C.; et al. Mesenchymal stromal cell-based therapies as promising treatments for muscle regeneration after snakebite envenoming. Front. Immunol. 2021, 11, 609961. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, M.; Almeida, J.R.; Rajan, E.; Williams, H.F.; Townsend, F.; Cornish, E.; Mitchell, R.D.; Patel, K.; Vaiyapuri, S. Intramuscular bleeding and formation of microthrombi during skeletal muscle damage caused by a snake venom metalloprotease and a cardiotoxin. Toxins 2023, 15, 530. [Google Scholar] [CrossRef]
- Hernández, R.; Cabalceta, C.; Saravia-Otten, P.; Chaves, A.; Gutiérrez, J.M.; Rucavado, A. Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: Alterations in microvasculature and nerves. PLoS ONE 2011, 6, e19834, Correction in PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Han, M.S.; Kim, J.E.; Shin, H.I.; Kim, I.S. Expression patterns of betaig-h3 in chondrocyte differentiation during endochondral ossification. Exp. Mol. Med. 2008, 40, 453–460. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, R.G.; Bezverkov, K.I.; Zimber, M.P.; Pavelec, R.; Skonier, J.; Purchio, A.F. Beta IG-H3, a novel secretory protein inducible by transforming growth factor-beta, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J. Investig. Dermatol. 1995, 104, 844–849. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, E.H.; Kim, I.S.; Kim, J.E. Tgfbi deficiency leads to a reduction in skeletal size and degradation of the bone matrix. Calcif. Tissue Int. 2015, 96, 56–64. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.J.; Lee, B.H.; Park, R.W.; Kim, K.S.; Kim, I.S. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, betaig-h3. J. Biol. Chem. 2000, 275, 30907–30915. [Google Scholar] [CrossRef] [PubMed]
- Runager, K.; Enghild, J.J.; Klintworth, G.K. Focus on molecules: Transforming growth factor beta induced protein (TGFBIp). Exp. Eye Res. 2008, 87, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Toupet, K.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noël, D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials 2020, 226, 119544. [Google Scholar] [CrossRef] [PubMed]
- Schüler, S.C.; Liu, Y.; Dumontier, S.; Grandbois, M.; Le Moal, E.; Cornelison, D.; Bentzinger, C.F. Extracellular matrix: Brick and mortar in the skeletal muscle stem cell niche. Front. Cell Dev. Biol. 2022, 10, 1056523. [Google Scholar] [CrossRef]
- Kano, J.; Wang, H.; Zhang, H.; Noguchi, M. Roles of DKK3 in cellular adhesion, motility, and invasion through extracellular interaction with TGFBI. FEBS J. 2022, 289, 6385–6399. [Google Scholar] [CrossRef]
- Steitz, A.M.; Steffes, A.; Finkernagel, F.; Unger, A.; Sommerfeld, L.; Jansen, J.M.; Wagner, U.; Graumann, J.; Müller, R.; Reinartz, S. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis. 2020, 11, 249. [Google Scholar] [CrossRef]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef]
- Ito, N.; Miyagoe-Suzuki, Y.; Takeda, S.; Kudo, A. Periostin is required for the maintenance of muscle fibers during muscle regeneration. Int. J. Mol. Sci. 2021, 22, 3627. [Google Scholar] [CrossRef]
- Cheong, S.; Peng, Y.; Lu, F.; He, Y. Structural extracellular matrix-mediated molecular signaling in wound repair and tissue regeneration. Biochimie 2025, 229, 58–68. [Google Scholar] [CrossRef]
- Lecker, L.S.M.; Berlato, C.; Maniati, E.; Delaine-Smith, R.; Pearce, O.M.T.; Heath, O.; Nichols, S.J.; Trevisan, C.; Novak, M.; McDermott, J.; et al. TGFBI production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res. 2021, 81, 5706–5719. [Google Scholar] [CrossRef]
- Ogawa, Y.; Yamamoto, M.; Sato, M.; Odaka, K.; Kasahara, M.; Hinata, N.; Ochi, H.; Uezumi, A.; Tsuchida, K.; Nakashima, K.; et al. Localization of T-cell factor 4 positive fibroblasts and CD206-positive macrophages during skeletal muscle regeneration in mice. Ann. Anat. 2021, 235, 151694. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Dye, D.E.; Kinnear, B.F.; van Kuppevelt, T.H.; Grounds, M.D.; Coombe, D.R. Interactions between skeletal muscle myoblasts and their extracellular matrix revealed by a serum free culture system. PLoS ONE 2015, 10, e0127675. [Google Scholar] [CrossRef] [PubMed]
- Bin Haidar, H.; Almeida, J.R.; Williams, J.; Guo, B.; Bigot, A.; Senthilkumaran, S.; Vaiyapuri, S.; Patel, K. Differential effects of the venoms of Russell’s viper and Indian cobra on human myoblasts. Sci. Rep. 2024, 14, 3184. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Thapa, N.; Kim, J.E.; Yang, J.D.; Cho, B.C.; Kim, I.S. Recombinant tetra-cell adhesion motifs supports adhesion, migration and proliferation of keratinocytes/fibroblasts, and promotes wound healing. Exp. Mol. Med. 2007, 39, 663–672. [Google Scholar] [CrossRef]
- Schwander, M.; Leu, M.; Stumm, M.; Dorchies, O.M.; Ruegg, U.T.; Schittny, J.; Müller, U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell 2003, 4, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim. Biophys. Sin. 2009, 41, 263–272. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Klingler, W.; Jurkat-Rott, K.; Lehmann-Horn, F.; Schleip, R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012, 31, 184–195. [Google Scholar] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, N.R.; Kim, J.E. Bioinformatics of differentially expressed genes in phorbol 12-myristate 13-acetate-induced megakaryocytic differentiation of K562 cells by microarray analysis. Int. J. Mol. Sci. 2022, 23, 4221. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, N.R.; Jin, S.-Y.; Kim, S.-Y.; Lee, S.-H.; Kim, I.-S.; Kim, J.-E. TGFBI Facilitates Myogenesis and Limits Fibrosis in Mouse Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2025, 26, 9042. https://doi.org/10.3390/ijms26189042
Park NR, Jin S-Y, Kim S-Y, Lee S-H, Kim I-S, Kim J-E. TGFBI Facilitates Myogenesis and Limits Fibrosis in Mouse Skeletal Muscle Regeneration. International Journal of Molecular Sciences. 2025; 26(18):9042. https://doi.org/10.3390/ijms26189042
Chicago/Turabian StylePark, Na Rae, So-Yeon Jin, Soon-Young Kim, Seung-Hoon Lee, In-San Kim, and Jung-Eun Kim. 2025. "TGFBI Facilitates Myogenesis and Limits Fibrosis in Mouse Skeletal Muscle Regeneration" International Journal of Molecular Sciences 26, no. 18: 9042. https://doi.org/10.3390/ijms26189042
APA StylePark, N. R., Jin, S.-Y., Kim, S.-Y., Lee, S.-H., Kim, I.-S., & Kim, J.-E. (2025). TGFBI Facilitates Myogenesis and Limits Fibrosis in Mouse Skeletal Muscle Regeneration. International Journal of Molecular Sciences, 26(18), 9042. https://doi.org/10.3390/ijms26189042






