Antibacterial and Antibiofilm Properties of Postbiotics Derived from Lactiplantibacillus pentosus B1
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of LAB Isolates
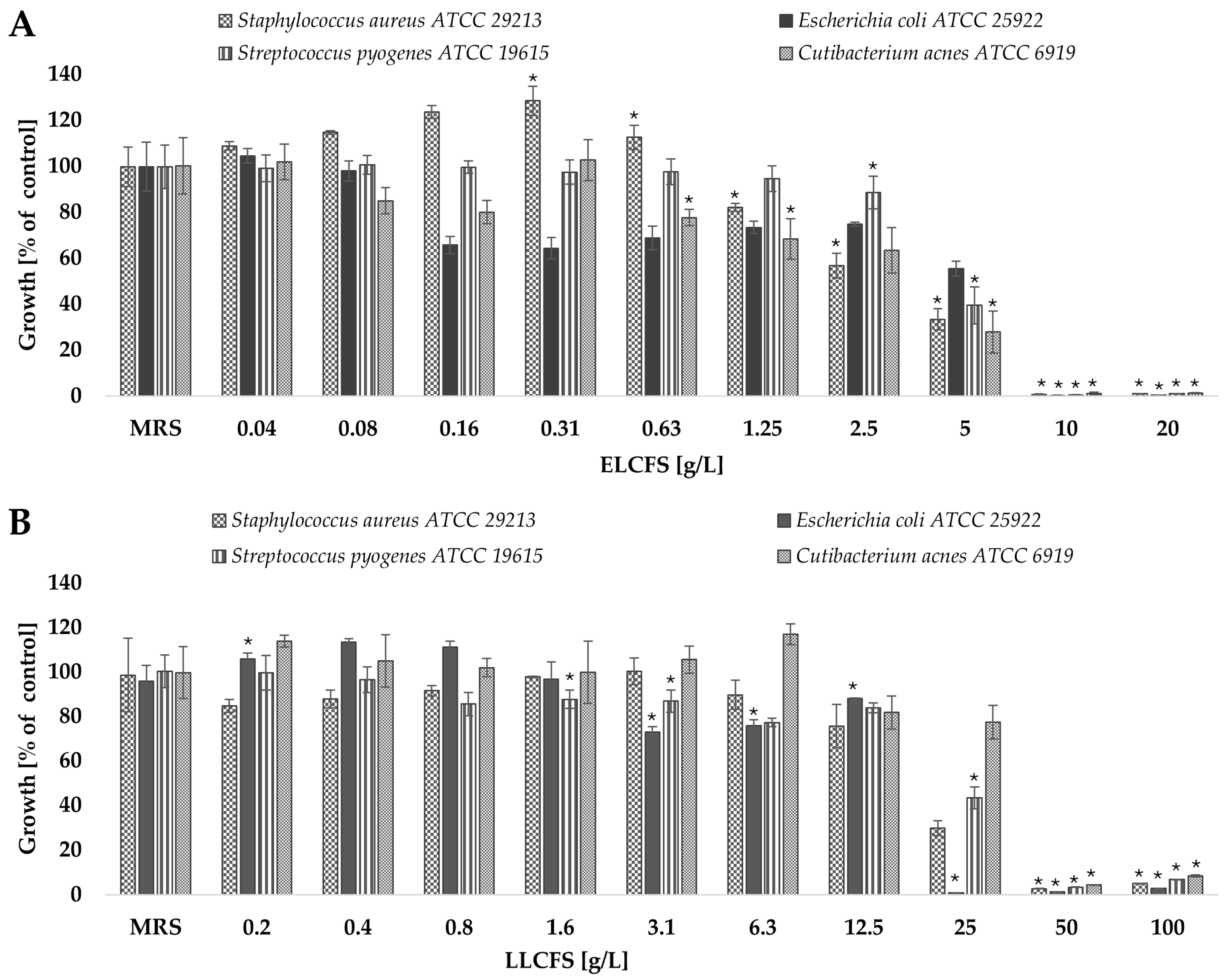
2.2. Antimicrobial Activity of L. pentosus Postbiotics
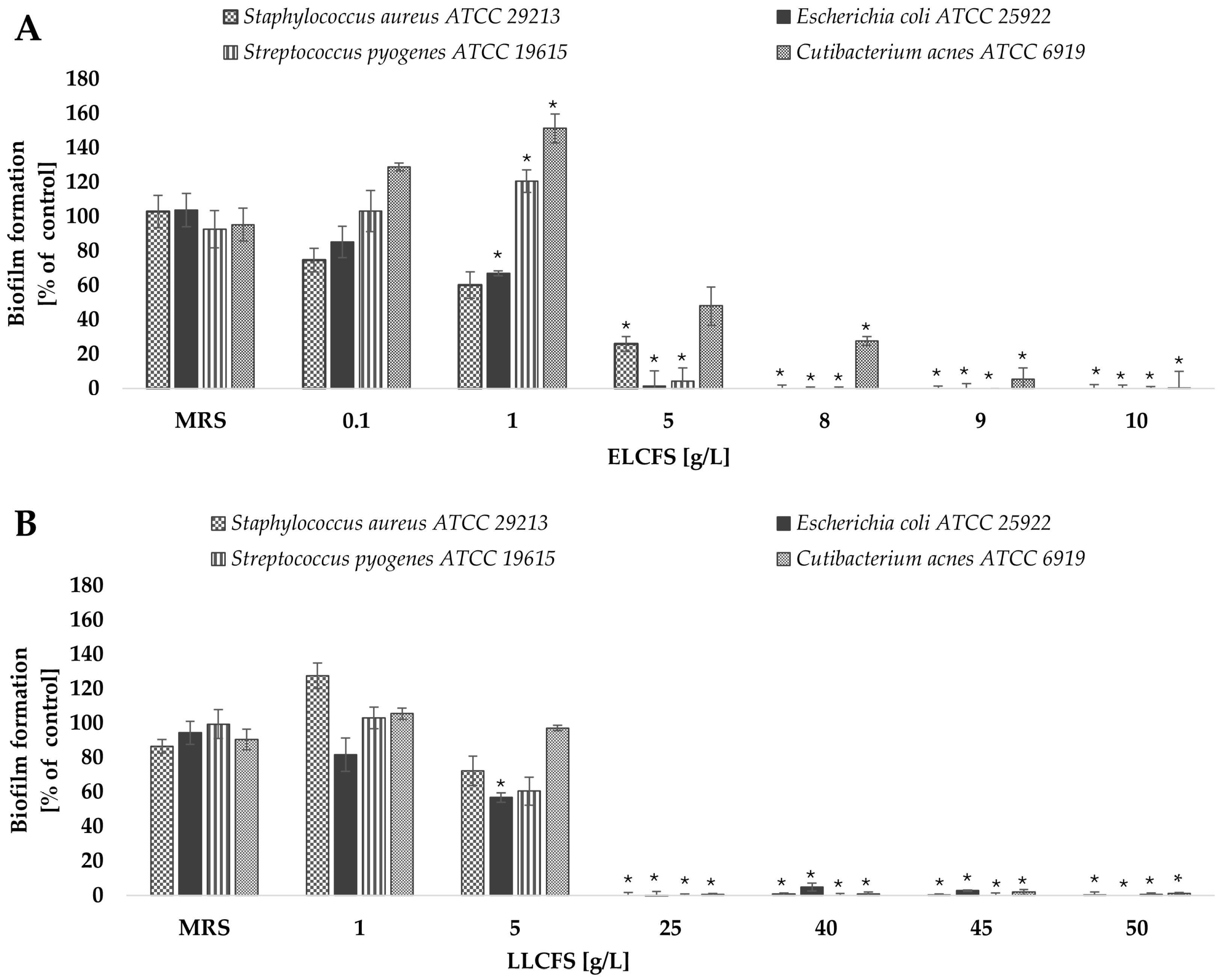
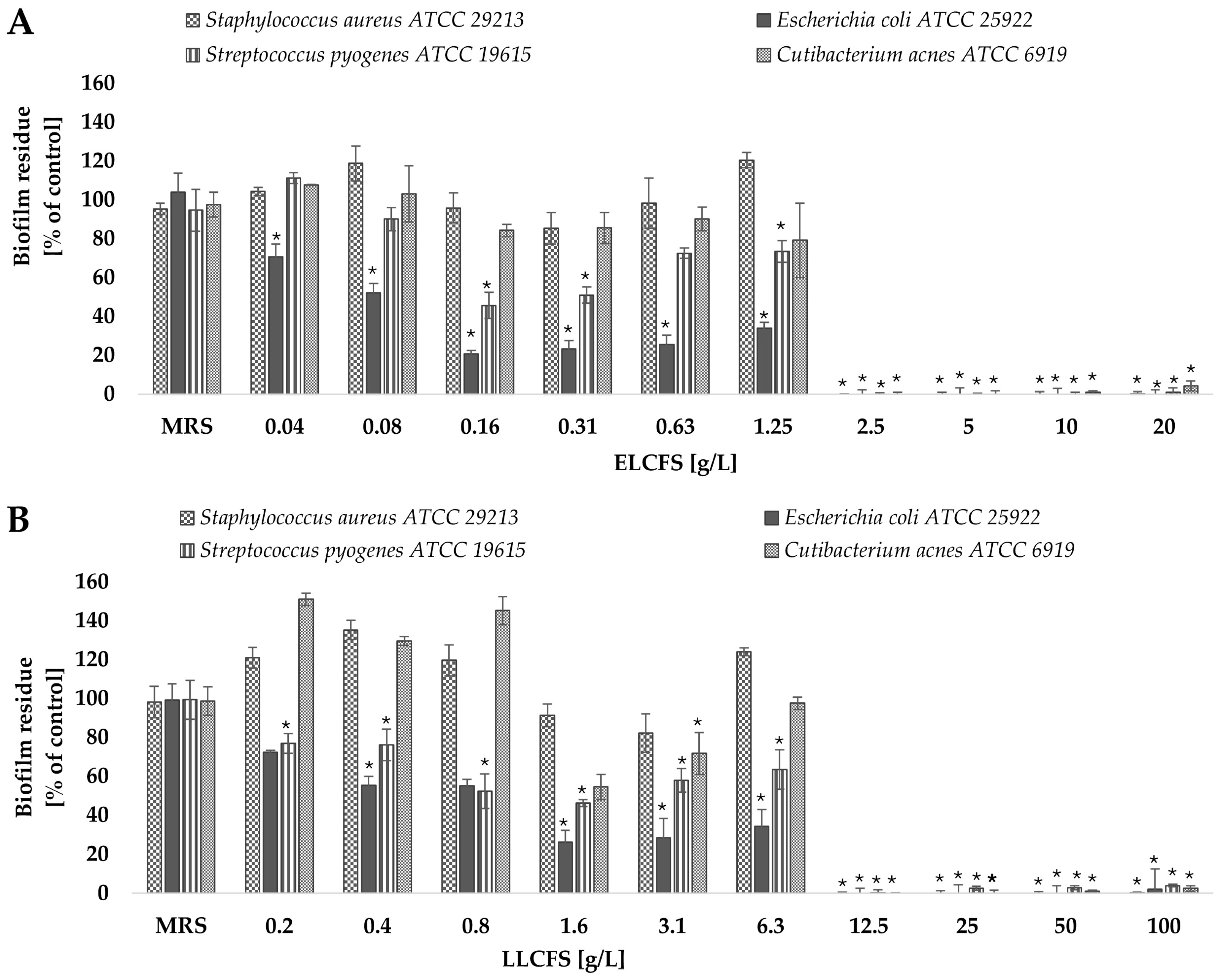
2.3. Antibiofilm Activity of Postbiotics of L. pentosus
2.3.1. Biofilm Inhibition
2.3.2. Biofilm Eradication
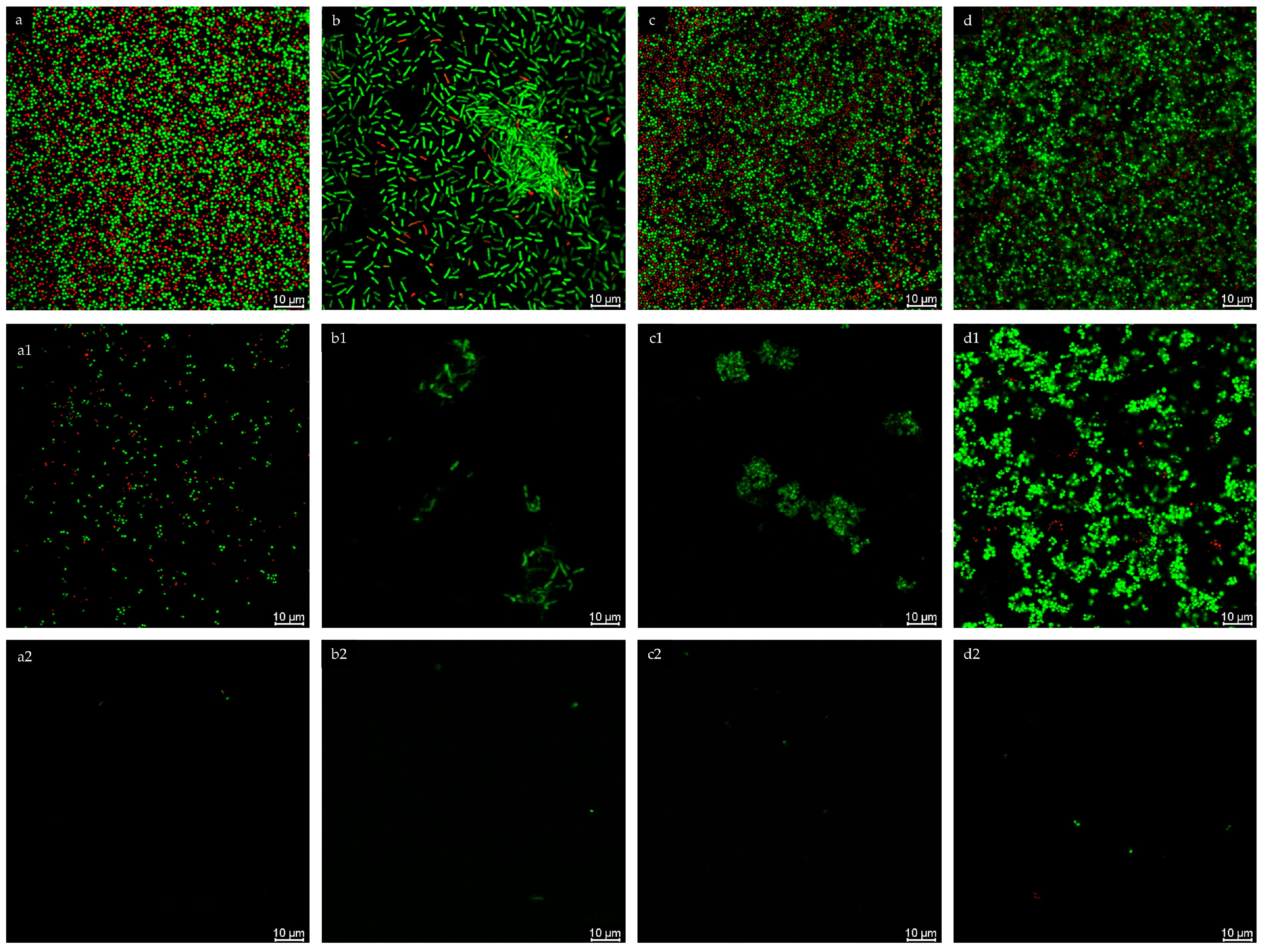
2.3.3. Confocal Microscopy
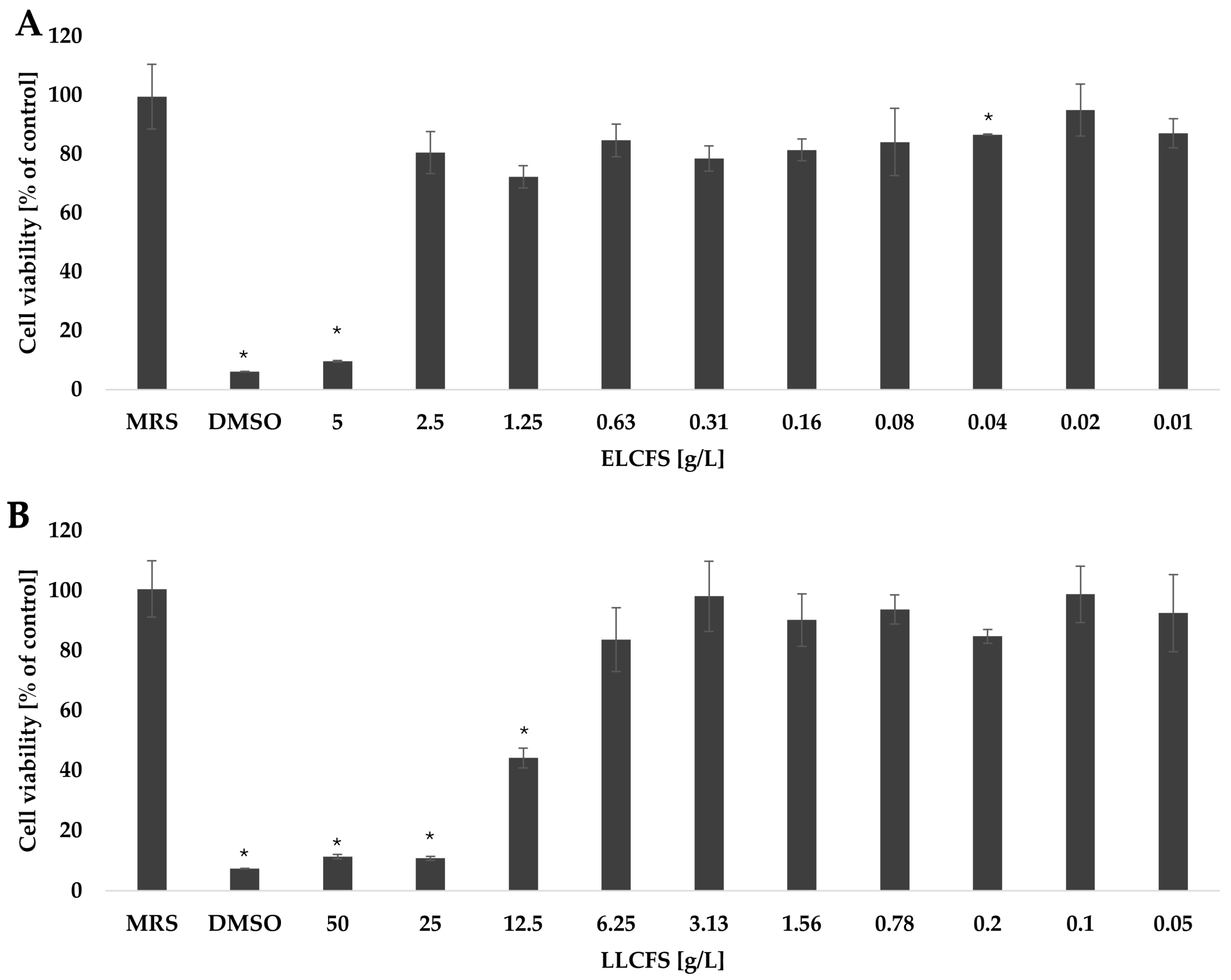
2.4. Cytotoxic Activity of Postbiotics
2.5. Production of Postbiotics and Identification of Organic Acids
3. Materials and Methods
3.1. LAB Isolation and Identification
3.2. Postbiotic Preparation
3.3. Identification of Organic Acids Using GC-MS
3.4. Antimicrobial Activity of Postbiotics
3.5. Long-Time and Thermal Stability of Postbiotics
3.6. Biofilm Inhibition and Eradication
3.7. Biofilm Visualization by Confocal Microscopy
3.8. Cytotoxicity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SanMiguel, A.; Grice, E.A. Interactions between Host Factors and the Skin Microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef]
- Singh, A.K.; Gaur, V.; Singh, S.K. Biofilm-Mediated Skin Infections. In Biofilms in Human Diseases: Treatment and Control; Kumar, S., Chandra, N., Singh, L., Hashmi, M.Z., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 215–231. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin Microbiota–Host Interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Schmid, B.; Künstner, A.; Fähnrich, A.; Busch, H.; Glatz, M.; Bosshard, P.P. Longitudinal Characterization of the Fungal Skin Microbiota in Healthy Subjects Over a Period of 1 Year. J. Investig. Dermatol. 2022, 142, 2766–2772.e8. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Crandall, K.A. Spatial Diversity of the Skin Bacteriome. Front. Microbiol. 2023, 14, 1257276. [Google Scholar] [CrossRef] [PubMed]
- Mekadim, C.; Skalnikova, H.K.; Cizkova, J.; Cizkova, V.; Palanova, A.; Horak, V.; Mrazek, J. Dysbiosis of Skin Microbiome and Gut Microbiome in Melanoma Progression. BMC Microbiol. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.L.; Bhate, K. A Global Perspective on the Epidemiology of Acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef]
- Vlassova, N.; Han, A.; Zenilman, J.M.; James, G.; Lazarus, G.S. New Horizons for Cutaneous Microbiology: The Role of Biofilms in Dermatological Disease. Br. J. Dermatol. 2011, 165, 751–759. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Nakamura, Y.; Núñez, G. Role of the Microbiota in Skin Immunity and Atopic Dermatitis. Allergol. Int. 2017, 66, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef]
- Mustari, A.P.; Agarwal, I.; Das, A.; Vinay, K. Role of Cutaneous Microbiome in Dermatology. Indian J. Dermatol. 2023, 68, 303–312. [Google Scholar] [CrossRef]
- Gonzalez, T.; Stevens, M.L.; Kyzy, A.B.; Alarcon, R.; He, H.; Kroner, J.W.; Spagna, D.; Grashel, B.; Sidler, E.; Martin, L.J.; et al. Biofilm Propensity of Staphylococcus aureus Skin Isolates Is Associated with Increased Atopic Dermatitis Severity and Barrier Dysfunction in the MPAACH Pediatric Cohort. Allergy 2021, 76, 302–313. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial Biofilm in Chronic Lesions of Hidradenitis Suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B.; et al. Skin Dysbiosis and Cutibacterium acnes Biofilm in Inflammatory Acne Lesions of Adolescents. Sci. Rep. 2022, 12, 21104. [Google Scholar] [CrossRef]
- Kravvas, G.; Veitch, D.; Al-Niaimi, F. The Increasing Relevance of Biofilms in Common Dermatological Conditions. J. Dermatol. Treat. 2018, 29, 202–207. [Google Scholar] [CrossRef]
- Belizário, J.E.; Napolitano, M. Human Microbiomes and Their Roles in Dysbiosis, Common Diseases, and Novel Therapeutic Approaches. Front. Microbiol. 2015, 6, 151578. [Google Scholar] [CrossRef]
- Devi, L.S.; Joshi, S.R. Antimicrobial and Synergistic Effects of Silver Nanoparticles Synthesized Using Soil Fungi of High Altitudes of Eastern Himalaya. Mycobiology 2012, 40, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.A.; Faiesal, A.A.; Gamar, G.M.; El Dougdoug, N.K. Efficacy of Bacteriophages with Aloe Vera Extract in Formulated Cosmetics to Combat Multidrug-Resistant Bacteria in Skin Diseases. Sci. Rep. 2025, 15, 4335. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Lee, H.; Kim, M.; Lee, J.W.; Saeed, M.; Lee, H.; Jung, S.-H.; Shim, J.-J.; Lee, J.-L.; Heo, K.; et al. Development and Metabolic Profiling of a Postbiotic Complex Exhibiting Antibacterial Activity against Skin Microorganisms and Anti-Inflammatory Effect on Human Keratinocytes. Food Sci. Biotechnol. 2022, 31, 1325–1334. [Google Scholar] [CrossRef]
- WHO; FAO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations and World Health Organization Working Group: Geneva, Switzerland. 2002. [Google Scholar]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective Study of Probiotic Supplementation Results in Immune Stimulation and Improvement of Upper Respiratory Infection Rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Ye, M.; Luo, F.; Hu, B.; Wang, A.; Chen, J.; Yan, J.; He, Z.; Chen, F.; Qian, C.; et al. Probiotics Treatment Improves Cognitive Impairment in Patients and Animals: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 120, 159–172. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, P.; Zhou, X.; Yuan, J.; Chen, Q. Probiotics Therapy Show Significant Improvement in Obesity and Neurobehavioral Disorders Symptoms. Front. Cell. Infect. Microbiol. 2023, 13, 1178399. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.; Csongradi, C.; Aucamp, M.; Du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef]
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health Effects of Probiotics on the Skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Rossoni, R.D.; de Barros, P.P.; Mendonça, I.d.C.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The Postbiotic Activity of Lactobacillus paracasei 28.4 Against Candida Auris. Front. Cell. Infect. Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef]
- D’Auria, E.; Panelli, S.; Lunardon, L.; Pajoro, M.; Paradiso, L.; Beretta, S.; Loretelli, C.; Tosi, D.; Perini, M.; Bedogni, G.; et al. Rice Flour Fermented with Lactobacillus paracasei CBA L74 in the Treatment of Atopic Dermatitis in Infants: A Randomized, Double- Blind, Placebo- Controlled Trial. Pharmacol. Res. 2021, 163, 105284. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of Antifungal Metabolites Produced by Novel Lactic Acid Bacterium and Their Potential Application as Food Biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Breza-Boruta, B.; Ligocka, A.; Bauza-Kaszewska, J. Natural Bioactive Compounds in Organic and Conventional Fermented Food. Molecules 2022, 27, 4084. [Google Scholar] [CrossRef]
- Jedut, P.; Szwajgier, D.; Glibowski, P.; Iłowiecka, K. Some Plant Food Products Present on the Polish Market Are a Source of Vitamin B12. Appl. Sci. 2021, 11, 3601. [Google Scholar] [CrossRef]
- Wafula, E.N.; Kuja, J.O.; Wekesa, T.B.; Wanjala, P.M. Isolation and Identification of Autochthonous Lactic Acid Bacteria from Commonly Consumed African Indigenous Leafy Vegetables in Kenya. Bacteria 2023, 2, 1–20. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, J.Y.; Jeon, C.O. Source Tracking and Succession of Kimchi Lactic Acid Bacteria during Fermentation. J. Food Sci. 2015, 80, M1871–M1877. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Nyakudya, E.; Jeong, Y.S. Fermentation: Food Products. Encycl. Agric. Food Syst. 2014, 113–123. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; da Costa, W.C.A.; de Oliveira, K.S.; Franco, O.L.; de Morais Júnior, M.A.; Lucena, B.T.L.; Picão, R.C.; Magnani, M.; et al. Identification of Lactic Acid Bacteria in Fruit Pulp Processing Byproducts and Potential Probiotic Properties of Selected Lactobacillus Strains. Front. Microbiol. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-KIomkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Mojgani, N.; Haghighat, S.; Sanjabi, M.R.; Sarem-Nezhad, S. Investigation of Antimicrobial and Antioxidant Properties of Postbiotics Produced by Lactobacillus rhamnosus and Limosilactobacillus reuteri and Their Potential Application in Surface Decontamination of Red Meat. LWT 2024, 209, 116758. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zeng, H. Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis. Molecules 2022, 27, 7627. [Google Scholar] [CrossRef]
- Almeida, M.E.; Sodré, M.M.D.; Oliveira, S.S.; de Carvalho, L.D.; Apolônio, A.C.M.; Rocha, V.N.; Rezende, R.P.; Romano, C.C. Antimicrobial and Antibiofilm Activities of Supernatants of Lactiplantibacillus plantarum A2 and Lactiplantibacillus plantarum 2.1 against Escherichia coli ATCC 25922. PREPRINT (Version 1) available at Research Square 2024. [Google Scholar] [CrossRef]
- Rather, I.A.; Wani, M.Y.; Kamli, M.R.; Sabir, J.S.M.; Hakeem, K.R.; Firoz, A.; Park, Y.H.; Hor, Y.Y. Lactiplantibacillus plantarum KAU007 Extract Modulates Critical Virulence Attributes and Biofilm Formation in Sinusitis Causing Streptococcus Pyogenes. Pharmaceutics 2022, 14, 2702. [Google Scholar] [CrossRef]
- Chae, M.; Kim, B.J.; Na, J.; Kim, S.Y.; Lee, J.O.; Kim, Y.J.; Lee, E.; Cho, D.; Roh, J.; Kim, W. Antimicrobial Activity of Lactiplantibacillus Plantarum APsulloc 331261 and APsulloc 331266 against Pathogenic Skin Microbiota. Front. Biosci. Elite 2021, 13, 237–248. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Song, J.; Wang, C.; Xu, W.; Tan, H.; Suo, H. Antibacterial Activity and Mechanism of Cell-Free Supernatants of Lacticaseibacillus paracasei against Propionibacterium acnes. Microb. Pathog. 2024, 189, 106598. [Google Scholar] [CrossRef]
- Jin, X.; Zheng, Q.; Nguyen, T.T.M.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Assessment of Antimicrobial Activity and Safety of Pediococcus Pentosaceus Isolated from Ginseng as a Functional Cosmetic Ingredient. Microorganisms 2025, 13, 1093. [Google Scholar] [CrossRef]
- Isaac-Bamgboye, F.J.; Mgbechidinma, C.L.; Onyeaka, H.; Isaac-Bamgboye, I.T.; Chukwugozie, D.C. Exploring the Potential of Postbiotics for Food Safety and Human Health Improvement. J. Nutr. Metab. 2024, 2024, 1868161. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, J.-S.; Kim, H.-S.; Kim, Y.-Y.; Oh, J.-K.; Jung, H.-W.; Park, D.-S.; Bae, K.-H. Lactobacillus reuteri AN417 Cell-Free Culture Supernatant as a Novel Antibacterial Agent Targeting Oral Pathogenic Bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, W.; Xie, T.; Zhang, R.; Lu, C.; Han, X.; Zhong, Q. Anti-biofilm Potential of Kefir-derived Lactobacillus paracasei L10 against Vibrio Parahaemolyticus. Lett. Appl. Microbiol. 2021, 73, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nzekoue, F.K.; Wang, J.; Attili, A.R.; Coman, M.M.; Verdenelli, M.C.; Fiorini, D.; Rossi, G.; Marchini, C.; Miceli, C.; et al. A Study of Bioactivities and Composition of a Cocktail of Supernatants Derived from Lactic Acid Bacteria for Potential Food Applications. Probiotics Antimicrob. Proteins 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- ISO/EN10993-5; ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. International Standard Organization–ISO: Geneva, Switzerland, 2009.
- Kim, H.J.; Youn, H.Y.; Moon, J.S.; Kim, H.; Seo, K.H. Comparative Anti-Microbial and Anti-Biofilm Activities of Postbiotics Derived from Kefir and Normal Raw Milk Lactic Acid Bacteria against Bovine Mastitis Pathogens. LWT 2024, 191, 115699. [Google Scholar] [CrossRef]
- Azami, S.; Arefian, E.; Kashef, N. Postbiotics of Lactobacillus casei Target Virulence and Biofilm Formation of Pseudomonas Aeruginosa by Modulating Quorum Sensing. Arch. Microbiol. 2022, 204, 157. [Google Scholar] [CrossRef]
- da Costa, W.K.A.; de Souza, G.T.; Brandão, L.R.; de Lima, R.C.; Garcia, E.F.; dos Santos Lima, M.; de Souza, E.L.; Saarela, M.; Magnani, M. Exploiting Antagonistic Activity of Fruit-Derived Lactobacillus to Control Pathogenic Bacteria in Fresh Cheese and Chicken Meat. Food Res. Int. 2018, 108, 172–182. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Broad-Spectrum Antifungal-Producing Lactic Acid Bacteria and Their Application in Fruit Models. Folia Microbiol. 2013, 58, 291–299. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Ji, Q.Y.; Wang, W.; Yan, H.; Qu, H.; Liu, Y.; Qian, Y.; Gu, R. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods 2023, 12, 3011. [Google Scholar] [CrossRef]
- Sorathiya, K.B.; Melo, A.; Hogg, M.C.; Pintado, M. Organic Acids in Food Preservation: Exploring Synergies, Molecular Insights, and Sustainable Applications. Sustainability 2025, 17, 3434. [Google Scholar] [CrossRef]
- Hu, C.-H.; Ren, L.-Q.; Zhou, Y.; Ye, B.-C. Characterization of Antimicrobial Activity of Three Lactobacillus plantarum Strains Isolated from Chinese Traditional Dairy Food. Food Sci. Nutr. 2019, 7, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Saidi, N.; Saderi, H.; Owlia, P.; Soleimani, M. Anti-Biofilm Potential of Lactobacillus casei and Lactobacillus rhamnosus Cell-Free Supernatant Extracts Against Staphylococcus aureus. Adv. Biomed. Res. 2023, 12, 50. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, C.J.; Ren, B.B.; Li, Q.K.; Luo, Y.Y.; Yang, E.; Li, X.R. Comparison of Antibacterial Activity and Major Organic Acids in Fermentation Broths of Different Lactobacillus plantarum Strains. Shipin Kexue Food Sci. 2019, 40, 8–16. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Kefir Characteristics and Antibacterial Properties—Potential Applications in Control of Enteric Bacterial Infection. Int. Dairy J. 2021, 118, 105021. [Google Scholar] [CrossRef]
- Chotigarpa, R.; Lampang, K.N.; Pikulkaew, S.; Okonogi, S.; Ajariyakhajorn, K.; Mektrirat, R. Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel against Pathogenic Bacteria Causing Bovine Mastitis. Sci. Pharm. 2018, 86, 29. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of Organic Acids on Biofilm Formation and Quorum Signaling of Pathogens from Fresh Fruits and Vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Sakko, M.; Rautemaa-Richardson, R.; Sakko, S.; Richardson, M.; Sorsa, T. Antibacterial Activity of 2-Hydroxyisocaproic Acid (HICA) Against Obligate Anaerobic Bacterial Species Associated With Periodontal Disease. Microbiol. Insights 2021, 14, 11786361211050086. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, X.; Yan, R.; Huang, M.; Chen, D. Isolation, Identification and Antibacterial Mechanism of the Main Antibacterial Component from Pickled and Dried Mustard (Brassica Juncea Coss. Var. Foliosa Bailey). Molecules 2022, 27, 2418. [Google Scholar] [CrossRef]
- Bai, J.-R.; Zhong, K.; Wu, Y.-P.; Elena, G.; Gao, H. Antibiofilm Activity of Shikimic Acid against Staphylococcus aureus. Food Control. 2019, 95, 327–333. [Google Scholar] [CrossRef]
- Du, H.; Li, S.; Yao, H.; Wang, N.; Zhao, R.; Meng, F. Bacteriocin Mining in Lactiplantibacillus pentosus PCZ4 with Broad-Spectrum Antibacterial Activity and Its Biopreservative Effects on Snakehead Fish. Foods 2024, 13, 3863. [Google Scholar] [CrossRef]
- Nowak-Lange, M.; Niedziałkowska, K.; Bernat, P.; Lisowska, K. In Vitro Study of the Ecotoxicological Risk of Methylisothiazolinone and Chloroxylenol towards Soil Bacteria. Sci. Rep. 2022, 12, 19068. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Giordani, B.; Parolin, C.; De Gregorio, P.R.; Foschi, C.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Luppi, B. Lactobacillus crispatus BC1 Biosurfactant Delivered by Hyalurosomes: An Advanced Strategy to Counteract Candida Biofilm. Antibiotics 2021, 10, 33. [Google Scholar] [CrossRef]
| Strain | MIC [g/L] | MBC [g/L] | ||||||
|---|---|---|---|---|---|---|---|---|
| ELCFS | LLCFS | ELCFS | LLCFS | |||||
| Fresh | Stored | Fresh | Stored | Fresh | Stored | Fresh | Stored | |
| S. aureus ATCC 29213 | 10 | 10 | 50 | 50 | 20 | 20 | 100 | 100 |
| E. coli ATCC 25922 | 10 | 10 | 25 | 25 | 10 | 10 | 50 | 50 |
| S. pyogenes ATCC 19615 | 10 | 10 | 50 | 50 | 20 | 20 | - | - |
| C. acnes ATCC 6919 | 10 | 10 | 50 | 50 | 20 | 20 | 100 | 100 |
| ELCFS [10 g/L] | ||||
| Strain | 20 °C | 40 °C | 60 °C | 80 °C |
| S. aureus ATCC 29213 | 99.98 ± 0.02% | 99.96 ± 0.15% | 99.75 ± 0.33% | 99.84 ± 0.08% |
| E. coli ATCC 25922 | 99.98 ± 0.01% | 100.04 ± 0.01% | 99.67 ± 0.12% | 99.78 ± 0.00% |
| S. pyogenes ATCC 19615 | 99.99 ± 0.07% | 99.45 ± 0.03% | 99.61 ± 0.22% | 99.55 ± 0.05% |
| C. acnes ATCC 6919 | 100.04 ± 0.12% | 29.66 ± 19.13% * | 30.77 ± 11.90% * | 50.29 ± 2.88% * |
| LLCFS [50 g/L] | ||||
| Strain | 20 °C | 40 °C | 60 °C | 80 °C |
| S. aureus ATCC 29213 | 100.31 ± 0.07% | 100.02 ± 0.08% | 99.03 ± 0.06% * | 99.44 ± 0.22% * |
| E. coli ATCC 25922 | 99.98 ± 0.28% | 100.05 ± 0.26% | 100.11 ± 0.08% | 100.05 ± 0.08% |
| S. pyogenes ATCC 19615 | 99.68 ± 0.58% | 100.43 ± 0.15% | 100.10 ± 0.27% | 100.07 ± 0.00% |
| C. acnes ATCC 6919 | 99.55 ± 0.91% | 97.81 ± 0.15% * | 95.84 ± 0.66% * | 96.40 ± 0.31% * |
| [mg/L] | ||
| Organic Acid | ELCFS | LLCFS |
| Hydroxyisocaproic acid | 11.63 ± 2.47 | 1.33 ± 0.07 |
| Lactic acid | 350.64 ± 53.35 | 98.53 ± 30.21 |
| Malonic acid | 12.63 ± 2.13 | 3.10 ± 0.02 |
| Shikimic acid | 1.56 ± 0.01 | 1.59 ± 0.04 |
| Succinic acid | 4.45 ± 1.10 | 0.66 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak-Lange, M.; Niedziałkowska, K.; Tończyk, A.; Parolin, C.; Vitali, B.; Lisowska, K. Antibacterial and Antibiofilm Properties of Postbiotics Derived from Lactiplantibacillus pentosus B1. Int. J. Mol. Sci. 2025, 26, 8169. https://doi.org/10.3390/ijms26178169
Nowak-Lange M, Niedziałkowska K, Tończyk A, Parolin C, Vitali B, Lisowska K. Antibacterial and Antibiofilm Properties of Postbiotics Derived from Lactiplantibacillus pentosus B1. International Journal of Molecular Sciences. 2025; 26(17):8169. https://doi.org/10.3390/ijms26178169
Chicago/Turabian StyleNowak-Lange, Marta, Katarzyna Niedziałkowska, Aleksandra Tończyk, Carola Parolin, Beatrice Vitali, and Katarzyna Lisowska. 2025. "Antibacterial and Antibiofilm Properties of Postbiotics Derived from Lactiplantibacillus pentosus B1" International Journal of Molecular Sciences 26, no. 17: 8169. https://doi.org/10.3390/ijms26178169
APA StyleNowak-Lange, M., Niedziałkowska, K., Tończyk, A., Parolin, C., Vitali, B., & Lisowska, K. (2025). Antibacterial and Antibiofilm Properties of Postbiotics Derived from Lactiplantibacillus pentosus B1. International Journal of Molecular Sciences, 26(17), 8169. https://doi.org/10.3390/ijms26178169







