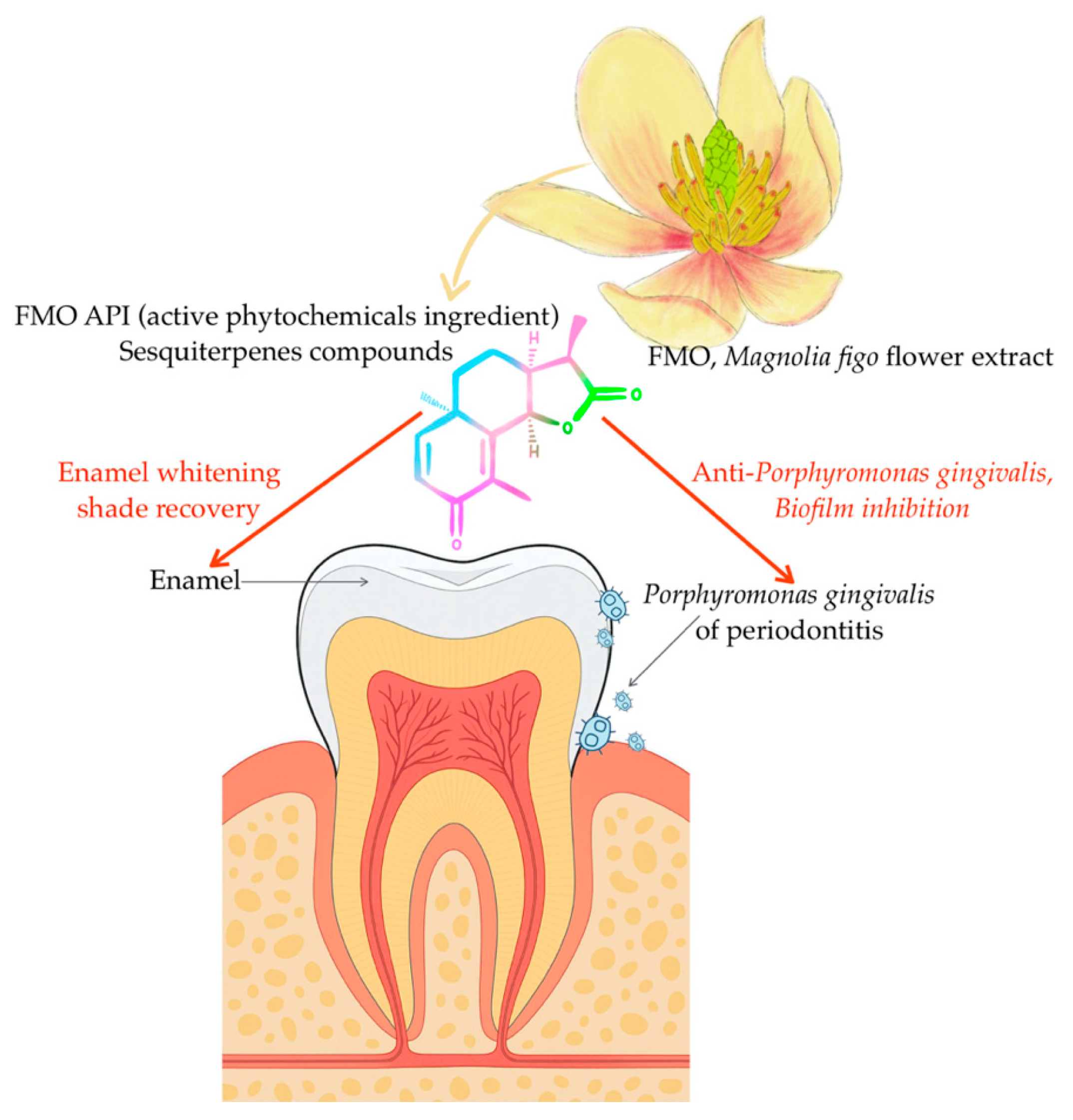
Magnolia figo Extract Induces Enamel Shade Recovery and Inhibits Porphyromonas gingivalis Biofilm Formation: An In Vitro, Dual-Action Natural Therapeutic Approach
Abstract
1. Introduction
2. Results
2.1. Effects of FMO on Enamel Shade Recovery
2.2. FTIR Spectroscopy of FMO Functional Groups and Structures
2.3. Scanning Electron Microscopy (SEM) Analysis of Bovine Tooth with 3% FMO
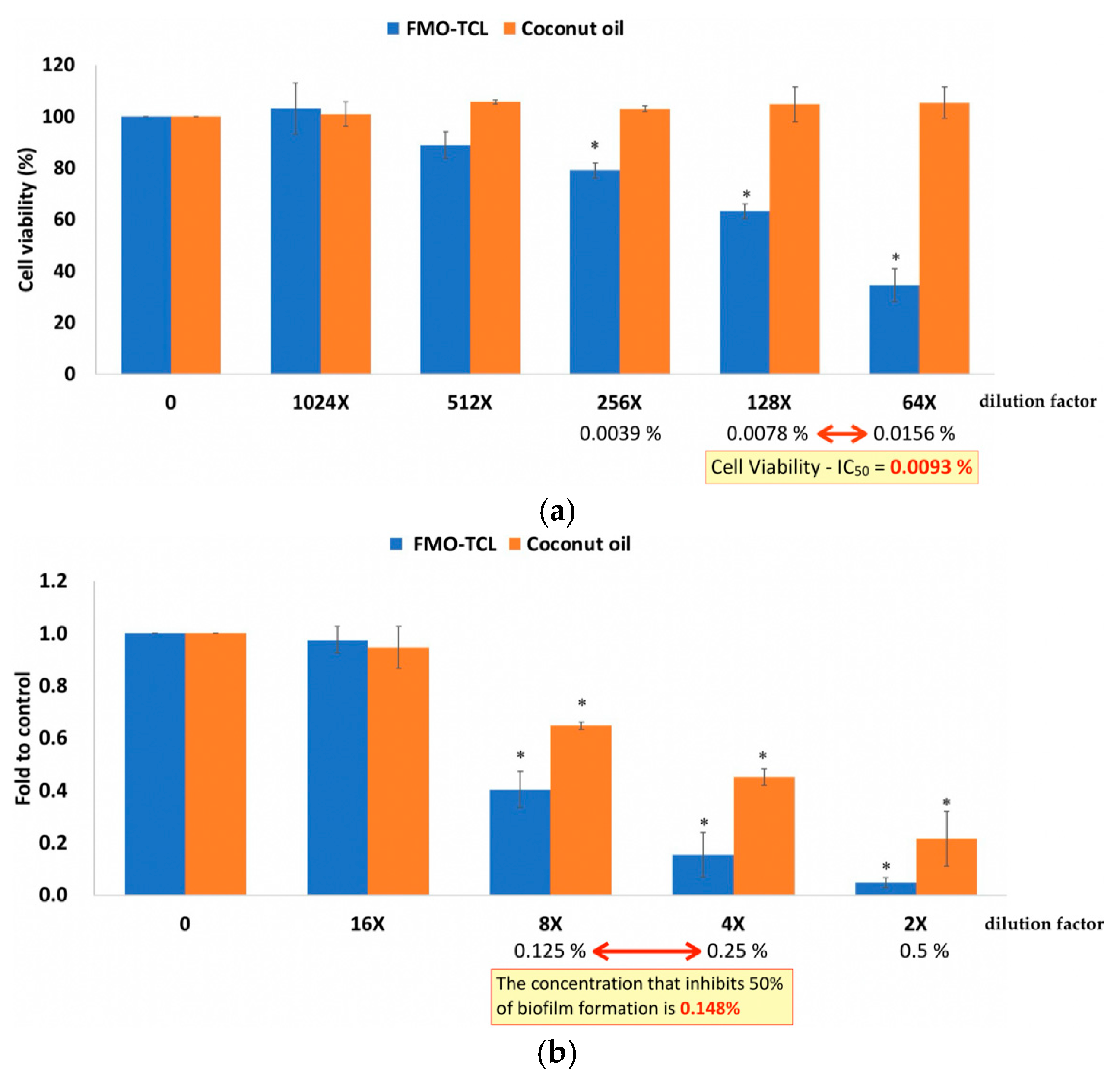
2.4. Effects of FMO on Porphyromonas gingivalis
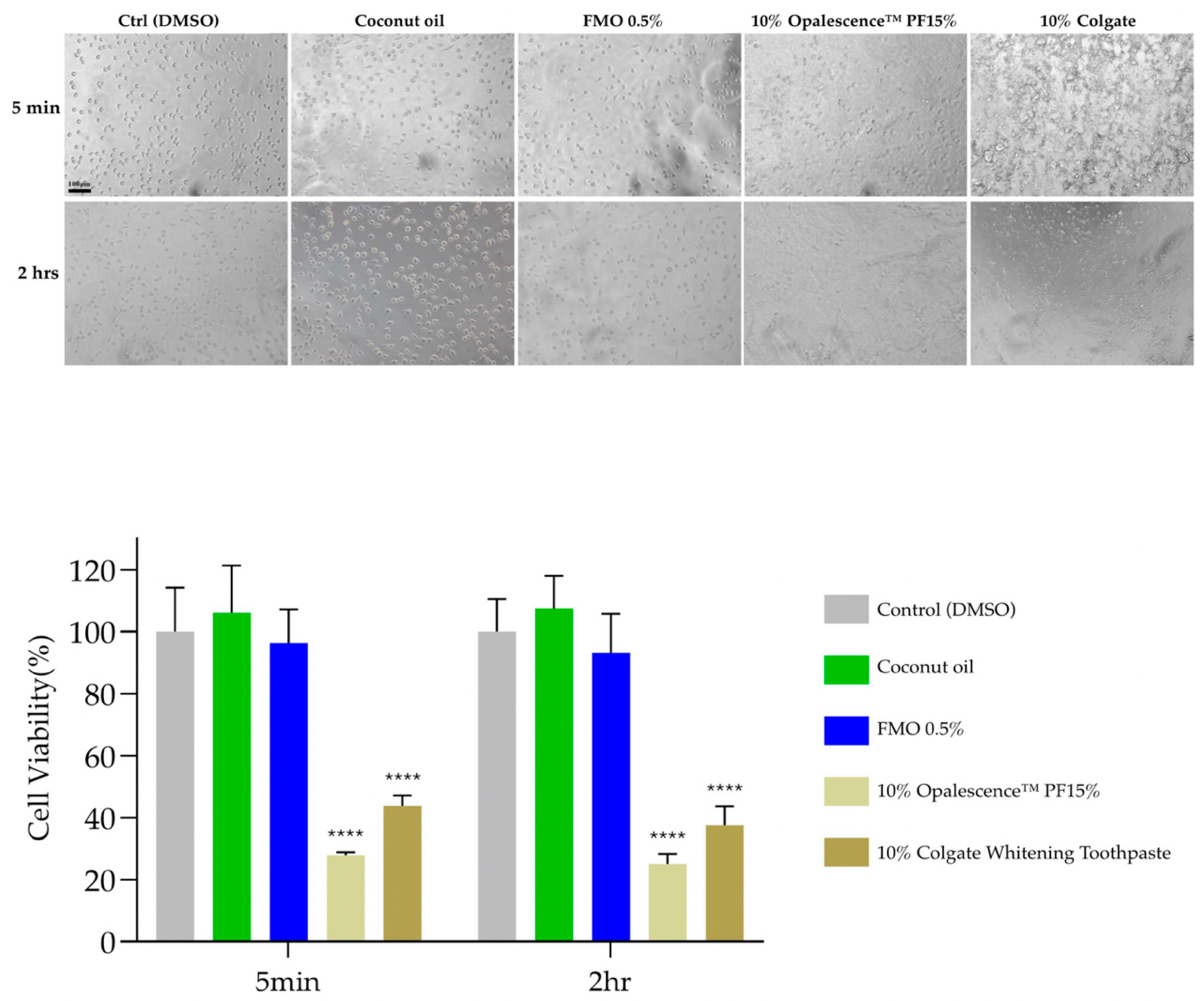
2.5. Cytocompatibility of FMO
3. Discussion
4. Materials and Methods
4.1. Preparation of Magnolia figo Flower Extract (FMO) and Other Experimental Materials
4.2. Specimen Preparation of Bovine Tooth and Enamel Standardization
4.3. Spectrophotometric Color Measurement
4.4. FTIR Spectroscopy
4.5. SEM Observation
4.6. FMO Verification with HGF and Biofilm Formation Assays on Porphyromonas gingivalis
4.7. Cell Culture and Cytotoxicity Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Kaya, S.; Schumann, S.; Steiger, M.; Bjelopavlovic, M.; Deschner, J.; Al-Nawas, B.; Lehmann, K.M. Effectiveness and safety of over-the-counter tooth-whitening agents compared to hydrogen peroxide in vitro. Int. J. Mol. Sci. 2023, 24, 1956. [Google Scholar] [CrossRef] [PubMed]
- Algharbi, M.; E Alenezi, Y.; Alshammari, F.F.; Alkhalaf, S.A.; A Siddiqui, A. Knowledge, Attitudes, and Practice about Teeth Bleaching Among Subpopulation in Saudi Arabia: A Cross-sectional Study. Open Dent. J. 2025, 19. [Google Scholar] [CrossRef]
- Kheradmand, E.; Daneshkazemi, A.; Davari, A.; Kave, M.; Ghanbarnejad, S. Effect of hydrogen peroxide and its combination with nano-hydroxyapatite or nano-bioactive glass on the enamel demineralization and tooth color: An in vitro study. Dent. Res. J. 2023, 20, 85. [Google Scholar] [CrossRef]
- Bizhang, M.; Seemann, R.; Duve, G.; Römhild, G.; Altenburger, M.J.; Jahn, K.R.; Zimmer, S. Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper. Dent. 2006, 31, 705–709. [Google Scholar] [CrossRef]
- Eachempati, P.; Nagraj, S.K.; Krishanappa, S.K.K.; Gupta, P.; Yaylali, I.E. Home-based chemically-induced whitening (bleaching) of teeth in adults. Cochrane Database Syst. Rev. 2018, 12, CD006202. [Google Scholar] [CrossRef]
- Eric, J.; Ciobanu, O.; Abdallah, M.N.; Nelea, V.D.; Gupta, N.; Abotaleb, A.; Tamimi, F. The effect of Hydrogen Peroxide treatments on dental enamel porosity and protein structure and its long-term implications on tooth hardness and optical properties. J. Dent. 2025, 156, 105714. [Google Scholar] [CrossRef] [PubMed]
- Bogdanoviciene, I.; Beganskiene, A.; Tõnsuaadu, K.; Glaser, J.; Meyer, H.-J.; Kareiva, A. Calcium hydroxyapatite, Ca10(PO4)6(OH)2 ceramics prepared by aqueous sol–gel processing. Mater. Res. Bull. 2006, 41, 1754–1762. [Google Scholar] [CrossRef]
- Shimabayashi, S.; Tanaka, H.; Nakagaki, M. Effect of added salt on the adsorption of dodecyl sulfate ion and concurrent release of phosphate and calcium ions at the surface of hydroxyapatite. Chem. Pharm. Bull. 1987, 35, 2171–2176. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef]
- Abedi, M.; Ghasemi, Y.; Nemati, M.M. Nanotechnology in toothpaste: Fundamentals, trends, and safety. Heliyon 2024, 10, e24949. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The evolving microbiome of dental caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Sun, Y.; Wang, G.; Sun, J.; Dong, S.; Ding, J. Advanced biomaterials for targeting mature biofilms in periodontitis therapy. Bioact. Mater. 2025, 48, 474–492. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.T.; Babiker, R.; Padmanabhan, V.; Ahmed, A.T.; Chaitanya, N.C.S.K.; Mohammed, R.; Priya, S.P.; Ahmed, A.; El Bahra, S.; Islam, S.; et al. The Global Burden of Periodontal Disease: A Narrative Review on Unveiling Socioeconomic and Health Challenges. Int. J. Environ. Res. Public Health 2025, 22, 624. [Google Scholar] [CrossRef]
- Villoria, G.E.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontology 2000 2024, 96, 7–19. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Budala, D.G.; Martu, M.-A.; Maftei, G.-A.; Diaconu-Popa, D.A.; Danila, V.; Luchian, I. The role of natural compounds in optimizing contemporary dental treatment—Current status and future trends. J. Funct. Biomater. 2023, 14, 273. [Google Scholar] [CrossRef]
- Refaey, M.S.; Abosalem, E.F.; El-Basyouni, R.Y.; Elsheriri, S.E.; Elbehary, S.H.; Fayed, M.A. Exploring the therapeutic potential of medicinal plants and their active principles in dental care: A comprehensive review. Heliyon 2024, 10, e37641. [Google Scholar] [CrossRef]
- Al-Rawi, R.; Bashir, Y.; Mustafa, A.; Omar, M.; Al-Rawi, N.; Saeed, M.; Uthman, A.; Al-Rawi, N.H. Teeth whitening and antibacterial effects of Juglans regia bark: A preliminary study. Int. J. Dent. 2021, 2021, 6685437. [Google Scholar] [CrossRef]
- Schönknecht, K.; Surdacka, A.; Rudenko, L. Effectiveness of composed herbal extract in the treatment of gingivitis and oral and pharyngeal mucosa–review of studies. Wiadomości Lek. 2021, 74, 1737–1749. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Nadri, M.H.; Othman, N.Z.; Rashid, S.N.A.A.; Lim, Y.-C.; Leong, H.-Y. Phytochemistry, bioactivities and traditional uses of Michelia× alba. Molecules 2022, 27, 3450. [Google Scholar] [CrossRef]
- Kuo, C.-S.; Chen, S.-Y.; Tsai, J.-C. Effects of the Supercritical Fluid Extract of Magnolia figo on Inducing the Apoptosis of Human Non-Small-Cell Lung Cancer Cells. Molecules 2023, 28, 7445. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Kang, Y.; Zou, H.; Cheng, X.; Xie, T.; Shi, L.; Zhang, H. β-elemene attenuates macrophage activation and proinflammatory factor production via crosstalk with Wnt/β-catenin signaling pathway. Fitoterapia 2018, 124, 92–102. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Structure and function of SARS-CoV and SARS-CoV-2 main proteases and their inhibition: A comprehensive review. Eur. J. Med. Chem. 2023, 260, 115772. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez Mdel, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef]
- Alghazali, N.; Burnside, G.; Moallem, M.; Smith, P.; Preston, A.; Jarad, F.D. Assessment of perceptibility and acceptability of color difference of denture teeth. J. Dent. 2012, 40, e10–e17. [Google Scholar] [CrossRef]
- Douglas, R.D.; Steinhauer, T.J.; Wee, A.G. Intraoral determination of the tolerance of dentists for perceptibility and acceptability of shade mismatch. J. Prosthet. Dent. 2007, 97, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Doleżyńska-Sewerniak, E.; Zygier, E. Chemical analysis of historic art and painting conservation materials (19th and 20th centuries) from the National Museum in Krakow by gas chromatography–mass spectrometry and Fourier transform infrared spectroscopy. Spectrosc. Lett. 2014, 47, 57–75. [Google Scholar] [CrossRef]
- Dekermenjian, M. FTIR analysis of aerosol formed in the ozone oxidation of sesquiterpenes. Aerosol Sci. Technol. 1999, 30, 349–363. [Google Scholar] [CrossRef]
- El Feky, S.E.; El Hafez, M.S.M.A.; El Moneim, N.A.A.; Ibrahim, H.A.H.; Okbah, M.A.; Ata, A.; El Sedfy, A.S.; Hussein, A. Cytotoxic and antimicrobial activities of two new sesquiterpenoids from red sea brittle star Ophiocoma dentata. Sci. Rep. 2022, 12, 8209. [Google Scholar] [CrossRef] [PubMed]
- Ameh, A.; Olakunle, M.; Shehu, H.; Oyegoke, T. Kinetics of the extraction of oleoresin from ginger: Influence of particle size and extraction time effects. NIPES-J. Sci. Technol. Res. 2020, 2, 142. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in dental applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Huang, X.-L.; Liu, M.-D.; Li, J.-Y.; Zhou, X.-D.; Cate, J.M.T. Chemical composition of Galla chinensis extract and the effect of its main component (s) on the prevention of enamel demineralization in vitro. Int. J. Oral Sci. 2012, 4, 146–151. [Google Scholar] [CrossRef]
- Liu, C.; Xu, M.; Wang, Y.; Yin, Q.; Hu, J.; Chen, H.; Sun, Z.; Liu, C.; Li, X.; Zhou, W.; et al. Exploring the potential of hydroxyapatite-based materials in biomedicine: A comprehensive review. Mater. Sci. Eng. R Rep. 2024, 161, 100870. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Liu, Z.; Zeng, Q.; Jiang, G.; Yang, M. Probing the surface structure of hydroxyapatite through its interaction with hydroxyl: A first-principles study. RSC Adv. 2018, 8, 3716–3722. [Google Scholar] [CrossRef]
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: Whitening efficacy using BI and WI D and side effects—An in vitro study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. The association between biofilm formation and antimicrobial resistance with possible ingenious bio-remedial approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’aDdona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 11405:2015; Dentistry—Testing of Adhesion to Tooth Structure. ISO: Geneva, Switzerland, 2015.
- Zeczkowski, M.; Tenuta, L.M.A.; Ambrosano, G.M.B.; Aguiar, F.H.B.; Lima, D.A.N.L. Effect of different storage conditions on the. physical properties of bleached enamel: An in vitro vs. in situ study. J. Dent. 2015, 43, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Kielbassa, A.M. Remineralization of bovine enamel subsurface lesions: Effects of different calcium-phosphate saturations in buffered aqueous solutions. Quintessence Int. 2011, 42, 501–514. [Google Scholar] [PubMed]
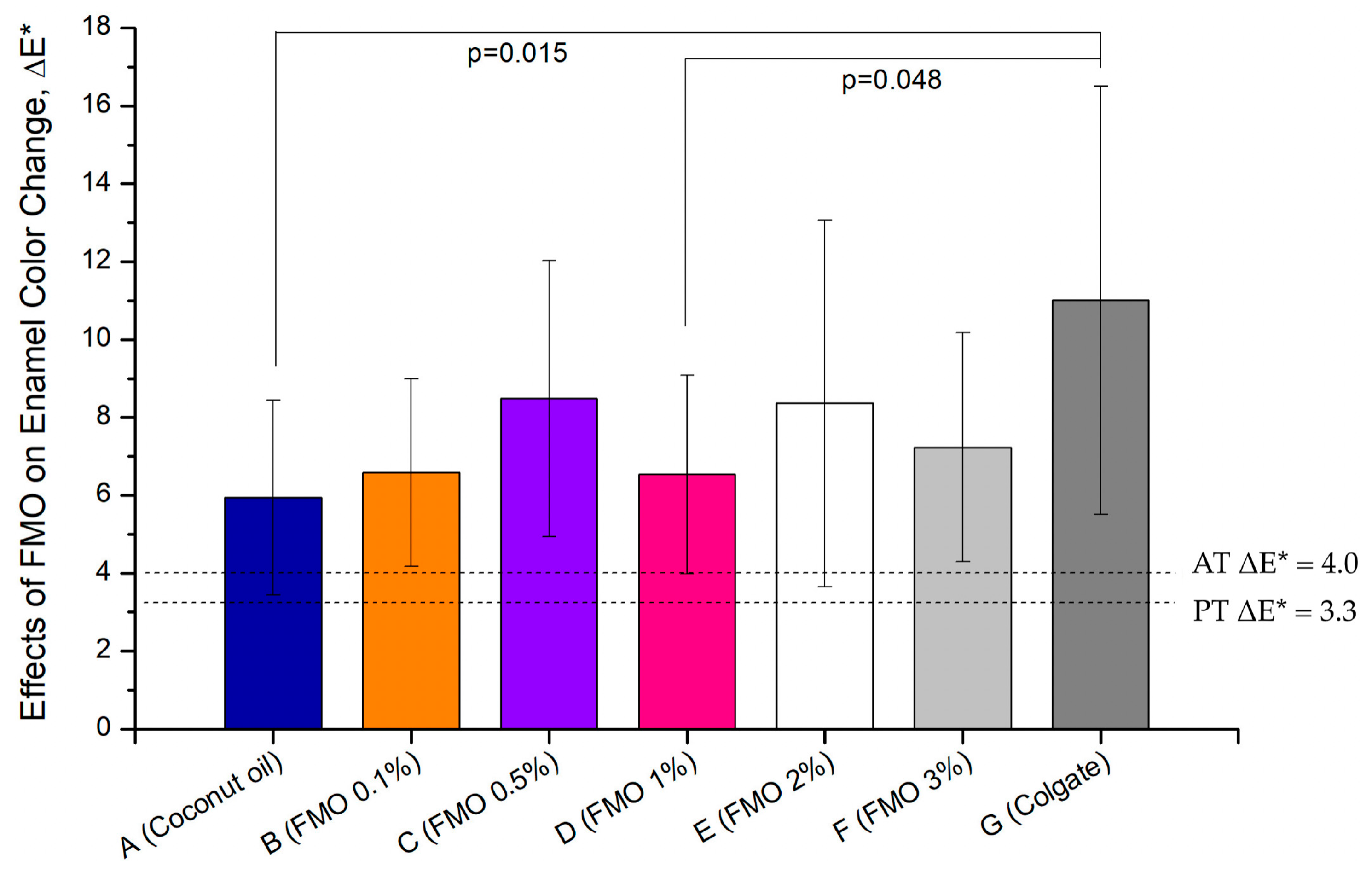



| ΔL | Δa | Δb | ΔE* | |
|---|---|---|---|---|
| A (Coconut oil) | 4.66 ± 2.69 | −1.10 ± 1.00 | −2.64 ± 2.00 | 5.94 ± 2.50 |
| B (FMO 0.1%) | 4.40 ± 2.20 | −1.41 ± 0.38 | −4.53 ± 1.63 | 6.59 ± 2.41 |
| C (FMO 0.5%) | 6.71 ± 4.64 | −2.14 ± 0.77 | −2.74 ± 2.69 | 8.49 ± 3.55 |
| D (FMO 1%) | 4.06 ± 4.01 | −2.52 ± 1.09 | −1.61 ± 2.90 | 6.53 ± 2.56 |
| E (FMO 2%) | 6.24 ± 5.47 | −2.09 ± 1.37 | −3.09 ± 3.06 | 8.36 ± 4.71 |
| F (FMO 3%) | 4.68 ± 3.69 | −1.78 ± 0.79 | −4.33 ± 1.96 | 7.23 ± 2.94 |
| G (Colgate) | 8.49 ± 5.39 | −2.83 ± 1.27 | −5.87 ± 2.66 | 11.01 ± 5.50 |
| FTIR Absorption Wavenumber (cm−1) | FTIR Analysis of Indicating Functional Groups/Structural Features (References [28,29,30,31]) | Corresponding GC-MS Composition (Reference [21]) | Phytochemical Structural Correspondence Description |
|---|---|---|---|
| 3468–3400 | O–H stretching (hydroxyl) | Spathulenol, Ledene Alcohol, Caryophyllene Oxide | Sesquiterpenols contain hydroxyl (–OH) structures |
| 2950–2850 | Aliphatic C–H stretching (aliphatic CH3, CH2) | β-Elemene, γ-Elemene, Caryophyllene Oxide | All C15; sesquiterpenes contain alkyl side chains |
| 1740–1635 | C=O, C=C stretching (keto or olefin double bond) | Caryophyllene Oxide, Copaene-8-ol, (1R,3E,7E,11R)-… | Double bond, or oxidation structure, corresponding to epoxy group/olefin structure |
| 1450–1370 | CH3, CH2 bending (methylene) | All sesquiterpenes | Common absorption for aliphatic chains and alkyl side chains |
| 1230–1100 | C–O stretching (ether or alcohol) | Spathulenol, Ledene Oxide (II), Ledene Alcohol | Corresponding to the alcohol and ether functional groups in sesquiterpenol |
| <900 | C–H out-of-plane bending (olefins) | β-Elemene, γ-Elemene, Copaene-8-ol | Indicating substituted olefin skeleton structures, such as double bonded benzene rings or cyclohexene structures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-S.; Lin, C.-W.; Hsu, Y.-M.; Tsai, J.-C.; Lin, D.-J. Magnolia figo Extract Induces Enamel Shade Recovery and Inhibits Porphyromonas gingivalis Biofilm Formation: An In Vitro, Dual-Action Natural Therapeutic Approach. Int. J. Mol. Sci. 2025, 26, 8157. https://doi.org/10.3390/ijms26178157
Kuo C-S, Lin C-W, Hsu Y-M, Tsai J-C, Lin D-J. Magnolia figo Extract Induces Enamel Shade Recovery and Inhibits Porphyromonas gingivalis Biofilm Formation: An In Vitro, Dual-Action Natural Therapeutic Approach. International Journal of Molecular Sciences. 2025; 26(17):8157. https://doi.org/10.3390/ijms26178157
Chicago/Turabian StyleKuo, Chun-Sheng, Cheng-Wen Lin, Yuan-Man Hsu, Jen-Chieh Tsai, and Dan-Jae Lin. 2025. "Magnolia figo Extract Induces Enamel Shade Recovery and Inhibits Porphyromonas gingivalis Biofilm Formation: An In Vitro, Dual-Action Natural Therapeutic Approach" International Journal of Molecular Sciences 26, no. 17: 8157. https://doi.org/10.3390/ijms26178157
APA StyleKuo, C.-S., Lin, C.-W., Hsu, Y.-M., Tsai, J.-C., & Lin, D.-J. (2025). Magnolia figo Extract Induces Enamel Shade Recovery and Inhibits Porphyromonas gingivalis Biofilm Formation: An In Vitro, Dual-Action Natural Therapeutic Approach. International Journal of Molecular Sciences, 26(17), 8157. https://doi.org/10.3390/ijms26178157






