Dextromethorphan Enhances Apoptosis and Suppresses EMT in PANC-1 Pancreatic Cancer Cells: Synergistic Effects with Gemcitabine
Abstract
1. Introduction
2. Results
2.1. Cell Viability Analysis
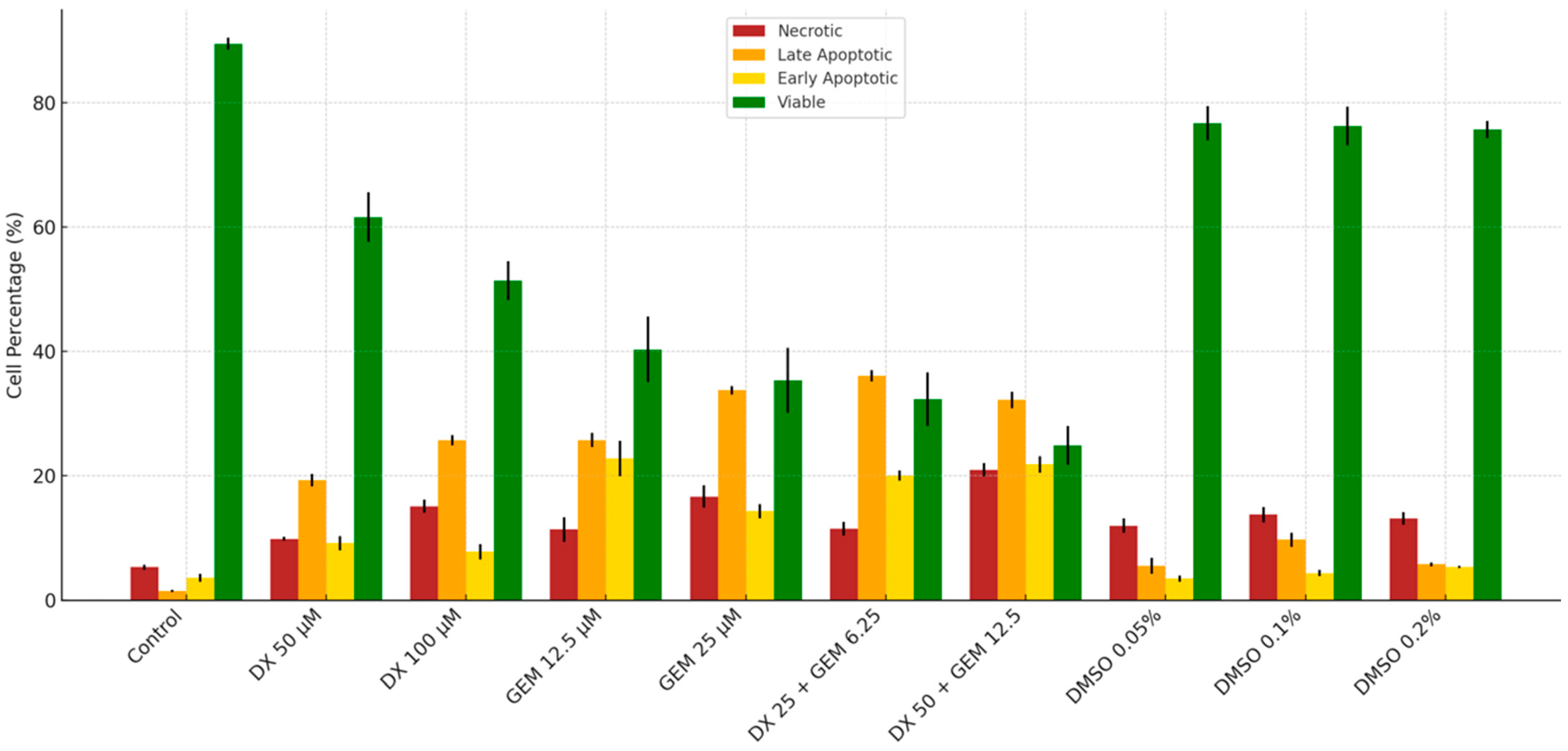
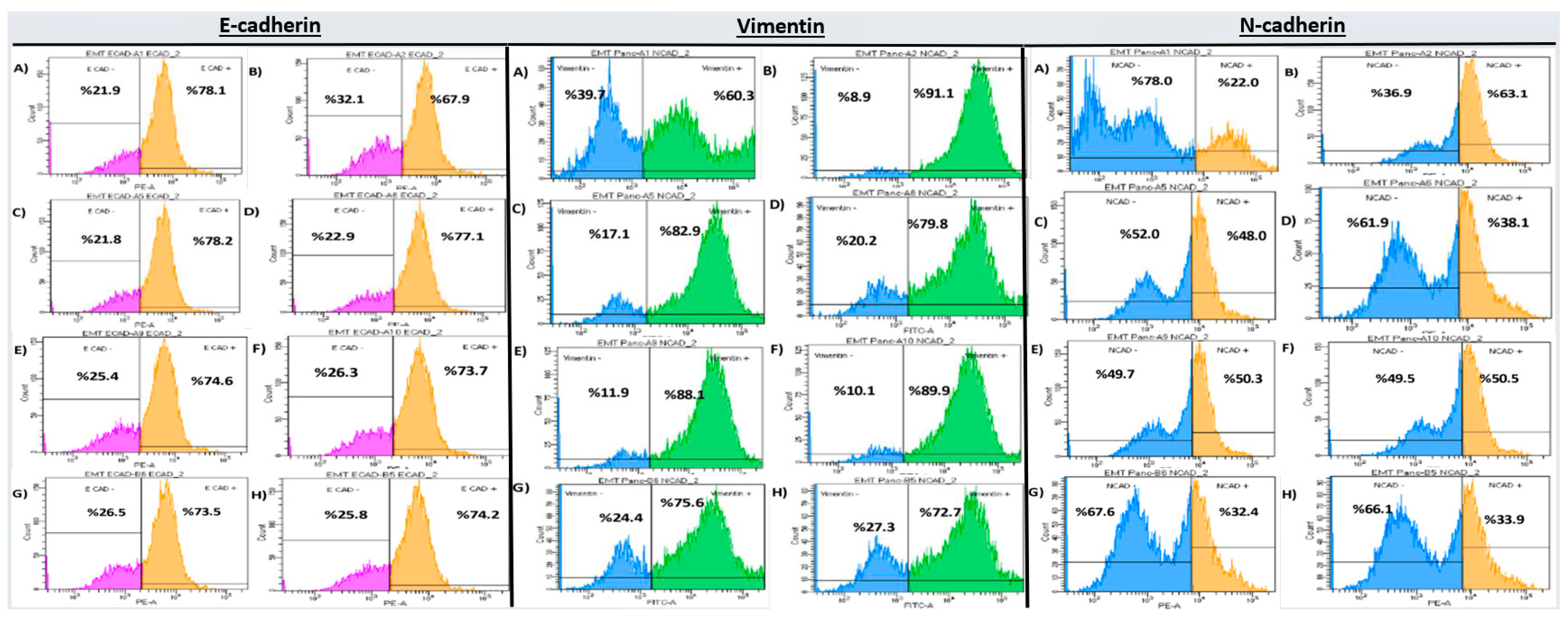
2.2. Flow Cytometry Analysis
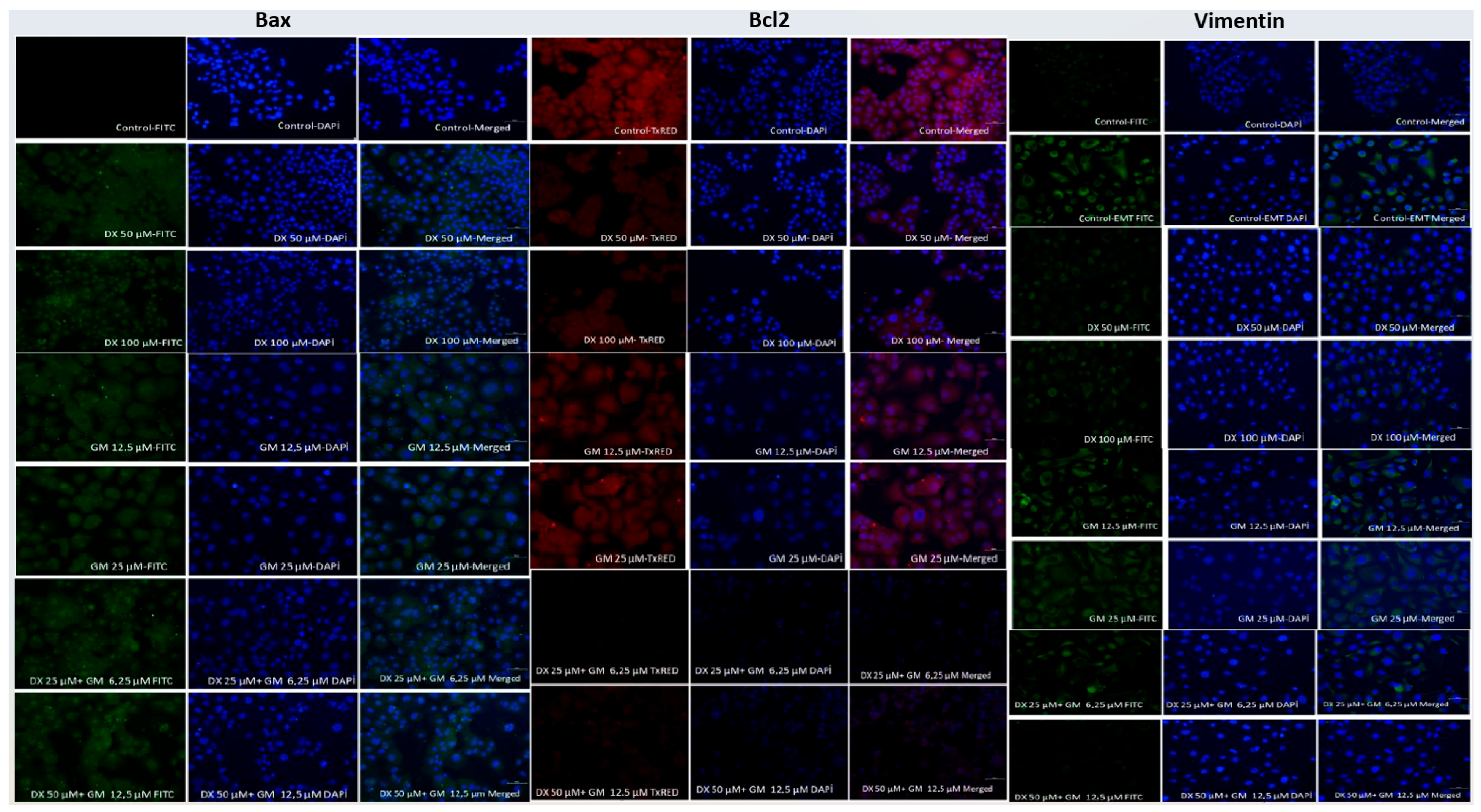
2.3. Immunocytochemical (ICC) Analysis
2.4. qRT-PCR Gene Expression Analysis
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design and Cell Culture
4.2. Drug Preparation
- Dextromethorphan: 42.5 mM in DMSO;
- Gemcitabine: 20 mM in DPBS;
- 5-FU: diluted to 2.5 mM in culture medium from a 384 mM stock.
4.3. Cytotoxicity Assay (MTT)
4.4. Experimental Groups
- DX alone (IC50 and ½ IC50);
- Gemcitabine alone (IC50 and ½ IC50);
- DX + gemcitabine combination (½, ¼, and ⅛ of IC50 values);
- Control and TGF-β-induced EMT groups.
4.5. EMT Induction
4.6. Flow Cytometry for Apoptosis
4.7. Flow Cytometry for EMT Markers
- Vimentin-FITC (1:250);
- N-cadherin-PE (1:200);
- E-cadherin-PE (1:250).
4.8. Immunocytochemistry (ICC)
- Bax (1:200, Bioss Inc., Woburn, MA, USA);
- Bcl-2 (1:50, Thermo Fisher Scientific Inc., Waltham, MA, USA).
- Donkey anti-Rb 488 for Bax (1:500);
- Goat anti-Rb 594 for Bcl-2 (1:500).
- Vimentin-FITC, N-cadherin-PE, and E-cadherin-PE.
4.9. Gene Expression Analysis (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Kramer, S.P.; Tonelli, C.; Luchette, F.A.; Swanson, J.; Abdelsattar, Z.; Cohn, T.; Baker, M.S. Locally advanced pancreatic cancer: Is surgical palliation associated with improved clinical outcome relative to medical palliation? Am. J. Surg. 2024, 230, 73–77. [Google Scholar] [CrossRef]
- Jobu, Y.; Nishigawa, M.; Furihata, K.; Furihata, M.; Uchida, K.; Taniuchi, K. Inhibitory effects of the combination of rapamycin with gemcitabine plus paclitaxel on the growth of pancreatic cancer tumors. Hum. Cell 2025, 38, 44. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.C.; Piper, M.; Goodspeed, A.; Bhuvane, S.; Williams, J.S.; Bhatia, S.; Phan, A.V.; Van Court, B.; Zolman, K.L.; Pena, B.; et al. Induction of ADAM10 by Radiation Therapy Drives Fibrosis, Resistance, and Epithelial-to-Mesenchyal Transition in Pancreatic Cancer. Cancer Res. 2021, 81, 3255–3269. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, J.; Zhao, J.; Li, J.; Li, D.; Popp, M.; Popp, F.; Alakus, H.; Kong, B.; Dong, Q.; et al. Inflammatory IFIT3 renders chemotherapy resistance by regulating post-translational modification of VDAC2 in pancreatic cancer. Theranostics 2020, 10, 7178–7192. [Google Scholar] [CrossRef] [PubMed]
- Issagholian, L.; Tabaie, E.; Reddy, A.J.; Ghauri, M.S.; Patel, R. Expression of E-cadherin and N-cadherin in Epithelial-to-Mesenchymal Transition of Osteosarcoma: A Systematic Review. Cureus 2023, 15, e49521. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Q.; You, L.; Chen, S.; Zhu, M.; Miao, C. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur. J. Pharmacol. 2017, 795, 150–159. [Google Scholar] [CrossRef]
- Dong, W.; Sun, S.J.; Qin, J.J.; Liu, G.M. Fyn stimulates the progression of pancreatic cancer via Fyn-GluN2b-AKT axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 109–121. [Google Scholar] [CrossRef]
- Gallo, S.; Vitacolonna, A.; Crepaldi, T. NMDA Receptor and Its Emerging Role in Cancer. Int. J. Mol. Sci. 2023, 24, 2540. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Dragnev, K.H.; Demidenko, E. Small-cell lung cancer growth inhibition: Synergism between NMDA receptor blockade and chemotherapy. Clin. Pharmacol. Adv. Appl. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.; de Graeff, A.; Jongen, J.L.; Dijkstra, D.; Mostovaya, I.; Vissers, K.C.; National Guideline Working Group “Diagnosis Treatment of Cancer Pain”. Pharmacological Treatment of Pain in Cancer Patients: The Role of Adjuvant Analgesics, a Systematic Review. Pain Pract. 2017, 17, 409–419. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Lin, L.Z.; Tian, R.; Akerman, B. NMDA receptors are important regulators of pancreatic cancer and are potential targets for treatment. Clin. Pharmacol. Adv. Appl. 2017, 9, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Egunlusi, A.O.; Joubert, J. NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders. Pharmaceuticals 2024, 17, 639. [Google Scholar] [CrossRef]
- Khan, M.M.; Galea, G.; Jung, J.; Zukowska, J.; Lauer, D.; Tuechler, N.; Halavatyi, A.; Tischer, C.; Haberkant, P.; Stein, F.; et al. Dextromethorphan inhibits collagen and collagen-like cargo secretion to ameliorate lung fibrosis. Sci. Transl. Med. 2024, 16, eadj3087. [Google Scholar] [CrossRef]
- Eskandari, K.; Bélanger, S.-M.; Lachance, V.; Kourrich, S. Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders. Pharmaceuticals 2025, 18, 700. [Google Scholar] [CrossRef]
- Wang, L.; Du, L.; Xiong, X.; Lin, Y.; Zhu, J.; Yao, Z.; Wang, S.; Guo, Y.; Chen, Y.; Geary, K.; et al. Repurposing dextromethorphan and metformin for treating nicotine-induced cancer by directly targeting CHRNA7 to inhibit JAK2/STAT3/SOX2 signaling. Oncogene 2021, 40, 1974–1987. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, L.; Chan, W.Y.; Nagrial, A.; Chantrill, L.A.; Sim, H.W.; Yip, D.; Chin, V. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 2024, 12, CD011044. [Google Scholar] [CrossRef]
- Jin, M.; Liu, H.L.; Xue, J.; Ma, H.; Liu, J.L.; Lin, Z.Y.; Wang, J.; Bao, L.Q.; Luo, Z.G.; Yu, X.J.; et al. Nab-paclitaxel plus S-1 versus nab-paclitaxel plus gemcitabine in patients with advanced pancreatic cancer: A multicenter, randomized, phase II study. Oncologist 2024, 29, e1406–e1418. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Ung, A.K.Y. Update on management of pancreatic cancer: A literature review. Chin. Clin. Oncol. 2024, 13, 41. [Google Scholar] [CrossRef]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, J.; Park, M.N.; Kim, B. Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells. Int. J. Mol. Sci. 2023, 24, 15236. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Tenebro, C.P.; Sabido, E.M.; Suarez, A.F.L.; Paderog, M.J.V.; Reyes-Salarda, R.; Saludes, J.P. Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies. Mar. Drugs 2024, 22, 114. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, X.; Chen, Y.; Selfridge, J.E.; Gorityala, S.; Du, Z.; Wang, J.M.; Hao, Y.; Cioffi, G.; Conlon, R.A.; et al. 5-Fluorouracil Enhances the Antitumor Activity of the Glutaminase Inhibitor CB-839 against PIK3CA-Mutant Colorectal Cancers. Cancer Res. 2020, 80, 4815–4827. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Sung, Y.S.; Wey, M.; Wang, Y.; Alatrash, N.; Berthod, A.; MacDonnell, F.M.; Armstrong, D.W. Roles of N-methyl-D-aspartate receptors and D-amino acids in cancer cell viability. Mol. Biol. Rep. 2020, 47, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Hu, J.; Ju, X.; Sun, G.; Shao, S.; Tang, R.X.; Zheng, K.Y.; Yan, J. The role of glutamate receptors in the regulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1123841. [Google Scholar] [CrossRef] [PubMed]
- Maniam, G.; Mai, C.W.; Zulkefeli, M.; Fu, J.Y. Co-encapsulation of gemcitabine and tocotrienols in nanovesicles enhanced efficacy in pancreatic cancer. Nanomedicine 2021, 16, 373–389. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Yin, C.; Hu, S.; Liu, P. The role of the mTOR pathway in breast cancer stem cells (BCSCs): Mechanisms and therapeutic potentials. Stem Cell Res. Ther. 2025, 16, 156. [Google Scholar] [CrossRef]
- Chuang, K.T.; Chiou, S.S.; Hsu, S.H. Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial-Mesenchymal Transition. Cancers 2023, 15, 3338. [Google Scholar] [CrossRef]
- Luo, F.; Lu, F.T.; Qiu, M.Z.; Zhou, T.; Ma, W.J.; Luo, M.; Zeng, K.M.; Luo, Q.Y.; Pan, W.T.; Zhang, L.; et al. Gemcitabine and APG-1252, a novel small molecule inhibitor of BCL-2/BCL-XL, display a synergistic antitumor effect in nasopharyngeal carcinoma through the JAK-2/STAT3/MCL-1 signaling pathway. Cell Death Dis. 2021, 12, 772. [Google Scholar] [CrossRef]
- Yu, W.; Srivastava, R.; Srivastava, S.; Ma, Y.; Shankar, S.; Srivastava, R.K. Oncogenic Role of SATB2 In Vitro: Regulator of Pluripotency, Self-Renewal, and Epithelial-Mesenchymal Transition in Prostate Cancer. Cells 2024, 13, 962. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, X.; Liang, L.; Chen, B.Z.; Liu, C.; Wang, Y. A stimulus responsive microneedle-based drug delivery system for cancer therapy. Biomater. Sci. 2024, 12, 6274–6283. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Su, T.; Ding, J.; Chen, F.; Mo, J.; Li, J.; Wang, Z.; Han, L.; Wu, Z.; Wu, S. Chlorophyllin exerts synergistic anti-tumor effect with gemcitabine in pancreatic cancer by inducing cuproptosis. Mol. Med. 2025, 31, 126. [Google Scholar] [CrossRef]
- Sakhaee, E.; Ostadhadi, S.; Khan, M.I.; Yousefi, F.; Norouzi-Javidan, A.; Akbarian, R.; Chamanara, M.; Zolfaghari, S.; Dehpour, A.R. The role of NMDA receptor and nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effect of dextromethorphan in mice forced swimming test and tail suspension test. Biomed. Pharmacother. 2017, 85, 627–634. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Perelroizen, R.; Yakovchuk, A.; Banyas, E.; Mayo, L.; David, S.; Benbenishty, A.; Blinder, P.; Shalom, M.; Ruban, A. Blood glutamate scavengers increase pro-apoptotic signaling and reduce metastatic melanoma growth in-vivo. Sci. Rep. 2021, 11, 14644. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar] [CrossRef]
- Glebov, O.O. Tonic NMDA receptor signalling shapes endosomal organisation in mammalian cells. Sci. Rep. 2020, 10, 9315. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, H.; Ho, A.C.Y. Ulinastatin, A Multivalent Serine Protease Inhibitor, Improves Recovery of Pancreatic Cancer Patients Undergoing Laparoscopic Surgery. Curr. Top. Nutraceutical Res. 2023, 21, 228–234. [Google Scholar] [CrossRef]
- Xu, D.; Shen, H.; Tian, M.; Chen, W.; Zhang, X. Cucurbitacin I inhibits the proliferation of pancreatic cancer through the JAK2/STAT3 signalling pathway in vivo and in vitro. J. Cancer 2022, 13, 2050–2060. [Google Scholar] [CrossRef]
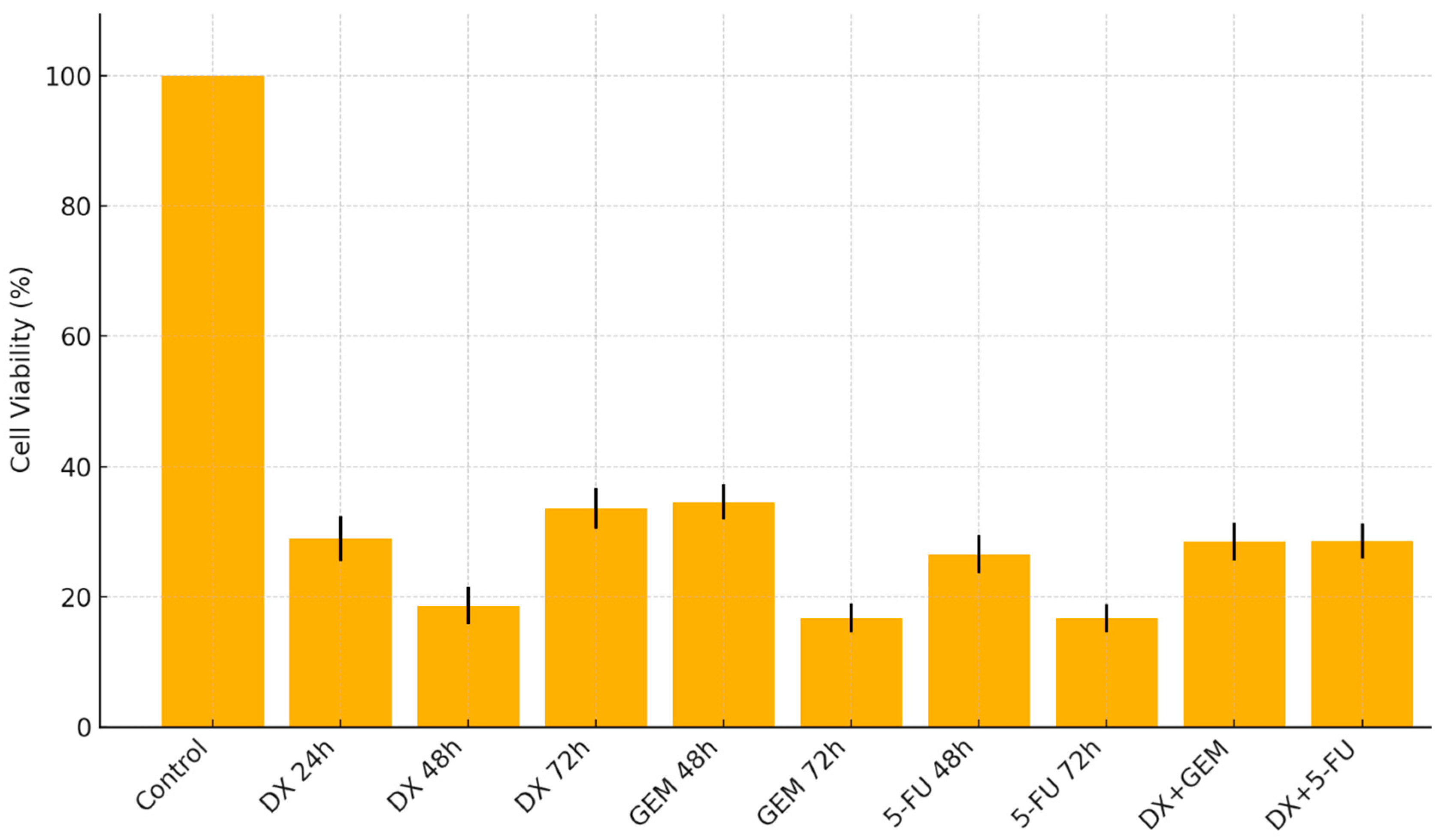
| Treatment | Viability (Mean ± SD) | Most Cytotoxic Conc. (µM) | Statistical Significance |
|---|---|---|---|
| DX (24 h) | 28.99 ± 3.5 | 400 | p < 0.001 |
| DX (48 h) | 18.67 ± 2.8 | 500 | p < 0.001 |
| DX (72 h) | 33.62 ± 3.1 | 200 | p < 0.001 |
| GEM (24 h) | — | — | n.s. |
| GEM (48 h) | 34.58 ± 2.7 | 500 | p < 0.001 |
| GEM (72 h) | 16.72 ± 2.2 | 425 | p < 0.001 |
| 5-FU (48 h) | 26.57 ± 3.0 | 375 | p < 0.001 |
| 5-FU (72 h) | 16.72 ± 2.1 | 425 | p < 0.001 |
| DX + GEM (72 h) | 28.5 ± 2.9 | DX 50 + GEM 12.5 | p < 0.001 |
| DX + 5-FU (72 h) | 28.6 ± 2.7 | DX 50 + 5-FU 12.5 | p < 0.001 |
| Agent | IC50 (24 h, µM) | IC50 (48 h, µM) | IC50 (72 h, µM) |
|---|---|---|---|
| Dextromethorphan | 280.4 | 163.2 | 105.6 |
| Gemcitabine | – | – | 57.53 |
| 5-FU | – | 340.1 | 43.24 |
| Group | Necrotic Cells | Late Apoptotic Cells | Early Apoptotic Cells | Viable Cells |
|---|---|---|---|---|
| Mean ± SD | ||||
| Control | 5.33 ± 0.40 a | 1.50 ± 0.17 a | 3.60 ± 0.61 a | 89.53 ± 0.97 a |
| DX 50 µM | 9.87 ± 0.32 b | 19.32 ± 0.99 b | 9.20 ± 1.15 b | 61.63 ± 4.00 b |
| DX 100 µM | 15.10 ± 1.04 c | 25.73 ± 0.85 b | 7.80 ± 1.25 b | 51.40 ± 3.10 b |
| GEM 12.5 µM | 11.37 ± 1.94 bc | 25.75 ± 1.13 b | 22.77 ± 2.84 c | 40.33 ± 5.26 c |
| GEM 25 µM | 16.67 ± 1.80 c | 33.75 ± 0.66 c | 14.30 ± 1.14 b | 35.33 ± 5.22 c |
| DX 25 + GEM 6.25 µM | 11.50 ± 1.10 b | 36.10 ± 0.93 c | 20.03 ± 0.81 c | 32.33 ± 4.32 c |
| DX 50 + GEM 12.5 µM | 20.97 ± 1.04 d | 32.20 ± 1.33 c | 21.83 ± 1.32 c | 24.93 ± 3.12 d |
| DMSO %0.05 | 12.00 ± 1.15 b | 5.57 ± 1.29 a | 3.50 ± 0.50 a | 76.73 ± 2.78 a |
| DMSO %0.1 | 13.77 ± 1.26 b | 9.74 ± 1.15 a | 4.37 ± 0.53 a | 76.32 ± 3.12 a |
| DMSO %0.2 | 13.17 ± 1.02 b | 5.77 ± 0.31 a | 5.37 ± 0.21 a | 75.70 ± 1.39 a |
| p-value (vs. Control) | <0.001 | <0.001 | <0.001 | <0.001 |
| Group | Bax | Bcl-2 | Vimentin |
|---|---|---|---|
| Cells/Unit Area | |||
| Control | 15.0 a | 60.0 a | 70.0 a |
| DX 50 µM | 42.0 b | 32.0 b | 45.0 b |
| DX 100 µM | 51.0 c | 28.0 b | 41.0 b |
| GEM 12.5 µM | 47.0 c | 29.0 b | 50.0 b |
| GEM 25 µM | 52.0 c | 25.0 b | 48.0 b |
| DX 25 + GEM 6.25 µM | 57.0 d | 21.0 c | 34.0 c |
| DX 50 + GEM 12.5 µM | 62.0 e | 19.0 c | 28.0 c |
| p-value (vs. Control) | <0.001 | <0.001 | <0.001 |
| Group | E-Cadherin | Vimentin | N-Cadherin |
|---|---|---|---|
| Mean ± SD | |||
| Control EMT | 68.97 ± 0.97 a | 91.00 ± 1.85 a | 62.47 ± 0.93 a |
| DX 50 µM | 78.27 ± 1.01 b | 83.93 ± 2.03 b | 48.71 ± 1.02 b |
| DX 100 µM | 78.61 ± 1.02 b | 78.95 ± 2.94 b | 45.36 ± 1.08 b |
| GEM 12.5 µM | 76.15 ± 1.67 b | 80.37 ± 3.01 a | 42.90 ± 1.04 b |
| GEM 25 µM | 76.17 ± 1.35 b | 80.71 ± 2.93 a | 40.77 ± 0.94 b |
| DX 25 + GEM 6.25 µM | 80.43 ± 0.74 b | 73.44 ± 1.03 b | 32.07 ± 0.73 b |
| DX 50 + GEM 12.5 µM | 83.84 ± 0.65 b | 71.04 ± 1.17 b | 30.47 ± 0.72 b |
| p-value (vs. EMT control) | <0.001 | <0.001 | <0.001 |
| Group | Bax | Bcl-2 | XIAP | E-Cadherin | N-Cadherin | Vimentin | Snail |
|---|---|---|---|---|---|---|---|
| EMT control | 1.0 a | 1.0 a | 1.00 a | 1.0 a | 1.00 a | 1.00 a | 1.00 a |
| DX 50 µM | 1.2 a | 0.8 a | 0.95 a | 1.4 a | 0.85 a | 0.90 a | 0.95 a |
| DX 100 µM | 1.5 a | 0.7 a | 0.90 a | 1.5 a | 0.80 a | 0.85 a | 0.90 a |
| GEM 12.5 µM | 1.6 a | 0.6 a | 0.85 a | 1.6 a | 0.75 a | 0.80 a | 0.85 a |
| GEM 25 µM | 1.7 a | 0.5 a | 0.75 a | 1.7 a | 0.70 a | 0.75 a | 0.80 a |
| DX 25 + GEM 6.25 µM | 1.9 a | 0.4 a | 0.65 a | 1.9 a | 0.65 a | 0.70 a | 0.75 a |
| DX 50 + GEM 12.5 µM | 2.1 a | 0.3 a | 0.60 b | 2.0 a | 0.60 a | 0.65 a | 0.70 a |
| p-value (DX + GEM vs. EMT control) | >0.05 | >0.05 | <0.001 | >0.05 | >0.05 | >0.05 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medet, G.; Inal, A. Dextromethorphan Enhances Apoptosis and Suppresses EMT in PANC-1 Pancreatic Cancer Cells: Synergistic Effects with Gemcitabine. Int. J. Mol. Sci. 2025, 26, 8151. https://doi.org/10.3390/ijms26178151
Medet G, Inal A. Dextromethorphan Enhances Apoptosis and Suppresses EMT in PANC-1 Pancreatic Cancer Cells: Synergistic Effects with Gemcitabine. International Journal of Molecular Sciences. 2025; 26(17):8151. https://doi.org/10.3390/ijms26178151
Chicago/Turabian StyleMedet, Gulsah, and Ahmet Inal. 2025. "Dextromethorphan Enhances Apoptosis and Suppresses EMT in PANC-1 Pancreatic Cancer Cells: Synergistic Effects with Gemcitabine" International Journal of Molecular Sciences 26, no. 17: 8151. https://doi.org/10.3390/ijms26178151
APA StyleMedet, G., & Inal, A. (2025). Dextromethorphan Enhances Apoptosis and Suppresses EMT in PANC-1 Pancreatic Cancer Cells: Synergistic Effects with Gemcitabine. International Journal of Molecular Sciences, 26(17), 8151. https://doi.org/10.3390/ijms26178151






