Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy
Abstract
1. Introduction
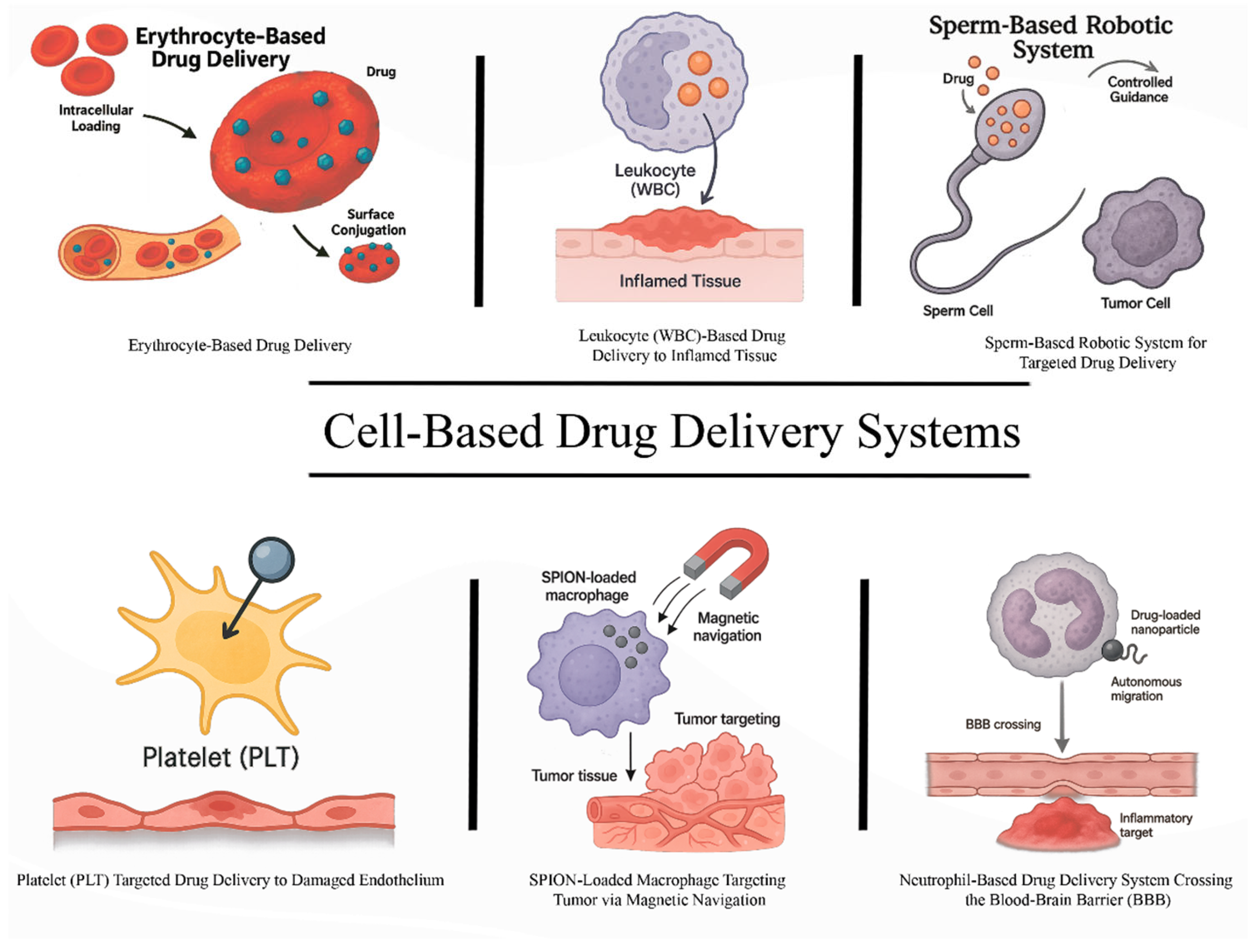
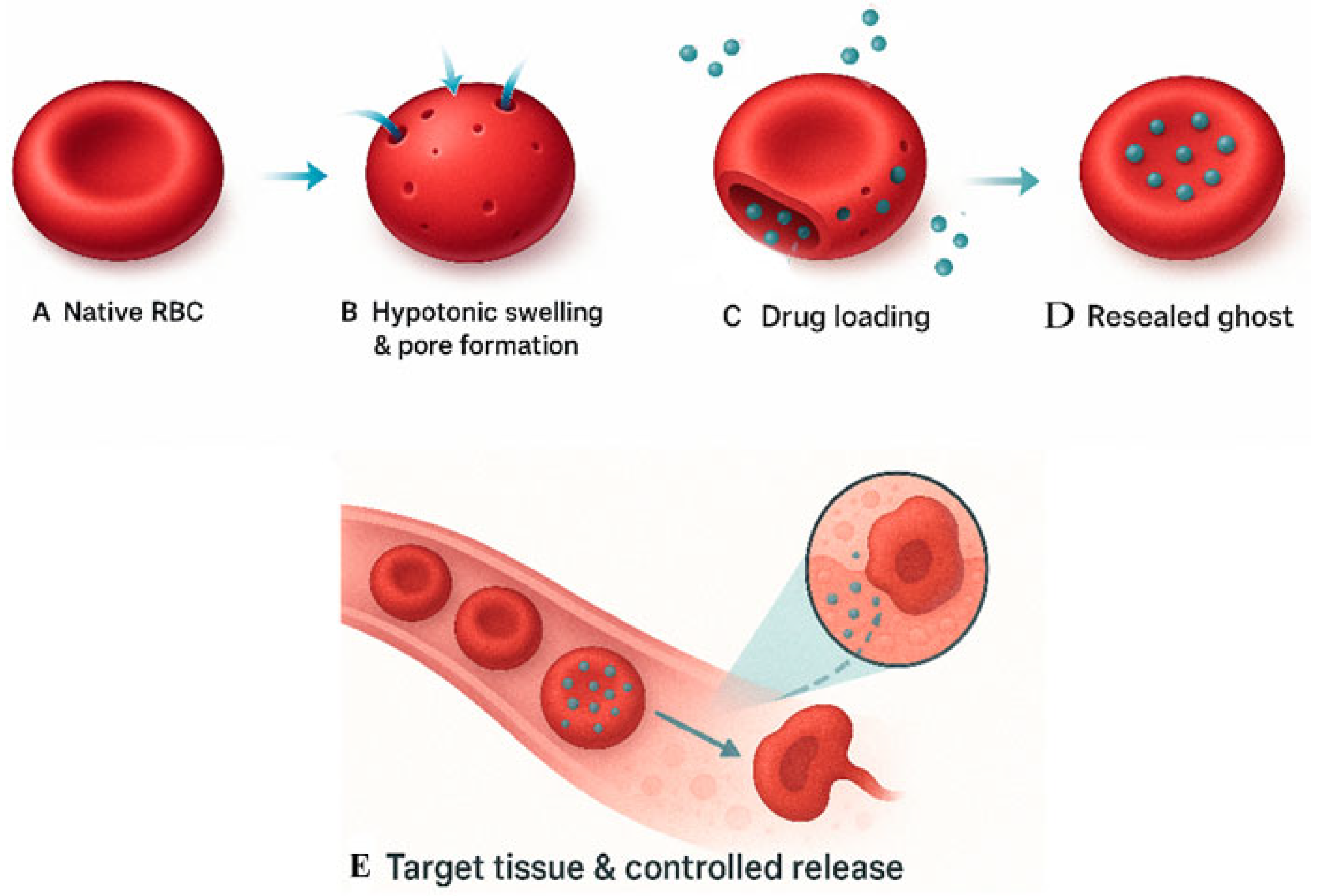
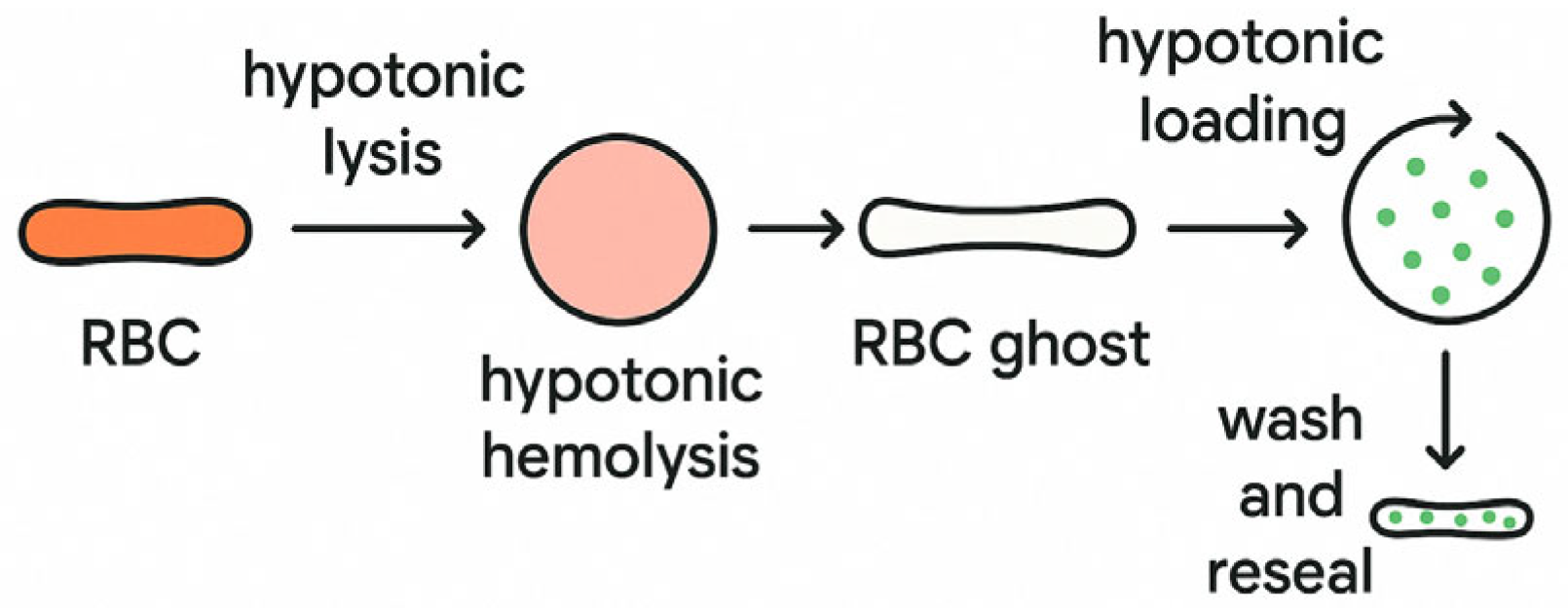
2. Erythrocyte-Based Drug Delivery Systems
Advantages and Strategies of Erythrocyte-Based Drug Delivery
3. Platelet-Based Drug Delivery Systems
4. Leukocyte-Based Drug Delivery Systems
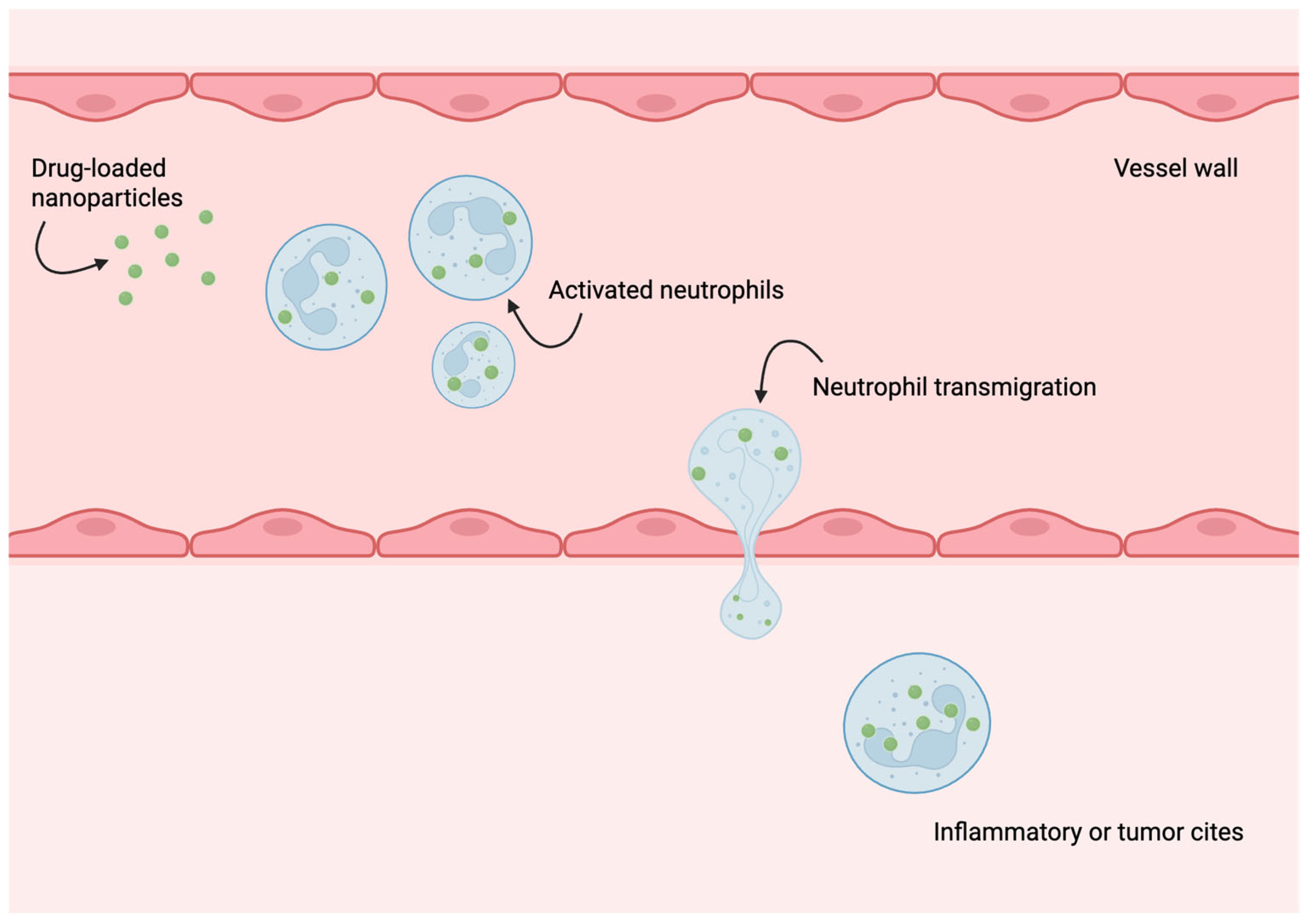
5. Neutrophils as Drug Delivery Vehicles and Components of Biohybrid Microrobots
6. Macrophages as Multifunctional Therapeutic Microrobots and Carriers
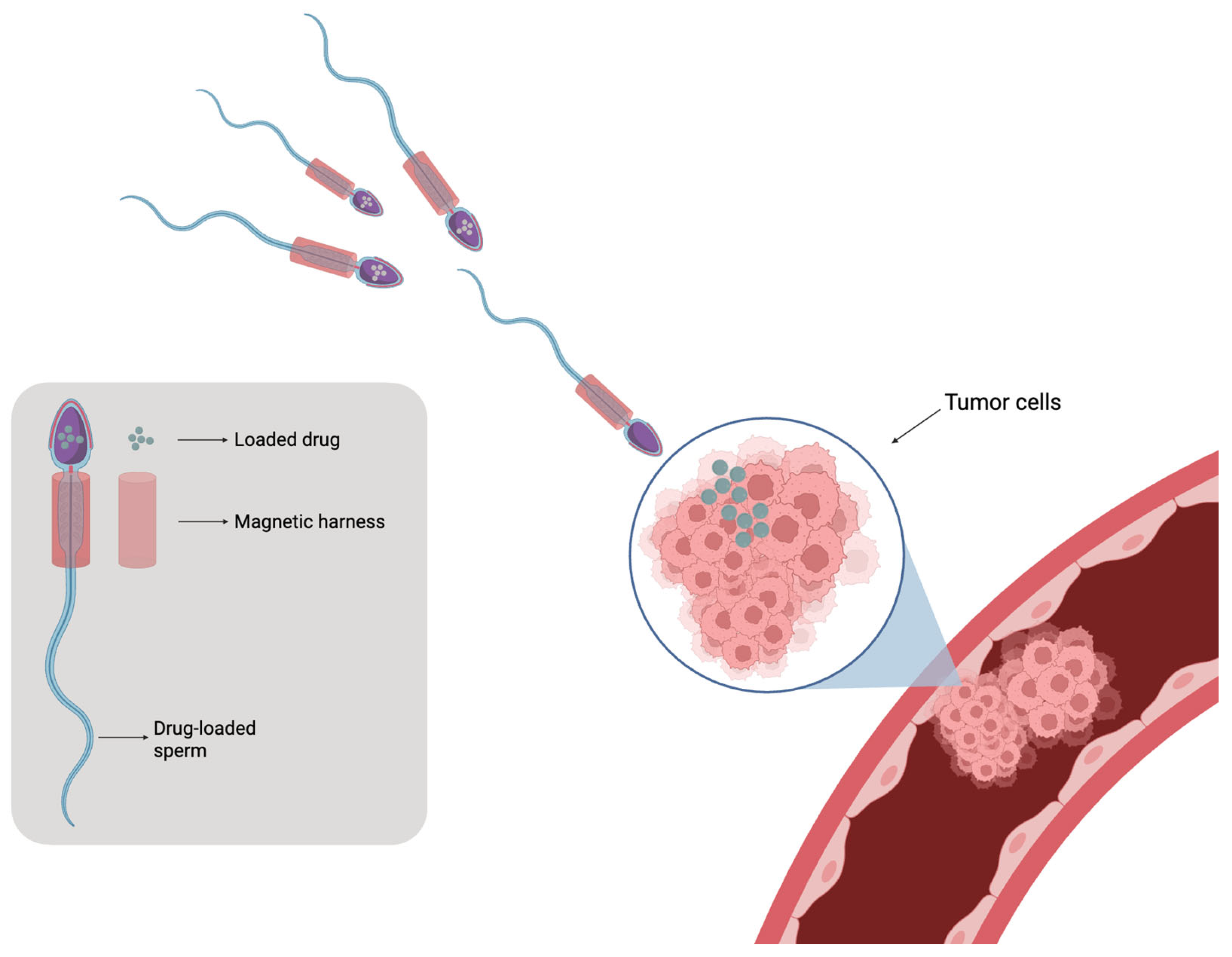
7. Sperm-Based Microrobots and Emerging Biohybrid Micromotors
8. Useful Life of Cell-Based Drug Delivery Systems
9. Bioactive Molecules for Cell-Based Drug Delivery Systems and Effect of Their Physicochemical Characteristics
9.1. Small Molecule Drugs
9.2. Protein, Peptides, and Nucleic Acids
9.3. Physicochemical Properties
10. Comparative and Critical Examination of Cell-Based Drug Delivery Systems
Advantages of Cell-Based Drug Delivery Systems over Polymer and Liposomal Systems
11. Clinical Trials and Translation of Cell-Based DDS
12. Discussion
13. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, L.; Xu, D.; Chen, H.; Wang, L.; Zhao, Y. Designing Bioactive Micro-/Nanomotors for Engineered Regeneration. Eng. Regen. 2021, 2, 109–115. [Google Scholar] [CrossRef]
- dos Santos Ramos, M.A.; de Toledo, L.G.; Spósito, L.; Marena, G.D.; de Lima, L.C.; Fortunato, G.C.; Araújo, V.H.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-Based Lipid Systems Applied to Resistant Bacterial Control: A Review of Their Use in the Past Two Decades. Int. J. Pharm. 2021, 603, 120706. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Alharthi, S.; Alavi, S.Z.; Raza, A.; Ebrahimi Shahmabadi, H. Bioresponsive Drug Delivery Systems. Drug Discov. Today 2024, 29, 103849. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F. The Emerging Role of Silk Fibroin for the Development of Novel Drug Delivery Systems. Biomimetics 2024, 9, 295. [Google Scholar] [CrossRef]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef]
- Ihler, G.M.; Glew, R.H.; Schnure, F.W. Enzyme Loading of Erythrocytes. Proc. Natl. Acad. Sci. USA 1973, 70, 2663–2666. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Herráez, A.; Murciano, J.C.; Jordán, J.A.; Díez, J.C.; Tejedor, M.C. In Vivo Survival and Organ Uptake of Loaded Carrier Rat Erythrocytes. J. Biochem. 1996, 120, 286–291. [Google Scholar] [CrossRef][Green Version]
- Pérez, M.T.; Alvarez, F.J.; García-Pérez, A.I.; Lucas, L.; Tejedor, M.C.; Sancho, P. Heterogeneity of Hypotonically Loaded Rat Erythrocyte Populations as Detected by Counter-Current Distribution in Aqueous Polymer Two-Phase Systems. J. Chromatogr. B Biomed. Appl. 1996, 677, 45–51. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, E.J.; Hou, J.H.; Kim, J.M.; Choi, H.G.; Shim, C.K.; Oh, Y.K. Opsonized Erythrocyte Ghosts for Liver-Targeted Delivery of Antisense Oligodeoxynucleotides. Biomaterials 2009, 30, 959–967. [Google Scholar] [CrossRef]
- Berikkhanova, K.; Omarbaev, R.; Gulyayev, A.; Shulgau, Z.; Ibrasheva, D.; Adilgozhina, G.; Sergazy, S.; Zhumadilov, Z.; Askarova, S. Red Blood Cell Ghosts as Promising Drug Carriers to Target Wound Infections. Med. Eng. Phys. 2016, 38, 877–884. [Google Scholar] [CrossRef]
- Bax, B.E.; Bain, M.D.; Fairbanks, L.D.; Webster, A.D.B.; Chalmers, R.A. In Vitro and in Vivo Studies with Human Carrier Erythrocytes Loaded with Polyethylene Glycol-Conjugated and Native Adenosine Deaminase. Br. J. Haematol. 2000, 109, 549–554. [Google Scholar] [CrossRef]
- Berikkhanova, K.; Taigulov, E.; Bokebaev, Z.; Kusainov, A.; Tanysheva, G.; Yedrissov, A.; Seredin, G.; Baltabayeva, T.; Zhumadilov, Z. Drug-Loaded Erythrocytes: Modern Approaches for Advanced Drug Delivery for Clinical Use. Heliyon 2024, 10, e23451. [Google Scholar] [CrossRef]
- Hadi Barhaghtalab, R.; Tanimowo Aiyelabegan, H.; Maleki, H.; Mirzavi, F.; Gholizadeh Navashenaq, J.; Abdi, F.; Ghaffari, F.; Vakili-Ghartavol, R. Recent Advances with Erythrocytes as Therapeutics Carriers. Int. J. Pharm. 2024, 665, 124658. [Google Scholar] [CrossRef]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjug Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.A.; Antane, J.T.; Roberts, J.L.; Lorentz, K.M.; Zuerndorfer, S.; Dunaif, A.C.; Bailey, L.J.; Tremain, A.C.; Nguyen, M.; de Loera, R.C.; et al. Persistent Antigen Exposure via the Eryptotic Pathway Drives Terminal T Cell Dysfunction. Sci. Immunol. 2021, 6, eabe1801. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Raza, F.; Liu, Y.; Wei, Y.; Rong, R.; Zheng, M.; Yuan, W.; Su, J.; Qiu, M.; Li, Y.; et al. Clinical Progress and Advanced Research of Red Blood Cells Based Drug Delivery System. Biomaterials 2021, 279, 121202. [Google Scholar] [CrossRef]
- Chen, Z.A.; Wu, S.H.; Chen, P.; Chen, Y.P.; Mou, C.Y. Critical Features for Mesoporous Silica Nanoparticles Encapsulated into Erythrocytes. ACS Appl. Mater. Interfaces 2019, 11, 4790–4798. [Google Scholar] [CrossRef]
- Harisa, G.I.; Badran, M.M.; AlQahtani, S.A.; Alanazi, F.K.; Attia, S.M. Pravastatin Chitosan Nanogels-Loaded Erythrocytes as a New Delivery Strategy for Targeting Liver Cancer. Saudi Pharm. J. 2016, 24, 74–81. [Google Scholar] [CrossRef]
- Rossi, L.; Pierigè, F.; Antonelli, A.; Bigini, N.; Gabucci, C.; Peiretti, E.; Magnani, M. Engineering Erythrocytes for the Modulation of Drugs’ and Contrasting Agents’ Pharmacokinetics and Biodistribution. Adv. Drug Deliv. Rev. 2016, 106, 73–87. [Google Scholar] [CrossRef]
- Zhang, E.; Phan, P.; Algarni, H.A.; Zhao, Z. Red Blood Cell Inspired Strategies for Drug Delivery: Emerging Concepts and New Advances. Pharm. Res. 2022, 39, 2673–2698. [Google Scholar] [CrossRef]
- Muzykantov, V.R.; Smirnov, M.D.; Zaltzman, A.B.; Samokhin, G.P. Tannin-Mediated Attachment of Avidin Provides Complement-Resistant Immunoerythrocytes That Can Be Lysed in the Presence of Activator of Complement. Anal. Biochem. 1993, 208, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.M.; Hood, E.D.; Ferguson, L.T.; Zhao, Z.; Siegel, D.L.; Mitragotri, S.; Brenner, J.S.; Muzykantov, V.R. Red Blood Cells: The Metamorphosis of a Neglected Carrier into the Natural Mothership for Artificial Nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. [Google Scholar] [CrossRef] [PubMed]
- Muzykantov, V.R.; Smirnov, M.D.; Samokhin, G.P. Avidin Acylation Prevents the Complement-Dependent Lysis of Avidin-Carrying Erythrocytes. Biochem. J. 1991, 273, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.H.; Pan, D.C.; Johnston, I.H.; Greineder, C.F.; Walsh, L.R.; Hood, E.D.; Cines, D.B.; Poncz, M.; Siegel, D.L.; Muzykantov, V.R. Biocompatible Coupling of Therapeutic Fusion Proteins to Human Erythrocytes. Blood Adv. 2018, 2, 165–176. [Google Scholar] [CrossRef]
- Flower, R.; Peiretti, E.; Magnani, M.; Rossi, L.; Serafini, S.; Gryczynski, Z.; Gryczynski, I. Observation of Erythrocyte Dynamics in the Retinal Capillaries and Choriocapillaris Using ICG-Loaded Erythrocyte Ghost Cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5510–5516. [Google Scholar] [CrossRef]
- Kontos, S.; Hubbell, J.A. Improving Protein Pharmacokinetics by Engineering Erythrocyte Affinity. Mol. Pharm. 2010, 7, 2141–2147. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L. Approaches to Erythrocyte-Mediated Drug Delivery. Expert. Opin. Drug Deliv. 2014, 11, 677–687. [Google Scholar] [CrossRef]
- Antonelli, A.; Pacifico, S.; Sfara, C.; Tamma, M.; Magnani, M. Ferucarbotran-Loaded Red Blood Cells as Long Circulating MRI Contrast Agents: First in Vivo Results in Mice. Nanomedicine 2018, 13, 675–687. [Google Scholar] [CrossRef]
- Markov, D.E.; Boeve, H.; Gleich, B.; Borgert, J.; Antonelli, A.; Sfara, C.; Magnani, M. Human Erythrocytes as Nanoparticle Carriers for Magnetic Particle Imaging. Phys. Med. Biol. 2010, 55, 6461–6473. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional Theranostic Red Blood Cells for Magnetic-Field-Enhanced in Vivo Combination Therapy of Cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Agola, J.O.; Serda, R.; Franco, S.; Lei, Q.; Wang, L.; Minster, J.; Croissant, J.G.; Butler, K.S.; Zhu, W.; et al. Biomimetic Rebuilding of Multifunctional Red Blood Cells: Modular Design Using Functional Components. ACS Nano 2020, 14, 7847–7859. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Esteban-Fernández De Ávila, B.; Martín, A.; Christianson, C.; Gao, W.; Thamphiwatana, S.K.; Escarpa, A.; He, Q.; Zhang, L.; Wang, J. RBC Micromotors Carrying Multiple Cargos towards Potential Theranostic Applications. Nanoscale 2015, 7, 13680–13686. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lin, Z.; Wang, D.; Wu, Z.; Xie, H.; He, Q. Red Blood Cell-Mimicking Micromotor for Active Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23392–23400. [Google Scholar] [CrossRef]
- Yu, H.; Piao, Y.; Zhang, Y.; Xiang, J.; Shao, S.; Tang, J.; Zhou, Z.; Shen, Y. Cell-Selective Binding Zwitterionic Polymeric Micelles Boost the Delivery Efficiency of Antibiotics. ACS Nano 2023, 17, 22430–22443. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Liu, Q.; Tang, F.; Zhang, X.; Yang, S.; Wang, Q.; Yang, Q.; Li, S.; Liu, J.; et al. RBC-Hitchhiking PLGA Nanoparticles Loading β-Glucan as a Delivery System to Enhance in Vitro and in Vivo Immune Responses in Mice. Front. Vet. Sci. 2024, 11, 1462518. [Google Scholar] [CrossRef]
- Brenner, J.S.; Mitragotri, S.; Muzykantov, V.R. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu. Rev. Biomed. Eng. 2021, 23, 225–248. [Google Scholar] [CrossRef]
- Zinger, A.; Cooke, J.P.; Taraballi, F. Biomimetic Nano Drug Delivery Carriers for Treating Cardiovascular Diseases. Nanomedicine 2021, 33, 102360. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering Nanoparticles to Lungs While Avoiding Liver and Spleen through Adsorption on Red Blood Cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef]
- Marcos-Contreras, O.A.; Brenner, J.S.; Kiseleva, R.Y.; Zuluaga-Ramirez, V.; Greineder, C.F.; Villa, C.H.; Hood, E.D.; Myerson, J.W.; Muro, S.; Persidsky, Y.; et al. Combining Vascular Targeting and the Local First Pass Provides 100-Fold Higher Uptake of ICAM-1-Targeted vs. Untargeted Nanocarriers in the Inflamed Brain. J. Control. Release 2019, 301, 54–61. [Google Scholar] [CrossRef]
- Zhumadilov, Z.S.; Makarenkova, R.V. Features of Antibiotic Inclusion into Erythrocyte Ghosts—A System for Targeted Chemotherapeutic Drug Delivery. Antibiot. Chemother. 1990, 35, 54–56. [Google Scholar]
- Wu, Z.; Li, T.; Gao, W.; Xu, T.; Jurado-Sánchez, B.; Li, J.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Cell-Membrane-Coated Synthetic Nanomotors for Effective Biodetoxification. Adv. Funct. Mater. 2015, 25, 3881–3887. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, D.; Fang, R.H.; Zhang, L. Biomimetic Strategies for Targeted Nanoparticle Delivery. Bioeng. Transl. Med. 2016, 1, 30–46. [Google Scholar] [CrossRef]
- Fliervoet, L.A.L.; Mastrobattista, E. Drug Delivery with Living Cells. Adv. Drug Deliv. Rev. 2016, 106, 63–72. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Li, J.; Gao, W.; Xu, T.; Christianson, C.; Gao, W.; Galarnyk, M.; He, Q.; Zhang, L.; et al. Turning Erythrocytes into Functional Micromotors. ACS Nano 2014, 8, 12041–12048. [Google Scholar] [CrossRef]
- Fei, Z.; Fan, Q.; Dai, H.; Zhou, X.; Xu, J.; Ma, Q.; Maruyama, A.; Wang, C. Physiologically Triggered Injectable Red Blood Cell-Based Gel for Tumor Photoablation and Enhanced Cancer Immunotherapy. Biomaterials 2021, 271, 120724. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red Blood Cells: Supercarriers for Drugs, Biologicals, and Nanoparticles and Inspiration for Advanced Delivery Systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Jin, K.; Zhang, B.; Peng, S.; Nayak, A.K.; Pang, Z. Recent Advances in Erythrocyte Membrane-Camouflaged Nanoparticles for the Delivery of Anti-Cancer Therapeutics. Expert. Opin. Drug Deliv. 2022, 19, 965–984. [Google Scholar] [CrossRef]
- Lorant, T.; Wilton, J.; Olausson, M.; Tufveson, G.; Johnsson, C. Oral Administration of Xenogeneic Erythrocytes Induces Production of Antibodies That Are Capable of Inducing Hyperacute Rejection of Concordant Vascularized Xenografts. Transplantation 2004, 77, 1100–1103. [Google Scholar] [CrossRef]
- Niimi, M.; Shirasugi, N.; Hamano, K.; Esato, K.; Matsumoto, K.; Ikeda, Y.; Shatari, T.; Takami, H.; Kodaira, S. Oral Delivery of Xeno-Antigen Combined with Non-Depleting Anti-CD4 Monoclonal Antibody Induces Significantly Prolonged Survival of Concordant Skin Xenograft. Xenotransplantation 2001, 8, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, Q.; Jiang, C.; Gu, Z. Platelet for Drug Delivery. Curr. Opin. Biotechnol. 2019, 58, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhao, T.; Song, N.; Pan, K.; Yang, Y.; Zhu, X.; Chen, P.; Zhang, J.; Xia, C. Platelets and Platelet Extracellular Vesicles in Drug Delivery Therapy: A Review of the Current Status and Future Prospects. Front. Pharmacol. 2022, 13, 1026386. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. Int. J. Nanomed. 2023, 18, 509–525. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, G.; Cui, H.; Tian, Z.; Li, B.; Han, Y.; Wu, J.X.; Sun, J.; Zhao, Y.; Chen, T.; et al. Theoretical Basis, State and Challenges of Living Cell-Based Drug Delivery Systems. Theranostics 2024, 14, 5152–5183. [Google Scholar] [CrossRef]
- Ward, S.R.; Guzman, L.A.; Sutton, J.M.; Forudi, F.; Wendt, M.; Brewer, L.; Topol, E.J.; Crawford, N. 984-30 Use of Electroporated Platelets as a Novel Drug Delivery System in Preventing Complications of Coronary Angioplasty. J. Am. Coll. Cardiol. 1995, 25, 303A–304A. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Ma, L.; Wang, W.; Liu, H.; Wan, D.; Liu, J.-F.; Li, A.; Guo, S.-S.; Zhang, L.; et al. Platelet-Facilitated Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Angew. Chem. 2018, 130, 998–1003. [Google Scholar] [CrossRef]
- Ji, J.; Lian, W.; Zhang, Y.; Lin, D.; Wang, J.; Mo, Y.; Xu, X.; Hou, C.; Ma, C.; Zheng, Y.; et al. Preoperative Administration of a Biomimetic Platelet Nanodrug Enhances Postoperative Drug Delivery by Bypassing Thrombus. Int. J. Pharm. 2023, 636, 122851. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, L.; Mu, Y.; Xiao, Y. Targeting Macrophage Endocytosis via Platelet Membrane Coating for Advanced Osteoimmunomodulation. iScience 2022, 25, 105196. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Fang, R.H.; Luk, B.T.; Kroll, A.V.; Dehaini, D.; Zhou, J.; Kim, H.W.; Gao, W.; Lu, W.; et al. Nanoparticles Camouflaged in Platelet Membrane Coating as an Antibody Decoy for the Treatment of Immune Thrombocytopenia. Biomaterials 2016, 111, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, C. Platelet-Derived Extracellular Vesicles for Drug Delivery. Biomater. Sci. 2023, 11, 5758–5768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Chen, X.; Chen, Q.; Zhou, W.; Guo, Q.; Lu, Y.; Li, C.; Zhang, Y.; Liang, D.; et al. Activated Platelets-Targeting Micelles with Controlled Drug Release for Effective Treatment of Primary and Metastatic Triple Negative Breast Cancer. Adv. Funct. Mater. 2019, 29, 1806620. [Google Scholar] [CrossRef]
- Li, J.; Sharkey, C.C.; Wun, B.; Liesveld, J.L.; King, M.R. Genetic Engineering of Platelets to Neutralize Circulating Tumor Cells. J. Control. Release 2016, 228, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Schroeder, J.A.; Jing, W.; Gurski, C.; Williams, C.B.; Wang, S.; Dittel, B.N.; Shi, Q. Targeting Transmembrane-Domain-Less MOG Expression to Platelets Prevents Disease Development in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2022, 13, 1029356. [Google Scholar] [CrossRef]
- Kona, S.; Dong, J.F.; Liu, Y.; Tan, J.; Nguyen, K.T. Biodegradable Nanoparticles Mimicking Platelet Binding as a Targeted and Controlled Drug Delivery System. Int. J. Pharm. 2012, 423, 516–524. [Google Scholar] [CrossRef]
- Spragg, D.D.; Alford, D.R.; Greferath, R.; Larsen, C.E.; Lee, K.D.; Gurtner, G.C.; Cybulsky, M.I.; Tosi, P.F.; Nicolau, C.; Gimbrone, M.A. Immunotargeting of Liposomes to Activated Vascular Endothelial Cells: A Strategy for Site-Selective Delivery in the Cardiovascular System. Proc. Natl. Acad. Sci. USA 1997, 94, 8795–8800. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Platelet Membrane Camouflaged Nanoparticles: Biomimetic Architecture for Targeted Therapy. Int. J. Pharm. 2021, 598, 120395. [Google Scholar] [CrossRef]
- Gulyayev, A.E.; Lokhvytsky, S.V.; Zubtsov, V.N.; Zhaugasheva, S.K.; Sheptunov, Y.M. Targeted Drug Delivery: Possibilities for Use in Purulent Surgery. In Pharmacokinetics and Pharmacodynamics of Drugs in Experiment and Clinic; Collection of Scientific Papers; News of the Kyiv Commercial Institute: Almaty, Kazakhstan, 1992; pp. 82–83. [Google Scholar]
- Nurgozhin, T.; Gulyayev, A.; Lokhvytsky, S.; Yermekbayeva, B.; Sergazy, S.; Shulgau, Z.; Berikkhanova, K. Pharmacokinetics of Ceftriaxone Included In Cellular Transport System. Clin. Ther. 2015, 37, e62. [Google Scholar] [CrossRef]
- Munerati, M.; Cortesi, R.; Ferrari, D.; Di Virgilio, F.; Nastruzzi, C. Macrophages Loaded with Doxorubicin by ATP-Mediated Permeabilization: Potential Carriers for Antitumor Therapy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1994, 1224, 269–276. [Google Scholar] [CrossRef]
- Sofias, A.M.; Toner, Y.C.; Meerwaldt, A.E.; Van Leent, M.M.T.; Soultanidis, G.; Elschot, M.; Gonai, H.; Grendstad, K.; Flobak, Å.; Neckmann, U.; et al. Tumor Targeting by Avβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano 2020, 14, 7832–7846. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lu, Y.; Shi, Y.; Jiang, M.; Shan, X.; Li, X.; Zhang, J.; Qin, B.; Liu, X.; Guo, X.; et al. Neutrophil Hitchhiking for Drug Delivery to the Bone Marrow. Nat. Nanotechnol. 2023, 18, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, D.; Qiao, Z.; Zhuang, Y.; Zhang, Y.; Jiang, Q.; Liu, M.; An, Q.; Shen, D. Neutrophil-Targeted Mn3O4 Nanozyme Treats Myocardial Ischemia Reperfusion Injury by Scavenging Reactive Oxygen Species; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Najer, A.; Blakney, A.K.; McKay, P.F.; Bellahcene, M.; Winter, C.W.; Sintou, A.; Tang, J.; Keane, T.J.; Schneider, M.D.; et al. Neutrophils Enable Local and Non-Invasive Liposome Delivery to Inflamed Skeletal Muscle and Ischemic Heart. Adv. Mater. 2020, 32, e2003598. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J. Bacteria and Bacterial Derivatives as Drug Carriers for Cancer Therapy. J. Control. Release 2020, 326, 396–407. [Google Scholar] [CrossRef]
- Visser, J.G.; Van Staden, A.D.P.; Smith, C. Harnessing Macrophages for Controlled-Release Drug Delivery: Lessons From Microbes. Front. Pharmacol. 2019, 10, 22. [Google Scholar] [CrossRef]
- Bush, L.M.; Healy, C.P.; Javdan, S.B.; Emmons, J.C.; Deans, T.L. Biological Cells as Therapeutic Delivery Vehicles. Trends Pharmacol. Sci. 2021, 42, 106–118. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Q.; Wu, S.; Qin, H.; Zhang, T.; Zheng, X.; Li, B. Optically Manipulated Neutrophils as Native Microcrafts In Vivo. ACS Cent. Sci. 2022, 8, 1017–1027. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Zhang, M.; Li, Y.; Tang, B.Z. Neutrophil-like Biomimic AIE Nanoparticles with High-Efficiency Inflammatory Cytokine Targeting Enable Precise Photothermal Therapy and Alleviation of Inflammation. ACS Nano 2023, 17, 7394–7405. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-Guided Hybrid Neutrophil Micromotors for Targeted Drug Transport. Angew. Chem. Int. Ed. 2017, 56, 12935–12939. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-Responsive Biohybrid Neutrobots for Active Target Delivery. Sci. Robot. 2020, 6, eaaz9519. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Gao, J.; Wang, Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano 2015, 9, 11800–11811. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, H. Neutrobots Smuggle Drugs across Biological Barriers. Sci. Robot. 2020, 6, eabh0286. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Wei, W.; Jin, Y.; Wang, S.; Zhao, D.; Wang, S.; Gao, X.; Qiao, C.; Yue, H.; Ma, G.; et al. Biomimetic Immuno-Magnetosomes for High-Performance Enrichment of Circulating Tumor Cells. Adv. Mater. 2016, 28, 7929–7935. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil Membrane-Coated Nanoparticles Inhibit Synovial Inflammation and Alleviate Joint Damage in Inflammatory Arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Lu, G.H.; Nie, W.; Zhang, L.; Lv, Y.; Bao, W.; Gao, X.; Wei, W.; Pu, K.; et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano 2019, 13, 5662–5673. [Google Scholar] [CrossRef]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef]
- Li, Y.; Cong, Z.; Xie, L.; Tang, S.; Ren, C.; Peng, X.; Tang, D.; Wan, F.; Han, H.; Zhang, X.; et al. Magnetically Powered Immunogenic Macrophage Microrobots for Targeted Multimodal Cancer Therapy. Small 2023, 19, e2301489. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The Complex Role of Tumor-Infiltrating Macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, R.; Luo, Y.; Guo, X.; Yang, G.; Li, X.; Zhou, S. A Hierarchical Structured Fiber Device Remodeling the Acidic Tumor Microenvironment for Enhanced Cancer Immunotherapy. Adv. Mater. 2023, 35, e2300216. [Google Scholar] [CrossRef]
- Hou, M.; Wei, Y.; Zhao, Z.; Han, W.; Zhou, R.; Zhou, Y.; Zheng, Y.; Yin, L. Immuno-Engineered Nanodecoys for the Multi-Target Anti-Inflammatory Treatment of Autoimmune Diseases. Adv. Mater. 2022, 34, e2108817. [Google Scholar] [CrossRef]
- Song, X.; Qian, R.; Li, T.; Fu, W.; Fang, L.; Cai, Y.; Guo, H.; Xi, L.; Cheang, U.K. Imaging-Guided Biomimetic M1 Macrophage Membrane-Camouflaged Magnetic Nanorobots for Photothermal Immunotargeting Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 56548–56559. [Google Scholar] [CrossRef]
- Fan, J.X.; Li, Z.H.; Liu, X.H.; Zheng, D.W.; Chen, Y.; Zhang, X.Z. Bacteria-Mediated Tumor Therapy Utilizing Photothermally-Controlled TNF-α Expression via Oral Administration. Nano Lett. 2018, 18, 2373–2380. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, Y.; Choi, Y.J.; Cho, S.; Jung, H.E.; Zheng, S.; Park, B.J.; Ko, S.Y.; Park, J.O.; Park, S. Monocyte-Based Microrobot with Chemotactic Motility for Tumor Theragnosis. Biotechnol. Bioeng. 2014, 111, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Liu, Y.; Yang, G.; Zhao, C.X. Macrophage-Mediated Cancer Drug Delivery. Mater. Today Sustain. 2021, 11–12, 100055. [Google Scholar] [CrossRef]
- Luo, C.H.; Huang, C.T.; Su, C.H.; Yeh, C.S. Bacteria-Mediated Hypoxia-Specific Delivery of Nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016, 16, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.; Wang, Y.M. Macrophages as a “Weapon” in Anticancer Cellular Immunotherapy. Kaohsiung J. Med. Sci. 2021, 37, 749–758. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Park, J.O.; Choi, E. Macrophage-Based Microrobots for Anticancer Therapy: Recent Progress and Future Perspectives. Biomimetics 2023, 8, 553. [Google Scholar] [CrossRef]
- Sitti, M. Miniature Devices: Voyage of the Microrobots. Nature 2009, 458, 1121–1122. [Google Scholar] [CrossRef]
- Leung, M.R.; Zeng, J.; Wang, X.; Roelofs, M.C.; Huang, W.; Zenezini Chiozzi, R.; Hevler, J.F.; Heck, A.J.R.; Dutcher, S.K.; Brown, A.; et al. Structural Specializations of the Sperm Tail. Cell 2023, 186, 2880–2896.e17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Sukhov, A.; Hauri, D.; Rodrigue, D.; Maranta, G.; Harting, J.; Nelson, B.J. Bioinspired Acousto-Magnetic Microswarm Robots with Upstream Motility. Nat. Mach. Intell. 2021, 3, 116–124. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, C., Jr. Mensuration of the Heads of Bull Spermatozoa. Mikroskopie 1960, 14, 256–276. [Google Scholar]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef]
- Magdanz, V.; Khalil, I.S.M.; Simmchen, J.; Furtado, G.P.; Mohanty, S.; Gebauer, J.; Xu, H.; Klingner, A.; Aziz, A.; Medina-Sánchez, M.; et al. IRONSperm: Sperm-Templated Soft Magnetic Microrobots. Sci. Adv. 2020, 6, 5855–5863. [Google Scholar] [CrossRef]
- Zhou, M.; Yin, Y.; Zhao, J.; Zhou, M.; Bai, Y.; Zhang, P. Applications of Microalga-Powered Microrobots in Targeted Drug Delivery. Biomater. Sci. 2023, 11, 7512–7530. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Chen, C.; Luan, H.; Fang, R.H.; Zhang, L.; Wang, J. Biohybrid Microalgae Robots: Design, Fabrication, Materials, and Applications. Adv. Mater. 2024, 36, e2303714. [Google Scholar] [CrossRef]
- Mathur, D.; Bhatia, D. Bio-Propulsion Techniques for Bio-Micro/Nano-Robots. Lect. Notes Data Eng. Commun. Technol. 2021, 61, 431–439. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Liang, S.; Dai, Y.; Bai, X.; Song, B.; Zhang, D.; Chen, H.; Feng, L. Recent Advances in Field-Controlled Micro–Nano Manipulations and Micro–Nano Robots. Adv. Intell. Syst. 2022, 4, 2100116. [Google Scholar] [CrossRef]
- Chen, B.; Sun, H.; Zhang, J.; Xu, J.; Song, Z.; Zhan, G.; Bai, X.; Feng, L. Cell-Based Micro/Nano-Robots for Biomedical Applications: A Review. Small 2024, 20, e2304607. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, J.; Li, Z.; Gong, H.; de Ávila, B.E.F.; Duan, Y.; Zhang, Q.; Zhou, J.; Yin, L.; Karshalev, E.; et al. Nanoparticle-Modified Microrobots for in Vivo Antibiotic Delivery to Treat Acute Bacterial Pneumonia. Nat. Mater. 2022, 21, 1324–1332. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Chen, B.; Gao, J.; Li, T.; Ye, Y.; Tian, H.; Wang, S.; Wang, F.; Jiang, J.; et al. Magnetically Actuated Biohybrid Microswimmers for Precise Photothermal Muscle Contraction. ACS Nano 2022, 16, 6515–6526. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, O.K.; Kim, J.; Shin, K.; Pack, C.G.; Kim, K.; Ko, G.; Lee, N.; Kwon, S.H.; Hyeon, T. Deep Tumor Penetration of Drug-Loaded Nanoparticles by Click Reaction-Assisted Immune Cell Targeting Strategy. J. Am. Chem. Soc. 2019, 141, 13829–13840. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, J.; Yang, J. Advances in Artificial Cells Based on Microfluidic Chips. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2024, 40, 2100–2119. [Google Scholar] [CrossRef]
- Fasciano, S.; Wang, S. Recent Advances of Droplet-Based Microfluidics for Engineering Artificial Cells. SLAS Technol. 2024, 29, 100090. [Google Scholar] [CrossRef] [PubMed]
- Ngocho, K.; Yang, X.; Wang, Z.; Hu, C.; Yang, X.; Shi, H.; Wang, K.; Liu, J. Synthetic Cells from Droplet-Based Microfluidics for Biosensing and Biomedical Applications. Small 2024, 20, e2400086. [Google Scholar] [CrossRef] [PubMed]
- Rijal, D.; Ariana, A.; Wight, A.; Kim, K.; Alturki, N.A.; Aamir, Z.; Ametepe, E.S.; Korneluk, R.G.; Tiedje, C.; Menon, M.B.; et al. Differentiated Macrophages Acquire a Pro-Inflammatory and Cell Death–Resistant Phenotype Due to Increasing XIAP and P38-Mediated Inhibition of RipK1. J. Biol. Chem. 2018, 293, 11913–11927. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, C.; Qin, J.; Han, L.; Li, R.; Hong, C.; He, H.; Wang, J. A Novel Strategy to Achieve Effective Drug Delivery: Exploit Cells as Carrier Combined with Nanoparticles. Drug Deliv. 2017, 24, 83–91. [Google Scholar] [CrossRef]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human Neutrophil Kinetics: Modeling of Stable Isotope Labeling Data Supports Short Blood Neutrophil Half-Lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, H.; Li, Y.; Wu, J.; Hou, J.; Liu, K.; Zhang, X. Photo-Driven Sperm-Inspired Microrobots Serving in Liquid Environments. Adv. Intell. Syst. 2024, 6, 2400004. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid. Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-Mediated Targeting Drugs Delivery Systems. Drug Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Lam, D.; Cabrales, P. Doxorubicin-Loaded Red Blood Cells Reduced Cardiac Toxicity and Preserved Anticancer Activity. Drug Deliv. 2019, 26, 433. [Google Scholar] [CrossRef]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-Loaded Platelets as a Smart Drug Delivery System: An Improved Therapy for Lymphoma. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef]
- Xu, P.; Zuo, H.; Zhou, R.; Wang, F.; Liu, X.; Ouyang, J.; Chen, B.; Xu, P.; Zuo, H.; Zhou, R.; et al. Doxorubicin-Loaded Platelets Conjugated with Anti-CD22 MAbs: A Novel Targeted Delivery System for Lymphoma Treatment with Cardiopulmonary Avoidance. Oncotarget 2017, 8, 58322–58337. [Google Scholar] [CrossRef]
- Yousfan, A.; Moursel, N.; Hanano, A. Encapsulation of Paclitaxel into Date Palm Lipid Droplets for Enhanced Brain Cancer Therapy. Sci. Rep. 2024, 14, 32057. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-Mediated Anticancer Drug Delivery for Suppression of Postoperative Malignant Glioma Recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Tanaka, H.; Horioka, K.; Hasebe, T.; Sawada, K.; Nakajima, S.; Konishi, H.; Isozaki, S.; Goto, M.; Fujii, Y.; Kamikokura, Y.; et al. Treatment of Hepatocellular Carcinoma with Autologous Platelets Encapsulating Sorafenib or Lenvatinib: A Novel Therapy Exploiting Tumor-Platelet Interactions. Int. J. Cancer 2022, 150, 1640–1653. [Google Scholar] [CrossRef]
- Rossi, L.; Pierigè, F.; Bregalda, A.; Magnani, M. Preclinical Developments of Enzyme-Loaded Red Blood Cells. Expert. Opin. Drug Deliv. 2021, 18, 43–54. [Google Scholar] [CrossRef]
- Magnani, M.; Laguerre, M.; Rossi, L.; Bianchi, M.; Ninfali, P.; Mangani, F.; Ropars, C. In vivo accelerated acetaldehyde metabolism using acetaldehyde dehydrogenase-loaded erythrocytes. Alcohol. Alcohol. 1990, 25, 627–637. [Google Scholar] [CrossRef]
- Wayne, E.C.; Long, C.; Haney, M.J.; Batrakova, E.V.; Leisner, T.M.; Parise, L.V.; Kabanov, A.V. Targeted Delivery of SiRNA Lipoplexes to Cancer Cells Using Macrophage Transient Horizontal Gene Transfer. Adv. Sci. 2019, 6, 1900582. [Google Scholar] [CrossRef]
- Vauquelin, G. Cell Membranes… and How Long Drugs May Exert Beneficial Pharmacological Activity In Vivo. Br. J. Clin. Pharmacol. 2016, 82, 673–682. [Google Scholar] [CrossRef]
- Bourgeaux, V.; Lanao, J.M.; Bax, B.E.; Godfrin, Y. Drug-Loaded Erythrocytes: On the Road toward Marketing Approval. Drug Des. Devel Ther. 2016, 10, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Mukthavaram, R.; Shi, G.; Kesari, S.; Simberg, D. Targeting and Depletion of Circulating Leukocytes and Cancer Cells by Lipophilic Antibody-Modified Erythrocytes. J. Control. Release 2014, 183, 146–153. [Google Scholar] [CrossRef]
- Dou, H.; Destache, C.J.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a Macrophage-Based Nanoparticle Platform for Antiretroviral Drug Delivery. Blood 2006, 108, 2827. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Zhang, W.; Seaton, M.P.H.; Brison, D.R.; Edmondson, R.J.; Taylor, S.S.; Nelson, L.; Zeng, K.; Bagley, S.; et al. Human Spermbots for Patient-Representative 3D Ovarian Cancer Cell Treatment. Nanoscale 2020, 12, 20467–20481. [Google Scholar] [CrossRef]
- Li, T.; Feng, J.; Ding, W.; Zhang, Z. Targeted Treatment of Myocardial Infarction by Macrophage Membrane Coated with Resveratrol Nanoparticles. ACS Omega 2024, 9, 47145–47155. [Google Scholar] [CrossRef]
- Zargar, S.M.; Hafshejani, D.K.; Eskandarinia, A.; Rafienia, M.; Kharazi, A.Z. A Review of Controlled Drug Delivery Systems Based on Cells and Cell Membranes. J. Med. Signals Sens. 2019, 9, 181–189. [Google Scholar] [CrossRef]
- Wang, Q.H.; Cheng, S.; Han, C.Y.; Yang, S.; Gao, S.R.; Yin, W.Z.; Song, W.Z. Tailoring Cell-Inspired Biomaterials to Fuel Cancer Therapy. Mater. Today Bio 2025, 30, 101381. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Millán, C.; Bravo, D.G.; Lanao, J.M. New Erythrocyte-Related Delivery Systems for Biomedical Applications. J. Drug Deliv. Sci. Technol. 2017, 42, 38–48. [Google Scholar] [CrossRef]
- Xie, L.; Gan, F.; Hu, Y.; Zheng, Y.; Lan, J.; Liu, Y.; Zhou, X.; Zheng, J.; Zhou, X.; Lou, J. From Blood to Therapy: The Revolutionary Application of Platelets in Cancer-Targeted Drug Delivery. J. Funct. Biomater. 2025, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In Vivo Labeling with 2H2O Reveals a Human Neutrophil Lifespan of 5.4 Days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Y.; Xu, Y.; Peng, J. Cell-Based Drug Delivery Systems and Their in Vivo Fate. Adv. Drug Deliv. Rev. 2022, 187, 114394. [Google Scholar] [CrossRef]
- Sairam, A.B.; Sanmugam, A.; Pushparaj, A.; Mahesh Kumar, G.; Sundarapandian, N.; Balaji, S.; Nallal, M.; Park, K.H. Toxicity of Polymeric Nanodrugs as Drug Carriers. ACS Chem. Health Saf. 2023, 30, 236–250. [Google Scholar] [CrossRef]
- Taher, M.; Susanti, D.; Haris, M.S.; Rushdan, A.A.; Widodo, R.T.; Syukri, Y.; Khotib, J. PEGylated Liposomes Enhance the Effect of Cytotoxic Drug: A Review. Heliyon 2023, 9, e13823. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Ervolino, E.; Paulo, J.; Issa, M.; Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef] [PubMed]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the Application of Liposomes as Drug Delivery Systems for the Diagnosis and Treatment of Cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef] [PubMed]
- Haroon, H.B.; Hunter, A.C.; Farhangrazi, Z.S.; Moghimi, S.M. A Brief History of Long Circulating Nanoparticles. Adv. Drug Deliv. Rev. 2022, 188, 114396. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hong, G.; Zhao, G.; Pei, H.; Qu, J.; Chun, C.; Huang, Z.; Lu, Z. Red Blood Cell Membrane-Camouflaged PLGA Nanoparticles Loaded With Basic Fibroblast Growth Factor for Attenuating Sepsis-Induced Cardiac Injury. Front. Pharmacol. 2022, 13, 881320. [Google Scholar] [CrossRef]
- Baruchel, A.; Bertrand, Y.; Thomas, X.; Blin, N.; Tavernier, E.; Ducassou, S.; Vey, N.; Gandemer, V.; Cacheux, V.; Mazingue, F.; et al. Updated Clinical Activity of Graspa Versus Native L-Asparaginase in Combination with Cooprall Regimen in Phase 3 Randomized Trial in Patients with Relapsed Acute Lymphoblastic Leukemia (NCT01518517). Blood 2015, 126, 3723. [Google Scholar] [CrossRef]
- Hammel, P.; Fabienne, P.; Mineur, L.; Metges, J.P.; Andre, T.; De La Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. Erythrocyte-Encapsulated Asparaginase (Eryaspase) Combined with Chemotherapy in Second-Line Treatment of Advanced Pancreatic Cancer: An Open-Label, Randomized Phase IIb Trial. Eur. J. Cancer 2020, 124, 91–101. [Google Scholar] [CrossRef]
- Hammel, P.; El-Hariry, I.; Macarulla, T.; Garcia-Carbonero, R.; Metges, J.-P.; Bouché, O.; Portales, F.; Cid, R.A.P.; Mineur, L.; Gracian, A.M.C.; et al. Trybeca-1: A Randomized, Phase 3 Study of Eryaspase in Combination with Chemotherapy versus Chemotherapy Alone as Second-Line Treatment in Patients with Advanced Pancreatic Adenocarcinoma (NCT03665441). J. Clin. Oncol. 2022, 40, 518. [Google Scholar] [CrossRef]
- Yu, B.; Xue, X.; Yin, Z.; Cao, L.; Li, M.; Huang, J. Engineered Cell Membrane-Derived Nanocarriers: The Enhanced Delivery System for Therapeutic Applications. Front. Cell Dev. Biol. 2022, 10, 844050. [Google Scholar] [CrossRef]
- Liu, J.; Liew, S.S.; Wang, J.; Pu, K. Bioinspired and Biomimetic Delivery Platforms for Cancer Vaccines. Adv. Mater. 2022, 34, e2103790. [Google Scholar] [CrossRef] [PubMed]
- Waeterschoot, J.; Gosselé, W.; Lemež, Š.; Casadevall i Solvas, X. Artificial Cells for in Vivo Biomedical Applications through Red Blood Cell Biomimicry. Nat. Commun. 2024, 15, 2504. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Raza, F.; Liu, Y.; Yuan, W.; Su, J.; Qiu, M. Blood Cell-Based Drug Delivery Systems: A Biomimetic Platform for Antibacterial Therapy. Eur. J. Pharm. Biopharm. 2022, 177, 273–288. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Y.; Wen, Y.; Zou, Y.; Nie, K.; Liu, Z.; Li, X.; Zou, H.; Wang, Y. Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles. Pharmaceutics 2025, 17, 481. [Google Scholar] [CrossRef]
- Li, Y.T.; Nishikawa, T.; Kaneda, Y. Platelet-Cytokine Complex Suppresses Tumour Growth by Exploiting Intratumoural Thrombin-Dependent Platelet Aggregation. Sci. Rep. 2016, 6, 25077. [Google Scholar] [CrossRef]
- Hu, Y.L.; Fu, Y.H.; Tabata, Y.; Gao, J.Q. Mesenchymal Stem Cells: A Promising Targeted-Delivery Vehicle in Cancer Gene Therapy. J. Control. Release 2010, 147, 154–162. [Google Scholar] [CrossRef]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022, 28, 2124–2132, Erratum in Nat. Med. 2023, 29, 2956. https://doi.org/10.1038/s41591-022-02091-9. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Van Morris, K.; Vo, H.H.; Eck, S.; Lin, Y.F.; Rivas, J.M.; Andersson, B.S. T-Cell Receptor-Based Therapy: An Innovative Therapeutic Approach for Solid Tumors. J. Hematol. Oncol. 2021, 14, 102. [Google Scholar] [CrossRef]
- Chagnon, F.; Thompson-Snipes, L.; Elhilali, M.; Tanguay, S. Murine Renal Cell Carcinoma: Evaluation of a Dendritic-Cell Tumour Vaccine. BJU Int. 2001, 88, 418–424. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-Macrophage: An Extensive Immune Enhancer to Fight Cancer. EBioMedicine 2022, 76, 103873. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
| Type | Key Characteristics | Therapeutic Applications | Challenges | Examples of In Vivo/In Vitro Studies |
|---|---|---|---|---|
| Erythrocytes | Long circulation time, biocompatible | Cancer, infections, autoimmune diseases | Fast leakage of specific drugs, large size | Targeting and depletion of circulating leukocytes and cancer cells by Rituximab-loaded erythrocytes [139] |
| Platelets | Selectively adhere to tumor tissues, expresses CD47 | Cancer, infectious diseases, gene therapy | Risk of thrombosis, tumor growth, sensitive to environment | Doxorubicin-loaded platelets enhanced the antitumor activity of DOX by regulating the expression of apoptosis-related genes and successfully reduced the growth of the lymphoma Raji cells in BALB/c nude mice [127] |
| Neutrophils | Rapid response toward inflammation, phagocytosis | Glioma therapy, brain inflammation | Short lifespan, fast intracellular degradation | Treatment with neutrophil-carrying liposomes that contain paclitaxel helped to significantly inhibit tumor recurrence in surgically treated glioma mouse models [130] |
| Macrophages | Phagocytosis, ability to cross biological barriers | Tumor immunotherapy, BBB delivery | Limited payload, interactions with non-target tissues, heterogeneity | HIV-1 suppression in mice was achieved by using bone marrow-derived macrophages loaded with indinavir-encapsulated nanoparticles [140] |
| Sperm Cells | Active motion, high drug encapsulation capability | Cancer, reproductive medicine | Ethical issues, motility varies between individuals, risk, accumulation in undesired tissues | Cervical cancer HeLa cells were successfully treated by doxorubicin hydrochloride encapsulated in human sperm [141] |
| Membrane-Coated NPs | Mimic surface properties of the parent cell, immune escape | Cancer, infection, cardiovascular disorders | Coating efficiency, targeting capability | Resveratrol nanoparticles coated with macrophage membrane effectively targeted damaged myocardial sites, improved cardiac function, and reduced infarct size in MI mice [142] |
| Parameter | CB-DDS | Liposomes | Polymer NPs |
|---|---|---|---|
| Typical sizes | Ranging from 2 to 50 μm | 50 to 500 nm [153] | 10 to 1000 nm [154] |
| Targeting mechanism | Active | Passive/active (depends on the presence of a ligand) [153] | Passive/Active (depends on the presence of a ligand) [155] |
| Circulation | Hours–months (depends on the type of cell: red blood cells, macrophages, etc.) | Hours–days (require PEGylation modification) [156] | Minutes–hours [157] |
| Toxicity | Low | Low [156] | Moderate (depends on the polymer type; immune reactions and accumulation might happen) [151] |
| Advantages |
|
|
|
| Challenges |
|
|
|
| Cell Type | Type of Bioactive Cargo | Application | ClinicalTrials.Gov Identifier and Phase No. | Phase No. | Status |
|---|---|---|---|---|---|
| Red blood cells | L-Asparaginase | Pancreatic cancer | NCT02195180 | Phase II | Completed |
| NCT03665441 | Phase III | Completed | |||
| Leukemia | NCT01518517 | Phase II/III | Completed | ||
| NCT00723346 | Phase I/II | Completed | |||
| NCT01523782 | Phase II | Completed | |||
| Leukemia (for individuals with ALL and hypersensitivity to PEG-asparaginase) | NCT03267030 | Phase II | Completed | ||
| Dexamethasone 21-phosphate | Steroid-dependent ulcerative colitis | NCT01171807 | Phase II | Completed | |
| Dexamethasone | Steroid-dependent Crohn’s disease | NCT01277289 | Phase III | Completed | |
| Dexamethasone sodium phosphate | Ataxia telangiectasia | NCT01255358 | Phase II | Completed | |
| NCT03563053 | N/A | Terminated by sponsor | |||
| L-asparaginase | Leukemia | NCT01518517 | Phase II/III | Completed | |
| Macrophages | Anti-HER2 CAR-M | HER2-positive adenocarcinoma | NCT04660929 | Phase I | Active, not recruiting |
| Monocytes | CMV pp65-LAMP mRNA | Glioblastoma | NCT04741984 | Phase I | Withdrawn |
| Neutrophils | Albumin-bound paclitaxel | Breast cancer | NCT06496724 | N/A | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergazy, S.; Berikkhanova, K.; Gulyayev, A.; Shulgau, Z.; Maikenova, A.; Bilal, R.; Terzic, M.; Zhumadilov, Z.; Aljofan, M. Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 8143. https://doi.org/10.3390/ijms26178143
Sergazy S, Berikkhanova K, Gulyayev A, Shulgau Z, Maikenova A, Bilal R, Terzic M, Zhumadilov Z, Aljofan M. Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(17):8143. https://doi.org/10.3390/ijms26178143
Chicago/Turabian StyleSergazy, Shynggys, Kulzhan Berikkhanova, Alexandr Gulyayev, Zarina Shulgau, Assiya Maikenova, Ruslan Bilal, Milan Terzic, Zhaxybay Zhumadilov, and Mohamad Aljofan. 2025. "Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy" International Journal of Molecular Sciences 26, no. 17: 8143. https://doi.org/10.3390/ijms26178143
APA StyleSergazy, S., Berikkhanova, K., Gulyayev, A., Shulgau, Z., Maikenova, A., Bilal, R., Terzic, M., Zhumadilov, Z., & Aljofan, M. (2025). Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy. International Journal of Molecular Sciences, 26(17), 8143. https://doi.org/10.3390/ijms26178143






