Claudin-1 Contributes to Gastrointestinal Stromal Tumors (GIST) Resistance to Imatinib Mesylate (IM) via Regulation of FGFR-Signaling
Abstract
1. Introduction
2. Results
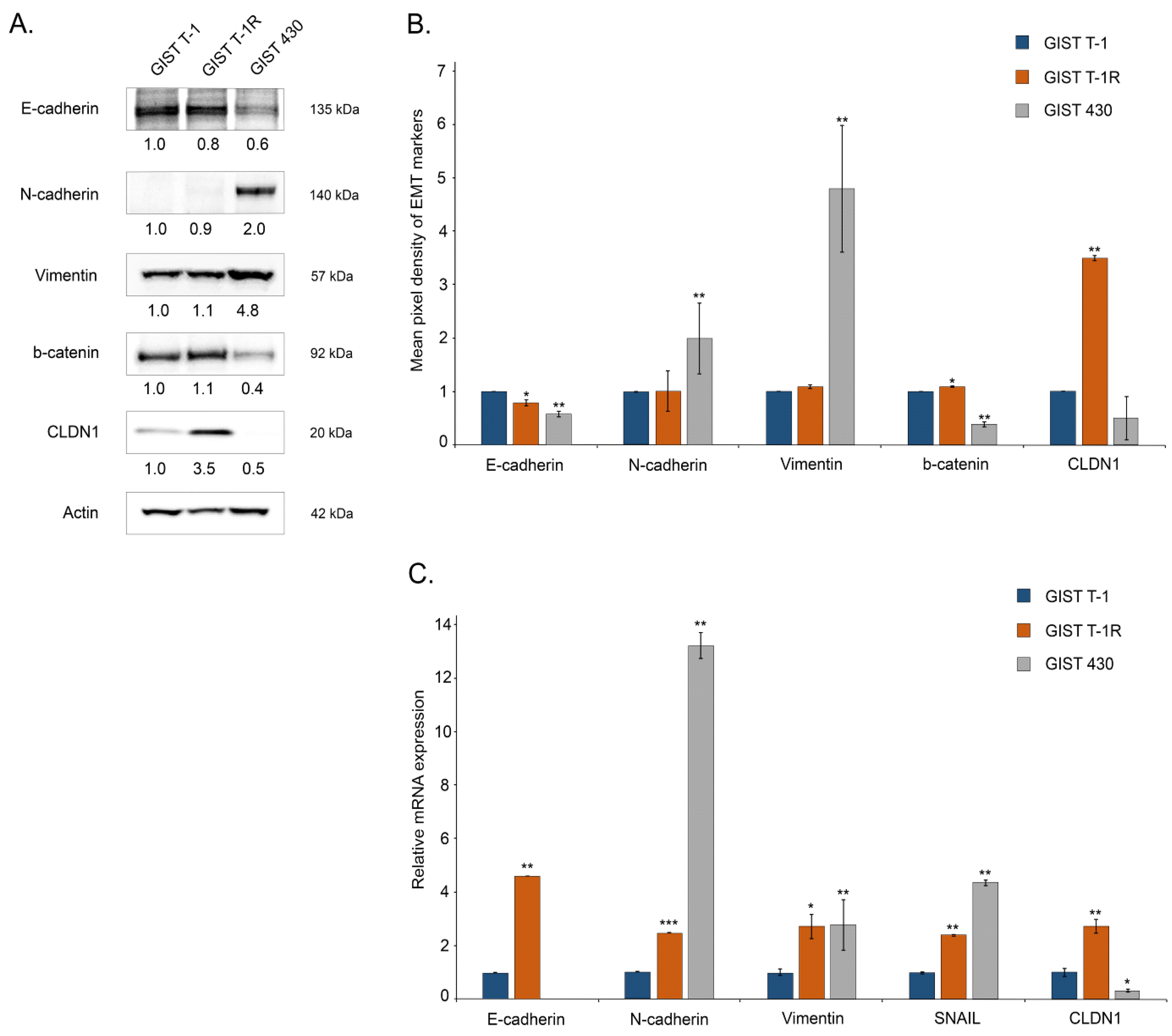
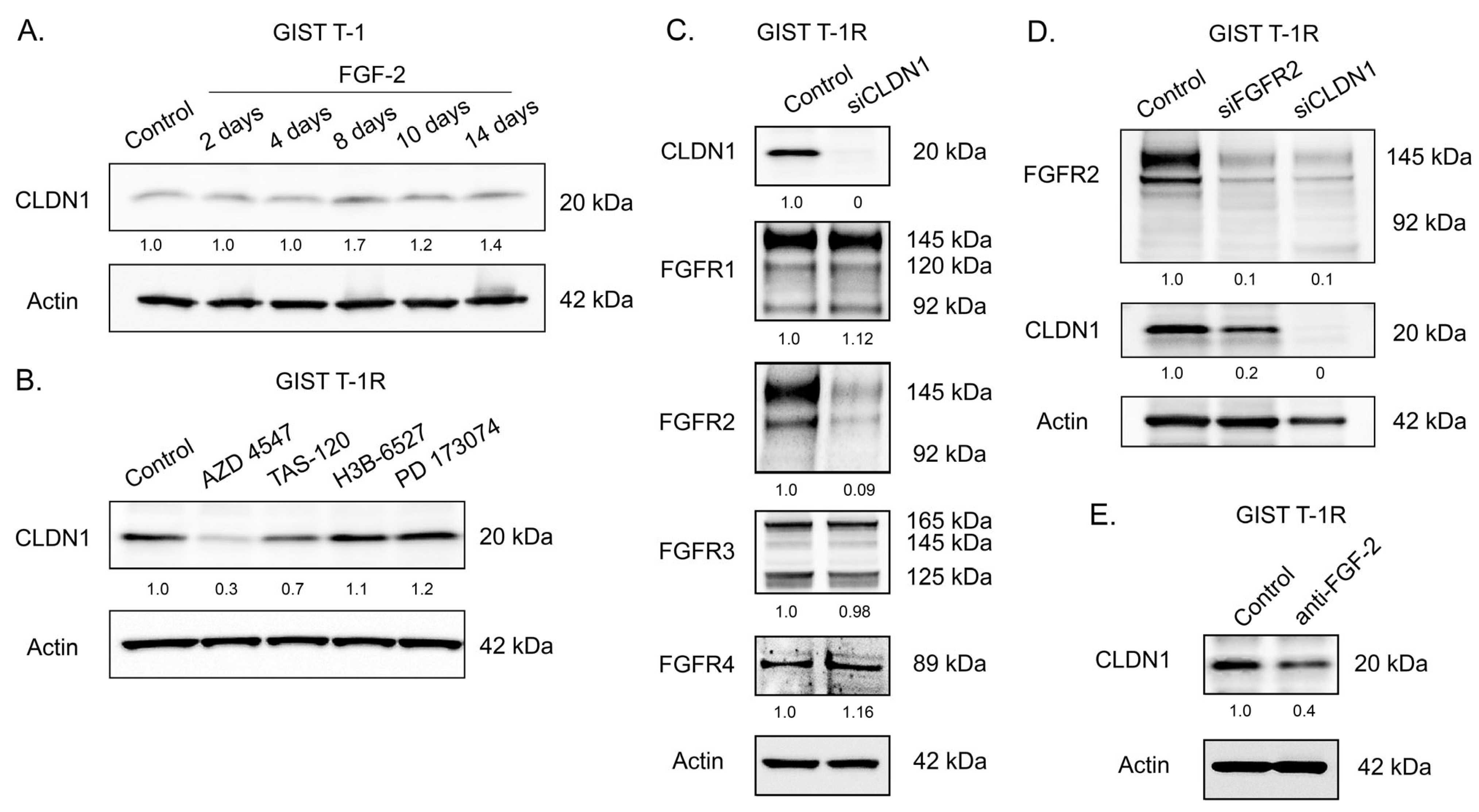
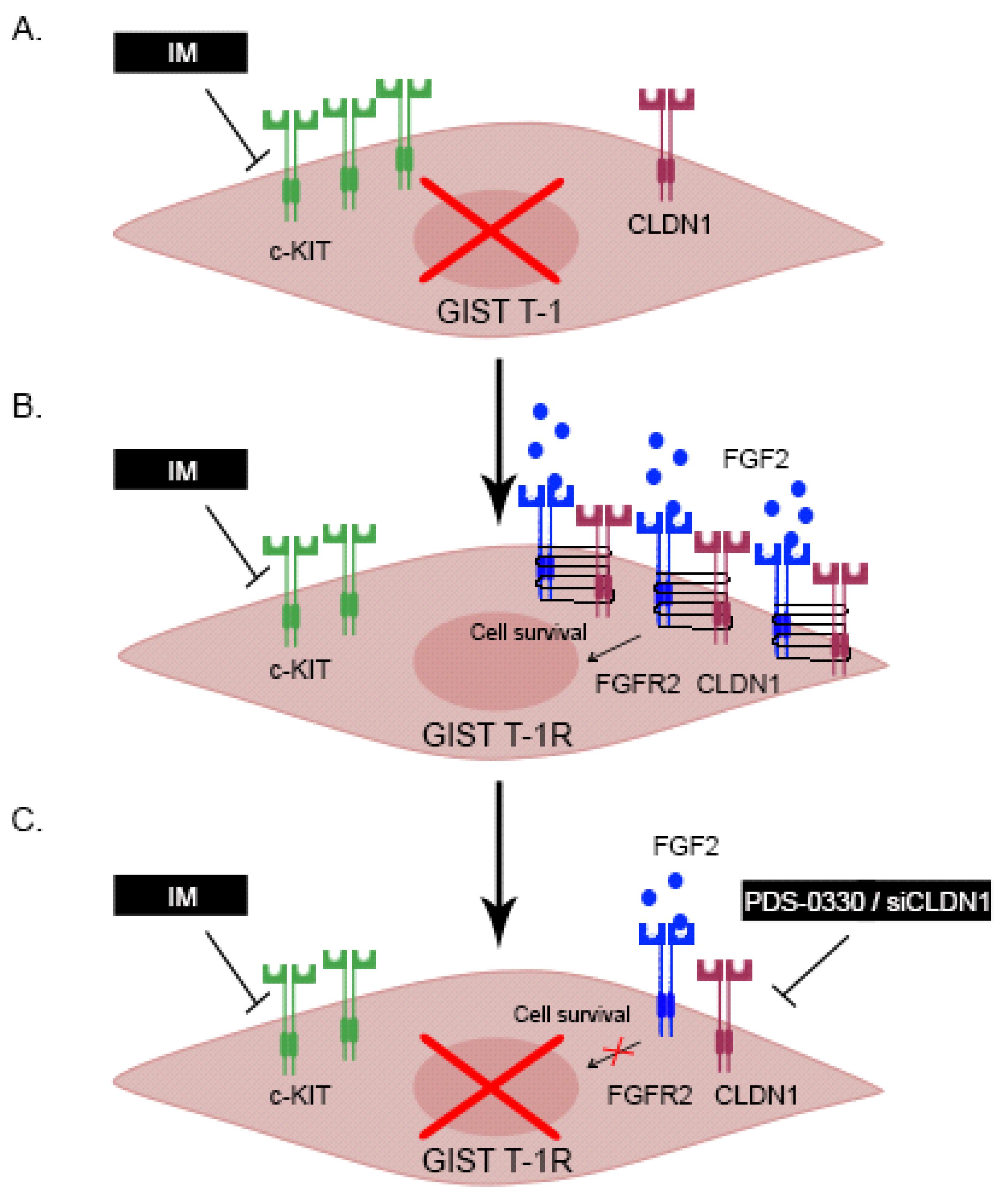
2.1. An Increased Expression of CLDN1 in GIST T-1 Cells Contributes to IM’s Resistance
2.2. Functional Cross-Talk Between CLDN1 and FGFR-Signaling in GIST
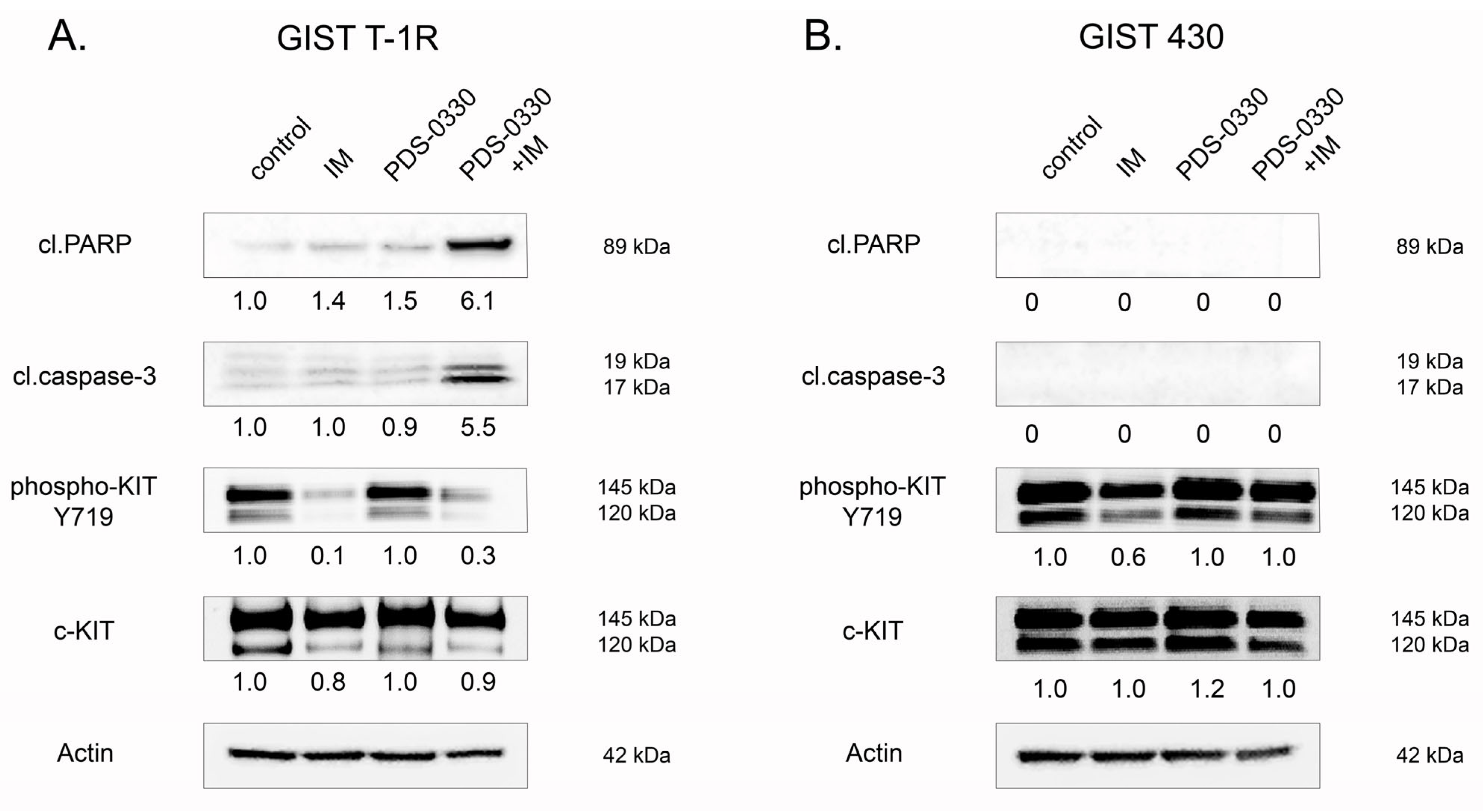
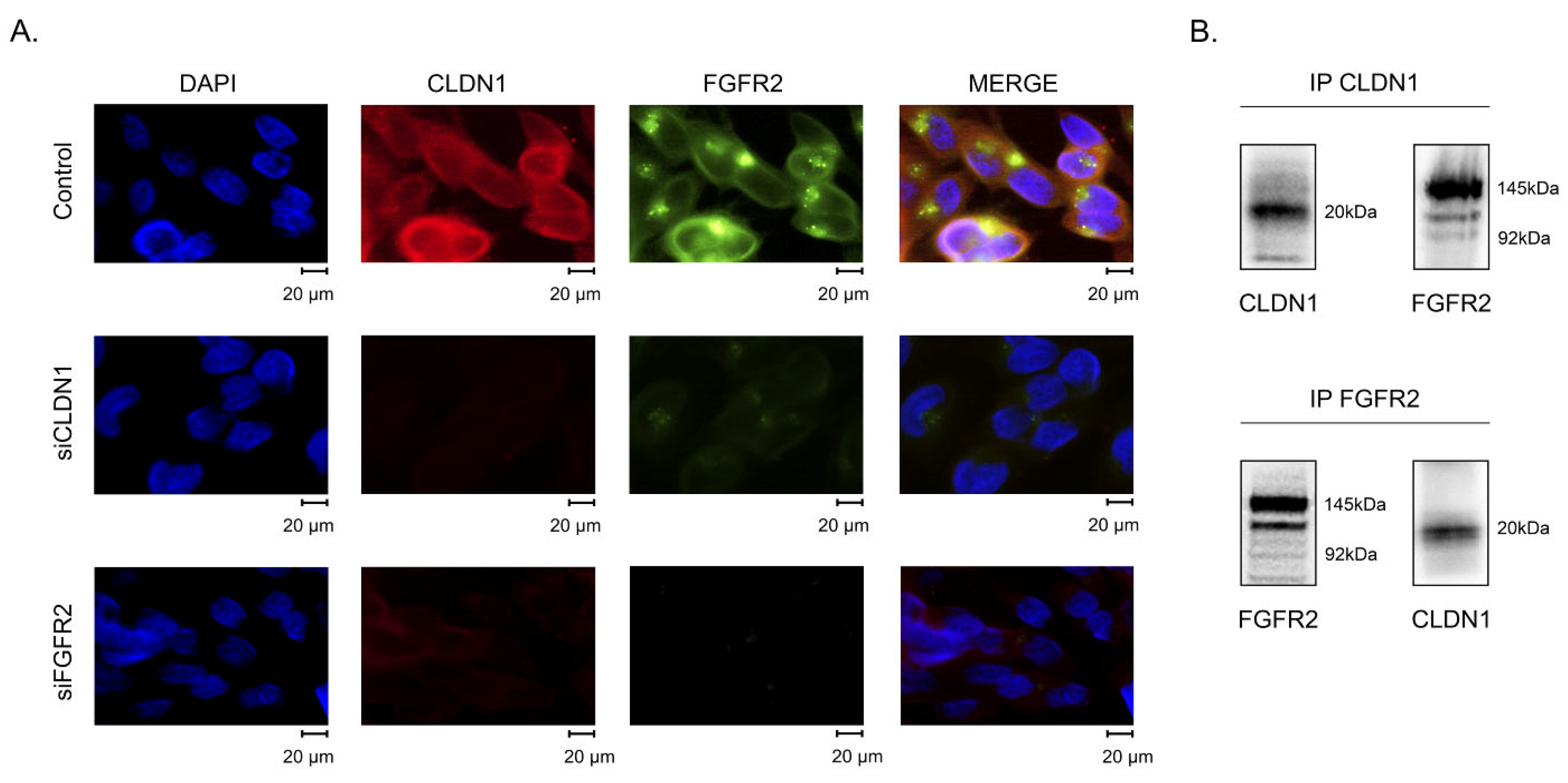
2.3. CLDN1 Interacts with FGFR2 and Regulates FGFR-Signaling in IM-Resistant GIST
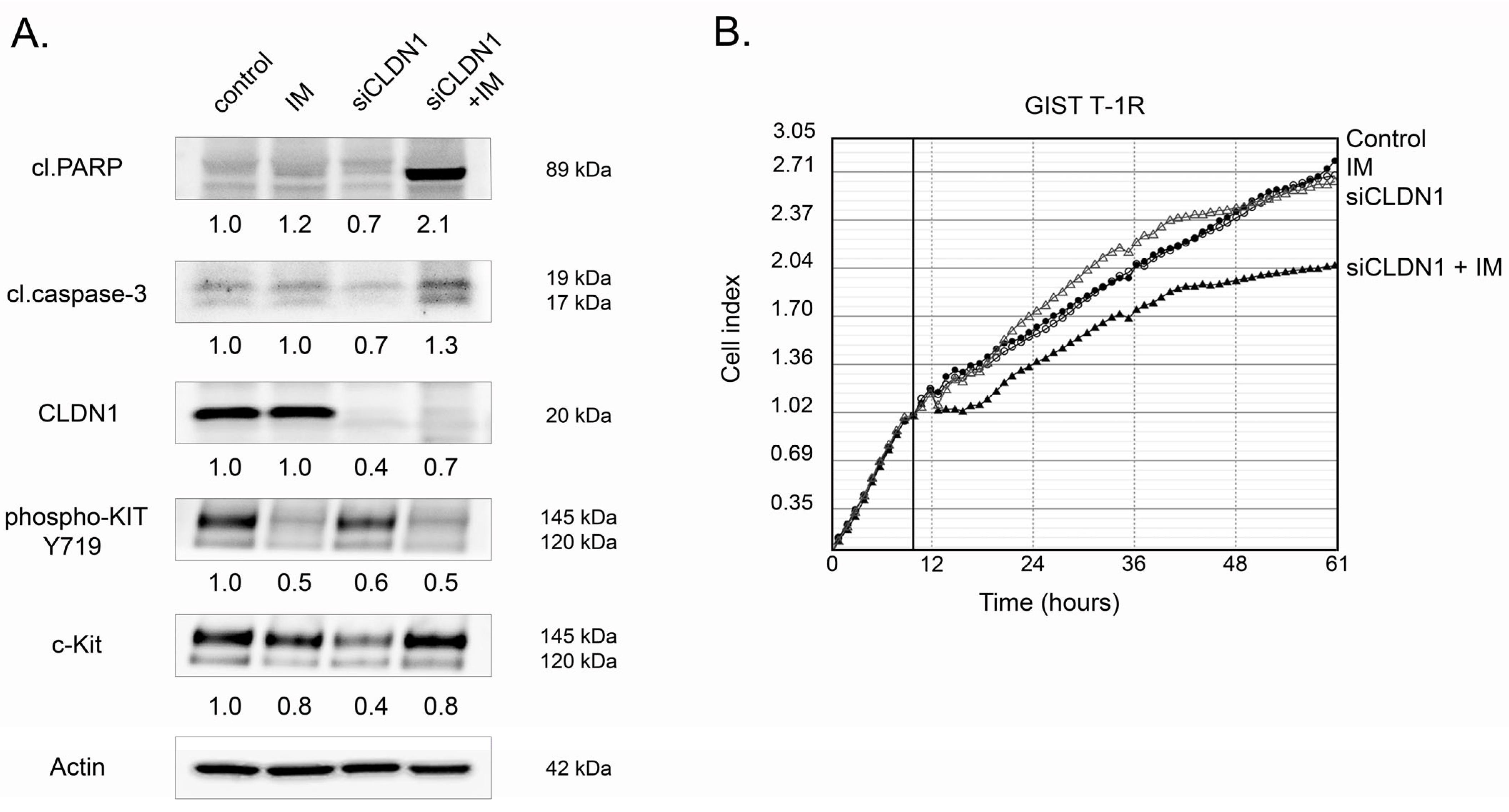
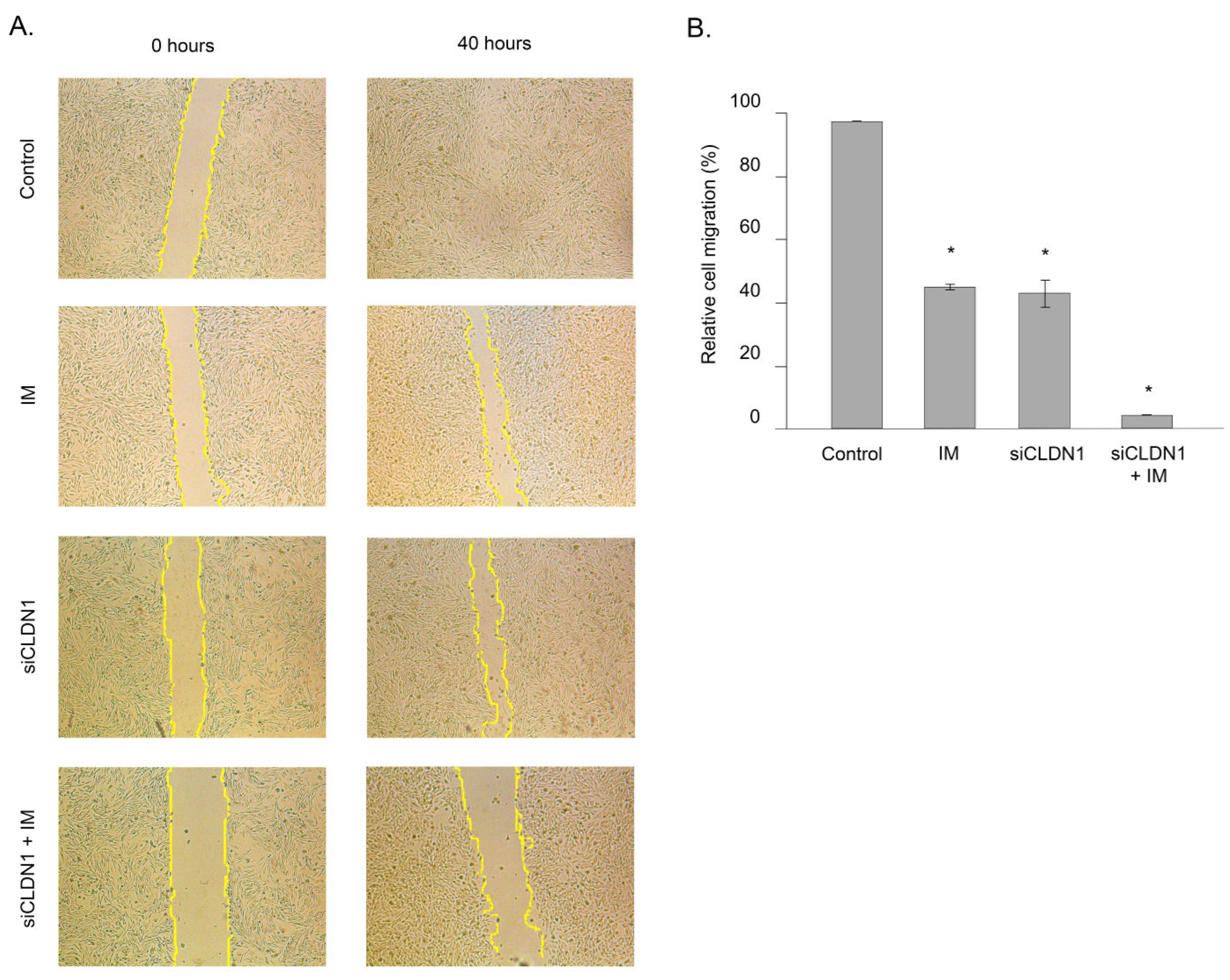
2.4. CLDN1 Regulates the Motility of IM-Resistant GIST
2.5. Subcellular Distribution of CLDN1 in IM-Resistant GIST
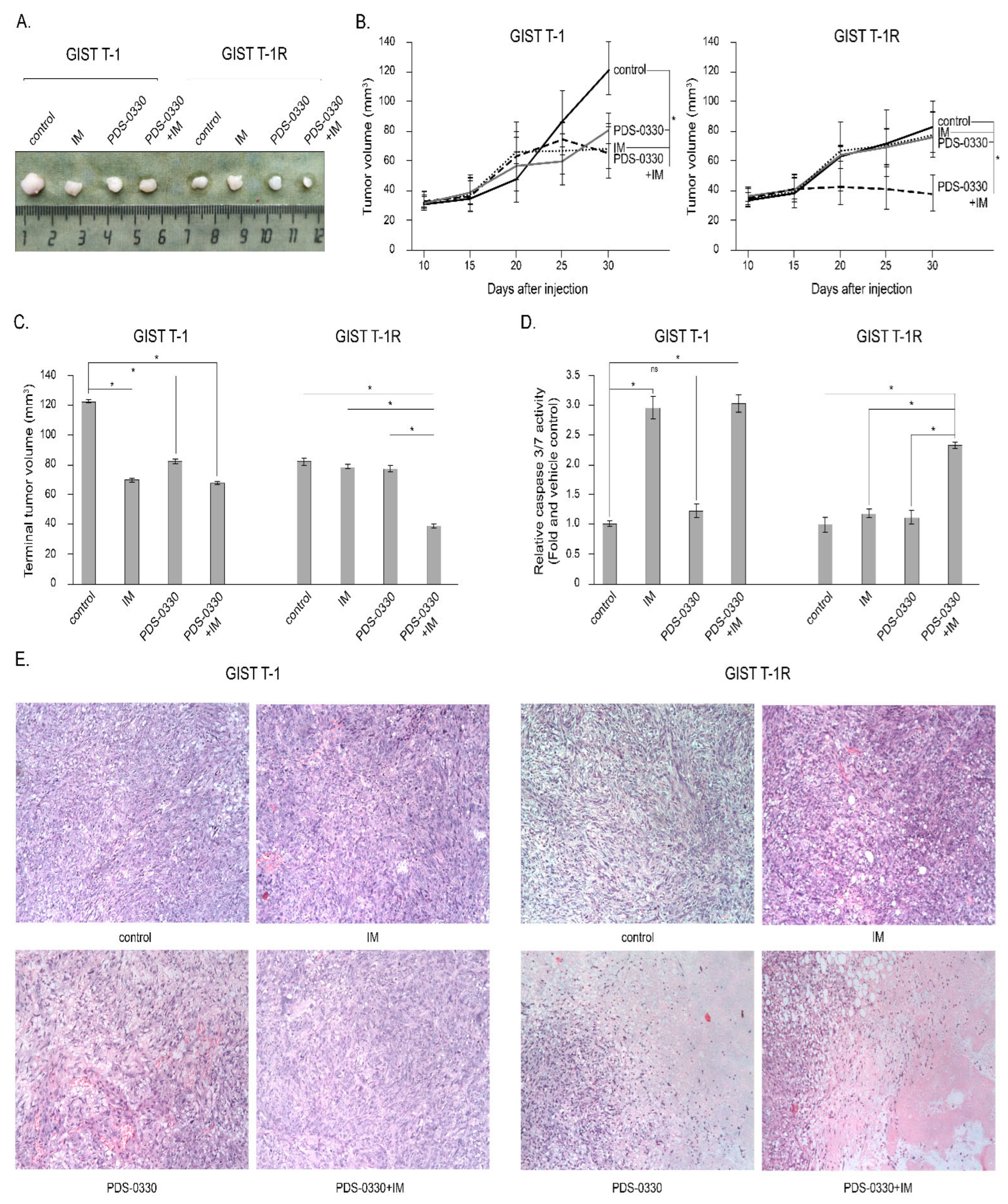
2.6. Targeting of CLDN1 In Vivo Inhibits the Growth of KIT-Inhibited IM-Resistant Xenografts
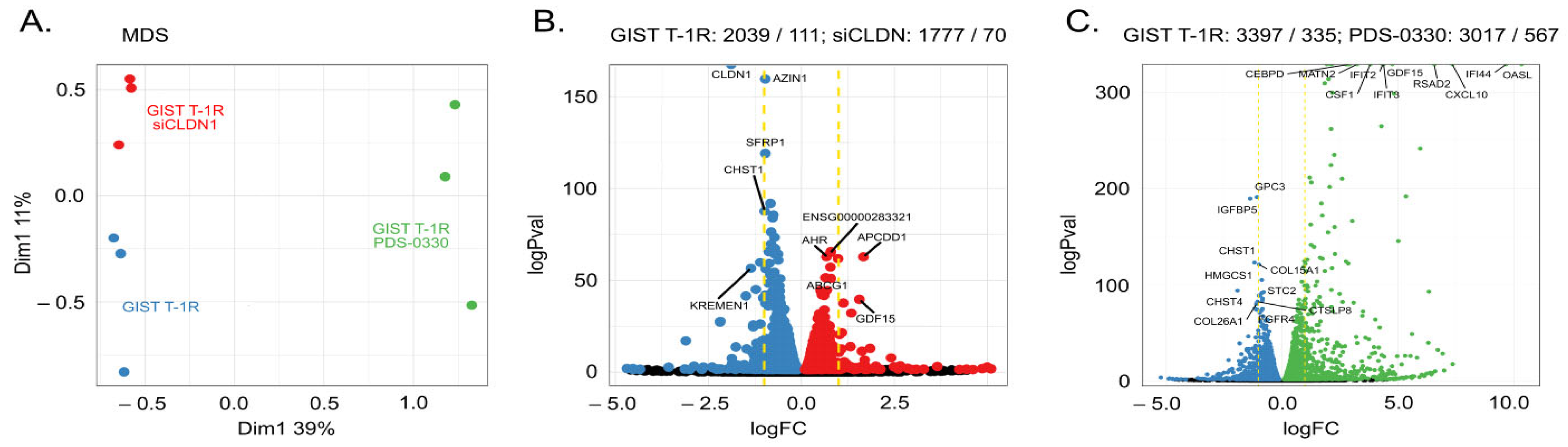
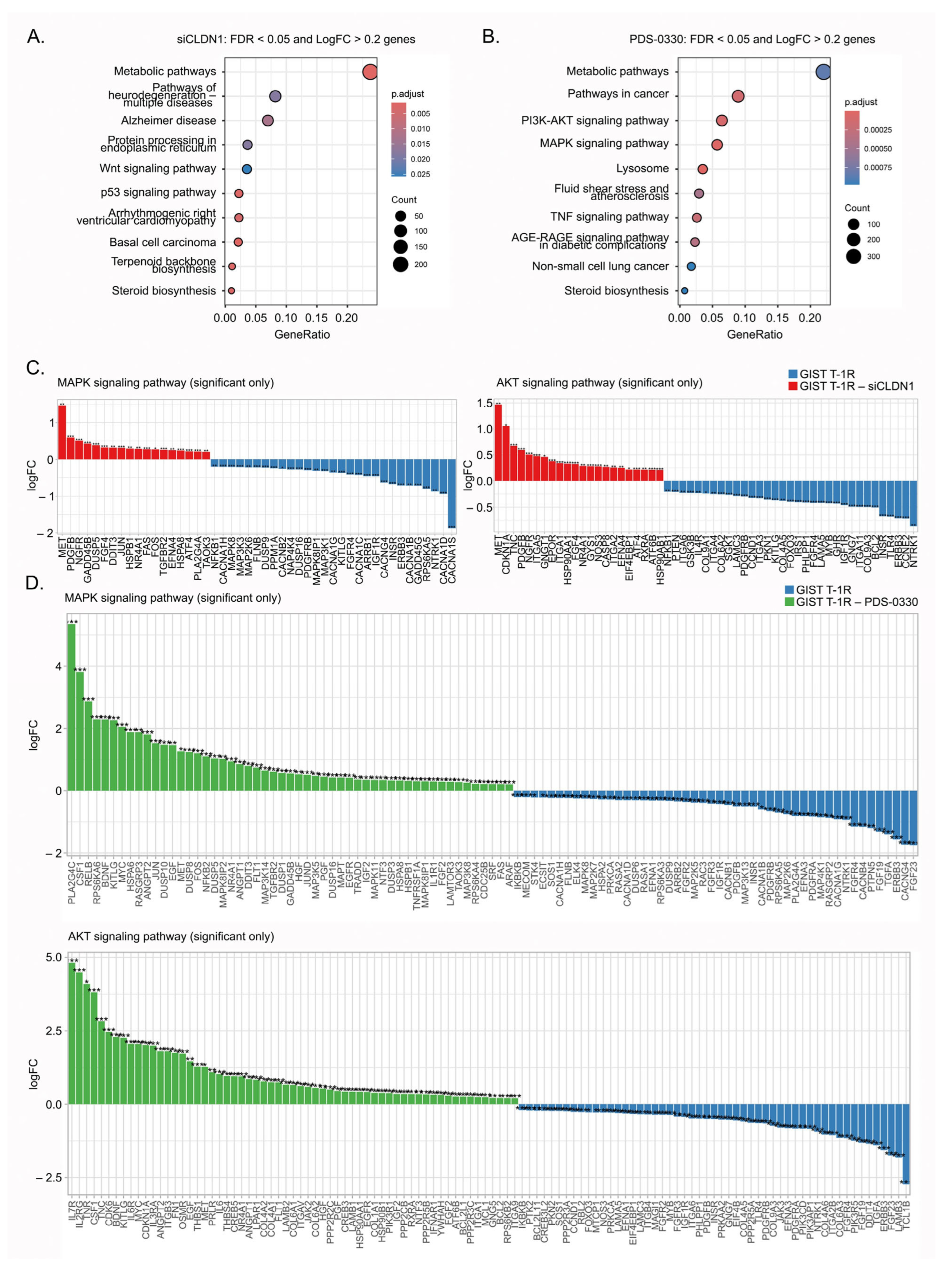
2.7. Molecular Profiling of IM-Resistant GIST Cells with Inhibited CLDN1 Signaling
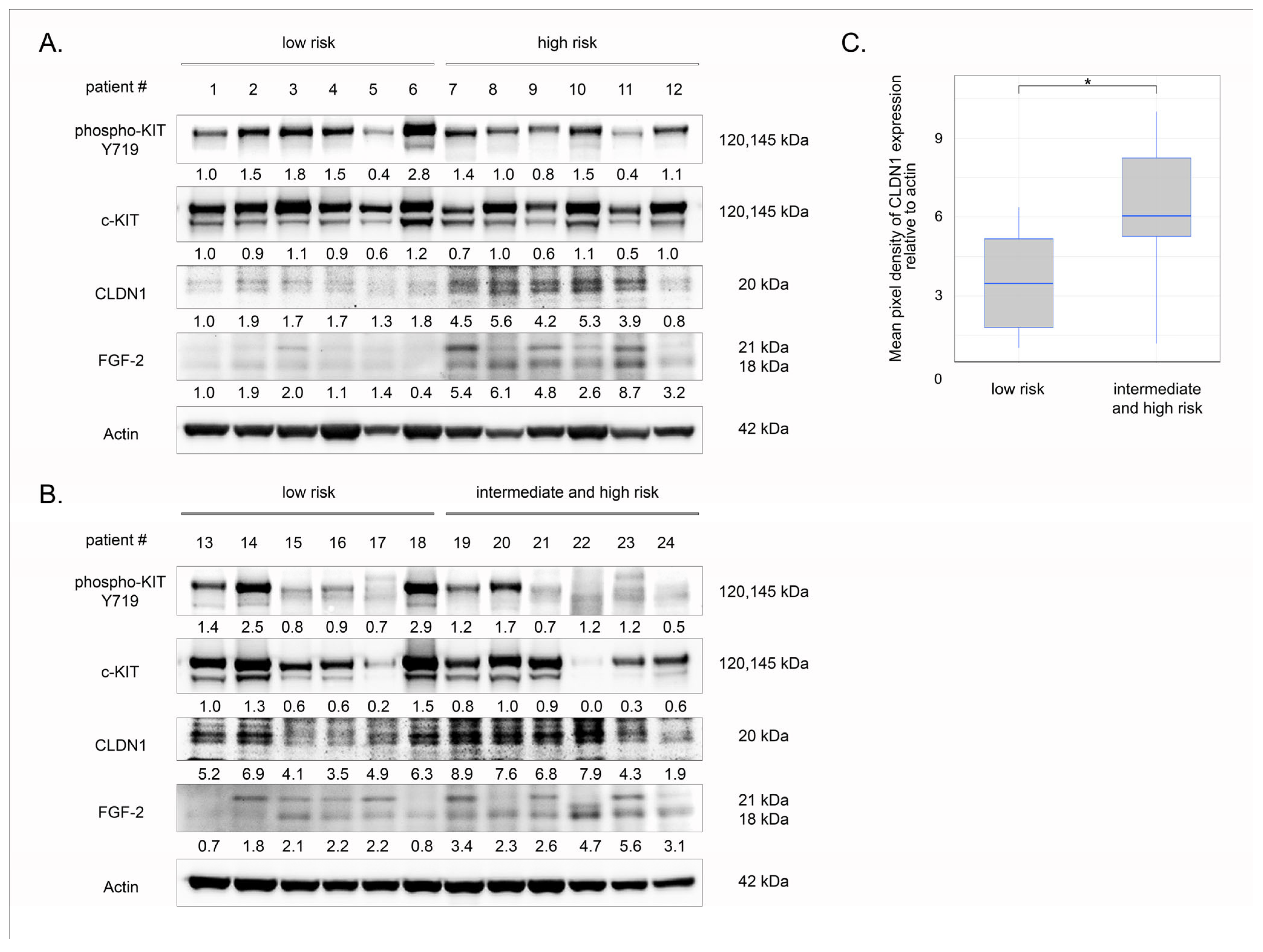
2.8. Clinical Significance of CLDN1 in Primary GIST
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Antibodies
4.3. Cell Lines and Culture Conditions
4.4. Cellular Survival MTS-Based Assay
4.5. Real-Time Monitoring of Cell Proliferation
4.6. Crystal Violet Staining
4.7. Subcellular Fractionation
4.8. siRNA-Mediated Knockdown of CLDN1 and FGFR2
4.9. Western Blotting
4.10. Co-Immunoprecipitation (Co-IP)
4.11. Immunofluorescence Staining
4.12. RNA Extraction and Real-Time Quantitative PCR
4.13. RNA-Seq Library Preparation, Sequencing, and Bioinformatics Pipeline
4.14. GIST Xenograft Studies
4.15. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Rubin, B.P.; Singer, S.; Tsao, C.; Duensing, A.; Lux, M.L.; Ruiz, R.; Hibbard, M.K.; Chen, C.-J.; Xiao, S.; Tuveson, D.A. KIT Activation Is a Ubiquitous Feature of Gastrointestinal Stromal Tumors. Cancer Res. 2001, 61, 8118–8121. [Google Scholar]
- Heinrich, M.C.; Corless, C.L.; Duensing, A.; McGreevey, L.; Chen, C.-J.; Joseph, N.; Singer, S.; Griffith, D.J.; Haley, A.; Town, A. PDGFRA Activating Mutations in Gastrointestinal Stromal Tumors. Science 2003, 299, 708–710. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M. Gain-of-Function Mutations of c-Kit in Human Gastrointestinal Stromal Tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Gronchi, A.; Blay, J.; Trent, J.C. The Role of High-dose Imatinib in the Management of Patients with Gastrointestinal Stromal Tumor. Cancer 2010, 116, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Gutierrez Sainz, L.; Chi, P. The Management of Metastatic GIST: Current Standard and Investigational Therapeutics. J. Hematol. Oncol. 2021, 14, 2. [Google Scholar] [CrossRef]
- Verweij, J.; van Oosterom, A.; Blay, J.-Y.; Judson, I.; Rodenhuis, S.; van der Graaf, W.; Radford, J.; Le Cesne, A.; Hogendoorn, P.C.W.; Di Paola, E.D. Imatinib Mesylate (STI-571 Glivec®, GleevecTM) Is an Active Agent for Gastrointestinal Stromal Tumours, but Does Not Yield Responses in Other Soft-Tissue Sarcomas That Are Unselected for a Molecular Target: Results from an EORTC Soft Tissue and Bone Sarcom. Eur. J. Cancer 2003, 39, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Heinrich, M.C.; George, S.; Zalcberg, J.R.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Serrano, C.; Jones, R.L.; Attia, S. 1540P Ripretinib as ≥4th-Line Treatment in Patients with Advanced Gastrointestinal Stromal Tumor: Long-Term Update from the Phase III INVICTUS Study. Ann. Oncol. 2021, 32, S1120–S1121. [Google Scholar] [CrossRef]
- Smith, B.D.; Kaufman, M.D.; Lu, W.-P.; Gupta, A.; Leary, C.B.; Wise, S.C.; Rutkoski, T.J.; Ahn, Y.M.; Al-Ani, G.; Bulfer, S.L. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738–751. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Zhang, X.; Jones, R.L.; George, S.; Serrano, C.; Deng, Y.; Bauer, S.; Cai, S.; Wu, X.; Zhou, Y. Clinical Benefit of Avapritinib in KIT-Mutant Gastrointestinal Stromal Tumors: A Post Hoc Analysis of the Phase I NAVIGATOR and Phase I/II CS3007–001 Studies. Clin. Cancer Res. 2024, 30, 719–728. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Bauer, S.; Kang, Y.-K.; Schoffski, P.; Eskens, F.; Mir, O.; Cassier, P.A.; Serrano, C. Clinical Activity of Avapritinib in ≥ Fourth-Line (4L+) and PDGFRA Exon 18 Gastrointestinal Stromal Tumors (GIST). J. Clin. Oncol. 2019, 37, 11022. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.-K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D. Avapritinib in Advanced PDGFRA D842V-Mutant Gastrointestinal Stromal Tumour (NAVIGATOR): A Multicentre, Open-Label, Phase 1 Trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Galembikova, A.; Dunaev, P.; Valeeva, E.; Shagimardanova, E.; Gusev, O.; Khaiboullina, S. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules 2017, 22, 2152. [Google Scholar] [CrossRef]
- Mahadevan, D.; Cooke, L.; Riley, C.; Swart, R.; Simons, B.; Della Croce, K.; Wisner, L.; Iorio, M.; Shakalya, K.; Garewal, H. A Novel Tyrosine Kinase Switch Is a Mechanism of Imatinib Resistance in Gastrointestinal Stromal Tumors. Oncogene 2007, 26, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huynh, H.; Li, X.; Ruddy, D.A.; Wang, Y.; Ong, R.; Chow, P.; Qiu, S.; Tam, A.; Rakiec, D.P. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov. 2015, 5, 438–451. [Google Scholar] [CrossRef]
- Tarn, C.; Rink, L.; Merkel, E.; Flieder, D.; Pathak, H.; Koumbi, D.; Testa, J.R.; Eisenberg, B.; von Mehren, M.; Godwin, A.K. Insulin-like Growth Factor 1 Receptor Is a Potential Therapeutic Target for Gastrointestinal Stromal Tumors. Proc. Natl. Acad. Sci. USA 2008, 105, 8387–8392. [Google Scholar] [CrossRef]
- Javidi-Sharifi, N.; Traer, E.; Martinez, J.; Gupta, A.; Taguchi, T.; Dunlap, J.; Heinrich, M.C.; Corless, C.L.; Rubin, B.P.; Druker, B.J. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res. 2015, 75, 880–891. [Google Scholar] [CrossRef]
- Sakurama, K.; Noma, K.; Takaoka, M.; Tomono, Y.; Watanabe, N.; Hatakeyama, S.; Ohmori, O.; Hirota, S.; Motoki, T.; Shirakawa, Y. Inhibition of Focal Adhesion Kinase as a Potential Therapeutic Strategy for Imatinib-Resistant Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2009, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Dunaev, P.; Mustafin, I.; Mani, S.; Syuzov, K.; Valeeva, E.; Bikinieva, F.; Galembikova, A. Infigratinib (BGJ 398), a Pan-FGFR Inhibitor, Targets P-Glycoprotein and Increases Chemotherapeutic-Induced Mortality of Multidrug-Resistant Tumor Cells. Biomedicines 2022, 10, 601. [Google Scholar] [CrossRef]
- Beretta, G.L.; Cassinelli, G.; Pennati, M.; Zuco, V.; Gatti, L. Overcoming ABC Transporter-Mediated Multidrug Resistance: The Dual Role of Tyrosine Kinase Inhibitors as Multitargeting Agents. Eur. J. Med. Chem. 2017, 142, 271–289. [Google Scholar] [CrossRef]
- Anreddy, N.; Gupta, P.; Kathawala, R.J.; Patel, A.; Wurpel, J.N.D.; Chen, Z.-S. Tyrosine Kinase Inhibitors as Reversal Agents for ABC Transporter Mediated Drug Resistance. Molecules 2014, 19, 13848–13877. [Google Scholar] [CrossRef]
- Rastogi, I.; Rajanna, S.; Webb, A.; Chhabra, G.; Foster, B.; Webb, B.; Puri, N. Mechanism of C-Met and EGFR Tyrosine Kinase Inhibitor Resistance through Epithelial Mesenchymal Transition in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2016, 477, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Takeuchi, S.; Arai, S.; Katayama, R.; Nanjo, S.; Tanimoto, A.; Nishiyama, A.; Nakagawa, T.; Taniguchi, H.; Suzuki, T. Epithelial-to-Mesenchymal Transition Is a Mechanism of ALK Inhibitor Resistance in Lung Cancer Independent of ALK Mutation Status. Cancer Res. 2019, 79, 1658–1670. [Google Scholar] [CrossRef]
- Clement, M.S.; Gammelgaard, K.R.; Nielsen, A.L.; Sorensen, B.S. Epithelial-to-Mesenchymal Transition Is a Resistance Mechanism to Sequential MET-TKI Treatment of MET-Amplified EGFR-TKI Resistant Non-Small Cell Lung Cancer Cells. Transl. Lung Cancer Res. 2020, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.R.; Demuth, C.; Madsen, A.T.; Hussmann, D.; Vad-Nielsen, J.; Nielsen, A.L.; Sorensen, B.S. MET Amplification and Epithelial-to-Mesenchymal Transition Exist as Parallel Resistance Mechanisms in Erlotinib-Resistant, EGFR-Mutated, NSCLC HCC827 Cells. Oncogenesis 2017, 6, e307. [Google Scholar] [CrossRef]
- Thomson, S.; Buck, E.; Petti, F.; Griffin, G.; Brown, E.; Ramnarine, N.; Iwata, K.K.; Gibson, N.; Haley, J.D. Epithelial to Mesenchymal Transition Is a Determinant of Sensitivity of Non–Small-Cell Lung Carcinoma Cell Lines and Xenografts to Epidermal Growth Factor Receptor Inhibition. Cancer Res. 2005, 65, 9455–9462. [Google Scholar] [CrossRef]
- Dela Cruz, M.C.P.; Medina, P.M.B. Epithelial-Mesenchymal Transition (EMT) and Its Role in Acquired Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor (EGFR-TKI) Chemoresistance in Non-Small Cell Lung Cancer (NSCLC). Cancer Pathog. Ther. 2024, 2, E001–E051. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, L.; Niu, X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gowrikumar, S.; Primeaux, M.; Pravoverov, K.; Wu, C.; Szeglin, B.C.; Sauve, C.-E.G.; Thapa, I.; Bastola, D.; Chen, X.S.; Smith, J.J. A Claudin-Based Molecular Signature Identifies High-Risk, Chemoresistant Colorectal Cancer Patients. Cells 2021, 10, 2211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, J.; Jiang, Y.; Xu, W.; Li, X.; Jing, W. CLDN1 Increases Drug Resistance of Non-Small Cell Lung Cancer by Activating Autophagy via up-Regulation of ULK1 Phosphorylation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 2906. [Google Scholar] [CrossRef]
- Akizuki, R.; Maruhashi, R.; Eguchi, H.; Kitabatake, K.; Tsukimoto, M.; Furuta, T.; Matsunaga, T.; Endo, S.; Ikari, A. Decrease in Paracellular Permeability and Chemosensitivity to Doxorubicin by Claudin-1 in Spheroid Culture Models of Human Lung Adenocarcinoma A549 Cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 769–780. [Google Scholar] [CrossRef]
- Primeaux, M.; Liu, X.; Gowrikumar, S.; Fatima, I.; Fisher, K.W.; Bastola, D.; Vecchio, A.J.; Singh, A.B.; Dhawan, P. Claudin-1 Interacts with EPHA2 to Promote Cancer Stemness and Chemoresistance in Colorectal Cancer. Cancer Lett. 2023, 579, 216479. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yin, W.; Ma, H.; Elshoura, I.; Wang, L. Targeting Claudin-3 Suppresses Stem Cell-like Phenotype in Nonsquamous Non-Small-Cell Lung Carcinoma. Lung Cancer Manag. 2019, 8, LMT04. [Google Scholar] [CrossRef]
- Yoshida, H.; Sumi, T.; Zhi, X.; Yasui, T.; Honda, K.-I.; Ishiko, O. Claudin-4: A Potential Therapeutic Target in Chemotherapy-Resistant Ovarian Cancer. Anticancer Res. 2011, 31, 1271–1277. [Google Scholar] [PubMed]
- Casagrande, F.; Cocco, E.; Bellone, S.; Richter, C.E.; Bellone, M.; Todeschini, P.; Siegel, E.; Varughese, J.; Arin-Silasi, D.; Azodi, M. Eradication of Chemotherapy-resistant CD44+ Human Ovarian Cancer Stem Cells in Mice by Intraperitoneal Administration of Clostridium Perfringens Enterotoxin. Cancer 2011, 117, 5519–5528. [Google Scholar] [CrossRef]
- Breed, C.; Hicks, D.A.; Webb, P.G.; Galimanis, C.E.; Bitler, B.G.; Behbakht, K.; Baumgartner, H.K. Ovarian Tumor Cell Expression of Claudin-4 Reduces Apoptotic Response to Paclitaxel. Mol. Cancer Res. 2019, 17, 741–750. [Google Scholar] [CrossRef]
- Shang, X.; Lin, X.; Manorek, G.; Howell, S.B. Claudin-3 and Claudin-4 Regulate Sensitivity to Cisplatin by Controlling Expression of the Copper and Cisplatin Influx Transporter CTR1. Mol. Pharmacol. 2013, 83, 85–94. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Ruan, Y.; Lu, Y.; Lin, D.; Xie, Y.; Dong, B.; Dang, Q.; Quan, C. CLDN6 Enhances Chemoresistance to ADM via AF-6/ERKs Pathway in TNBC Cell Line MDAMB231. Mol. Cell. Biochem. 2018, 443, 169–180. [Google Scholar] [CrossRef]
- Wang, D.-W.; Zhang, W.-H.; Danil, G.; Yang, K.; Hu, J.-K. The Role and Mechanism of Claudins in Cancer. Front. Oncol. 2022, 12, 1051497. [Google Scholar] [CrossRef]
- Hana, C.; Thaw Dar, N.N.; Galo Venegas, M.; Vulfovich, M. Claudins in Cancer: A Current and Future Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 4634. [Google Scholar] [CrossRef]
- Li, J. Targeting Claudins in Cancer: Diagnosis, Prognosis and Therapy. Am. J. Cancer Res. 2021, 11, 3406. [Google Scholar] [PubMed]
- Gyorffy, H. Study of claudins and prognostic factors in some gastrointestinal diseases. Magy. Onkol. 2009, 53, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, D.; Asano, H.; Okumura, R.; Takasawa, K.; Takasawa, A.; Konno, T.; Nakamori, Y.; Magara, K.; Ono, Y.; Imamura, M.; et al. The Role of Claudin-1 in Enhancing Pancreatic Cancer Aggressiveness and Drug Resistance via Metabolic Pathway Modulation. Cancers 2025, 17, 1469. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef]
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 2005, 29, 52–68. [Google Scholar] [CrossRef]
- Rädler, P.D.; Wehde, B.L.; Triplett, A.A.; Shrestha, H.; Shepherd, J.H.; Pfefferle, A.D.; Rui, H.; Cardiff, R.D.; Perou, C.M.; Wagner, K.-U. Highly Metastatic Claudin-Low Mammary Cancers Can Originate from Luminal Epithelial Cells. Nat. Commun. 2021, 12, 3742. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Deng, M.-S.; Liu, Y.-F.; Yao, J.; Xiao, Z.-Y. Tight Junction Protein CLDN17 Serves as a Tumor Suppressor to Reduce the Invasion and Migration of Oral Cancer Cells by Inhibiting Epithelial-Mesenchymal Transition. Arch. Oral Biol. 2022, 133, 105301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W. IRF2-induced Claudin-7 Suppresses Cell Proliferation, Invasion and Migration of Oral Squamous Cell Carcinoma. Exp. Ther. Med. 2022, 23, 7. [Google Scholar] [CrossRef]
- Cho Jin, S.; Jeong Young, B.; Yoon, S.-H.; Park Gyo, C.; Lee Young, H. Rab25 Suppresses Colon Cancer Cell Invasion through Upregulating Claudin-7 Expression. Oncol. Rep. 2024, 51, 26. [Google Scholar] [CrossRef]
- Xu, C.; Ding, Y.; Wang, K.; Hao, M.; Li, H.; Ding, L. Claudin-7 Deficiency Promotes Stemness Properties in Colorectal Cancer through Sox9-Mediated Wnt/β-Catenin Signalling. J. Transl. Med. 2021, 19, 311. [Google Scholar] [CrossRef]
- Lu, Y.; Dang, Q.; Bo, Y.; Su, X.; Wang, L.; Sun, J.; Wei, J.; Quan, C.; Li, Y. The Expression of CLDN6 in Hepatocellular Carcinoma Tissue and the Effects of CLDN6 on Biological Phenotypes of Hepatocellular Carcinoma Cells. J. Cancer 2021, 12, 5454. [Google Scholar] [CrossRef]
- Oku, N.; Sasabe, E.; Ueta, E.; Yamamoto, T.; Osaki, T. Tight Junction Protein Claudin-1 Enhances the Invasive Activity of Oral Squamous Cell Carcinoma Cells by Promoting Cleavage of Laminin-5 Gamma2 Chain via Matrix Metalloproteinase (MMP)-2 and Membrane-Type MMP-1. Cancer Res. 2006, 66, 5251–5257. [Google Scholar] [CrossRef]
- Paquet-Fifield, S.; Koh, S.L.; Cheng, L.; Beyit, L.M.; Shembrey, C.; Mølck, C.; Behrenbruch, C.; Papin, M.; Gironella, M.; Guelfi, S. Tight Junction Protein Claudin-2 Promotes Self-Renewal of Human Colorectal Cancer Stem-like Cells. Cancer Res. 2018, 78, 2925–2938. [Google Scholar] [CrossRef]
- Zhou, B.; Flodby, P.; Luo, J.; Castillo, D.R.; Liu, Y.; Yu, F.-X.; McConnell, A.; Varghese, B.; Li, G.; Chimge, N.-O. Claudin-18–Mediated YAP Activity Regulates Lung Stem and Progenitor Cell Homeostasis and Tumorigenesis. J. Clin. Investig. 2018, 128, 970–984. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, Y.; Yang, X.; Ma, P.; Li, Y.; Wu, Y.; Chen, X.; Deng, X.; Yang, T.; Mao, X.; et al. Claudin-2 Promotes Colorectal Cancer Growth and Metastasis by Suppressing NDRG1 Transcription. Clin. Transl. Med. 2021, 11, e667. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Kinugasa, T.; Wang, L.; Huang, J.; Zhao, J.; Shibaguchi, H.; Kuroki, M.; Tanaka, T.; Yamashita, Y.; Nabeshima, K. Claudin-1 Protein Is a Major Factor Involved in the Tumorigenesis of Colorectal Cancer. Anticancer Res. 2009, 29, 851–857. [Google Scholar]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.-R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 Regulates Cellular Transformation and Metastatic Behavior in Colon Cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Ouban, A. Expression of Claudin-1 in Laryngeal Squamous Cell Carcinomas (LSCCs) and Its Significance. Histol. Histopathol. 2021, 36, 437–446. [Google Scholar]
- Cherradi, S.; Martineau, P.; Gongora, C.; Del Rio, M. Claudin Gene Expression Profiles and Clinical Value in Colorectal Tumors Classified According to Their Molecular Subtype. Cancer Manag. Res. 2019, 11, 1337–1348. [Google Scholar] [CrossRef]
- Miskad, U.A.; Aswidah, A.; Dahlan, H.; Ikram, D.; Cangara, M.H.; Djimahit, T.; Kaelan, C. The Role of Claudin-1 Expression in Follicular and Papillary Thyroid Neoplasm. Asian Pac. J. Cancer Prev. 2022, 23, 4023–4027. [Google Scholar] [CrossRef]
- Zhou, B.; Moodie, A.; Blanchard, A.A.A.; Leygue, E.; Myal, Y. Claudin 1 in Breast Cancer: New Insights. J. Clin. Med. 2015, 4, 1960–1976. [Google Scholar] [CrossRef] [PubMed]
- Ouban, A.; Ameer, O.Z.; Quek, K.J.; Arafah, M.A.; Raddaoui, L. Detection of Increased Expression of Claudin-1 in Triple-Negative Breast Cancer: Analysis and Clinical-Pathological Correlation. Cureus 2023, 15, e36648. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-C.; Fang, C.-Y.; Yang, H.-Y.; Jian, Y.-J.; Wang, S.-C.; Liu, Y.-W. The Correlation of Epithelial-Mesenchymal Transition-Related Gene Expression and the Clinicopathologic Features of Colorectal Cancer Patients in Taiwan. PLoS ONE 2021, 16, e0254000. [Google Scholar] [CrossRef]
- Skálová, H.; Hájková, N.; Majerová, B.; Bártů, M.; Povýšil, C.; Tichá, I. Impact of Chemotherapy on the Expression of Claudins and Cadherins in Invasive Breast Cancer. Exp. Ther. Med. 2019, 18, 3014–3024. [Google Scholar] [CrossRef]
- Singh, P.; Toom, S.; Huang, Y. Anti-Claudin 18.2 Antibody as New Targeted Therapy for Advanced Gastric Cancer. J. Hematol. Oncol. 2017, 10, 105. [Google Scholar] [CrossRef]
- Schuler, M.H.; Zvirbule, Z.; Lordick, F.; Krilova, A.; Helbig, U.; Schulze-Bergkamen, H.; Thuss-Patience, P.C.; von Wichert, G.; Schulmann, K.; Trarbach, T. Safety, Tolerability, and Efficacy of the First-in-Class Antibody IMAB362 Targeting Claudin 18.2 in Patients with Metastatic Gastroesophageal Adenocarcinomas. J. Clin. Oncol. 2013, 31, 4080. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J. Zolbetuximab plus MFOLFOX6 in Patients with CLDN18. 2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Fatima, I.; Uppada, J.P.; Chhonker, Y.S.; Gowrikumar, S.; Barman, S.; Roy, S.; Tolentino, K.T.; Palermo, N.; Natarajan, A.; Beauchamp, D.R.; et al. Identification and Characterization of a First-Generation Inhibitor of Claudin-1 in Colon Cancer Progression and Metastasis. Biomed. Pharmacother. 2023, 159, 114255. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Calvo-Jiménez, E.; Cossard, A.; Na, Y.; Cooper, J.A.; Jossin, Y. N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration. eLife 2019, 8, e47673. [Google Scholar] [CrossRef] [PubMed]
- Bernemann, C.; Hülsewig, C.; Ruckert, C.; Schäfer, S.; Blümel, L.; Hempel, G.; Götte, M.; Greve, B.; Barth, P.J.; Kiesel, L.; et al. Influence of Secreted Frizzled Receptor Protein 1 (SFRP1) on Neoadjuvant Chemotherapy in Triple Negative Breast Cancer Does Not Rely on WNT Signaling. Mol. Cancer 2014, 13, 174. [Google Scholar] [CrossRef]
- Ren, J.; Wang, R.; Song, H.; Huang, G.; Chen, L. Secreted Frizzled Related Protein 1 Modulates Taxane Resistance of Human Lung Adenocarcinoma. Mol. Med. 2014, 20, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Menyhárt, O.; Fekete, J.T.; Győrffy, B. Gene Expression Indicates Altered Immune Modulation and Signaling Pathway Activation in Ovarian Cancer Patients Resistant to Topotecan. Int. J. Mol. Sci. 2019, 20, 2750. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, X.; Ren, Y.; Jia, L.; Zou, S.; Han, J.; Zhao, M.; Han, M.; Li, H.; Hua, Q.; et al. Targeting HDAC/OAZ1 Axis with a Novel Inhibitor Effectively Reverses Cisplatin Resistance in Non-Small Cell Lung Cancer. Cell Death Dis. 2019, 10, 400. [Google Scholar] [CrossRef]
- Yao, W.; Jiao, Y.; Zhou, Y.; Luo, X. KLF13 Suppresses the Proliferation and Growth of Colorectal Cancer Cells through Transcriptionally Inhibiting HMGCS1-Mediated Cholesterol Biosynthesis. Cell Biosci. 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Cheng, H.; Yang, G.; Tan, W. Stanniocalcin 2 Promotes Cell Proliferation and Cisplatin Resistance in Cervical Cancer. Biochem. Biophys. Res. Commun. 2015, 466, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kikuchi, H.; Iino, I.; Uehara, T.; Setoguchi, T.; Fujita, T.; Hiramatsu, Y.; Ohta, M.; Kamiya, K.; Kitagawa, K.; et al. Anti-VEGF Antibody Therapy Induces Tumor Hypoxia and Stanniocalcin 2 Expression and Potentiates Growth of Human Colon Cancer Xenografts. Int. J. Cancer 2014, 135, 295–307. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Tsai, M.-F.; Wu, S.-G.; Chang, T.-H.; Tsai, T.-H.; Gow, C.-H.; Chang, Y.-L.; Shih, J.-Y. Acquired Resistance to EGFR Tyrosine Kinase Inhibitors Is Mediated by the Reactivation of STC2/JUN/AXL Signaling in Lung Cancer. Int. J. Cancer 2019, 145, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhan, L.; Zhang, L.-L.; Wang, Q.; Liu, J.; Jiang, Z.-Y.; Hu, X.-M.; Yuan, X.-C. Stanniocalcin 2 Induces Oxaliplatin Resistance in Colorectal Cancer Cells by Upregulating P-Glycoprotein. Can. J. Physiol. Pharmacol. 2016, 94, 929–935. [Google Scholar] [CrossRef]
- Jiang, K.; Yin, X.; Zhang, Q.; Yin, J.; Tang, Q.; Xu, M.; Wu, L.; Shen, Y.; Zhou, Z.; Yu, H.; et al. STC2 Activates PRMT5 to Induce Radioresistance through DNA Damage Repair and Ferroptosis Pathways in Esophageal Squamous Cell Carcinoma. Redox Biol. 2023, 60, 102626. [Google Scholar] [CrossRef]
- Taguchi, T.; Sonobe, H.; Toyonaga, S.; Yamasaki, I.; Shuin, T.; Takano, A.; Araki, K.; Akimaru, K.; Yuri, K. Conventional and Molecular Cytogenetic Characterization of a New Human Cell Line, GIST-T1, Established from Gastrointestinal Stromal Tumor. Lab. Investig. 2002, 82, 663–665. [Google Scholar] [CrossRef]
- Berenbaum, M.C. What Is Synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [CrossRef]
- Loewe, S. The Problem of Synergism and Antagonism of Combined Drugs. Arzneim. Forsch. 1953, 3, 285–290. [Google Scholar]
- Bliss, C.I. The Toxicity of Poisons Applied Jointly 1. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose–Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Jiao, H.; Fang, F.; Fang, T.; You, Y.; Feng, M.; Wang, X.; Yin, Z.; Zhao, W. SOX13 Regulates Cancer Stem-like Properties and Tumorigenicity in Hepatocellular Carcinoma Cells. Am. J. Cancer Res. 2021, 11, 760. [Google Scholar] [PubMed]
- Zhong, G.; Zhao, Q.; Chen, Z.; Yao, T. TGF-β Signaling Promotes Cervical Cancer Metastasis via CDR1as. Mol. Cancer 2023, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Achari, C.; Winslow, S.; Larsson, C. Down Regulation of CLDND1 Induces Apoptosis in Breast Cancer Cells. PLoS ONE 2015, 10, e0130300. [Google Scholar] [CrossRef]
- Deng, X.; Zhou, S.; Hu, Z.; Gong, F.; Zhang, J.; Zhou, C.; Lan, W.; Gao, X.; Huang, Y. Nicotinic Acid-Mediated Modulation of Metastasis-Associated Protein 1 Methylation and Inflammation in Brain Arteriovenous Malformation. Biomolecules 2023, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
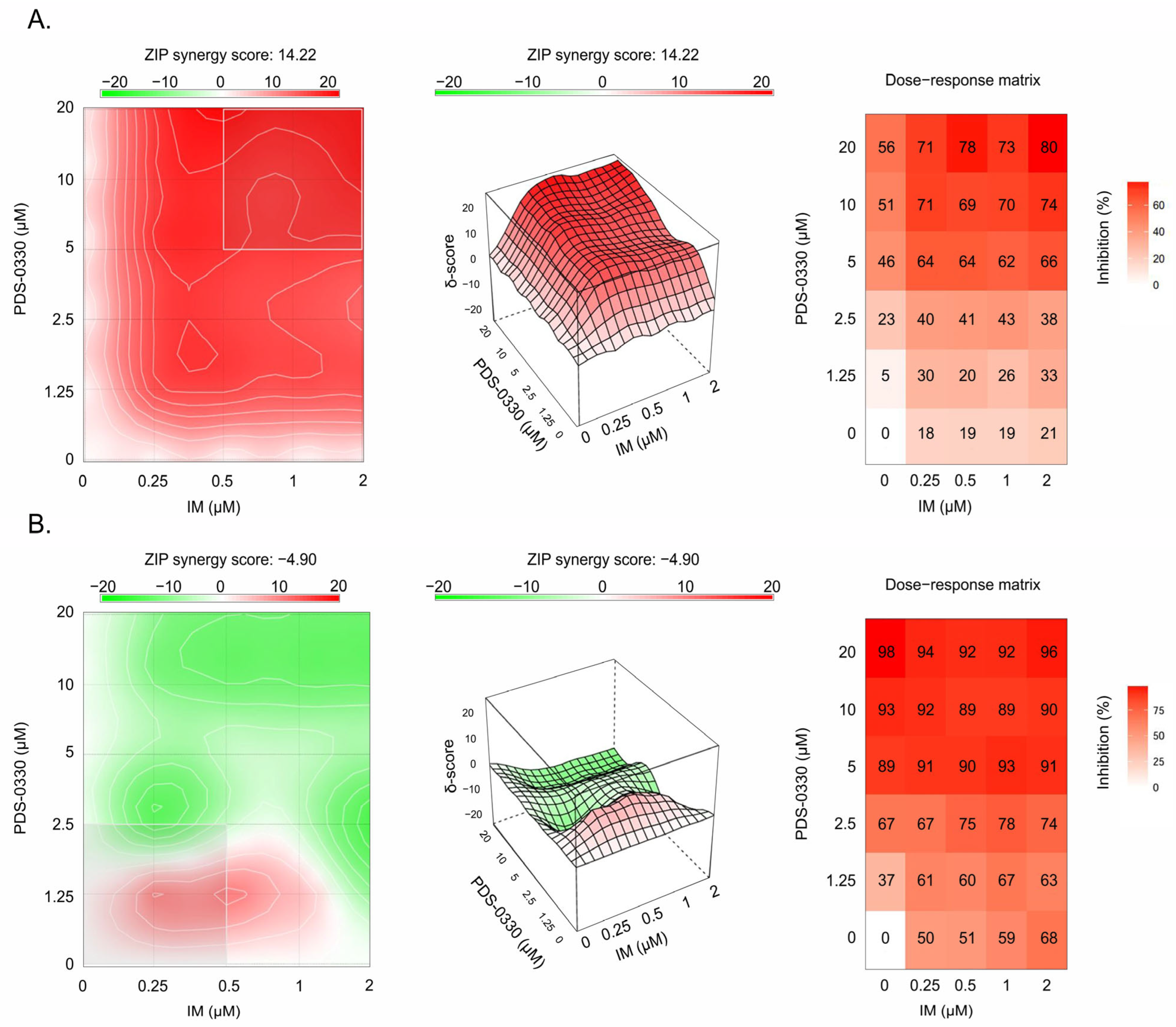

| Cell Line | ZIP | Bliss | Loewe | HSA |
|---|---|---|---|---|
| GIST T-1R | 14.22 | 14.20 | 19.38 | 19.03 |
| GIST 430 | 2.02 |
| Low Risk | Intermediate/High Risk | p | |
|---|---|---|---|
| Mean | 3.60 | 6.29 | 0.011 |
| Standard deviation | 2.03 | 2.65 | |
| Number of patients, n | 12 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boichuk, S.; Bikinieva, F.; Dunaev, P.; Galembikova, A.; Mikheeva, E.; Valeeva, E.; Mani, S.; Khromova, N.; Kopnin, P.; Shigapova, L.; et al. Claudin-1 Contributes to Gastrointestinal Stromal Tumors (GIST) Resistance to Imatinib Mesylate (IM) via Regulation of FGFR-Signaling. Int. J. Mol. Sci. 2025, 26, 8138. https://doi.org/10.3390/ijms26178138
Boichuk S, Bikinieva F, Dunaev P, Galembikova A, Mikheeva E, Valeeva E, Mani S, Khromova N, Kopnin P, Shigapova L, et al. Claudin-1 Contributes to Gastrointestinal Stromal Tumors (GIST) Resistance to Imatinib Mesylate (IM) via Regulation of FGFR-Signaling. International Journal of Molecular Sciences. 2025; 26(17):8138. https://doi.org/10.3390/ijms26178138
Chicago/Turabian StyleBoichuk, Sergei, Firyuza Bikinieva, Pavel Dunaev, Aigul Galembikova, Ekaterina Mikheeva, Elena Valeeva, Shinjit Mani, Natalia Khromova, Pavel Kopnin, Leyla Shigapova, and et al. 2025. "Claudin-1 Contributes to Gastrointestinal Stromal Tumors (GIST) Resistance to Imatinib Mesylate (IM) via Regulation of FGFR-Signaling" International Journal of Molecular Sciences 26, no. 17: 8138. https://doi.org/10.3390/ijms26178138
APA StyleBoichuk, S., Bikinieva, F., Dunaev, P., Galembikova, A., Mikheeva, E., Valeeva, E., Mani, S., Khromova, N., Kopnin, P., Shigapova, L., Deviatiiarov, R., Shagimardanova, E., Ryzhkin, S., & Sabirov, A. (2025). Claudin-1 Contributes to Gastrointestinal Stromal Tumors (GIST) Resistance to Imatinib Mesylate (IM) via Regulation of FGFR-Signaling. International Journal of Molecular Sciences, 26(17), 8138. https://doi.org/10.3390/ijms26178138









