Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells
Abstract
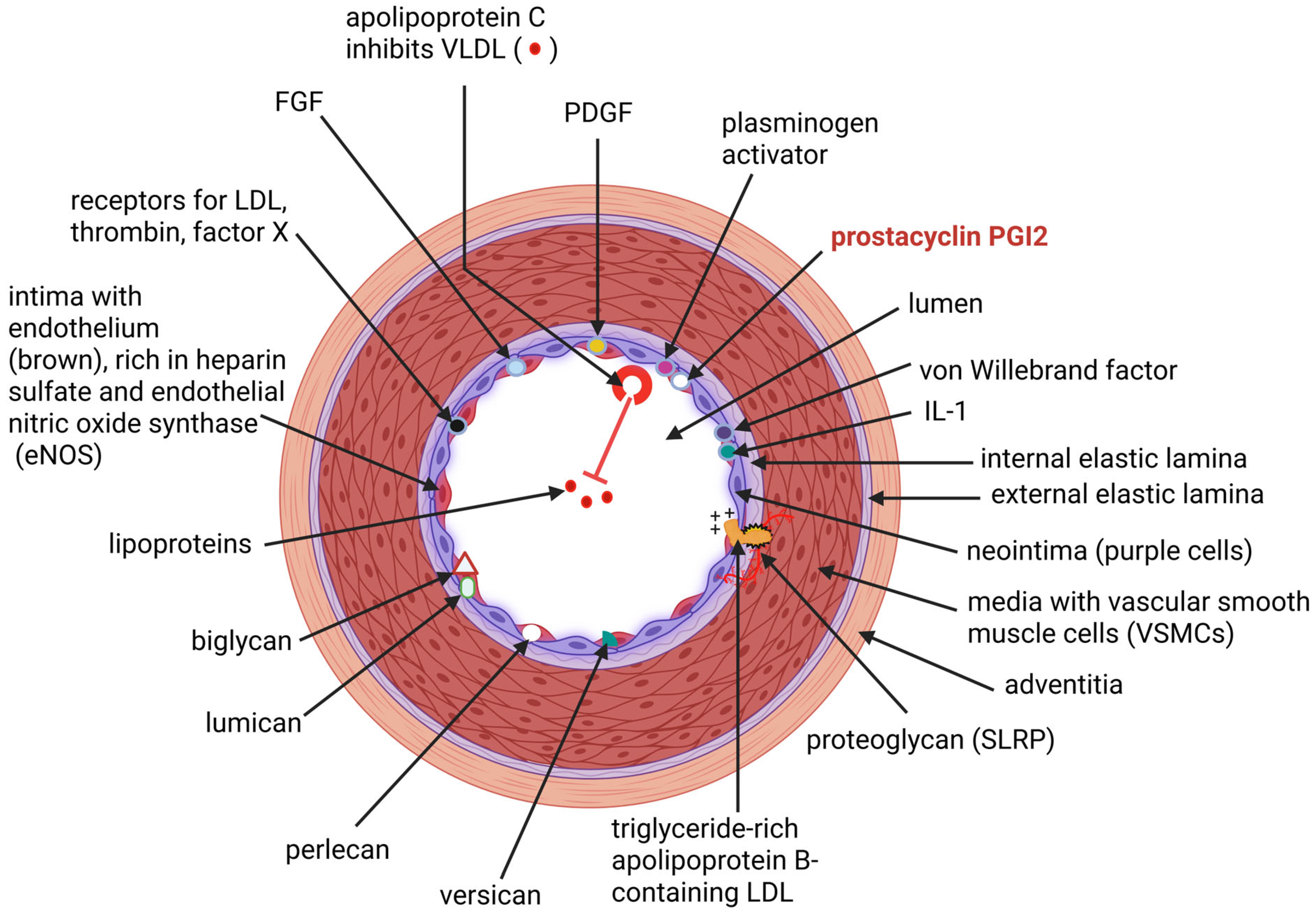
1. Introduction
2. Synthesis and Functioning of the Endothelium
3. Effect of a “Cytokine Storm” on the Endothelium
4. Alleviation of Endothelial Injuries
5. IMA Graft Patency and Clinical Implications
6. Conclusions
Funding
Conflicts of Interest
References
- Otsuka, F.; Yahagi, K.; Sakakura, K.; Virmani, R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann. Cardiothorac. Surg. 2013, 2, 519–526. [Google Scholar]
- Van Son, J.A.; Smedts, F.; De Wilde, P.C.; Pijls, N.H.; Wong-Alcala, L.; Kubat, K.; Tavilla, G.; Lacquet, L.K. Histological study of the internal mammary artery with emphasis on its suitability as a coronary artery bypass graft. Ann. Thorac. Surg. 1993, 55, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hobeika, M.J.; Thompson, R.W.; Muhs, B.E.; Brooks, P.C.; Gagne, P.J. Matrix metalloproteinases in peripheral vascular disease. J. Vasc. Surg. 2007, 45, 849–857. [Google Scholar] [CrossRef]
- Kraler, S.; Libby, P.; Evans, P.C.; Akhmedov, A.; Schmiady, M.O.; Reinehr, M.; Camici, G.G.; Lüscher, T.F. Resilience of the internal mammary artery to atherogenesis: Shifting from risk to resistance to address unmet needs. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, C.; Nastase, M.-V.; Zeng-Brouwers, J.; Roedig, H.; Hsieh, L.T.-H.; Michaelis, J.B.; Buhl, E.M.; Rezende, F.; Manavski, Y.; Bleich, A.; et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019, 95, 540–562. [Google Scholar] [CrossRef]
- Karamanou, K.; Perrot, G.; Maquart, F.-X.; Brézillon, S. Lumican as a multivalent effector in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 344–351. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Merrilees, M.J. Versican and the control of inflammation. Matrix Biol. 2014, 35, 152–161. [Google Scholar] [CrossRef]
- Hayes, A.J.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J.; Perlecan, A. Multi-functional, cell-instructive, matrix-stabilizing proteoglycan with roles in tissue development has relevance to connective tissue repair and regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK57149 (accessed on 10 August 2025).
- Titus, A.; Marappa-Ganeshan, R. Physiology, Endothelin; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK551627/ (accessed on 10 August 2025).
- Schiffrin, E.L.; Intengan, H.D.; Thibault, G.; Touyz, R.M. Clinical significance of endothelin in cardiovascular disease. Curr. Opin. Cardiol. 1997, 12, 354–367. [Google Scholar] [CrossRef]
- Banecki, K.M.R.M.; Dora, K.A. Endothelin-1 in health and disease. Int. J. Mol. Sci. 2023, 24, 11295. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 is increased in the plasma of patients hospitalised with Covid-19. J. Mol. Cell. Cardiol. 2022, 167, 92–96. [Google Scholar] [CrossRef]
- Russell, F.D.; Molenaar, P. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol. Sci. 2000, 21, 353–359. [Google Scholar] [CrossRef]
- Nilsson, D.; Gustafsson, L.; Wackenfors, A.; Gesslein, B.; Edvinsson, L.; Paulsson, P.; Ingemansson, R.; Malmsjö, M. Up-regulation of endothelin type B receptors in the human internal mammary artery in culture is dependent on protein kinase C and mitogen-activated kinase signaling pathways. BMC Cardiovasc. Disord. 2008, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Fujiwara, H.; Doyama, K.; Inada, T.; Ohtani, S.; Fujiwara, T.; Hosoda, K.; Nakao, K.; Sasayama, S. Endothelin-1-selective receptor in the arterial intima of patients with hypertension. J. Hypertension 1994, 23, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, J.; Grandjean, C.M.; Sasse, T.; Clozel, M.; Nayler, O. Slow receptor dissociation kinetics differentiate Macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS ONE 2012, 7, e47662. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; d’Uscio, L.V.; Shaw, S.; Takase, H.; Barton, M.; Lüscher, T.F. Angiotensin II increases tissue endothelin and induces vascular hypertrophy: Reversal by ET(A)-receptor antagonist. Circulation 1997, 96, 1593–1597. [Google Scholar] [CrossRef]
- Yamada, H.; Fabris, B.; Allen, A.M.; Jackson, B.; Johnston, C.I.; Mendelsohn, A.O. Localization of angiotensin converting enzyme in rat heart. Circ. Res. 1991, 68, 141–149. [Google Scholar] [CrossRef]
- Singh, B.; Cusick, A.S.; Goyal, A.; Patel, P. ACE Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK430896/ (accessed on 10 August 2025).
- Jaspard, E.; Costerousse, O.; Wei, L.; Corvol, P.; Alhenc-Gelas, F. The angiotensin I-converting enzyme (kininase II): Molecular and regulatory aspects. Agents Actions Suppl. 1992, 38, 349–358. [Google Scholar]
- He, G.-W.; Liu, Z.-G. Comparison of nitric oxide release and endothelium-derived hyperpolarizing factor-mediated hyperpolarization between human radial and internal mammary arteries. Circulation 2001, 104, 344–349. [Google Scholar] [CrossRef]
- Lonn, E.M.; Yusuf, S.; Dzavik, V.; Doris, C.I.; Yi, Q.; Smith, S.; Moore-Cox, A.; Bosch, J.; Riley, W.A.; Teo, K.K. Effects of ramipril and vitamin E on atherosclerosis: The study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001, 103, 919–925. [Google Scholar] [CrossRef]
- Chappel, M.C.; Ferrario, C.M. ACE and ACE2: Their role to balance the expression of angiotensin II and angiotensin-(1–7). Kidney Int. 2006, 70, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Gressenberger, P.; Muster, V.; Avian, A.; Meinitzer, A.; Strohmaier, H.; Sourij, H.; Raggam, R.B.; Stradner, M.H.; Demel, U.; et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection—A cross-sectional study. Front. Cardiovasc. Med. 2021, 8, 750887. [Google Scholar]
- Auguet, T.; Aragonès, G.; Guiu-Jurado, E.; Berlanga, A.; Curriu, M.; Martinez, S.; Alibalic, A.; Aguilar, C.; Camara, M.L.; Hernández, E.; et al. Adipo/cytokines in atherosclerotic secretomes: Increased visfatin levels in unstable carotid plaque. BMC Cardiovasc. Disord 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Mroueh, A.; Fakih, W.; Carmona, A.; Trimaille, A.; Matsushita, K.; Marchandot, B.; Qureshi, A.W.; Gong, D.-S.; Auger, C.; Sattler, L.; et al. COVID-19 promotes endothelial dysfunction and thrombogenicity: Role of proinflammatory cytokines/SGLT2 prooxidant pathway. J. Thromb. Haemost. 2024, 22, 286–299. [Google Scholar] [CrossRef]
- Chioh, W.; Fong, S.W.; Young, B.E.; Wu, K.X.; Siau, A.; Krishnan, S.; Chan, Y.H.; Carissimo, G.; Teo, L.L.; Gao, F.; et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife 2021, 10, e64909. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Butyrate produced by gut microbiota regulates atherosclerosis: A narrative review of the latest findings. Int. J. Mol. Sci. 2025, 26, 6744. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef]
- Dąbek, J.; Kułach, A.; Gaąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis. Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Park, S.H.; Amissi, S.; Algara-Suarez, P.; Gong, D.S.; Mroueh, A.; Belcastro, E.; Matsushita, K.; Bruckert, C.; Chaker, A.B.; Jesel, L.P.; et al. Sodium-glucose co-transporter 1 and 2 expression in the mammary artery of patients with bypass surgery: Role of the pro-inflammatory response and contribution to oxidative stress. Europ. Heart J. 2021, 42, ehab724.3440. [Google Scholar] [CrossRef]
- Yang, Z.; Ruschitzka, F.; Rabelink, T.J.; Noll, G.; Julmy, F.; Joch, H.; Gafner, V.; Aleksic, I.; Althaus, U.; Lüscher, T.F. Different effects of thrombin receptor activation on endothelium and smooth muscle cells of human coronary bypass vessels. Circulation 1997, 95, 1870–1876. [Google Scholar] [CrossRef]
- Zińczuk, J.; Zaręba, K.; Guzińska-Ustymowicz, K.; Kędra, B.; Kemona, A.; Pryczynicz, A. p16, p21, and p53 proteins play an important role in development of pancreatic intraepithelial neoplastic. Ir. J. Med. Sci. 2018, 187, 629–637. [Google Scholar] [CrossRef]
- Li, C.M.; Lingeman, R.G.; Haratipour, P.; Gu, L.; Jossart, J.; Perry, J.J.P.; Hickey, R.J.; Malkas, L.H. S phase. In Encyclopedia of Cell Biology, 2nd ed.; Bradshaw, R.A., Hart, G.W., Stahl, P.D., Eds.; Academic Press: New York, NY, USA, 2023; pp. 266–284. [Google Scholar]
- Wang, K.; Gheblawi, M.; Nikhanj, A.; Munan, M.; MacIntyre, E.; O’Neil, C.; Poglitsch, M.; Colombo, D.; Del Nonno, F.; Kassiri, Z.; et al. Dysregulation of ACE (angiotensin-converting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries. Hypertension 2022, 79, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.J.J.M.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system—Elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [PubMed]
- Ostergaard, L. SARS-CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Roh, J.D.; Kitchen, R.R.; Guseh, J.S.; McNeill, J.N.; Aid, M.; Martinot, A.J.; Yu, A.; Platt, C.; Rhee, J.; Weber, B.; et al. Plasma proteomics of COVID-19-associated cardiovascular complications: Implications for pathophysiology and therapeutics. JACC Basic Transl. Sci. 2022, 7, 425–441. [Google Scholar] [CrossRef]
- Calabretta, E.; Moraleda, J.M.; Iacobelli, M.; Jara, R.; Vlodavsky, I.; O’Gorman, P.; Pagliuca, A.; Mo, C.; Caron, R.M.; Aghemo, A.; et al. COVID-19-induced endotheliitis: Emerging evidence and possible therapeutic strategies. Br. J. Haematol. 2021, 193, 43–51. [Google Scholar] [CrossRef]
- Fisk, M.; Althage, M.; Moosmang, S.; Greasley, P.J.; Cope, A.P.; Jayne, D.R.; Galloway, J.; Hall, F.; Wilkinson, I.B.; Ambery, P.; et al. Endothelin antagonism and sodium glucose co-transporter 2 inhibition. A potential combination therapeutic strategy for COVID-19. Pulm. Pharmacol. Ther. 2021, 69, 102035. [Google Scholar] [CrossRef]
- Jalili, M.; Sayehmiri, K.; Ansari, N.; Pourhossein, B.; Fazeli, M.; Azizi Jalilian, F. Association between influenza and COVID-19 viruses and the risk of atherosclerosis: Meta-analysis study and systematic review. Adv. Respir. Med. 2022, 90, 338–348. [Google Scholar] [CrossRef]
- Saeed, S.; Tadic, M.; Larsen, T.H.; Grassi, G.; Mancia, G. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. J. Hypertens. 2021, 39, 1282–1292. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Grzegorowska, O.; Lorkowski, J. Possible correlations between atherosclerosis, acute coronary syndromes and COVID-19. J. Clin. Med. 2020, 9, 3746. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, V.; Paupe, V.; Herranz-Montoya, I.; Janssen, J.; Wortel, I.M.N.; Morris, J.L.; Ferguson, A.; Chowdury, S.R.; Segarra-Mondejar, M.; Costa, A.S.H.; et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 2023, 615, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef]
- Almeida, C.; Barata, P.; Fernandes, R. The influence of gut microbiota in cardiovascular diseases—A brief review. Porto Biomed. J. 2021, 6, e106. [Google Scholar] [CrossRef]
- Shaw, A.; Doherty, M.K.; Mutch, N.J.; MacRury, S.M.; Megson, I.L. Endothelial cell oxidative stress in diabetes: A key driver of cardiovascular complications? Biochem. Soc. Trans. 2014, 42, 928–933. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern concepts in cardiovascular disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.V.; Khamashta, M.A. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stemme, S.; Hansson, G.K. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am. J. Pathol. 1996, 149, 359–366. [Google Scholar] [PubMed]
- Davenport, P.; Tipping, P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef]
- Engelbertsen, D.; Andersson, L.; Ljungcrantz, I.; Wigren, M.; Hedblad, B.; Nilsson, J.; Bjorkbacka, H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 637–644. [Google Scholar] [CrossRef]
- Gee, T.; Farrar, E.; Wang, Y.; Wu, B.; Hsu, K.; Zhou, B.A.; Butcher, J. NFκB (nuclear factor κ-light-chain enhancer of activated B cells) activity regulates cell-type-specific and context-specific susceptibility to calcification in the aortic valve. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 638–655. [Google Scholar] [CrossRef]
- Stump, M.; Mukohda, M.; Hu, C.; Sigmund, C.D. PPARγ regulation in hypertension and metabolic syndrome. Curr. Hypertens. Rep. 2015, 17, 89. [Google Scholar] [CrossRef]
- Wang, T.; Baron, M.; Trump, D. An overview of Notch3 function in vascular smooth muscle cells. Prog. Biophys. Mol. Biol. 2008, 96, 499–509. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Tacconi, E.; Palma, G.; De Biase, D.; Luciano, A.; Barbieri, M.; De Nigris, F.; Bruzzese, F. Microbiota effect on trimethylamine N-oxide production: From cancer to fitness—A practical preventing recommendation and therapies. Nutrients 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Mulligan-Kehoe, M.J.; Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 2014, 129, 2557–2566. [Google Scholar] [CrossRef] [PubMed]
- Khattib, A.; Musa, S.; Halabi, M.; Hayek, T.; Khatib, S. Lyso-DGTS lipid derivatives enhance PON1 activities and prevent oxidation of LDL: A structure-activity relationship study. Antioxidants 2022, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Altenhöfer, S.; Witte, I.; Teiber, J.F.; Wilgenbus, P.; Pautz, A.; Li, H.; Daiber, A.; Witan, H.; Clement, A.M.; Förstermann, U.; et al. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010, 285, 24398–24403. [Google Scholar] [CrossRef]
- Wagner, C.; Zimmermann, S.; Brenner-Weiss, G.; Hug, F.; Prior, B.; Obst, U.; Hänsch, G.M. The quorum-sensing molecule N-3-oxododecanoyl homoserine lactone (3OC12-HSL) enhances the host defence by activating human polymorphonuclear neutrophils (PMN). Anal. Bioanal. Chem. 2007, 387, 481–487. [Google Scholar] [CrossRef]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of sumo proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Ruan, C.C.; Chen, D.R.; Zhang, K.; Yan, C.; Gao, P.J. Activating transcription factor 3 SUMOylation is involved in angiotensin II-induced endothelial cell inflammation and dysfunction. J. Mol. Cell. Cardiol. 2016, 92, 149–157. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Xiao, X.; Hu, C.-T.; Dai, Y.; Qu, S.-L.; Huang, L.; Zhang, C. SUMOylation in atherosclerosis. Clin. Chim. Acta 2020, 508, 228–233. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, Y.; Zhao, H.; Qin, L.; Shi, Y.; Zhu, X.; Song, L.; Zhou, X.; Chen, J.; Zhou, H.; et al. The critical role of SENP1-mediated GATA2 deSUMOylation in promoting endothelial activation in graft arteriosclerosis. Nat. Commun. 2017, 8, 15426. [Google Scholar] [CrossRef]
- Hsu, A.P.; McReynolds, L.J.; Holland, S.M. GATA2 deficiency. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 104–109. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Mu, N.; Lou, X.; Li, W.; Chen, Y.; Fan, D.; Tan, H. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab. Investig. 2017, 97, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.; John, S.W.; Liccardi, G.; Tenev, T.; Jaco, I.; Chen, C.-H.; Choi, J.; Kasperkiewicz, P.; Fernandes-Alnemri, T.; Alnemri, E.; et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 2018, 9, 3001. [Google Scholar]
- Rychli, K.; Huber, K.; Wojta, J. Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease. Expert Opin. Ther. Targets. 2009, 13, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Inagaki, Y.; Nakamura, K.; Abe, R.; Shimizu, T.; Yoshimura, A.; Imaizumi, T. Pigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generation. J. Mol. Cell. Cardiol. 2004, 37, 497–506. [Google Scholar] [CrossRef]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef]
- Ma, S.; Wang, S.; Li, M.; Zhang, Y.; Zhu, P. The effects of pigment epithelium-derived factor on atherosclerosis: Putative mechanisms of the process. Lipids Health Dis. 2018, 17, 240. [Google Scholar] [CrossRef]
- Chen, L.; DiPietro, L.A. Production and function of pigment epithelium-derived factor in isolated skin keratinocytes. Exp. Dermatol. 2014, 23, 436–438. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, Y.; Liu, R.; Zhang, K.; Yang, T.; Hu, C.; Gao, Y.; Lan, Q.; Liu, Y.; Yang, X.; et al. Pigment epithelium-derived factor (PEDF) plays anti-inflammatory roles in the pathogenesis of dry eye disease. Ocul. Surf. 2021, 20, 70–85. [Google Scholar] [CrossRef]
- Liu, J.; Yao, S.; Wang, S.; Jiao, P.; Song, G.; Yu, Y.; Zhu, P.; Qin, S. D-4F, an apolipoprotein A-I mimetic peptide, protects human umbilical vein endothelial cells from oxidized low-density lipoprotein-induced injury by preventing the downregulation of pigment epithelium-derived factor expression. J. Cardiovasc. Pharmacol. 2014, 63, 553–561. [Google Scholar] [CrossRef]
- Talamillo, A.; Ajuria, L.; Grillo, M.; Barroso-Gomila, O.; Mayor, U.; Barrio, R. SUMOylation in the control of cholesterol homeostasis. Open Biol. 2020, 10, 200054. [Google Scholar] [CrossRef]
- Fitzky, B.U.; Witsch-Baumgartner, M.; Erdel, M.; Lee, J.N.; Paik, Y.K.; Glossmann, H.; Utermann, G.; Fogarty, H.; Townsend, L.; Morrin, H.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.; Lee, L.; Nakamura, Y.; Wilkinson, K.A.; Henley, J.M. Protective role of the deSUMOylating enzyme SENP3 in myocardial ischemia-reperfusion injury. PLoS ONE 2019, 14, e0213331. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.-I.; Sandhu, U.G.; Hoang, N.M.; Thangam, M.; Quintana-Quezada, R.A.; Fujiwara, K.; Le, N.T. Coordination of cellular localization-dependent effects of SUMOylation in regulating cardiovascular and neurological diseases. Adv. Exp. Med. Biol. 2017, 963, 337–358. [Google Scholar] [PubMed]
- Zhao, W.; Zhang, X.; Rong, J. SUMOylation as a therapeutic target for myocardial infarction. Front. Cardiovasc. Med. 2021, 8, 701583. [Google Scholar] [CrossRef]
- Chang, E.; Abe, J.-I. Kinase-SUMO networks in diabetes-mediated cardiovascular disease. Metabolism 2016, 65, 623–633. [Google Scholar] [CrossRef]
- Jia, Y.; Claessens, L.A.; Vertegaal, A.C.O.; Ovaa, H. Chemical tools and biochemical assays for SUMO specific proteases (SENPs). ACS Chem. Biol. 2019, 14, 2389–2395. [Google Scholar] [CrossRef]
- Brackett, C.M.; Blagg, B.S.J. Current status of SUMOylation inhibitors. Curr. Med. Chem. 2021, 28, 3892–3912. [Google Scholar] [CrossRef]
- Ezzine, C.; Loison, L.; Montbrion, N.; Bôle-Feysot, C.l.; Déchelotte, P.; Coëffier, M.; Ribet, D. Fatty acids produced by the gut microbiota dampen host inflammatory responses by modulating intestinal SUMOylation. Gut Microbes 2022, 14, 2108280. [Google Scholar] [CrossRef]
- Johnson, E.S. Protein modification by SUMO. Annu. Rev. Biochem. 2004, 73, 355–382. [Google Scholar] [CrossRef]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar] [CrossRef]
- Favaloro, R.G. Bilateral internal mammary artery implants. Operative technique. A preliminary report. Clevel. Clin. Q. 1967, 34, 61. [Google Scholar] [CrossRef]
- Squiers, J.J.; Mack, M.J. Coronary artery bypass grafting—Fifty years of quality initiatives since Favaloro. Ann. Cardiothorac. Surg. 2018, 7, 516–520. [Google Scholar] [CrossRef]
- Ahmed, I.; Yandrapalli, S. Internal mammary artery bypass. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK560835/ (accessed on 10 August 2025).
- Khot, U.N.; Friedman, D.T.; Pettersson, G.; Smedira, N.G.; Li, J.; Ellis, S.G. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation 2004, 109, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.Y.; D’Hoore, W.; Papadatos, S.; Mairy, Y.; El Khoury, G.; Noirhomme, P.; Hanet, C.; Glineur, D. Five-year follow-up of drug-eluting stents implantation vs. minimally invasive direct coronary artery bypass for left anterior descending artery disease: A propensity score analysis. Eur. J. Cardiothoracic Surg. 2013, 44, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Seki, T.; Kawachi, K.; Morita, R.; Kawata, T.; Mizuguchi, K.; Kobayashi, S.; Fukutomi, M.; Nishii, T.; Kobayashi, H.; et al. Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery. New evidence for a “live” conduit. Circulation 1988, 78 Pt 2, I129–I139. [Google Scholar]
- Harskamp, R.E.; Alexander, J.H.; Ferguson, T.B., Jr.; Hager, R.; Mack, M.J.; Englum, B.; Wojdyla, D.; Schulte, P.J.; Kouchoukos, N.T.; de Winter, R.J.; et al. Frequency and predictors of internal mammary artery graft failure and subsequent clinical outcomes: Insights from the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV Trial. Circulation 2016, 133, 131–138. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boothroyd, D.B.; Reitz, B.A.; Shilane, D.A.; Baker, L.C.; Go, A.S. Adoption and effectiveness of internal mammary artery grafting in coronary artery bypass surgery among Medicare beneficiaries. J. Am. Coll. Cardiol. 2014, 63, 33–39. [Google Scholar] [CrossRef]
- Cameron, A.; Davis, K.B.; Green, G.E.; Myers, W.O.; Pettinger, M. Clinical implications of internal mammary artery bypass grafts: The Coronary Artery Surgery Study experience. Circulation 1988, 77, 815–819. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.M.T. Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 8142. https://doi.org/10.3390/ijms26178142
Dicks LMT. Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(17):8142. https://doi.org/10.3390/ijms26178142
Chicago/Turabian StyleDicks, Leon M. T. 2025. "Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells" International Journal of Molecular Sciences 26, no. 17: 8142. https://doi.org/10.3390/ijms26178142
APA StyleDicks, L. M. T. (2025). Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells. International Journal of Molecular Sciences, 26(17), 8142. https://doi.org/10.3390/ijms26178142





