Fully Automated Measurement of GFAP in CSF Using the LUMIPULSE® System: Implications for Alzheimer’s Disease Diagnosis and Staging
Abstract
1. Introduction
2. Results
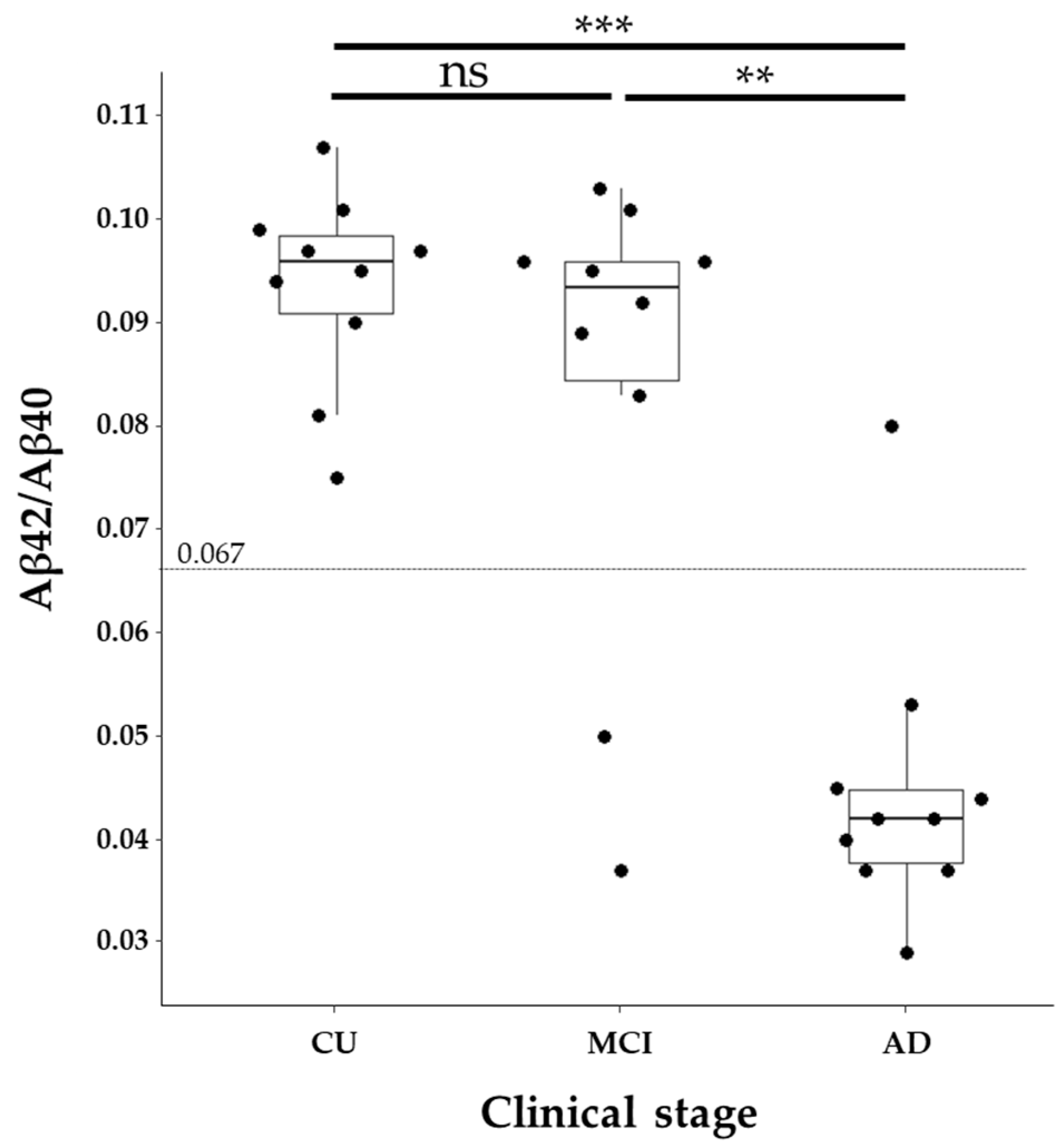
2.1. Specimens and Biomarker Characteristics
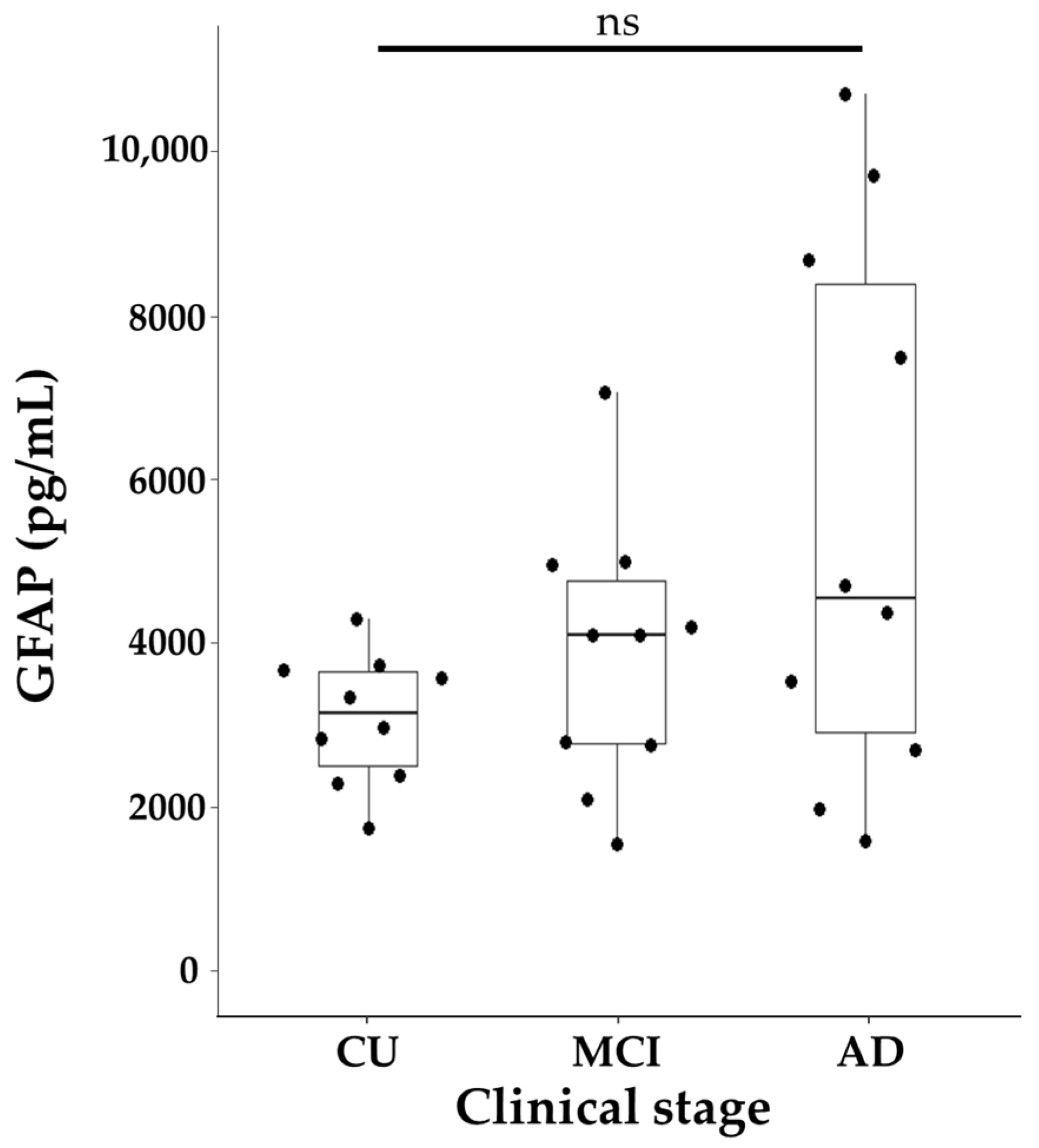
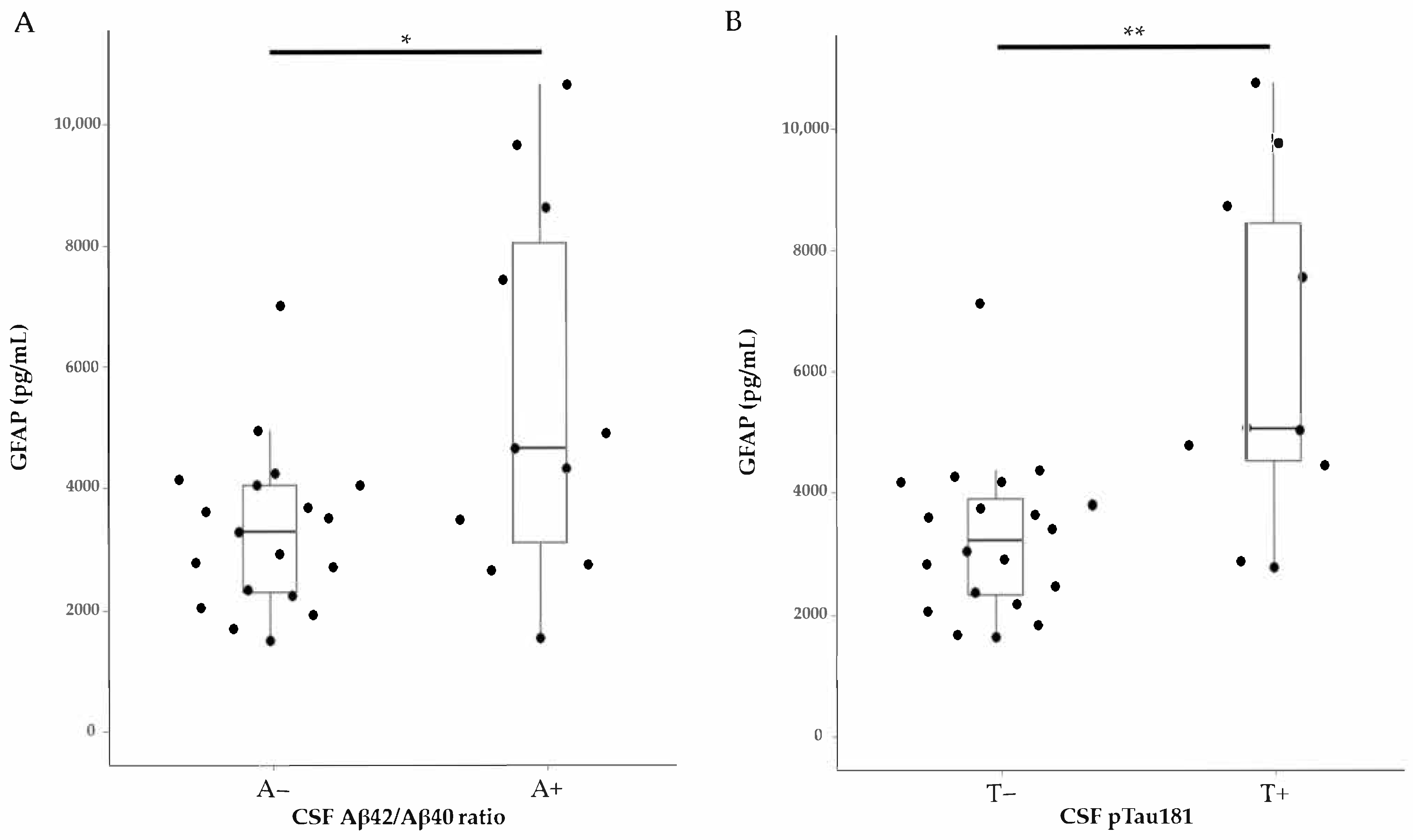
2.2. GFAP Levels in Relation to Clinical Diagnosis and Core AD Biomarkers
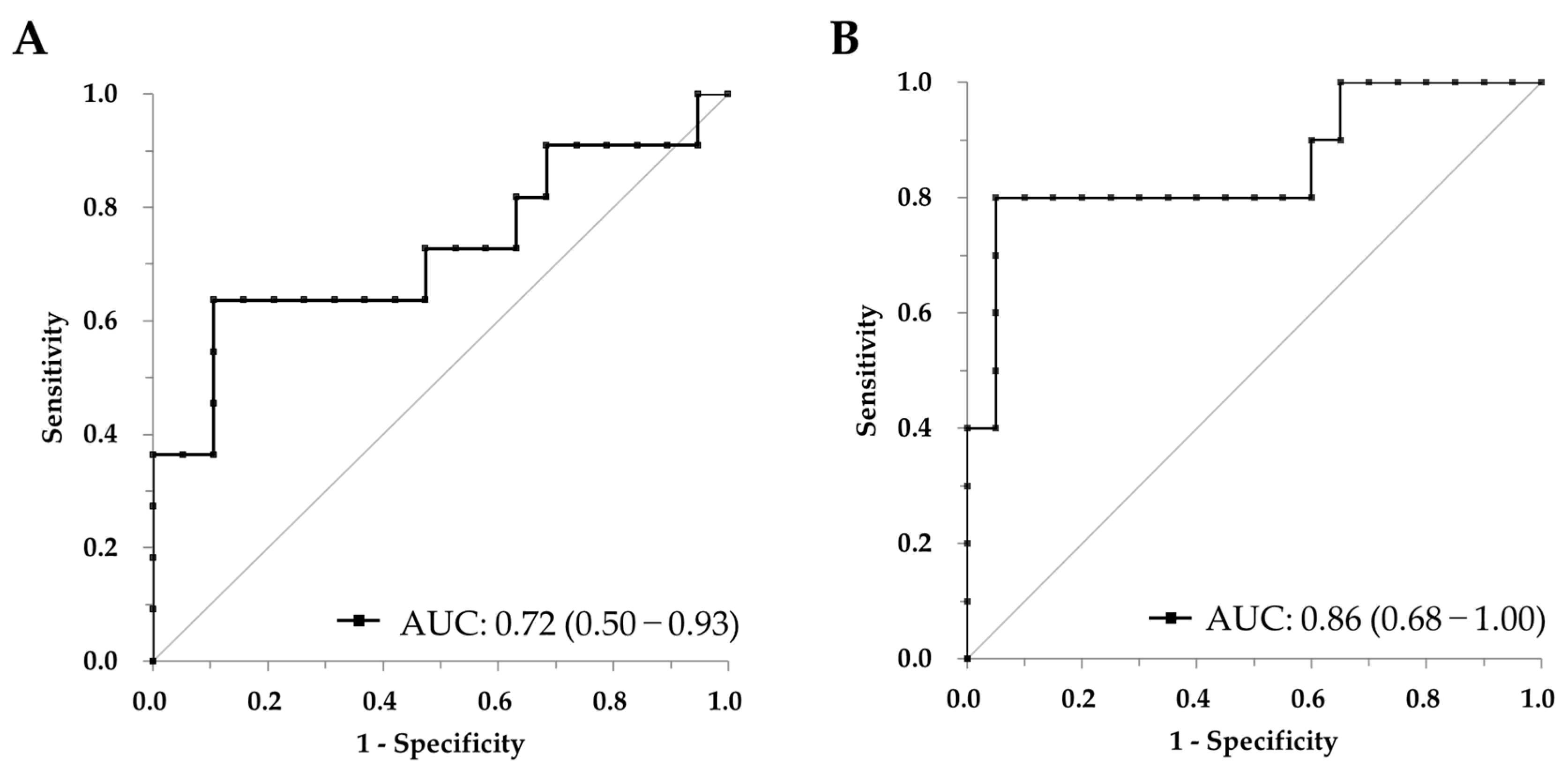
2.3. CSF GFAP Discriminates Aβ and pTau Positivity: ROC Curve Analysis
3. Discussion
4. Materials and Methods
4.1. CSF Samples
4.2. Sample Analysis
4.3. GFAP Assay Discription
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Leipp, F.; Vialaret, J.; Mohaupt, P.; Coppens, S.; Jaffuel, A.; Niehoff, A.C.; Lehmann, S.; Hirtz, C. Glial fibrillary acidic protein in Alzheimer’s disease: A narrative review. Brain Commun. 2024, 6, fcae396. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Lu, M.; Burnham, S.C.; Schade, A.E.; Dage, J.L.; Shcherbinin, S.; Collins, E.C.; Sims, J.R.; Mintun, M.A. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1250–1259. [Google Scholar] [CrossRef]
- Nojima, H.; Ito, S.; Kushida, A.; Abe, A.; Motsuchi, W.; Verbel, D.; Vandijck, M.; Jannes, G.; Vandenbroucke, I.; Aoyagi, K. Clinical utility of cerebrospinal fluid biomarkers measured by LUMIPULSE((R)) system. Ann. Clin. Transl. Neurol. 2022, 9, 1898–1909. [Google Scholar] [CrossRef]
- Blennow, K.; Mattsson, N.; Scholl, M.; Hansson, O.; Zetterberg, H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol. Sci. 2015, 36, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer’s Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, Y.; Ma, X.; Mu, D.; Zhong, J.; Ma, C.; Mao, C.; Yu, S.; Gao, J.; Qiu, L. CSF and blood glial fibrillary acidic protein for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2024, 101, 102485. [Google Scholar] [CrossRef] [PubMed]
- Kamada, J.; Hamanaka, T.; Oshimo, A.; Sato, H.; Nishii, T.; Fujita, M.; Makiguchi, Y.; Tanaka, M.; Aoyagi, K.; Nojima, H. Glial Fibrillary Acidic Protein’s Usefulness as an Astrocyte Biomarker Using the Fully Automated LUMIPULSE(®) System. Diagnostics 2024, 14, 2520. [Google Scholar] [CrossRef]
- Yuri, T.; Degrieck, R.; Minczakiewicz, D.; Sato, H.; Kamada, J.; Nakazawa, T.; Vandenbroucke, I.; Aoyagi, K.; Nojima, H. Estimation of the allelic status of apolipoprotein E4 isoforms with fully automated LUMIPULSE® assays. Explor. Neurosci. 2023, 2, 238–244. [Google Scholar] [CrossRef]
- Palmqvist, S.; Warmenhoven, N.; Anastasi, F.; Pilotto, A.; Janelidze, S.; Tideman, P.; Stomrud, E.; Mattsson-Carlgren, N.; Smith, R.; Ossenkoppele, R.; et al. Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nat. Med. 2025, 31, 2036–2043. [Google Scholar] [CrossRef]
- Barthel, H.; Sabri, O. Clinical Use and Utility of Amyloid Imaging. J. Nucl. Med. 2017, 58, 1711–1717. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer′s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Ishiki, A.; Kamada, M.; Kawamura, Y.; Terao, C.; Shimoda, F.; Tomita, N.; Arai, H.; Furukawa, K. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J. Neurochem. 2016, 136, 258–261. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Steinacker, P.; Polischi, B.; Mammana, A.; Bartoletti-Stella, A.; Oeckl, P.; Baiardi, S.; Zenesini, C.; Huss, A.; Cortelli, P.; et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimer′s Res. Ther. 2019, 12, 2. [Google Scholar] [CrossRef]
- Simrén, J.; Weninger, H.; Brum, W.S.; Khalil, S.; Benedet, A.L.; Blennow, K.; Zetterberg, H.; Ashton, N.J. Differences between blood and cerebrospinal fluid glial fibrillary Acidic protein levels: The effect of sample stability. Alzheimers Dement. 2022, 18, 1988–1992. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Mielke, M.L.; Gómez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Hottenrott, T.; Morenas-Rodríguez, E.; Suárez-Calvet, M.; Zettl, U.K.; Haass, C.; Meuth, S.G.; Rauer, S.; Otto, M.; Tumani, H.; et al. Glial Activation Markers in CSF and Serum From Patients With Primary Progressive Multiple Sclerosis: Potential of Serum GFAP as Disease Severity Marker? Front. Neurol. 2019, 10, 280. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Ciaccio, A.M.; Salemi, G.; Brighina, F.; Ragonese, P.; Piccoli, T.; Blandino, V.; Di Stefano, V.; Cacciabaudo, F.; et al. The value of serum glial fibrillary acidic protein as a biomarker of astrogliosis in different neurological diseases. Clin. Chim. Acta 2025, 572, 120248. [Google Scholar] [CrossRef] [PubMed]
- Marksteiner, J.; Humpel, C. Glial fibrillary acidic protein as a biomarker for diagnosis of Alzheimer’s disease in cerebrospinal fluid, plasma and saliva measured with Lumipulse technology: A narrative review. NeuroMarkers 2025, 2, 100038. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- de Rancher, M.R.; Oudart, J.B.; Maquart, F.X.; Monboisse, J.C.; Ramont, L. Evaluation of Lumipulse® G1200 for the measurement of six tumor markers: Comparison with AIA® 2000. Clin. Biochem. 2016, 49, 1302–1306. [Google Scholar] [CrossRef]
- Hampel, H.; Shaw, L.M.; Aisen, P.; Chen, C.; Lleo, A.; Iwatsubo, T.; Iwata, A.; Yamada, M.; Ikeuchi, T.; Jia, J.; et al. State-of-the-art of lumbar puncture and its place in the journey of patients with Alzheimer’s disease. Alzheimers Dement. 2022, 18, 159–177. [Google Scholar] [CrossRef]

| CU | MCI | AD | p-Value | |
| n | 10 | 10 | 10 | |
| Gender, Female, n (%) | 5 (50.0) | 5 (50.0) | 6 (60.0) | 1 |
| Age, years * | 63.50 [61.25, 66.75] | 70.50 [63.50, 80.25] | 72.50 [68.50, 77.25] | 0.182 |
| Aβ40 * | 9262 [7604, 11,847] pg/mL | 10,668 [10,158, 12,705] pg/mL | 9103.5 [6775, 11,239] pg/mL | 0.272 |
| Aβ42 * | 814 [620, 1136] pg/mL | 981 [670, 1003] pg/mL | 392 [319, 418] pg/mL | <0.001 |
| Aβ42/Aβ40 ratio * | 0.096 [0.091, 0.098] | 0.094 [0.084, 0.096] | 0.042 [0.038, 0.045] | <0.001 |
| pTau181 * | 29.6 [23.0, 32.7] pg/mL | 43.1 [37.2, 56.6] pg/mL | 91.0 [51.0, 113.0] pg/mL | <0.05 |
| GFAP * | 3212.5 [2549.0, 3710.5] pg/mL | 4176.5 [2825.0, 4847.2] pg/mL | 4621.5 [2970.2, 8523.2] pg/mL | 0.244 |
| Variable A | Variable B | Adjusted Variables | r (Partial) | p-Value |
|---|---|---|---|---|
| GFAP | Aβ42/Aβ40 | Age | –0.230 | 0.230 |
| GFAP | Aβ42/Aβ40 | Age, Sex | –0.233 | 0.232 |
| GFAP | pTau181 | Age | 0.564 | 0.001 |
| GFAP | pTau181 | Age, Sex | 0.551 | 0.002 |
| Cut Off | Sensitivity | Specificity | |
|---|---|---|---|
| Aβ Positivity | 4412.0 | 63.6% (30.8–89.1%) | 89.5% (66.9–98.7%) |
| pTau Positivity | 4412.0 | 80.0% (44.4–97.5%) | 95.0% (75.1–99.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojima, H.; Yamamoto, M.; Kamada, J.; Hamanaka, T.; Aoyagi, K. Fully Automated Measurement of GFAP in CSF Using the LUMIPULSE® System: Implications for Alzheimer’s Disease Diagnosis and Staging. Int. J. Mol. Sci. 2025, 26, 8134. https://doi.org/10.3390/ijms26178134
Nojima H, Yamamoto M, Kamada J, Hamanaka T, Aoyagi K. Fully Automated Measurement of GFAP in CSF Using the LUMIPULSE® System: Implications for Alzheimer’s Disease Diagnosis and Staging. International Journal of Molecular Sciences. 2025; 26(17):8134. https://doi.org/10.3390/ijms26178134
Chicago/Turabian StyleNojima, Hisashi, Mai Yamamoto, Jo Kamada, Tomohiro Hamanaka, and Katsumi Aoyagi. 2025. "Fully Automated Measurement of GFAP in CSF Using the LUMIPULSE® System: Implications for Alzheimer’s Disease Diagnosis and Staging" International Journal of Molecular Sciences 26, no. 17: 8134. https://doi.org/10.3390/ijms26178134
APA StyleNojima, H., Yamamoto, M., Kamada, J., Hamanaka, T., & Aoyagi, K. (2025). Fully Automated Measurement of GFAP in CSF Using the LUMIPULSE® System: Implications for Alzheimer’s Disease Diagnosis and Staging. International Journal of Molecular Sciences, 26(17), 8134. https://doi.org/10.3390/ijms26178134






