Non-Invasive vs. Invasive Markers in Ulcerative Colitis: A Systematic Review of Intestinal Ultrasound, Biopsy, and Faecal Calprotectin
Abstract
1. Introduction
2. Methods
2.1. Criteria for Considering Studies for This Review
2.1.1. Types of Studies
2.1.2. Participants
2.1.3. Index Tests
2.1.4. Target Conditions
2.1.5. Reference Standards
2.1.6. Exclusion Criteria
2.2. Search Methods for the Identification of Studies
2.2.1. Electronic Searches
2.2.2. Searching Other Resources
2.3. Data Collection and Analysis
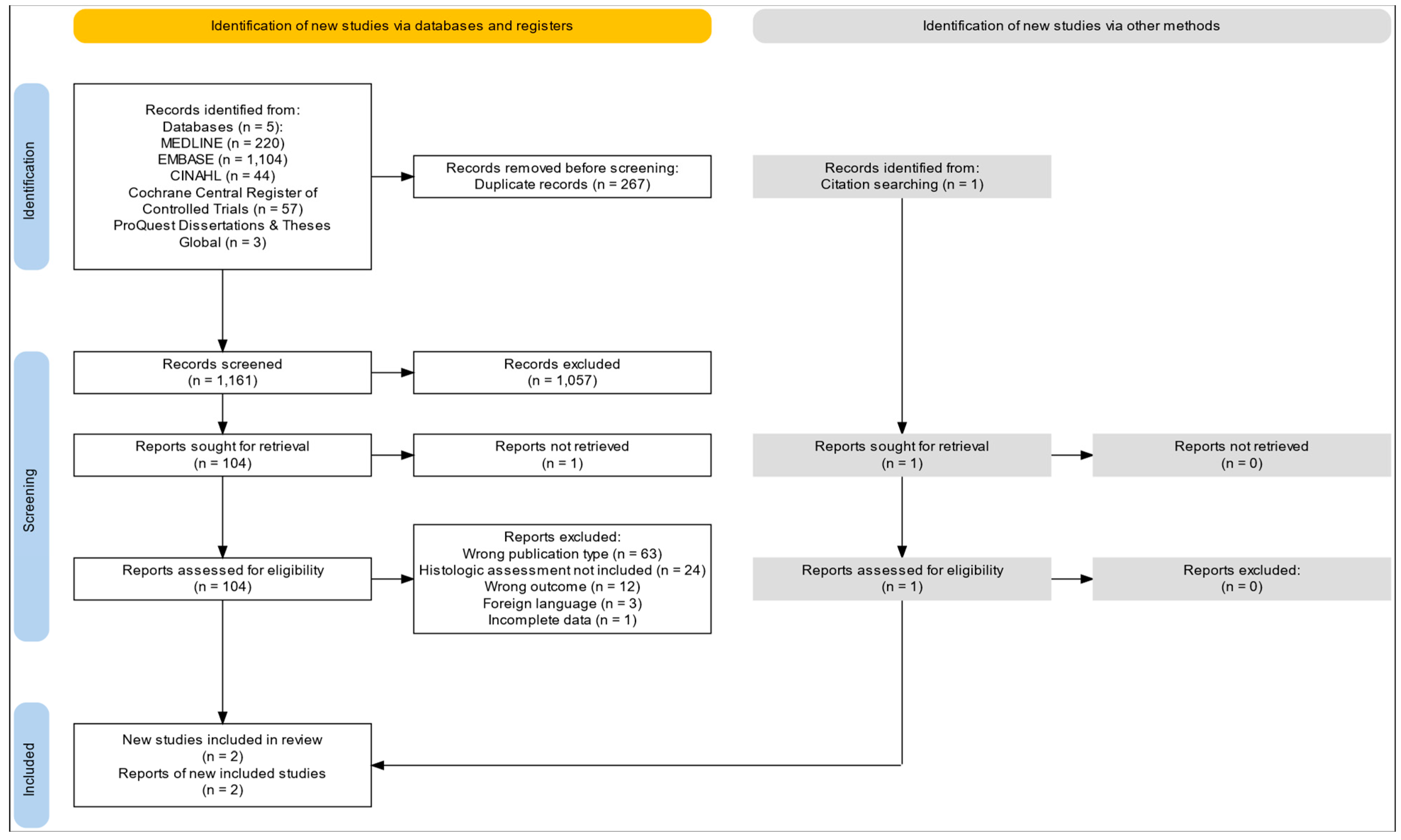
2.3.1. Selection of Studies
2.3.2. Data Extraction and Management
2.3.3. Assessment of Methodological Quality
2.3.4. Statistical Analysis and Data Synthesis
3. Results
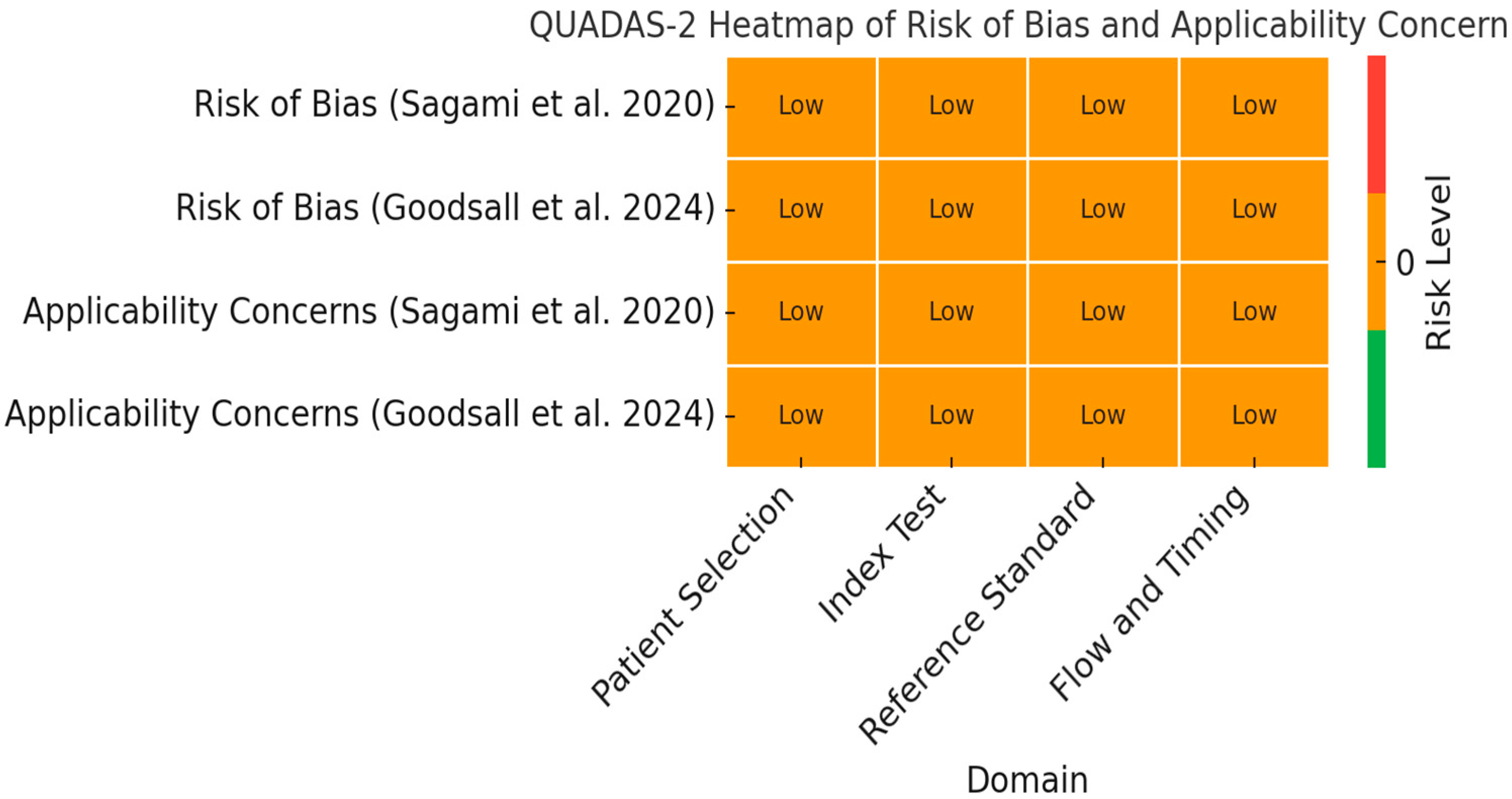
3.1. Risk of Bias
3.2. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Assessment
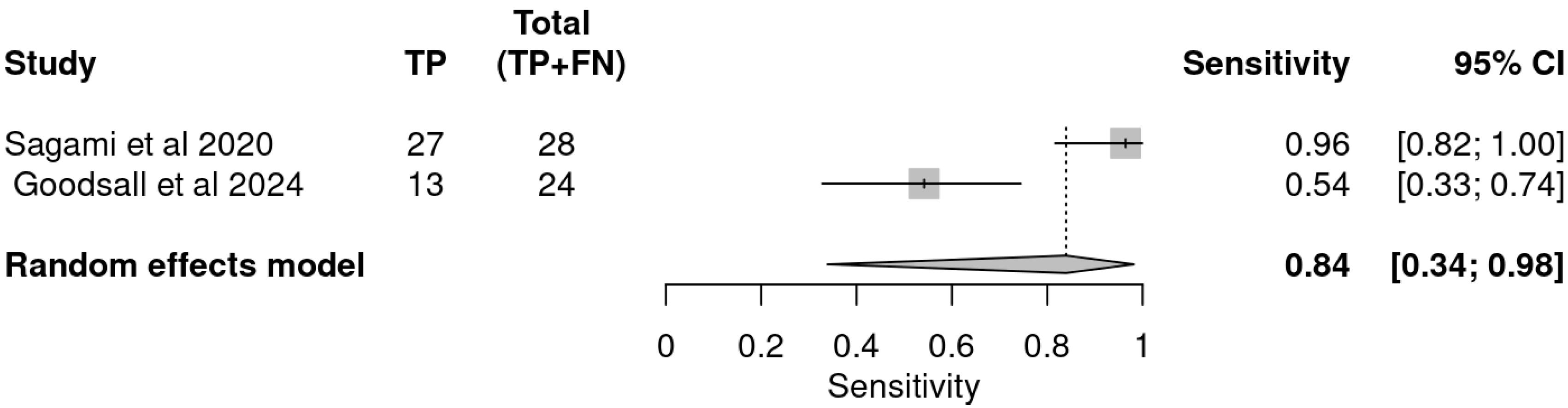
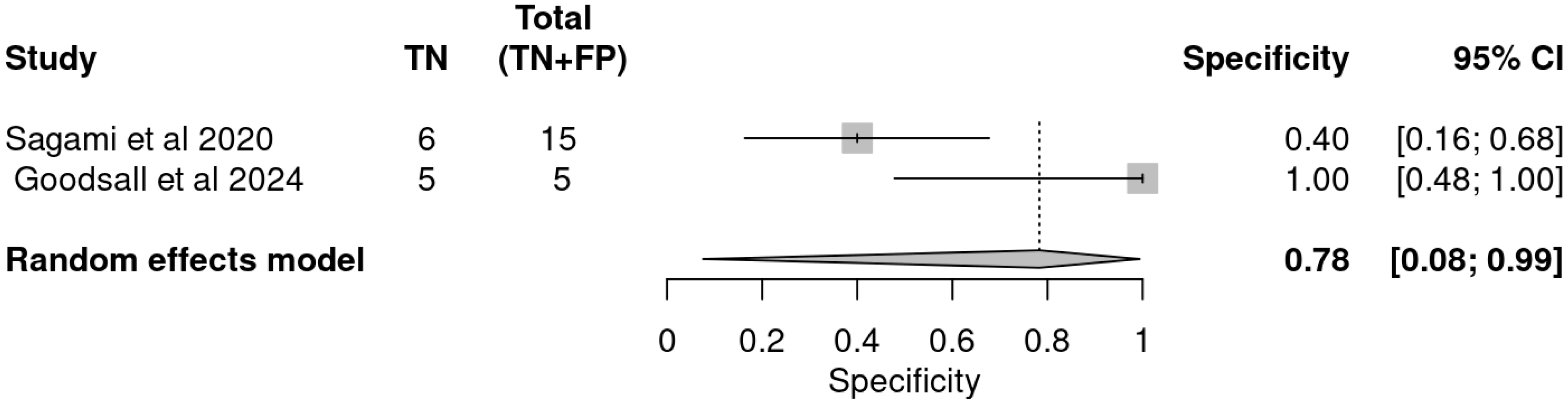
3.3. Diagnostic Performance of Included Studies
3.4. Pooled Accuracy Estimates
4. Discussion
5. Conclusions
Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Ulcerative Colitis |
| IBD | Inflammatory Bowel Disease |
| IUS | Intestinal Ultrasound |
| FC | Faecal Calprotectin |
| BWT | Bowel Wall Thickness |
| CDS | Color Doppler Signal |
| ECCO | The European Crohn’s And Colitis Organization |
| TPs | True Positives |
| FPs | False Positives |
| FNs | False Negatives |
| TNs | And True Negatives |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
References
- Chavannes, M.; Dolinger, M.T.; Cohen-Mekelburg, S.; Abraham, B. AGA Clinical Practice Update on the Role of Intestinal Ultrasound in Inflammatory Bowel Disease: Commentary. Clin. Gastroenterol. Hepatol. 2024, 22, 1790–1795.e1. [Google Scholar] [CrossRef]
- de Voogd, F.; van Wassenaer, E.A.; Mookhoek, A.; Bots, S.; van Gennep, S.; Löwenberg, M.; D’Haens, G.R.; Gecse, K.B. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients with Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology 2022, 163, 1569–1581. [Google Scholar] [CrossRef]
- Maaser, C.; Petersen, F.; Helwig, U.; Fischer, I.; Roessler, A.; Rath, S.; Lang, D.; Kucharzik, T. Intestinal Ultrasound for Monitoring Therapeutic Response in Patients with Ulcerative Colitis: Results from the TRUST&UC Study. Gut 2020, 69, 1629–1636. [Google Scholar] [CrossRef]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 Family of Calcium Sensor Proteins. Cell Calcium 2013, 53, 170–179. [Google Scholar] [CrossRef]
- Schäfer, B.W.; Heizmann, C.W. The S100 Family of EF-Hand Calcium-Binding Proteins: Functions and Pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Teigelkamp, S.; Bhardwaj, R.S.; Roth, J.; Meinardus-Hager, G.; Karas, M.; Sorg, C. Calcium-Dependent Complex Assembly of the Myeloic Differentiation Proteins MRP-8 and MRP-14. J. Biol. Chem. 1991, 266, 13462–13467. [Google Scholar] [CrossRef]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an Inflammatory Protein Complex from Neutrophils with a Broad Apoptosis-Inducing Activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From Biomarker to Biological Function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A Meta-Analysis of the Utility of C-Reactive Protein, Erythrocyte Sedimentation Rate, Fecal Calprotectin, and Fecal Lactoferrin to Exclude Inflammatory Bowel Disease in Adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef]
- Langhorst, J.; Kairey, L.; Oberle, A.; Boone, J.; Dobos, G.; Juette, H.; Tannapfel, A.; Rueffer, A. Assessing Histological Inflammatory Activity in Patients with Ulcerative Colitis: A Diagnostic Accuracy Study Testing Fecal Biomarkers Lactoferrin and Calprotectin. Crohns Colitis 360 2020, 2, otaa053. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Doherty, G.; Peyrin-Biroulet, L.; Svrcek, M.; Borralho, P.; Walsh, A.; Carneiro, F.; Rosini, F.; De Hertogh, G.; Biedermann, L.; et al. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J. Crohns Colitis 2020, 14, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Y.; Wang, H.H.X.; Feng, R.; Ye, Z.; Han, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Can Fecal Calprotectin Accurately Identify Histological Activity of Ulcerative Colitis? A Meta-Analysis. Ther. Adv. Gastroenterol. 2021, 14, 1756284821994741. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, S.J.; Yoon, H.; Park, J.; Oh, H.J.; Na, H.Y.; Lee, H.S.; Shin, C.M.; Park, Y.S.; Kim, N.; et al. Histologic Evaluation Using the Robarts Histopathology Index in Patients with Ulcerative Colitis in Deep Remission and the Association of Histologic Remission with Risk of Relapse. Inflamm. Bowel Dis. 2022, 28, 1709–1716. [Google Scholar] [CrossRef]
- Singh, A.; Bhardwaj, A.; Sharma, R.; Kaur Kahlon, B.; Dhaliwal, A.S.; Singh, D.; Kaur, S.; Jain, D.; Bansal, N.; Mahajan, R.; et al. Predictive Accuracy of Fecal Calprotectin for Histologic Remission in Ulcerative Colitis. Intest. Res. 2025, 23, 144–156. [Google Scholar] [CrossRef]
- Kawashima, K.; Oshima, N.; Kishimoto, K.; Kataoka, M.; Fukunaga, M.; Kotani, S.; Sonoyama, H.; Oka, A.; Mishima, Y.; Kazumori, H.; et al. Low Fecal Calprotectin Predicts Histological Healing in Patients with Ulcerative Colitis with Endoscopic Remission and Leads to Prolonged Clinical Remission. Inflamm. Bowel Dis. 2023, 29, 359–366, Correction in Bowel Dis. 2023, 29, 2013. [Google Scholar] [CrossRef]
- Stevens, T.W.; Gecse, K.; Turner, J.R.; de Hertogh, G.; Rubin, D.T.; D’Haens, G.R. Diagnostic Accuracy of Fecal Calprotectin Concentration in Evaluating Therapeutic Outcomes of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2333–2342. [Google Scholar] [CrossRef]
- Cannatelli, R.; Bazarova, A.; Zardo, D.; Nardone, O.M.; Shivaji, U.; Smith, S.C.L.; Gkoutos, G.; Ricci, C.; Gui, X.S.; Ghosh, S.; et al. Fecal Calprotectin Thresholds to Predict Endoscopic Remission Using Advanced Optical Enhancement Techniques and Histological Remission in IBD Patients. Inflamm. Bowel Dis. 2021, 27, 647–654. [Google Scholar] [CrossRef]
- D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Review Article: Faecal Calprotectin and Histologic Remission in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2020, 51, 689–698, Correction in Pharmacol. Ther. 2021, 53, 962–962. [Google Scholar] [CrossRef]
- Patel, A.; Panchal, H.; Dubinsky, M.C. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1600–1604. [Google Scholar] [CrossRef]
- Zittan, E.; Kelly, O.B.; Kirsch, R.; Milgrom, R.; Burns, J.; Nguyen, G.C.; Croitoru, K.; Van Assche, G.; Silverberg, M.S.; Steinhart, A.H. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 623–630. [Google Scholar] [CrossRef]
- Wilkens, R.; Dolinger, M.; Burisch, J.; Maaser, C. Point-of-Care Testing and Home Testing: Pragmatic Considerations for Widespread Incorporation of Stool Tests, Serum Tests, and Intestinal Ultrasound. Gastroenterology 2022, 162, 1476–1492. [Google Scholar] [CrossRef]
- Kucharzik, T.; Maaser, C.; Novak, K. Are We Ready to Use Activity Scores for Intestinal Ultrasound in Ulcerative Colitis? United Eur. Gastroenterol. J. 2021, 9, 423–424. [Google Scholar] [CrossRef]
- Palmela, C.; Maaser, C. The Use of Intestinal Ultrasound in Ulcerative Colitis—More Than a Mucosal Disease? Gastroenterology 2022, 163, 1485–1487. [Google Scholar] [CrossRef]
- Walsh, A.; Kormilitzin, A.; Hinds, C.; Sexton, V.; Brain, O.; Keshav, S.; Uhlig, H.; Geddes, J.; Goodwin, G.; Peters, M.; et al. Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis—A Prospective Analysis. J. Crohns Colitis 2019, 13, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Petersen, F.; Helwig, U.; Fischer, I.; Rath, S.; Kolterer, S.; Lang, D.; Kucharzik, T. Monitoring Response to Anti-TNF Therapy in Ulcerative Colitis Patients by Gastrointestinal Ultrasound: Sub-Analysis from TRUST&UC. J. Crohn’s Colitis 2019, 13, S063–S064. [Google Scholar]
- Smith, R.L.; Taylor, K.M.; Friedman, A.B.; Gibson, D.J.; Con, D.; Gibson, P.R. Early Sonographic Response to a New Medical Therapy Is Associated with Future Treatment Response or Failure in Patients with Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2022, 34, 613–621. [Google Scholar] [CrossRef]
- Ollech, J.E.; Eran-Banai, H.; Goren, I.; Sharar Fischler, T.; Avni-Biron, I.; Snir, Y.; Broitman, Y.; Cohen, S.; Friedenberg, A.; Pauker, M.H.; et al. Tofacitinib Is an Effective Treatment for Moderate to Severe Ulcerative Colitis, and Intestinal Ultrasound Can Discriminate Response from Non-Response: A Pragmatic Prospective Real-World Study. Ann. Med. 2024, 56, 2358183. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Mateen, M.A.; Pooja, K.; Marri, U.K.; Gupta, R.; Tandan, M.; Reddy, D.N. Leveraging Existing Mid-End Ultrasound Machine for Point-of-Care Intestinal Ultrasound in Low-Resource Settings: Prospective, Real-World Impact on Clinical Decision-Making. Aliment. Pharmacol. Ther. 2024, 60, 633–647. [Google Scholar] [CrossRef]
- Dubian, S.; Yzet, C.; Brazier, F.; Yzet, T.; Hautefeuille, V.; Decrombecque, C.; Bocquillon, Q.; Richard, N.; Buisson, A.; Meynier, J.; et al. Fecal Calprotectin, Intestinal Ultrasound, and Their Combination for the Diagnosis of Inflammatory Bowel Disease. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102549. [Google Scholar] [CrossRef]
- Goodsall, T.M.; Day, A.S.; Andrews, J.M.; Ruszkiewicz, A.; Ma, C.; Bryant, R.V. Composite Assessment Using Intestinal Ultrasound and Calprotectin Is Accurate in Predicting Histological Activity in Ulcerative Colitis: A Cohort Study. Inflamm. Bowel Dis. 2024, 30, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sagami, S.; Kobayashi, T.; Aihara, K.; Umeda, M.; Morikubo, H.; Matsubayashi, M.; Kiyohara, H.; Nakano, M.; Ohbu, M.; Hibi, T. Transperineal Ultrasound Predicts Endoscopic and Histological Healing in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2020, 51, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Theede, K.; Holck, S.; Ibsen, P.; Ladelund, S.; Nordgaard-Lassen, I.; Nielsen, A.M. Level of Fecal Calprotectin Correlates with Endoscopic and Histologic Inflammation and Identifies Patients with Mucosal Healing in Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 1929–1936.e1. [Google Scholar] [CrossRef] [PubMed]
- Malvão, L.D.R.; Madi, K.; Esberard, B.C.; De Amorim, R.F.; Silva, K.D.S.; Farias E Silva, K.; De Souza, H.S.P.; Carvalho, A.T.P. Fecal Calprotectin as a Non-invasive Test to Predict Deep Remission in Patients with Ulcerative Colitis. Medicine 2021, 100, E24058. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Cohen, B.L.; Velayos, F.S.; Weiss, J.M.; Sultan, S.; Siddique, S.M.; Adler, J.; Chachu, K.A. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- Yzet, C.; Meudjo, E.; Brazier, F.; Hautefeuille, V.; Moreau, C.; Robert, C.; Decrombecque, C.; Sarba, R.; Pichois, R.; Richard, N.; et al. Intestinal Ultrasound, Fecal Calprotectin, and Their Combination to Predict Endoscopic Mucosal Healing in Ulcerative Colitis: A Real-Life Cross-Sectional Study. Inflamm. Bowel Dis. 2024, 31, 1231–1236. [Google Scholar] [CrossRef]
- de Voogd, F.A.; Bots, S.J.; van Wassenaer, E.A.; de Jong, M.; Pruijt, M.J.; Haens, G.R.D.; Gecse, K.B. Early Intestinal Ultrasound Predicts Clinical and EndoscopicTreatment Response and Demonstrates Drug-Specific Kinetics in Moderate-to-Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2024, 30, 1992–2003. [Google Scholar] [CrossRef]
- Ghajarzadeh, M.; Aramesh, K. Ethics in Systematic Reviews. In Systematic Review and Meta-Analysis; Ghajarzadeh, M., Rezaei, N., Hanaei, S., Eds.; Academic Press: Cambridge, MA, USA, 2025; Chapter 18; pp. 209–213. [Google Scholar] [CrossRef]


| Feature | Goodsall et al. 2024 [32] | Sagami et al. 2020 [33] |
|---|---|---|
| Study Design | Cohort Study | Cross-sectional diagnostic accuracy study |
| Sample Size | 19 patients, 29 assessments | 53 patients (43 with histology) |
| Ultrasound Method | Transabdominal (Milan Criteria) | Transabdominal + Transperineal |
| Reference Standards | Nancy Histological Index, Mayo score | Nancy, Robarts, Geboes, Mayo |
| Main Innovation | Composite IUS + FC scoring | Targeted rectal assessment with TPUS |
| Validated Indices Used | MUC, NHI | Nancy, Robarts, Geboes |
| Blinding | Yes, all assessors were blinded | Yes, all assessors were blinded |
| Accuracy | 88% sensitivity, 80% specificity | 95.5% sensitivity, 41.6% specificity |
| GRADE Domain | Assessment Details |
|---|---|
| Outcome | Histological activity detection. |
| Study Design | Diagnostic accuracy studies (cross-sectional and cohort studies). |
| Risk of Bias | Low: Studies used blinded histology as a reference standard. |
| Inconsistency | Low: Similar results across studies despite ultrasound modality differences. |
| Indirectness | Low: Direct evaluation of UC patients using validated histological indices. |
| Imprecision | Moderate: Small sample sizes and wide CI in specificity. |
| Publication Bias | Low: All known relevant studies were included. |
| Certainty of Evidence | Moderate. |
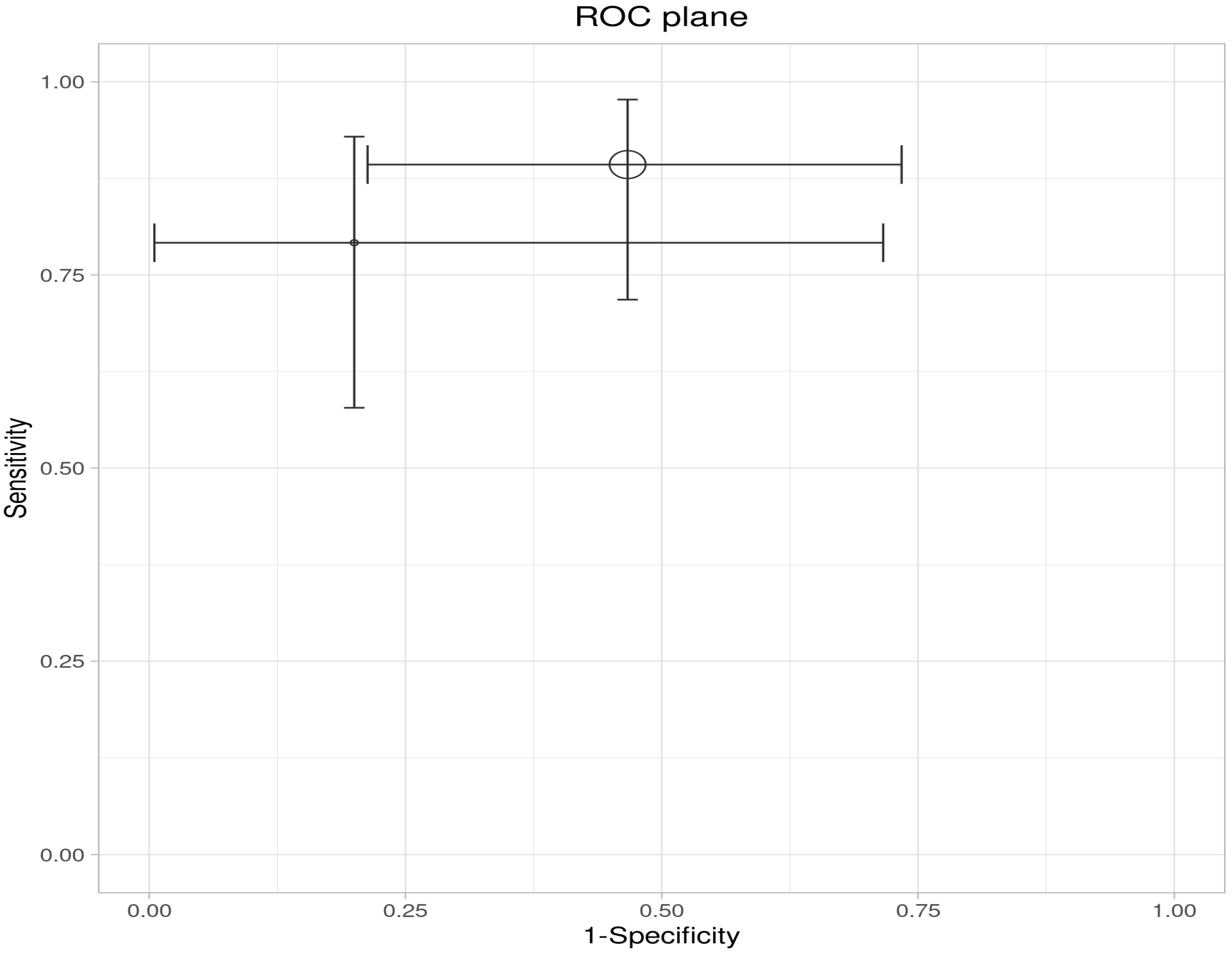
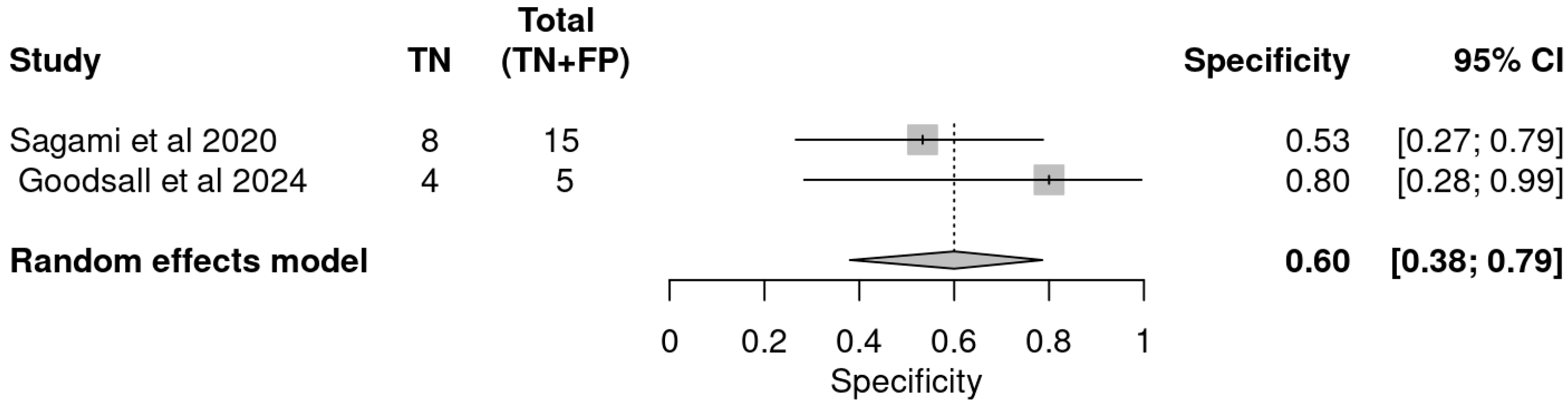
| Study | Indices | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NVP |
|---|---|---|---|---|---|---|---|---|---|
| Sagami et al. | IUS | 27 | 9 | 1 | 6 | 0.96 | 0.40 | 0.75 | 0.86 |
| FC ≥ 100 µg/g | 25 | 7 | 3 | 8 | 0.89 | 0.53 | 0.78 | 0.73 | |
| Goodsall et al. | IUS | 13 | 0 | 11 | 5 | 0.54 | 1.00 | 1.00 | 0.31 |
| FC > 100 µg/g | 19 | 1 | 5 | 4 | 0.79 | 0.80 | 0.95 | 0.44 | |
| IUS and FC > 100 | 21 | 1 | 3 | 4 | 0.88 | 0.80 | 0.95 | 0.57 |
| Estimate | 95% LCI | 95% UCI | |
|---|---|---|---|
| Sensitivity | 0.839 | 0.351 | 0.980 |
| Specificity | 0.788 | 0.068 | 0.995 |
| DOR | 19.352 | 0.208 | 1803.149 |
| LR+ | 3.959 | 0.175 | 89.568 |
| LR− | 0.205 | 0.026 | 1.624 |
| FPR | 0.212 | 0.005 | 0.932 |
| Estimate | 95% LCI | 95% UCI | |
|---|---|---|---|
| Sensitivity | 0.846 | 0.721 | 0.921 |
| Specificity | 0.600 | 0.380 | 0.786 |
| DOR | 8.250 | 2.562 | 26.569 |
| LR+ | 2.115 | 1.222 | 3.663 |
| LR− | 0.256 | 0.123 | 0.533 |
| FPR | 0.400 | 0.214 | 0.620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Izquierdo, V.; De Avila, J.; Gómez, O.; Barrero, N.; Duarte, M.; Romero-Sánchez, C. Non-Invasive vs. Invasive Markers in Ulcerative Colitis: A Systematic Review of Intestinal Ultrasound, Biopsy, and Faecal Calprotectin. Int. J. Mol. Sci. 2025, 26, 8129. https://doi.org/10.3390/ijms26178129
Parra-Izquierdo V, De Avila J, Gómez O, Barrero N, Duarte M, Romero-Sánchez C. Non-Invasive vs. Invasive Markers in Ulcerative Colitis: A Systematic Review of Intestinal Ultrasound, Biopsy, and Faecal Calprotectin. International Journal of Molecular Sciences. 2025; 26(17):8129. https://doi.org/10.3390/ijms26178129
Chicago/Turabian StyleParra-Izquierdo, Viviana, Juliette De Avila, Oscar Gómez, Nelson Barrero, Miguel Duarte, and Consuelo Romero-Sánchez. 2025. "Non-Invasive vs. Invasive Markers in Ulcerative Colitis: A Systematic Review of Intestinal Ultrasound, Biopsy, and Faecal Calprotectin" International Journal of Molecular Sciences 26, no. 17: 8129. https://doi.org/10.3390/ijms26178129
APA StyleParra-Izquierdo, V., De Avila, J., Gómez, O., Barrero, N., Duarte, M., & Romero-Sánchez, C. (2025). Non-Invasive vs. Invasive Markers in Ulcerative Colitis: A Systematic Review of Intestinal Ultrasound, Biopsy, and Faecal Calprotectin. International Journal of Molecular Sciences, 26(17), 8129. https://doi.org/10.3390/ijms26178129







