Paradoxical Use of Benralizumab in Reactive Hypereosinophilia from Toxocariasis and Tuberculosis Co-Infection—Case Report and Literature Review
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEC | Absolute Eosinophil Count |
| ADCC | Antibody-Dependent Cell-mediated Cytotoxicity |
| BMI | Body Mass Index |
| CBC | Complete Blood Count |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| ELISA | Enzyme Linked Immunosorbent Assey |
| ESR | Erythrocyte Sedimentation Rate |
| FEV1 | Forced Expiratory Volume in 1 s |
| FIP1L1 | Factor Interacting with PAPOLA and CPSF1 |
| FISH | Fluorescence In Situ Hybridization |
| GINA | Global Initiative for Asthma |
| HE | Hypereosinophilia |
| HES | Hypereosinophilic Syndrome |
| IL | Interleukine |
| LABA | Long-acting beta-agonists |
| LAMA | Long-Acting Muscarinic Antagonists |
| NK | Natural Killer cells |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| PDGFRB | Platelet-Derived Growth Factor Receptor Beta |
| Th1 | T-helper 1 cells |
| Th2 | T-helper 2 cells |
| TNF-α | Tumor Necrosis Factor-alpha |
| WHO | World Health Organisation |
References
- Rostami, A.; Riahi, S.M.; Holland, C.V.; Taghipour, A.; Khalili-Fomeshi, M.; Fakhri, Y.; Omrani, V.F.; Hotez, P.J.; Gasser, R.B. Seroprevalence Estimates for Toxocariasis in People Worldwide: A Systematic Review and Meta-Analysis. PLoS Neglected Trop. Dis. 2019, 13, e0007809. [Google Scholar] [CrossRef]
- Ma, G.; Rostami, A.; Wang, T.; Hofmann, A.; Hotez, P.J.; Gasser, R.B. Global and Regional Seroprevalence Estimates for Human Toxocariasis: A Call for Action. In Advances in Parasitology; Elseviser: New York, NY, USA, 2020; pp. 275–290. [Google Scholar]
- Chen, J.; Liu, Q.; Liu, G.-H.; Zheng, W.-B.; Hong, S.-J.; Sugiyama, H.; Zhu, X.-Q.; Elsheikha, H.M. Toxocariasis: A Silent Threat with a Progressive Public Health Impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.-K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human Toxocariasis. Lancet Infect. Dis. 2018, 18, e14–e24. [Google Scholar] [CrossRef]
- Loke, P.; Lee, S.C.; Oyesola, O.O. Effects of Helminths on the Human Immune Response and the Microbiome. Mucosal Immunol. 2022, 15, 1224–1233. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 5. [Google Scholar] [CrossRef]
- Trebuian, C.I.; Marza, A.M.; Cindrea, A.C.; Petrica, A.; Onea, S.; Sutoi, D.; Barsac, C.; Crintea-Najette, I.; Popa, D.; Chioibas, R.; et al. Risk Assessment of Venous Thromboembolism among Septic Shock Patients: Single versus Concurrent Insertion of Central Venous Catheters. Medicina 2024, 60, 785. [Google Scholar] [CrossRef] [PubMed]
- Leal-Silva, T.; Vieira-Santos, F.; Oliveira, F.M.S.; Padrão, L.d.L.S.; Kraemer, L.; da Paixão Matias, P.H.; de Almeida Lopes, C.; Loiola Ruas, A.C.; de Azevedo, I.C.; Nogueira, D.S.; et al. Detrimental Role of IL-33/ST2 Pathway Sustaining a Chronic Eosinophil-Dependent Th2 Inflammatory Response, Tissue Damage and Parasite Burden during Toxocara Canis Infection in Mice. PLoS Neglected Trop. Dis. 2021, 15, e0009639. [Google Scholar] [CrossRef]
- Ariyaratne, A.; Finney, C.A.M. Eosinophils and Macrophages within the Th2-Induced Granuloma: Balancing Killing and Healing in a Tight Space. Infect. Immun. 2019, 87, 10. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Shomali, W.; Gotlib, J. World Health Organization-Defined Eosinophilic Disorders: 2022 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2022, 97, 129–148. [Google Scholar] [CrossRef]
- Shomali, W.; Gotlib, J. World Health Organization and International Consensus Classification of Eosinophilic Disorders: 2024 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2024, 99, 946–968. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Klion, A.D.; Roufosse, F.; Simon, D.; Metzgeroth, G.; Leiferman, K.M.; Schwaab, J.; Butterfield, J.H.; Sperr, W.R.; Sotlar, K.; et al. Proposed Refined Diagnostic Criteria and Classification of Eosinophil Disorders and Related Syndromes. Allergy 2023, 78, 47–59. [Google Scholar] [CrossRef]
- Chusid, M.J.; Dale, D.C.; West, B.C.; Wolff, S.M. The Hypereosinophilic Syndrome: Analysis of Fourteen Cases with Review of the Literature. Medicine 1975, 54, 1–27. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Shomali, W.; Gotlib, J. Eosinophils and Their Disorders. In Williams Hematology, 10th ed.; Kaushansky, K., Prchal, J.T., Burns, L.J., Lichtman, M.A., Levi, M., Linch, D.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2021. [Google Scholar]
- Tefferi, A.; Patnaik, M.M.; Pardanani, A. Eosinophilia: Secondary, Clonal and Idiopathic. Br. J. Haematol. 2006, 133, 468–492. [Google Scholar] [CrossRef]
- Kahn, J.E.; Groh, M.; Lefèvre, G. (A Critical Appraisal of) Classification of Hypereosinophilic Disorders. Front. Med. 2017, 4, 216. [Google Scholar] [CrossRef]
- Ackerman, S.J.; Bochner, B.S. Mechanisms of Eosinophilia in the Pathogenesis of Hypereosinophilic Disorders. Immunol. Allergy Clin. N. Am. 2007, 27, 357–375. [Google Scholar] [CrossRef]
- Curtis, C.; Ogbogu, P. Hypereosinophilic Syndrome. Clin. Rev. Allergy Immunol. 2016, 50, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Requena, G.; Logie, J.; Gibbons, D.C.; Steinfeld, J.; Van Dyke, M.K. The Increasing Incidence and Prevalence of Hypereosinophilic Syndrome in the United Kingdom. Immun. Inflamm. Dis. 2021, 9, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Ibănescu, R.; Mîțu, D.-A.; Goje, I.-D.; Goje, G.-I.; Lighezan, D.-F. History of Heart Failure Definition. Card. Fail. Rev. 2025, 11, e07. [Google Scholar] [CrossRef]
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. 2022. Available online: www.ginasthma.org (accessed on 6 May 2025).
- Roufosse, F.; de Lavareille, A.; Schandené, L.; Cogan, E.; Georgelas, A.; Wagner, L.; Xi, L.; Raffeld, M.; Goldman, M.; Gleich, G.J.; et al. Mepolizumab as a Corticosteroid-Sparing Agent in Lymphocytic Variant Hypereosinophilic Syndrome. J. Allergy Clin. Immunol. 2010, 126, 828–835.e3. [Google Scholar] [CrossRef]
- Roufosse, F.E.; Kahn, J.-E.; Gleich, G.J.; Schwartz, L.B.; Singh, A.D.; Rosenwasser, L.J.; Denburg, J.A.; Ring, J.; Rothenberg, M.E.; Sheikh, J.; et al. Long-Term Safety of Mepolizumab for the Treatment of Hypereosinophilic Syndromes. J. Allergy Clin. Immunol. 2013, 131, 461–467.e5. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Law, M.A.; Noel, P.; Kim, Y.-J.; Haverty, T.P.; Nutman, T.B. Safety and Efficacy of the Monoclonal Anti–Interleukin-5 Antibody SCH55700 in the Treatment of Patients with Hypereosinophilic Syndrome. Blood 2004, 103, 2939–2941. [Google Scholar] [CrossRef]
- Kuang, F.L.; De Melo, M.S.; Makiya, M.; Kumar, S.; Brown, T.; Wetzler, L.; Ware, J.M.; Khoury, P.; Collins, M.H.; Quezado, M.; et al. Benralizumab Completely Depletes Gastrointestinal Tissue Eosinophils and Improves Symptoms in Eosinophilic Gastrointestinal Disease. J. Allergy Clin. Immunol. Pract. 2022, 10, 1598–1605.e2. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J.E. Type 1/Type 2 Immunity in Infectious Diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Noel, P.; Akin, C.; Law, M.A.; Gilliland, D.G.; Cools, J.; Metcalfe, D.D.; Nutman, T.B. Elevated Serum Tryptase Levels Identify a Subset of Patients with a Myeloproliferative Variant of Idiopathic Hypereosinophilic Syndrome Associated with Tissue Fibrosis, Poor Prognosis, and Imatinib Responsiveness. Blood 2003, 101, 4660–4666. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. Immunological Mechanisms by Which Concomitant Helminth Infections Predispose to the Development of Human Tuberculosis. Korean J. Parasitol. 2012, 50, 281–286. [Google Scholar] [CrossRef]
- Elias, D.; Mengistu, G.; Akuffo, H.; Britton, S. Are Intestinal Helminths Risk Factors for Developing Active Tuberculosis? Trop. Med. Int. Health 2006, 11, 551–558. [Google Scholar] [CrossRef]
- Frantz, F.G.; Rosada, R.S.; Turato, W.M.; Peres, C.M.; Coelho-Castelo, A.A.M.; Ramos, S.G.; Aronoff, D.M.; Silva, C.L.; Faccioli, L.H. The Immune Response to Toxocariasis Does Not Modify Susceptibility to Mycobacterium Tuberculosis Infection in BALB/c Mice. Am. J. Trop. Med. Hyg. 2007, 77, 691–698. [Google Scholar] [CrossRef][Green Version]
- Guila, A. Immunological Reactivity and Natural Resistance in Patients with Tuberculosis, Toxocariasis and Tuberculosis Associated with Toxocariasis. Bull. Acad. Sci. Mold. Med. Sci. 2024, 77, 239–247. [Google Scholar] [CrossRef]
- Kaneva, E.; Rainova, I.; Harizanov, R.; Kaftandjiev, I. Study of IgG Avidity and the Level of Specific IgA Antibodies and Their Significance in the Diagnosis of Human Toxocarosis. Exp. Parasitol. 2022, 236–237, 108236. [Google Scholar] [CrossRef]
- Bertici, N.S.; Tudoran, C.; Bertici, R.A.; Fira-Mladinescu, O.; Jianu, D.C.; Streian, C.G.; Staicu, R.E.; Manzur, A.R.; Lascu, A. Concomitance of Pericardial Tamponade and Pulmonary Embolism in an Invasive Mucinous Lung Adenocarcinoma with Atypical Presentation: Diagnostic and Therapeutic Pitfalls—Case Report and Literature Review. Int. J. Mol. Sci. 2024, 25, 8413. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Zheng, Y.-F.; Hu, Y.-Q.; Huang, J.-X.; Yuan, Z.-X.; Wu, Z.-Y.; Huang, L.-F.; Tang, C.-T.; Zhang, F.-Y.; Chen, Y.; et al. Diagnostic Accuracy of Xpert MTB/RIF Ultra for Detecting Pulmonary Tuberculosis and Rifampicin Resistance: A Systematic Review and Meta-Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 681–702. [Google Scholar] [CrossRef]
- Haftu, H.; Tadese, K.; Gebrehiwot, T.; Geberezgabiher, H. How Common Is Eosinophilia in Tuberculosis? Case Report. Pediatr. Health Med. Ther. 2020, 11, 59–63. [Google Scholar] [CrossRef]
- Garg, G.; Gogia, A.; Kakar, A.; Miglani, P. Persistent Marked Peripheral Eosinophilia Due to Tuberculosis: A Case Report. Iran. J. Med. Sci. 2017, 42, 102–105. [Google Scholar]
- Damera, A.R.; Gupta, P.; Farooqi, S.; Sanker, V.; Mathews, A.M.; Pampati, S.; Allala, M.R.; Dave, T. Tuberculosis Manifesting with Significant Peripheral Eosinophilia: A Case Report and Review of Literature. Clin. Case Rep. 2023, 11, e8085. [Google Scholar] [CrossRef]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a Humanized Anti–IL-5 Receptor α MAb with Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity Function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar] [CrossRef]
- Salimi Khorashad, A.; Shahraki, M.; Rahmati Balaghaleh, M.; Abdolahi Khabisi, S.; Rala, S.; Shafiei, R.; Mirahmadi, H. Seroprevalence of Toxocara Spp. in Children (3–13 Years Old) in Zahedan, Southeast of Iran. J. Parasit. Dis. 2021, 45, 449–453. [Google Scholar] [CrossRef]
- Mellado-Sola, I.; Rodríguez-Molino, P.; Armas, E.-A.; Nogueira López, J.; Falces-Romero, I.; Rey, C.C.; Grasa Lozano, C.; Mellado, M.J.; López-Hortelano, M.G.; Sainz, T. Impact of Coronavirus Pandemic on Tuberculosis and Other Imported Diseases Screening among Migrant Minors in Spain. Trop. Med. Infect. Dis. 2022, 8, 28. [Google Scholar] [CrossRef]
- Caminati, M.; Brussino, L.; Carlucci, M.; Carlucci, P.; Carpagnano, L.F.; Caruso, C.; Cosmi, L.; D’Amore, S.; Del Giacco, S.; Detoraki, A.; et al. Managing Patients with Hypereosinophilic Syndrome: A Statement from the Italian Society of Allergy, Asthma, and Clinical Immunology (SIAAIC). Cells 2024, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Saha, A.; Kuykendall, A.; Zhang, L. Clinical and Therapeutic Intervention of Hypereosinophilia in the Era of Molecular Diagnosis. Cancers 2024, 16, 1383. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D. Approach to the Patient with Suspected Hypereosinophilic Syndrome. Hematology 2022, 2022, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.S.; Wechsler, M.E. Eosinophilic Respiratory Disorders and the Impact of Biologics. Curr. Opin. Pulm. Med. 2023, 29, 202–208. [Google Scholar] [CrossRef]
- Pitlick, M.M.; Li, J.T.; Pongdee, T. Current and Emerging Biologic Therapies Targeting Eosinophilic Disorders. World Allergy Organ. J. 2022, 15, 100676. [Google Scholar] [CrossRef]
- Kuang, F.L.; Legrand, F.; Makiya, M.; Ware, J.; Wetzler, L.; Brown, T.; Magee, T.; Piligian, B.; Yoon, P.; Ellis, J.H.; et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N. Engl. J. Med. 2019, 380, 1336–1346. [Google Scholar] [CrossRef]
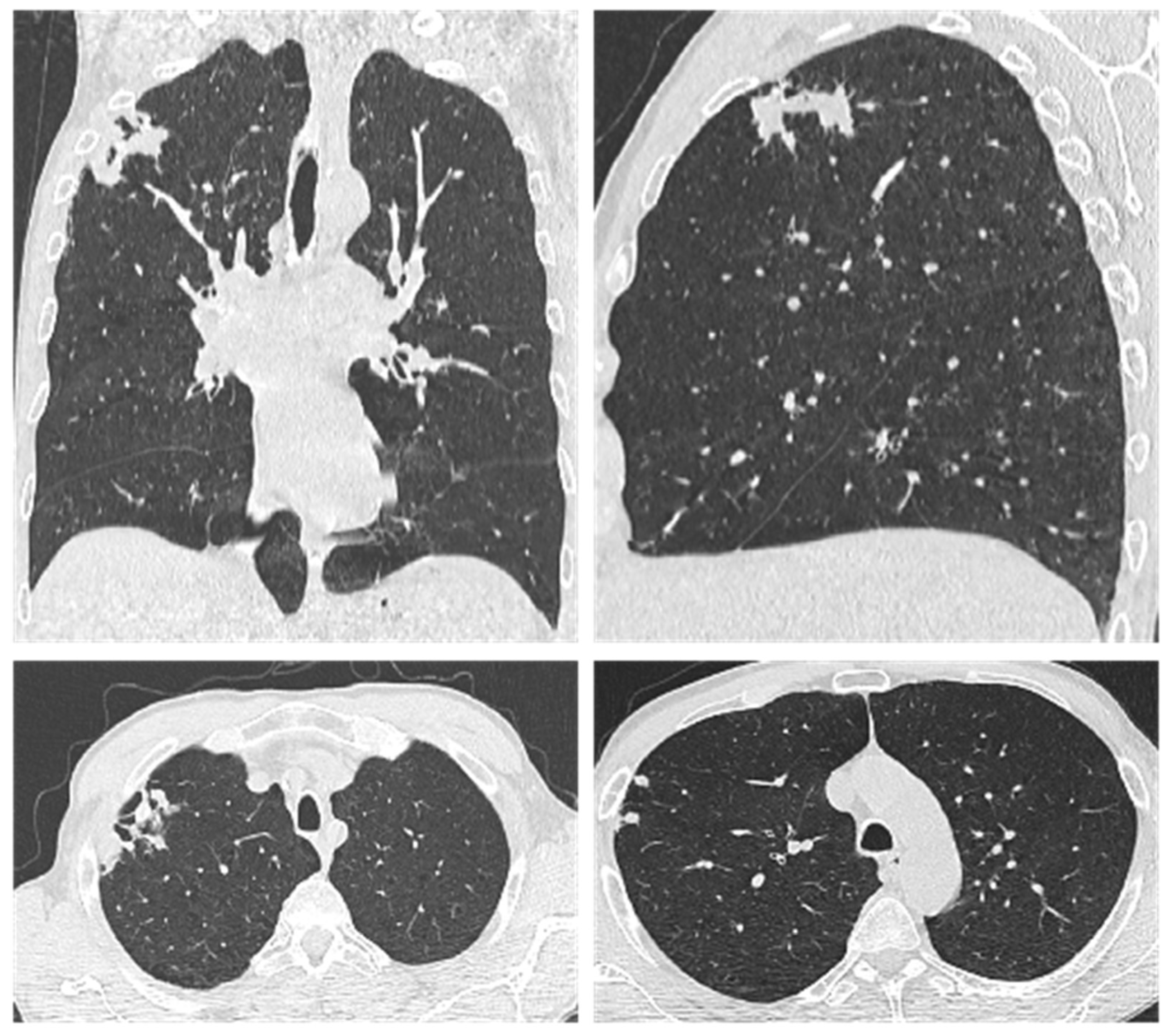
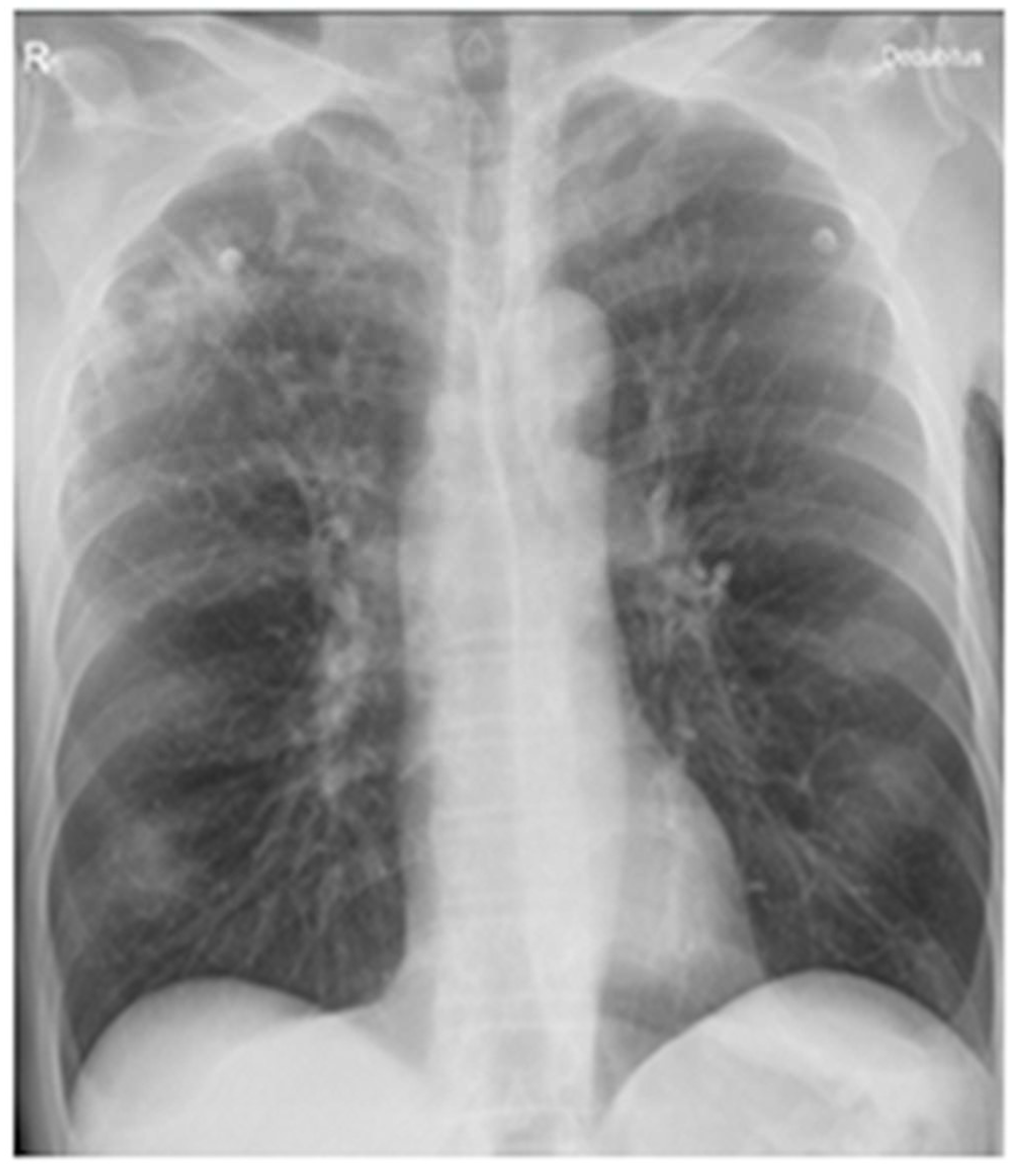
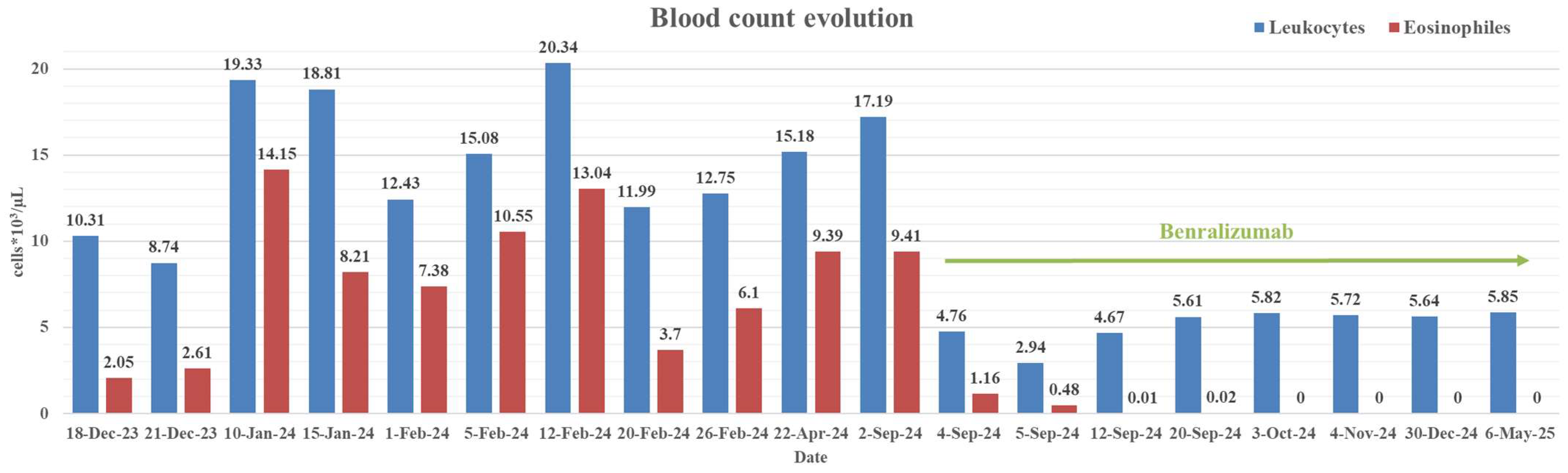

| Parameter (Normal Value) | 12 January 2023 | 18 December 2023 | 3 September 2024 | 6 May 2025 |
|---|---|---|---|---|
| BMI (kg/m2) | 20.9 | 19.59 | 21.22 | 21.79 |
| FEV1 (normal estimated 3.5 L) | 1.82 (52%) | - | 1.5 a (43%) | 1.86 a (53%) |
| CRP (0–5 mg/L) | 6.12 | 9.11 | 32.5 | 1.14 |
| ESR (3–10 mm/1 h) | 15 | 10 | 20 | 5 |
| Leucocytes (4–10 × 103/µL) | 6.2 | 10.31 | 17.19 | 5.85 |
| Eosinophils (0–0.7 × 103/µL) | 0.26 (3.7%) | 2.61 (29.9%) | 9.41 (54.7%) | 0 (0%) |
| Hemoglobin (14–17.2 g/dL) | 12.4 | 14.1 | 13.5 | 16 |
| IgE (0–100 UI/mL) | - | 2268.1 | 13,480.2 | >2500 b |
| Toxocara canis IgG (index < 0.9) | - | - | 3.892 | 4.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertici, N.S.; Cut, T.G.; Ridichie, A.; Manzur, A.R.; Bertici, R.A. Paradoxical Use of Benralizumab in Reactive Hypereosinophilia from Toxocariasis and Tuberculosis Co-Infection—Case Report and Literature Review. Int. J. Mol. Sci. 2025, 26, 8117. https://doi.org/10.3390/ijms26178117
Bertici NS, Cut TG, Ridichie A, Manzur AR, Bertici RA. Paradoxical Use of Benralizumab in Reactive Hypereosinophilia from Toxocariasis and Tuberculosis Co-Infection—Case Report and Literature Review. International Journal of Molecular Sciences. 2025; 26(17):8117. https://doi.org/10.3390/ijms26178117
Chicago/Turabian StyleBertici, Nicoleta Sorina, Talida Georgiana Cut, Amalia Ridichie, Andrei Raul Manzur, and Razvan Adrian Bertici. 2025. "Paradoxical Use of Benralizumab in Reactive Hypereosinophilia from Toxocariasis and Tuberculosis Co-Infection—Case Report and Literature Review" International Journal of Molecular Sciences 26, no. 17: 8117. https://doi.org/10.3390/ijms26178117
APA StyleBertici, N. S., Cut, T. G., Ridichie, A., Manzur, A. R., & Bertici, R. A. (2025). Paradoxical Use of Benralizumab in Reactive Hypereosinophilia from Toxocariasis and Tuberculosis Co-Infection—Case Report and Literature Review. International Journal of Molecular Sciences, 26(17), 8117. https://doi.org/10.3390/ijms26178117








