Safety and Efficacy of Diquafosol Compared to Artificial Tears for the Treatment of Dry Eye: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Databases and Information Sources
2.4. Search Methods
2.5. Study Eligibility Criteria
2.6. Outcome Measures
2.7. Shared Time Points and Outcomes
2.8. Data Collection and Analysis
2.9. Risk of Bias Assessment
2.10. Statistical Analysis
3. Results
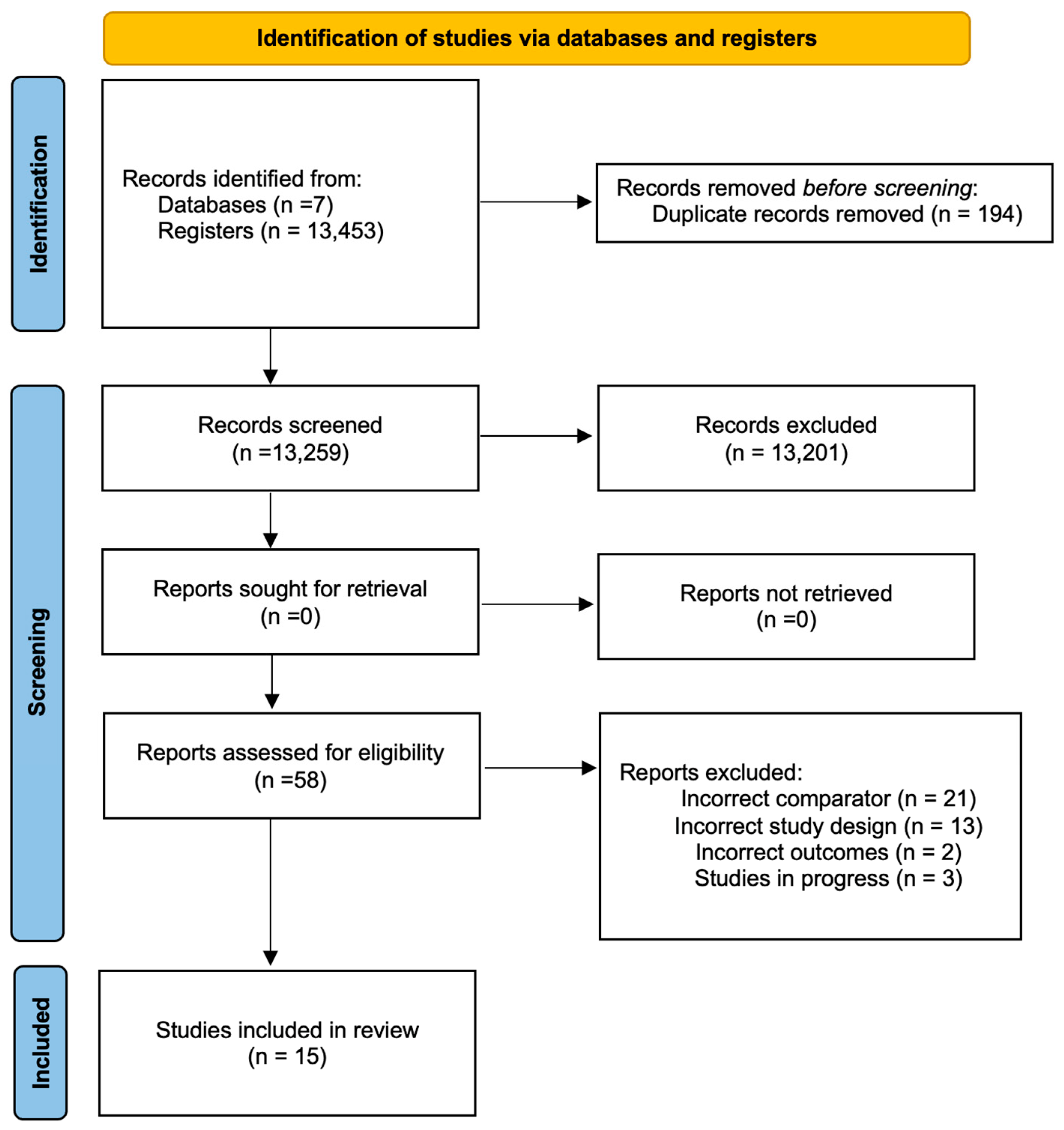
3.1. Literature Search
Risk-of-Bias Assessment
3.2. Interventions
3.2.1. Diquafosol 3% vs. Artificial Tears
3.2.2. Diquafosol 3% in Post-Cataract Patients
3.2.3. Pilocarpine vs. Artificial Tears
3.2.4. Cevimeline Trials
3.3. Effects of Interventions
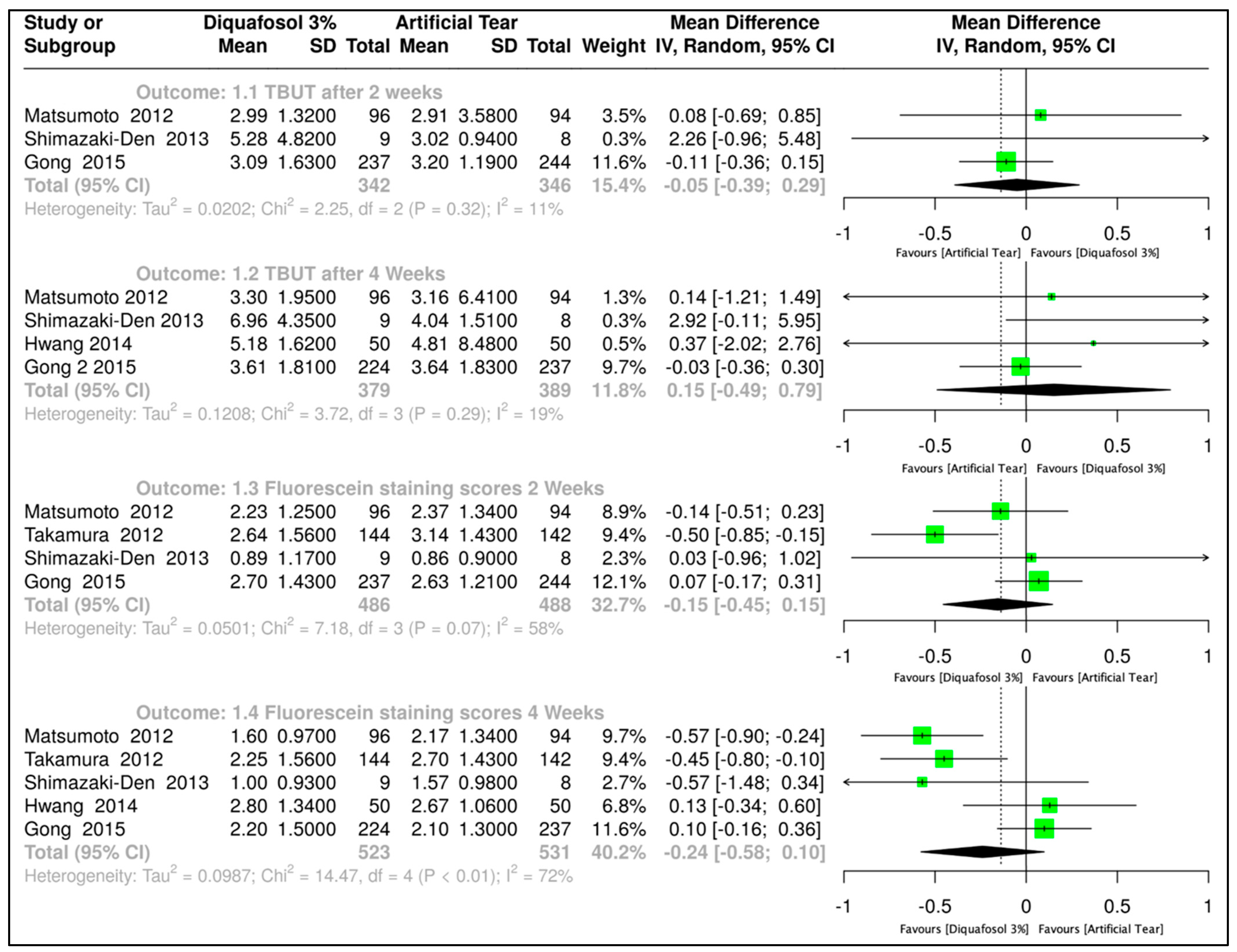
3.3.1. Diquafosol 3% vs. Artificial Tears
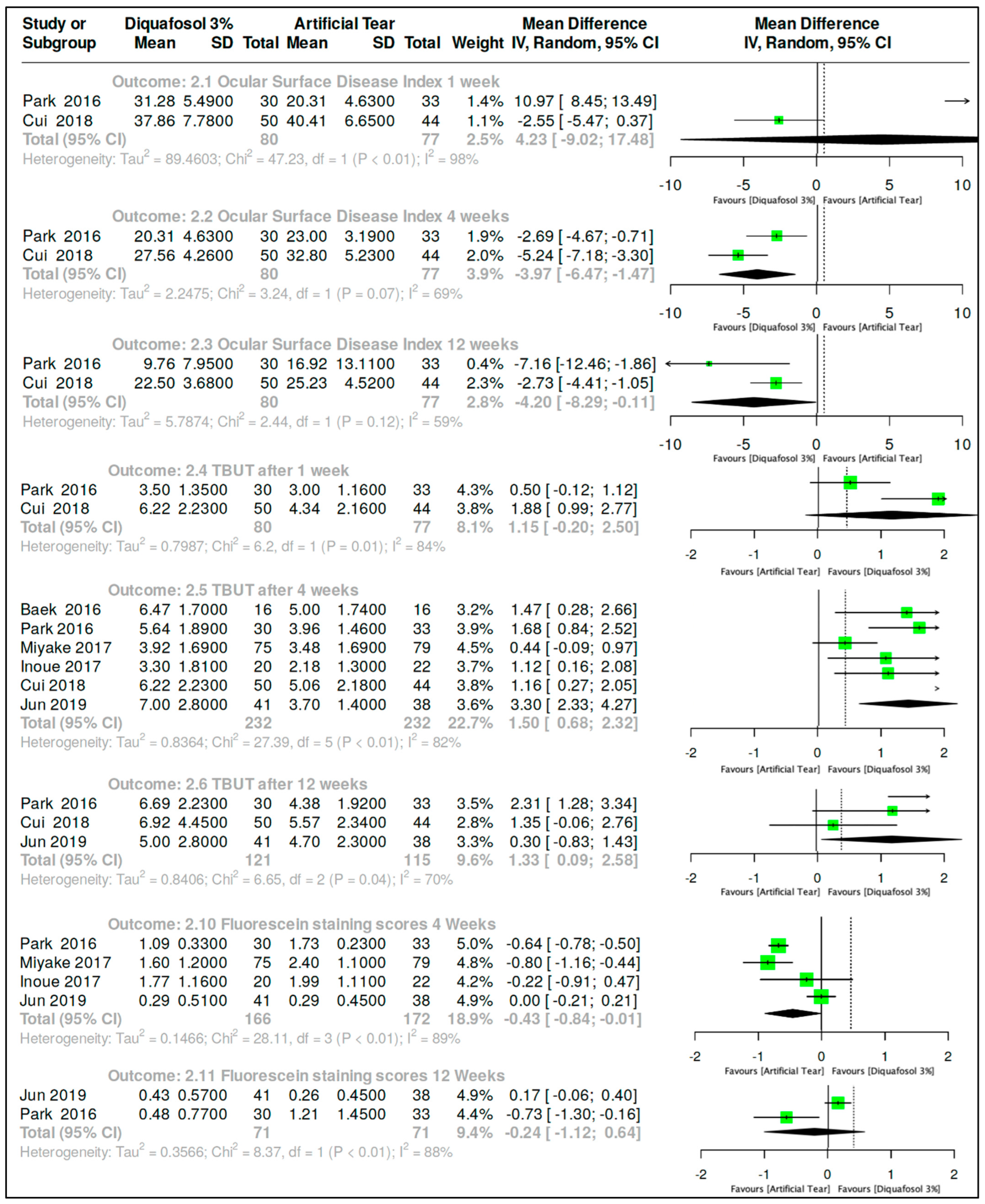
3.3.2. Diquafosol 3% vs. Artificial Tears After Cataract Surgery
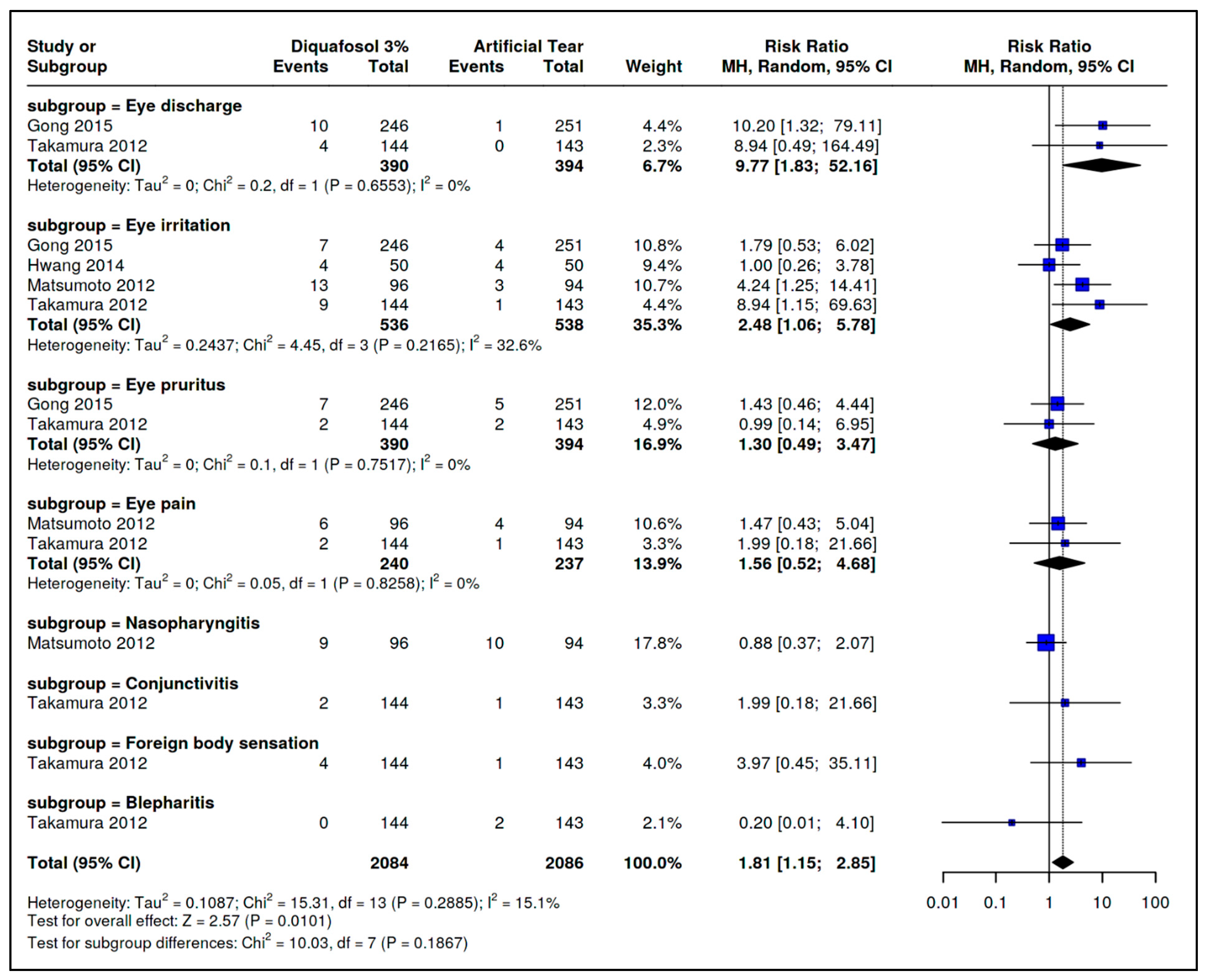
3.4. Safety Outcomes
4. Discussion
4.1. Quality of the Evidence
4.2. Overall Completeness and Applicability of Evidence
5. Conclusions
Implications for Research
- Compare treatment outcomes across clinically distinct subtypes of dry eye disease (e.g., aqueous-deficient vs. evaporative);
- Assess the dose–response relationships and adverse event profiles of pilocarpine and cevimeline;
- Investigate potential differences in efficacy and tolerability between systemic (oral) and topical administration routes;
- Incorporate relevant biological indicators (e.g., tear osmolarity, inflammatory cytokines, MMP-9) to better understand mechanisms of action;
- Include validated patient-centered outcomes such as vision-related quality of life and standardized symptom questionnaires;
- Prospectively register protocols and follow predefined methods and outcomes;
- Clearly describe randomization, allocation concealment, and masking procedures;
- Document handling of missing data and perform intention-to-treat analyses;
- Evaluate outcomes at standardized time points (e.g., 4, 12, and 24 weeks);
- Report adverse events in a stratified and comprehensive manner.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheppard, J.; Lee, B.S.; Periman, L.M. Dry eye disease: Identification and therapeutic strategies for primary care clinicians and clinical specialists. Ann. Med. 2022, 55, 241–252. [Google Scholar] [CrossRef]
- Bradley, J.L.; Stillman, I.Ö.; Pivneva, I.; Guerin, A.; Evans, A.M.; Dana, R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin. Ophthalmol. 2019, 13, 225–232. [Google Scholar] [CrossRef]
- Nichols, K.K. The International Workshop on Meibomian Gland Dysfunction: Introduction. Investig. Opthalmology Vis. Sci. 2011, 52, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Longo, D.L. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.Ö.; Evans, A.M.; Schaumberg, D.A. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Arch. Ophthalmol. 2019, 202, 47–54. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-Del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee. Investig. Opthalmology Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef]
- Şimşek, C.; Doğru, M.; Kojima, T.; Tsubota, K. Current Management and Treatment of Dry Eye Disease. Turk. J. Ophthalmol. 2018, 48, 309–313. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z. Multidimensional immunotherapy for dry eye disease: Current status and future directions. Front. Ophthalmol. 2024, 4, 1449283. [Google Scholar] [CrossRef]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, B.S.; Kabat, A.G.; Bacharach, J.; Karpecki, P.; Luchs, J. Managing Dry Eye Disease and Facilitating Realistic Patient Expectations: A Review and Appraisal of Current Therapies. Clin. Ophthalmol. 2020, 14, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; El-Hamid, B.N.A.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef] [PubMed]
- Rauz, S.; Koay, S.-Y.; Foot, B.; Kaye, S.B.; Figueiredo, F.; Burdon, M.A.; Dancey, E.; Chandrasekar, A.; Lomas, R. The Royal College of Ophthalmologists guidelines on serum eye drops for the treatment of severe ocular surface disease: Full report. Eye 2017, eye2017209. [Google Scholar] [CrossRef] [PubMed]
- Donthineni, P.R.; Doctor, M.B.; Shanbhag, S.; Kate, A.; Galor, A.; Djalilian, A.R.; Singh, S.; Basu, S. Aqueous-deficient dry eye disease: Preferred practice pattern guidelines on clinical approach, diagnosis, and management. Indian J. Ophthalmol. 2023, 71, 1332–1347. [Google Scholar] [CrossRef]
- Gupta, P.K.; Toyos, R.; Sheppard, J.D.; Toyos, M.; Mah, F.S.; Bird, B.; Theriot, P.; Higgins, D. Tolerability of Current Treatments for Dry Eye Disease: A Review of Approved and Investigational Therapies. Clin. Ophthalmol. 2024, 18, 2283–2302. [Google Scholar] [CrossRef]
- Park, D.H.; Chung, J.K.; Seo, D.R.; Lee, S.J. Clinical Effects and Safety of 3% Diquafosol Ophthalmic Solution for Patients with Dry Eye After Cataract Surgery: A Randomized Controlled Trial. Arch. Ophthalmol. 2016, 163, 122–131.e2. [Google Scholar] [CrossRef]
- Shimazaki, J.; Seika, D.; Saga, M.; Fukagawa, K.; Sakata, M.; Iwasaki, M.; Okano, T. A Prospective, Randomized Trial of Two Mucin Secretogogues for the Treatment of Dry Eye Syndrome in Office Workers. Sci. Rep. 2017, 7, 15210. [Google Scholar] [CrossRef]
- Ono, M.; Takamura, E.; Shinozaki, K.; Tsumura, T.; Hamano, T.; Yagi, Y.; Tsubota, K. Therapeutic effect of cevimeline on dry eye in patients with Sjögren’s syndrome: A randomized, double-blind clinical study. Arch. Ophthalmol. 2004, 138, 6–17. [Google Scholar] [CrossRef]
- Singer, M.C.; Marchal, F.; Angelos, P.; Bernet, V.; Boucai, L.; Buchholzer, S.; Burkey, B.; Eisele, D.; Erkul, E.; Faure, F.; et al. Salivary and lacrimal dysfunction after radioactive iodine for differentiated thyroid cancer: American Head and Neck Society Endocrine Surgery Section and Salivary Gland Section joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology. Head Neck 2020, 42, 3446–3459. [Google Scholar] [CrossRef]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vadlamudi, V.; Toshida, H.; Beuerman, R.W. Loss of parasympathetic innervation leads to sustained expression of pro-inflammatory genes in the rat lacrimal gland. Auton. Neurosci. 2006, 124, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Dota, A.; Sakamoto, A.; Nagano, T.; Murakami, T.; Matsugi, T. Effect of Diquafosol Ophthalmic Solution on Airflow-Induced Ocular Surface Disorder in Diabetic Rats. Clin. Ophthalmol. 2020, 14, 1019–1024. [Google Scholar] [CrossRef]
- Ohashi, Y.; Munesue, M.; Shimazaki, J.; Takamura, E.; Yokoi, N.; Watanabe, H.; Nomura, A.; Shimada, F. Long-Term Safety and Effectiveness of Diquafosol for the Treatment of Dry Eye in a Real-World Setting: A Prospective Observational Study. Adv. Ther. 2019, 37, 707–717. [Google Scholar] [CrossRef]
- Yang, C.-J.; Anand, A.; Huang, C.-C.; Lai, J.-Y. Unveiling the Power of Gabapentin-Loaded Nanoceria with Multiple Therapeutic Capabilities for the Treatment of Dry Eye Disease. ACS Nano 2023, 17, 25118–25135. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Serrano-Robles, J.G.; Vázquez, A.K.P.; Navas, A.; Graue-Hernandez, E.O.; Ramirez-Miranda, A.; Kahuam-López, N. Safety and efficacy of pilocarpine, cevimeline, and diquafosol compared to artificial tears for the treatment of dry eye: Protocol for a systematic review. Syst. Rev. 2022, 11, 105. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Covidence. Melbourne: Veritas Health Innovation. Covidence Systematic Review Software [Internet]. 28 June 2022. Available online: https://www.covidence.org/ (accessed on 13 October 2021).
- Matsumoto, Y.; Ohashi, Y.; Watanabe, H.; Tsubota, K. Efficacy and Safety of Diquafosol Ophthalmic Solution in Patients with Dry Eye Syndrome: A Japanese Phase 2 Clinical Trial. Ophthalmology 2012, 119, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki-Den, S.; Iseda, H.; Dogru, M.; Shimazaki, J. Effects of Diquafosol Sodium Eye Drops on Tear Film Stability in Short BUT Type of Dry Eye. Cornea 2013, 32, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Sun, X.; Ma, Z.; Wang, Q.; Xu, X.; Chen, X.; Shao, Y.; Yao, K.; Tang, L.; Gu, Y.; et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br. J. Ophthalmol. 2015, 99, 903–908. [Google Scholar] [CrossRef]
- Hwang, H.S.; Sung, Y.-M.; Lee, W.S.; Kim, E.C. Additive Effect of Preservative-free Sodium Hyaluronate 0.1% in Treatment of Dry Eye Syndrome with Diquafosol 3% Eye Drops. Cornea 2014, 33, 935–941. [Google Scholar] [CrossRef]
- Takamura, E.; Tsubota, K.; Watanabe, H.; Ohashi, Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 2012, 96, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, Y.; Lee, H.S.; Yang, J.M.; Choi, W.; Yoon, K.C. Effect of diquafosol tetrasodium 3% on the conjunctival surface and clinical findings after cataract surgery in patients with dry eye. Int. Ophthalmol. 2017, 38, 2021–2030. [Google Scholar] [CrossRef]
- Inoue, Y.; Ochi, S. Effects of 3% diquafosol sodium ophthalmic solution on higher-order aberrations in patients diagnosed with dry eye after cataract surgery. Clin. Ophthalmol. 2016, 11, 87–93. [Google Scholar] [CrossRef]
- Miyake, K.; Yokoi, N. Influence on ocular surface after cataract surgery and effect of topical diquafosol on postoperative dry eye: A multicenter prospective randomized study. Clin. Ophthalmol. 2017, 11, 529–540. [Google Scholar] [CrossRef]
- Jun, I.; Choi, S.; Lee, G.Y.; Choi, Y.J.; Lee, H.K.; Kim, E.K.; Seo, K.Y.; Kim, T.-I. Effects of Preservative-free 3% Diquafosol in Patients with Pre-existing Dry Eye Disease after Cataract Surgery: A Randomized Clinical Trial. Sci. Rep. 2019, 9, 12659. [Google Scholar] [CrossRef]
- Baek, J.; Doh, S.H.; Chung, S.K. The Effect of Topical Diquafosol Tetrasodium 3% on Dry Eye After Cataract Surgery. Curr. Eye Res. 2016, 41, 1281–1285. [Google Scholar] [CrossRef]
- Tsifetaki, N.; Kitsos, G.; Paschides, C.A.; Alamanos, Y.; Eftaxias, V.; Voulgari, P.V.; Psilas, K.; Drosos, A.A. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren’s syndrome: A randomised 12 week controlled study. Ann. Rheum. Dis. 2003, 62, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.; Condemi, J.J.; Fife, R.; Gluck, O.; Cohen, S.; Dalgin, P. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002, 46, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.M.; McMillan, A.S.; Wong, M.C.M.; Leung, W.K.; Mok, M.Y.; Lau, C.S. The efficacy of cevimeline hydrochloride in the treatment of xerostomia in Sjögren’s syndrome in southern Chinese patients: A randomised double-blind, placebo-controlled crossover study. Clin. Rheumatol. 2007, 27, 429–436. [Google Scholar] [CrossRef]
- Kaido, M.; Kawashima, M.; Shigeno, Y.; Yamada, Y.; Tsubota, K. Randomized Controlled Study to Investigate the Effect of Topical Diquafosol Tetrasodium on Corneal Sensitivity in Short Tear Break-Up Time Dry Eye. Adv. Ther. 2018, 35, 697–706. [Google Scholar] [CrossRef]
- Fukuoka, S.; Arita, R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: A randomized clinical study. Sci. Rep. 2019, 9, 9091. [Google Scholar] [CrossRef]
- Miyake, H.; Kawano, Y.; Tanaka, H.; Iwata, A.; Imanaka, T.; Nakamura, M. Tear volume estimation using a modified Schirmer test: A randomized, multicenter, double-blind trial comparing 3% diquafosol ophthalmic solution and artificial tears in dry eye patients. Clin. Ophthalmol. 2016, 10, 879–886. [Google Scholar] [CrossRef]
- Kim, S.; Shin, J.; Lee, J.E. A randomised, prospective study of the effects of 3% diquafosol on ocular surface following cataract surgery. Sci. Rep. 2021, 11, 9124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, Y.; Li, M.; Shi, Y.; Liu, L.; Sun, L.; Ye, H.; Zou, J. Combination Therapy with Diquafosol Sodium and Sodium Hyaluronate in Eyes with Dry Eye Disease After Small Incision Lenticule Extraction. Vivo 2023, 37, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, S.; Chen, Y. Comparison of the efficacy between topical diquafosol and artificial tears in the treatment of dry eye following cataract surgery. Medicine 2017, 96, e8174. [Google Scholar] [CrossRef] [PubMed]
| Comparison Diquafosol 3% vs. Artificial Tears | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Certainty Assessment | No. of Patients | Effect Size | Certainty | ||||||
| No Studies | Study Design | Risk of Bias | Inconsistency | Imprecision | [Diquafosol 3%] | [Artificial Tear] | Absolute (95% CI) | ||
| Tear Film Breakup Time after two weeks of treatment | |||||||||
| 3 | Randomized trials | Not serious | Not serious | Serious a | 342 | 346 | MD 0.05 (0.39–0.29) | ⨁⨁⨁◯ Moderate | |
| Tear Film Breakup Time after four weeks of treatment | |||||||||
| 4 | Randomized trials | Not serious | Not serious | Serious a | 379 | 389 | MD 0.15 (0.49–0.79) | ⨁⨁⨁◯ Moderate | |
| Fluorescein staining score two weeks of treatment | |||||||||
| 4 | Randomized trials | Not serious | Serious b | Serious a | 486 | 488 | MD 0.15 (0.45–0.15) | ⨁⨁◯◯ Low | |
| Fluorescein staining score four weeks of treatment | |||||||||
| 5 | Randomized trials | Not serious | Serious b | Serious a | 523 | 531 | MD 0.24 (0.58–0.1) | ⨁⨁◯◯ Low | |
| Comparison Diquafosol 3% vs. Artificial Tears in post-cataract surgery subjects | |||||||||
| Ocular Surface Disease Index after one week of treatment. | |||||||||
| 2 | Randomized trials | Not serious | Serious c | Serious d | 80 | 77 | MD 4.23 (9.02–17.48) | ⨁⨁◯◯ Low | |
| Ocular Surface Disease Index after four weeks of treatment. | |||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | 80 | 77 | MD 3.97 (6.47–1.47) | ⨁⨁⨁◯ Moderate | |
| Ocular Surface Disease Index after twelve weeks of treatment. | |||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | 80 | 77 | MD 4.2 (8.29–0.11) | ⨁⨁⨁◯ Moderate | |
| Tear Film Breakup Time after one week of treatment. | |||||||||
| 2 | Randomized trials | Not serious | Serious e | Serious e | 80 | 77 | MD 1.15 (0.2–2.5) | ⨁⨁◯◯ Low | |
| Tear Film Breakup Time after four weeks of treatment. | |||||||||
| 6 | Randomized trials | Serious c | Not serious | Not serious | 232 | 232 | MD 1.5 (0.68–2.32) | ⨁⨁⨁◯ Moderate | |
| Tear Film Breakup Time after twelve weeks of treatment. | |||||||||
| 3 | Randomized trials | Not serious | Serious c | Serious e | 121 | 115 | MD 1.33 (0.09–2.58) | ⨁⨁◯◯ Low | |
| Fluorescein staining after four weeks of treatment. | |||||||||
| 4 | Randomized trials | Serious e | Serious e | Serious b | 166 | 172 | MD 0.43 (0.84–0.01) | ⨁◯◯◯ Very low | |
| Fluorescein staining after four weeks of treatment. | |||||||||
| 2 | Randomized trials | Not serious | Serious e | Serious b | 71 | 71 | MD 0.24 (1.12–0.64) | ⨁⨁◯◯ Low | |
| Adverse Effects of the Comparison Diquafosol 3% vs. Artificial Tears | |||||||||
| Certainty Assessment | No. of Patients | Effect Size | Certainty | ||||||
| No Studies | Study Design | Inconsistency | Imprecision | Other Considerations | [Diquafosol 3%] | [Artificial Tear] | Relative (95% CI) | Absolute (95% CI) | |
| Adverse event: Ocular discharge | |||||||||
| 2 | Randomized trials | Not serious | Serious a | Strong association | 14/390 (3.6%) | 1/394 (0.3%) | RR 9.77 (1.83–52.16) | 22 more per 1000 (from 2 more to 130 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Eye Irritation | |||||||||
| 4 | Randomized trials | Serious c | Serious a | Strong association | 33/536 (6.2%) | 12/538 (2.2%) | RR 2.48 (1.06–5.78) | 33 more per 1000 (from 1 more to 107 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Ocular itching | |||||||||
| 2 | Randomized trials | Not serious | Serious a | None | 9/390 (2.3%) | 7/394 (1.8%) | RR 1.30 (0.49–3.47) | 5 more per 1000 (from 9 less to 44 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Eye pain | |||||||||
| 2 | Randomized trials | Not serious | Serious a | None | 8/240 (3.3%) | 5/237 (2.1%) | RR 1.56 (0.52–4.68) | 12 more per 1000 (from 10 less to 78 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Conjunctivitis | |||||||||
| 1 | Randomized trials | Not serious | Serious a | None | 2/144 (1.4%) | 1/143 (0.7%) | RR 1.99 (0.18–21.66) | 7 more per 1000 (from 6 less to 144 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Foreign body sensation | |||||||||
| 1 | Randomized trials | Not serious | Serious a | None | 4/144 (2.8%) | 1/143 (0.7%) | RR 3.97 (0.45–5.11) | 7 more per 1000 (from 6 less to 144 more) | ⨁⨁⨁◯ Moderate |
| Adverse event: Blepharitis | |||||||||
| 1 | Randomized trials | Not serious | Serious a | None | 0/144 (0.0%) | 2/143 (1.4%) | RR 0.20 (0.01–4.10) | 11 less per 1000 (from 14 less to 43 more) | ⨁⨁⨁◯ Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Robles, J.G.; Pérez-Vázquez, A.K.; Vera-Duarte, G.R.; Navas, A.; Ramirez-Miranda, A.; Graue-Hernandez, E.O.; Kahuam-López, N. Safety and Efficacy of Diquafosol Compared to Artificial Tears for the Treatment of Dry Eye: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 8113. https://doi.org/10.3390/ijms26178113
Serrano-Robles JG, Pérez-Vázquez AK, Vera-Duarte GR, Navas A, Ramirez-Miranda A, Graue-Hernandez EO, Kahuam-López N. Safety and Efficacy of Diquafosol Compared to Artificial Tears for the Treatment of Dry Eye: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(17):8113. https://doi.org/10.3390/ijms26178113
Chicago/Turabian StyleSerrano-Robles, José Gerardo, Ana Karen Pérez-Vázquez, Guillermo Raul Vera-Duarte, Alejandro Navas, Arturo Ramirez-Miranda, Enrique O. Graue-Hernandez, and Nicolás Kahuam-López. 2025. "Safety and Efficacy of Diquafosol Compared to Artificial Tears for the Treatment of Dry Eye: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 17: 8113. https://doi.org/10.3390/ijms26178113
APA StyleSerrano-Robles, J. G., Pérez-Vázquez, A. K., Vera-Duarte, G. R., Navas, A., Ramirez-Miranda, A., Graue-Hernandez, E. O., & Kahuam-López, N. (2025). Safety and Efficacy of Diquafosol Compared to Artificial Tears for the Treatment of Dry Eye: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(17), 8113. https://doi.org/10.3390/ijms26178113







