Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials
Abstract
1. Introduction
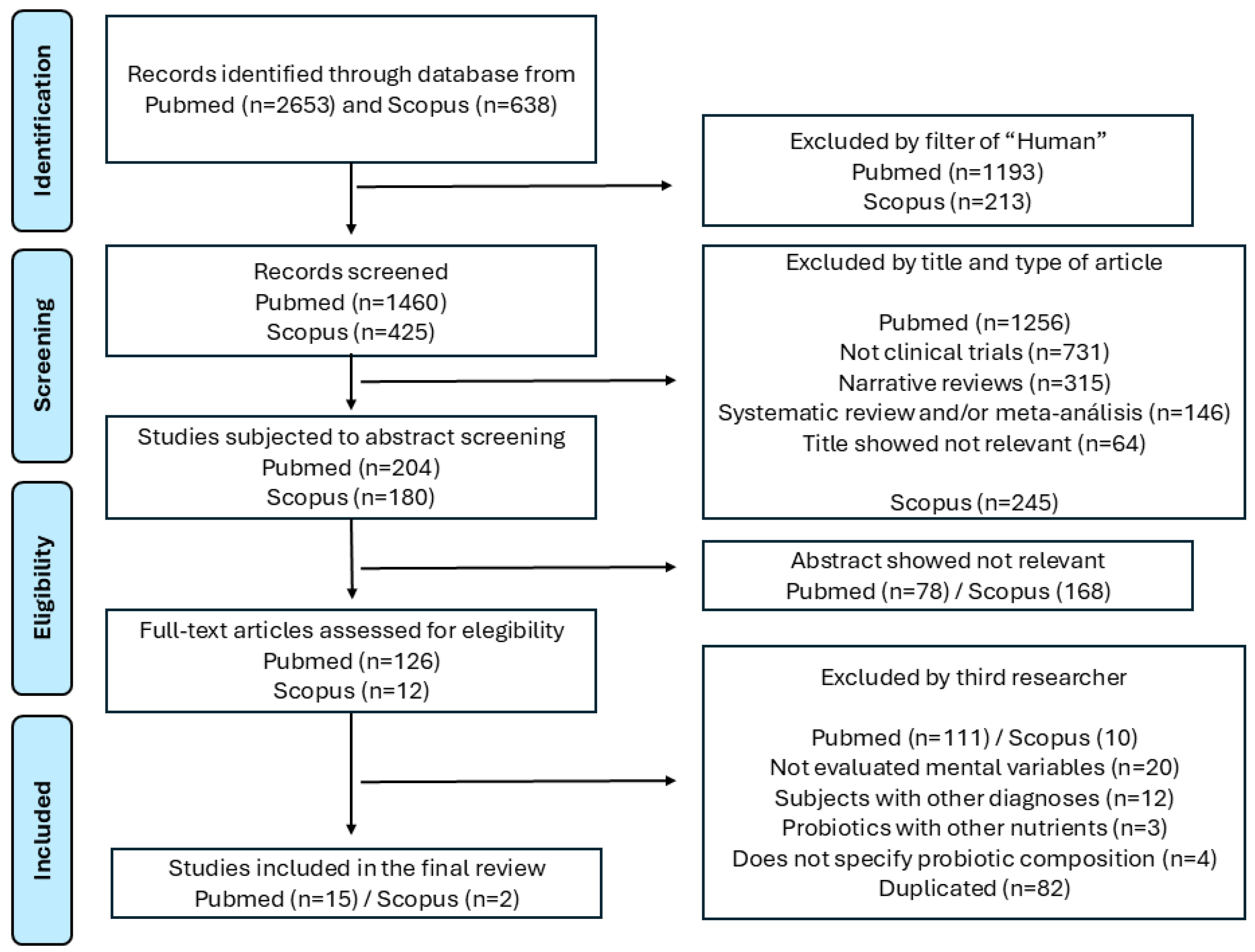
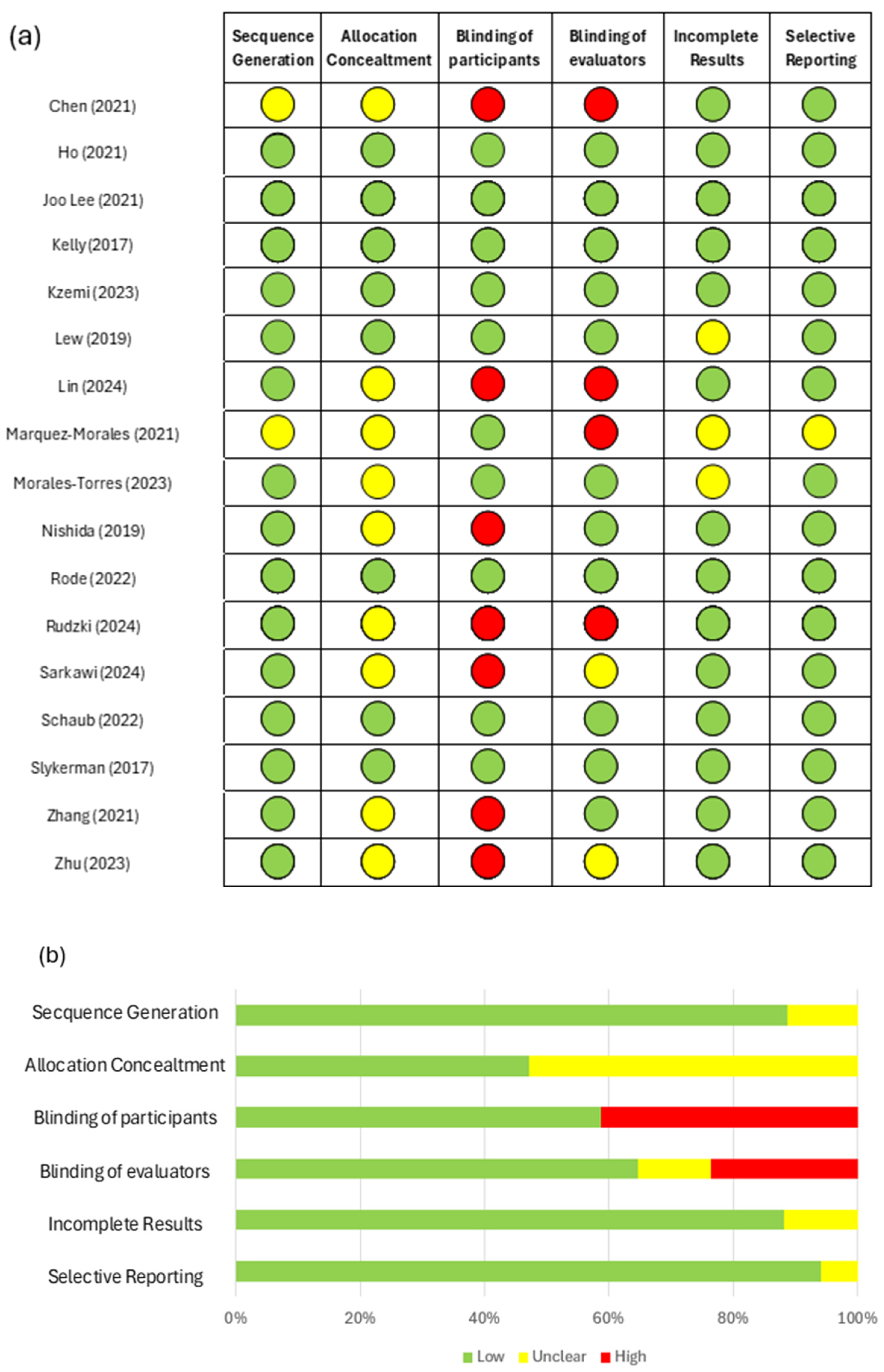
2. Methods
3. Results
3.1. Characteristics of the Included Studies
3.2. Efficacy of Formulations Exclusively Containing Lactobacillus spp.
3.3. Efficacy of Multi-Species Formulations Containing Lactobacillus spp.
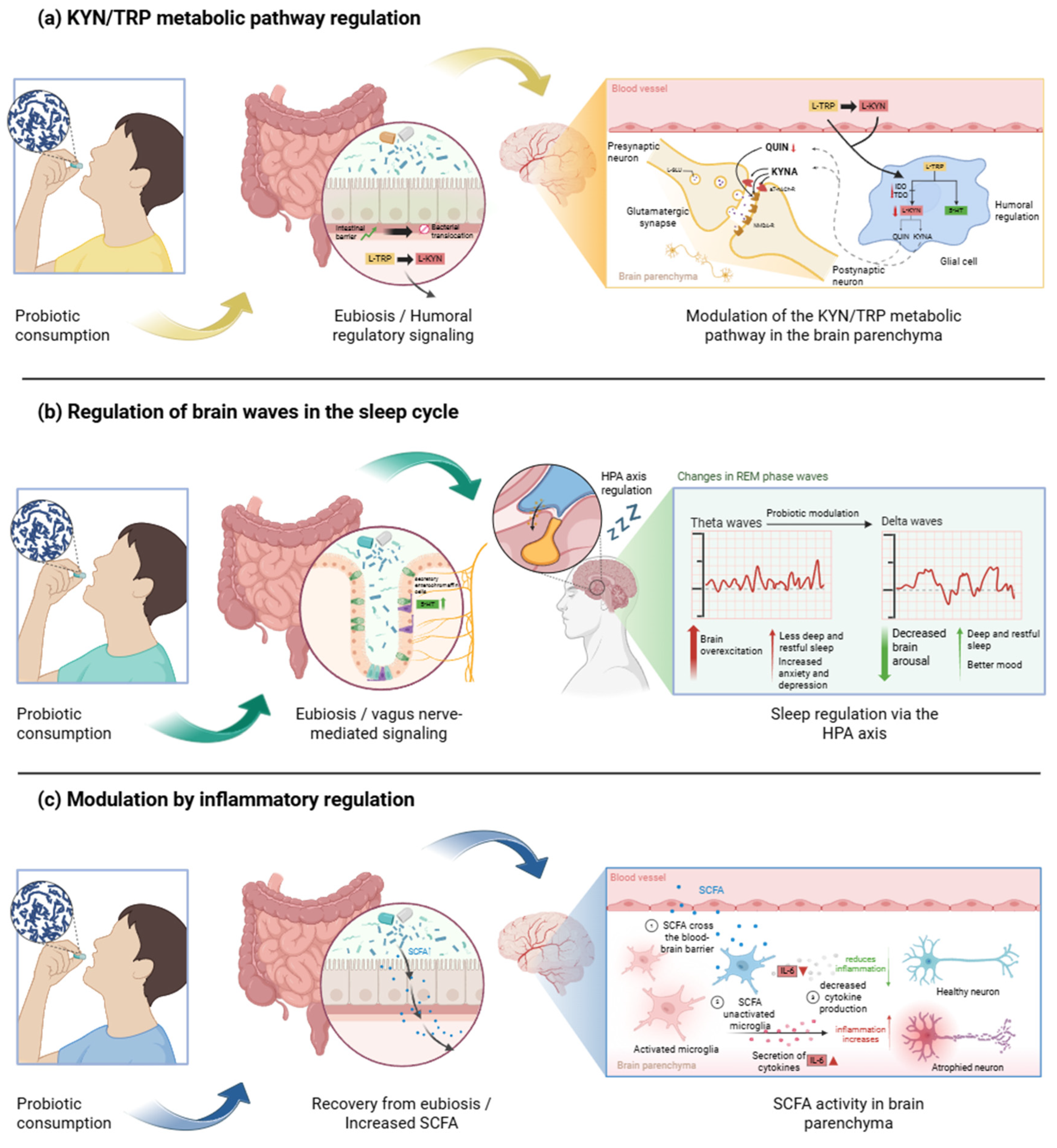
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Doenyas, C.; Clarke, G.; Cserjési, R. Gut–brain axis and neuropsychiatric health: Recent advances. Sci. Rep. 2025, 15, 3415. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Kanoujia, J.; Lakshmi, S.M.; Patil, C.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Lamers, F.; Tamayo, M.; Benito-Amat, C.; Molina-Mendoza, G.V.; Penninx, B.W.J.H.; Sanz, Y. The Gut Microbiome in Early Life Stress: A Systematic Review. Nutrients 2023, 15, 2566. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Woo, S.-Y.; Raza, S.; Ho, D.; Jeon, S.W.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Association between gut microbiota and anxiety symptoms: A large population-based study examining sex differences. J. Affect. Disord. 2023, 333, 21–29. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders. JAMA Psychiatry 2021, 78, 1343. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; García, J.J.B. Psicobióticos: Una nueva perspectiva para el tratamiento del estrés, de la ansiedad y de la depresión. Ansiedad Estrés 2024, 30, 79–93. [Google Scholar] [CrossRef]
- Kransel, M.S.S.; Zafra, J.J.J.; Diago, I.O.; Hernández, L.V.B. Depresión, ansiedad y microbiota intestinal: Mecanismos neurobiológicos. Acta Neurológica Colomb. 2024, 40. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.Y.; Siu, P.L.K.; Choy, C.T.; Chan, U.K.; Zhou, J.; Wong, C.H.; Lee, Y.W.; Chan, H.W.; Tsui, J.C.C.; Loo, S.K.F.; et al. Novel Multi-Strain E3 Probiotic Formulation Improved Mental Health Symptoms and Sleep Quality in Hong Kong Chinese. Nutrients 2023, 15, 5037. [Google Scholar] [CrossRef]
- Casertano, M.; Dekker, M.; Valentino, V.; De Filippis, F.; Fogliano, V.; Ercolini, D. Gaba-producing lactobacilli boost cognitive reactivity to negative mood without improving cognitive performance: A human Double-Blind Placebo-Controlled Cross-Over study. Brain Behav. Immun. 2024, 122, 256–265. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Am. Coll. Physicians 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Márquez-Morales, L.; El-Kassis, E.G.; Cavazos-Arroyo, J.; Rocha-Rocha, V.; Martínez-Gutiérrez, F.; Pérez-Armendáriz, B. Effect of the Intake of a Traditional Mexican Beverage Fermented with Lactic Acid Bacteria on Academic Stress in Medical Students. Nutrients 2021, 13, 1551. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.; Abdullah, M.F.I.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-T.; Tsai, Y.-C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef]
- Sarkawi, M.; Ali, R.A.R.; Wahab, N.A.; Rathi, N.D.A.; Mokhtar, N.M. A randomized, double-blinded, placebo-controlled clinical trial on Lactobacillus-containing cultured milk drink as adjuvant therapy for depression in irritable bowel syndrome. Sci. Rep. 2024, 14, 9478. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Chen, H.-M.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef]
- Lin, S.-K.K.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Chen, C.-H. The effects of Lactobacillus plantarum PS128 in patients with major depressive disorder: An eight-week double-blind, placebo-controlled study. Asian J. Psychiatr. 2024, 101, 104210. [Google Scholar] [CrossRef]
- Rode, J.; Carlman, H.M.T.E.; König, J.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Brummer, R.J. Multi-Strain Probiotic Mixture Affects Brain Morphology and Resting State Brain Function in Healthy Subjects: An RCT. Cells 2022, 11, 2922. [Google Scholar] [CrossRef]
- Morales-Torres, R.; Carrasco-Gubernatis, C.; Grasso-Cladera, A.; Cosmelli, D.; Parada, F.J.; Palacios-García, I. Psychobiotic Effects on Anxiety Are Modulated by Lifestyle Behaviors: A Randomized Placebo-Controlled Trial on Healthy Adults. Nutrients 2023, 15, 1706. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hong, J.K.; Kim, J.-K.; Kim, D.-H.; Jang, S.W.; Han, S.-W.; Yoon, I.-Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Abdill, R.J.; Adamowicz, E.M.; Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 2022, 20, e3001536. [Google Scholar] [CrossRef]
- Bouttell, J.; Heggie, R.; Oien, K.; Romaniuk, A.; VanSteenhouse, H.; von Delft, S.; Hawkins, N. Economic evaluation of genomic/genetic tests: A review and future directions. Int. J. Technol. Assess. Health Care 2022, 38, e67. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.M.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Biagioli, M.; Capobianco, D.; Carino, A.; Marchianò, S.; Fiorucci, C.; Ricci, P.; Distrutti, E.; Fiorucci, S. Divergent Effectiveness of Multispecies Probiotic Preparations on Intestinal Microbiota Structure Depends on Metabolic Properties. Nutrients 2019, 11, 325. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Renner, V.; Schellong, J.; Bornstein, S.; Petrowski, K. Stress-induced pro- and anti-inflammatory cytokine concentrations in female PTSD and depressive patient. Transl. Psychiatry 2022, 12, 158. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, T.; Shan, X.; Zhang, M.; Li, Y.; Qi, X.; Bian, Y.; Zhao, L. Pro-inflammatory cytokines in stress-induced depression: Novel insights into mechanisms and promising therapeutic strategies. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2024, 131, 110931. [Google Scholar] [CrossRef]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The Influence of Intestinal Microbiota on BDNF Levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.H.; Prus, A.J. The Discriminative Stimulus Properties of Drugs Used to Treat Depression and Anxiety. Brain Imaging Behav. Neurosci. 2018, 39, 289–320. [Google Scholar] [CrossRef]
- Sánchez, E.; Nieto, J.C.; Vidal, S.; Santiago, A.; Martinez, X.; Sancho, F.J.; Sancho-Bru, P.; Mirelis, B.; Corominola, H.; Juárez, C.; et al. Fermented milk containing Lactobacillus paracasei subsp. paracasei CNCM I-1518 reduces bacterial translocation in rats treated with carbon tetrachloride. Sci. Rep. 2017, 7, 45712. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Martinez, K.R.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Santiago, M.; Zielinska, K.; Scheiman, J.; Barsa, C.; Jäger, R.; Pinto, D.; Rinaldi, F.; Giuliani, G.; Senatore, T.; et al. A Lactobacillus consortium provides insights into the sleep-exercise-microbiome nexus in proof of concept studies of elite athletes and in the general population. Microbiome 2025, 13, 1. [Google Scholar] [CrossRef]
- Chu, A.; Samman, S.; Galland, B.; Foster, M. Daily consumption of Lactobacillus gasseri CP2305 improves quality of sleep in adults—A systematic literature review and meta-analysis. Clin. Nutr. 2023, 42, 1314–1321. [Google Scholar] [CrossRef]
- Vandekerckhove, M.; Cluydts, R. The emotional brain and sleep: An intimate relationship. Sleep Med. Rev. 2010, 14, 219–226. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Lemke, H.; Probst, S.; Warneke, A.; Waltemate, L.; Winter, A.; Thiel, K.; Meinert, S.; Enneking, V.; Breuer, F.; Klug, M.; et al. The Course of Disease in Major Depressive Disorder Is Associated With Altered Activity of the Limbic System During Negative Emotion Processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 323–332. [Google Scholar] [CrossRef]
- Mendoza-Alvarez, M.; Balthasar, Y.; Verbraecken, J.; Claes, L.; van Someren, E.; van Marle, H.J.; Vandekerckhove, M.; De Picker, L. Systematic review: REM sleep, dysphoric dreams and nightmares as transdiagnostic features of psychiatric disorders with emotion dysregulation—Clinical implications. Sleep Med. 2025, 127, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.S.; Christensen, K.S.; Prior, A. Variation in Psychometric Testing in General Practice—A Nationwide Cohort Study. Clin. Epidemiol. 2023, 15, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villena, A.R.; de La Fuente-Figuerola, V. Estandarización, adaptación y validación de pruebas psicométricas: Diferencias necesarias. An. Pediatr. 2020, 93, 353–354. [Google Scholar] [CrossRef]

| Author (Year) | Country | Subjects | Probiotics | Intervention | Treatment | Mental Outcomes | Gut Microbiota Composition | Biomarkers or Metabolites | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Kelly (2017) [17] | Ireland | Healthy male volunteers (n = 29) | Lactobacillus rhamnosus | Capsule (1 × 109 CFU) or placebo per day for 4 weeks | Unique | No significant difference between the groups | No significant difference between the groups | No significant differences were found | No observed |
| Nishida (2019) [18] | Japan | Healthy young adults (n = 60) | Lactobacillus gasseri | 1 × 1010 bacterial cells per 2 tablets or placebo for 24 weeks | Unique | ↓ STAI (p = 0.014) ↓ PSQI (p = 0.041) | CP2305 attenuates the decrease of Bifidobacterium spp. and increases Streptococcus spp. | No significant differences were found | ↓ Stressful irritability (p < 0.001) ↓ Abdominal discomfort (p < 0.001) ↓ Salival CgA (p = 0.039) |
| Márquez-Morales (2021) [19] | México | University students (n = 45) | Lactobacillus plantarum, Lactobacillus paracasei Lactobacillus brevis | 100 mL beverage (3 × 108 CFU/mL) or placebo per day for 8 weeks | Unique | ↓ SISCO (p = 0.001) | Increases Bacteroidetes and Firmicutes | Not evaluated | ↓ Environmental demands (p < 0.001) ↓ Physical factors (p < 0.001) ↓ Psychological factors (p < 0.001) |
| Author (Year) | Country | Subjects | Probiotics | Intervention | Treatment | Mental Outcomes | Gut Microbiota Composition | Biomarkers or Metabolites | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Slykerman (2017) [20] | New Zealand | Postpartum womens (n = 380) | Lactobacillus rhamnosus | 6 × 1010 or placebo per day from pregnancy to 6 months postpartum | Unique | ↓ EPDS (p = 0.037) ↓ STAI (p = 0.014) ↓ Rate anxiety (p = 0.002) | Not evaluated | Not evaluated | No observed |
| Lew (2019) [21] | Malaysia | Stressed Adults (n = 103) | Lactobacillus plantarum | 2 × 1010 CFU/sachet or placebo per day for 12 weeks | Unique | ↓ DASS42-A (p = 0.032) ↓ DASS42-S (p = 0.007) ↓ DASS42 (p = 0.048) | Not evaluated | ↓ IFN-γ (p < 0.001) ↓ TNF-α (p < 0.001) | IFN-γ and TNF-α correlated significantly with DASS-42 scores |
| Ho (2021) [22] | Taiwan | Patients with chronic primary insomnia (n = 40) | Lactobacillus plantarum | Capsule (3 × 1010 UFC) or placebo per day for 4 weeks | Unique | ↓ BDI-II (p < 0.05) | Not evaluated | Not evaluated | ↓ Fatigue levels ↓ Brainwave activity ↓ Awakenings during the deep sleep stage |
| Zhang (2021) [24] | China | Adults with constipation (n = 82) | Lactobacillus paracasei strain Shirota (LcS) | 100 mL of an LcS beverage (1 × 108 CFU) or placebo every day for 9 weeks | Coadyuvant at depression treatment | No significant difference between the groups | LcS increased Adlercreutzia, Megasphaera and Veillonella levels and decreased Rikenellaceae, Sutterella and Oscillibacter. | Not evaluated | ↓ Interleukin-6 (p < 0.05) |
| Zhu (2023) [23] | China | Anxious (n = 60) and healthy students (n = 30) | Lactobacillus plantarum | Powder sachet per day (1 g with 1.5 × 1010 CFU) or placebo for 3 weeks | Unique | ↓ HAMA (p = 0.000) ↓ HDRS (p = 0.000) ↓ AIS (p = 0.000) | JYLP-326 restore the disturbed Bacteroides, Bifidobacterium, Prevotella and Roseburia levels | No significant differences were found | No observed |
| Sarkawi (2024) [25] | Malasya | IBS patients with and without subthreshold depression (n = 110) | Lactobacillus acidophilus Lactobacillus paracasei | 2 bottles (125 mL) of cultured milk drinks (1 × 109 CFU) or placebo per day of 12 weeks | Unique | No significant difference between the groups | Not evaluated | ↑ Serotonine levels (p < 0.05) | No observed |
| Author (Year) | Country | Subjects | Probiotics | Intervention | Treatment | Mental Outcomes | Gut Microbiota Composition | Biomarkers or Metabolites | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Rudzki (2019) [26] | Poland | MDD patients (n = 79) | Lactobacillus plantarum | 2 capsules (10 × 109 CFU) or placebo per day for 8 weeks | Coadyuvant at depression treatment | No significant difference between the groups | Not evaluated | ↓ Kynurenine (p = 0.005) ↓ Anthranilic acid (p = 0.028) | ↑ Attention and Perceptivity Test (p = 0.006) ↑ Californian Verbal Learning Test (p = 0.023) |
| Chen (2021) [27] | Taiwan | MDD patients (n = 40) | Lactobacillus plantarum | 2 Capsules (3 × 1010 CFU) per day for 8 weeks | Coadyuvant at depression treatment | ↓ HAM-D (p = 0.01) ↓ DSSS (p < 0.001) | Akkermansia, Bifidobacterium, Enterococcus, Eggerthella, Megasphaera and Ruminococcus changed significantly | Not evaluated | Coprococcus and Lactobacillus, significantly correlated with both biomarkers and depressive symptoms. |
| Lin (2024) [28] | Taiwan | MDD patients (n = 32) | Lactobacillus plantarum | 2 capsules (3 × 1010 CFU) or placebo per day for 8 weeks | Coadyuvant at depression treatment | No significant difference between the groups | No significant difference between the groups | No significant differences were found | No observed |
| Author (Year) | Country | Subjects | Probiotics | Intervention | Treatment | Mental Outcomes | Gut Microbiota Composition | Biomarkers or Metabolites | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Rode (2022) [29] | Sweeden | Healthy adults (n = 22) | Bifidobacterium longum Lactobacillus helveticus Lactiplantibacillus plantarum | Probiotic mixture (3×109 CFU) or placebo per day for 4 weeks | Unique | No significant difference between the groups | Not evaluated | Not evaluated | ~ Gray matter (p < 0.0001) |
| Morales-Torres (2023) [30] | Chile | Healthy adults (n = 135) | Lactobacillus helveticus Bifidobacterium longum | Capsule of Cerebiome® (3 × 109 CFU) or placebo per day for 4 weeks | Unique | No significant difference between the groups | Not evaluated | Not evaluated | No observed |
| Author (Year) | Country | Subjects | Probiotics | Intervention | Treatment | Mental Outcomes | Gut Microbiota Composition | Biomarkers or Metabolites | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Kazemi (2019) [31] | Iran | Mild to moderate major depressed patients (n = 81) | Lactobacillus helveticus Bifidobacterium longum | Sachet of Probiotic (10 × 109 CFU) or Prebiotic (GOS) or placebo for 8 weeks | Coadyuvant at depression treatment | ↓ BDI (p = 0.042) | Not evaluated | ↓ Kynurenine/tryptophan (p = 0.048) ↑ Tryptophan/isoleucine (p = 0.023) | No observed |
| Lee (2021) [32] | Korea | Healthy adults with subclinical mental symptoms (n = 174) | Lactobacillus reuteri NK33 Bifidobacterium adolescentis NK98 | 500 mg capsule per day of 2.0 × 109 CFU of NK33 and 0.5 × 109 CFU of NK98, or placebo for 8 weeks | Coadyuvant at sleep treatment | ↓ BDI-II (p = 0.036) ↓ BAI (p = 0.014) | NVP-1704 increased Bifidobacteriaceae and Lactobacillaceae, whereas it decreased Enterobacteriaceae | Not evaluated | ↑ Quality Sleep (p = 0.006) ↓ Interleukin-6 (p = 0.041) |
| Schaub (2022) [33] | Switzerland | Patients with current depressive episodes (n = 90) | Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus helveticus | Vivomixx® (9 × 109 CFU) or placebo for 4 weeks | Coadyuvant at depressive treatment | ↓ HAM-D (p< 0.01) | PRO increased the abundance of the genus Lactobacillus | Not evaluated | ~ Inversed Simpson ~ Pielou’s evenness ~ Shannon index |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rodríguez, D.; Bravo, M.C.; Pizarro, M.; Vergara-Barra, P.; Hormazábal, M.J.; Leonario-Rodriguez, M. Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2025, 26, 8099. https://doi.org/10.3390/ijms26168099
Fernández-Rodríguez D, Bravo MC, Pizarro M, Vergara-Barra P, Hormazábal MJ, Leonario-Rodriguez M. Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials. International Journal of Molecular Sciences. 2025; 26(16):8099. https://doi.org/10.3390/ijms26168099
Chicago/Turabian StyleFernández-Rodríguez, Diego, María Consuelo Bravo, Marcela Pizarro, Pablo Vergara-Barra, María José Hormazábal, and Marcell Leonario-Rodriguez. 2025. "Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials" International Journal of Molecular Sciences 26, no. 16: 8099. https://doi.org/10.3390/ijms26168099
APA StyleFernández-Rodríguez, D., Bravo, M. C., Pizarro, M., Vergara-Barra, P., Hormazábal, M. J., & Leonario-Rodriguez, M. (2025). Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials. International Journal of Molecular Sciences, 26(16), 8099. https://doi.org/10.3390/ijms26168099






