Do Sex and Gender Interact with the Biological Actions of Taurine? A Critical Rereading of the Literature
Abstract
1. Introduction
2. Search Strategy
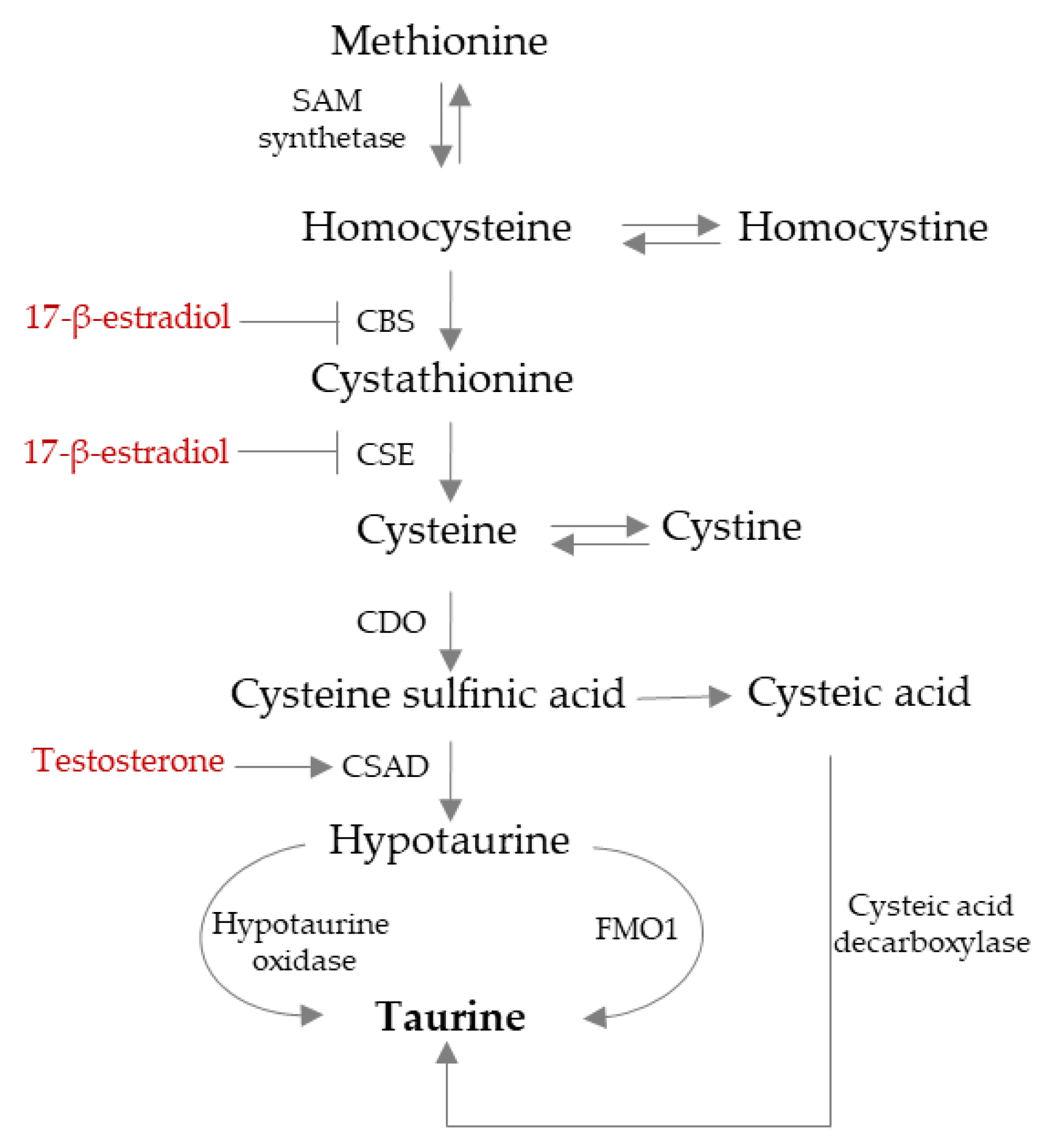
3. TAU Endogenous Synthesis Is Influenced by Sexual Hormones
4. TAU Absorption and Distribution
5. TAU Elimination
6. TAU Transporters: TAUT and Proton-Dependent Carrier (PAT1)
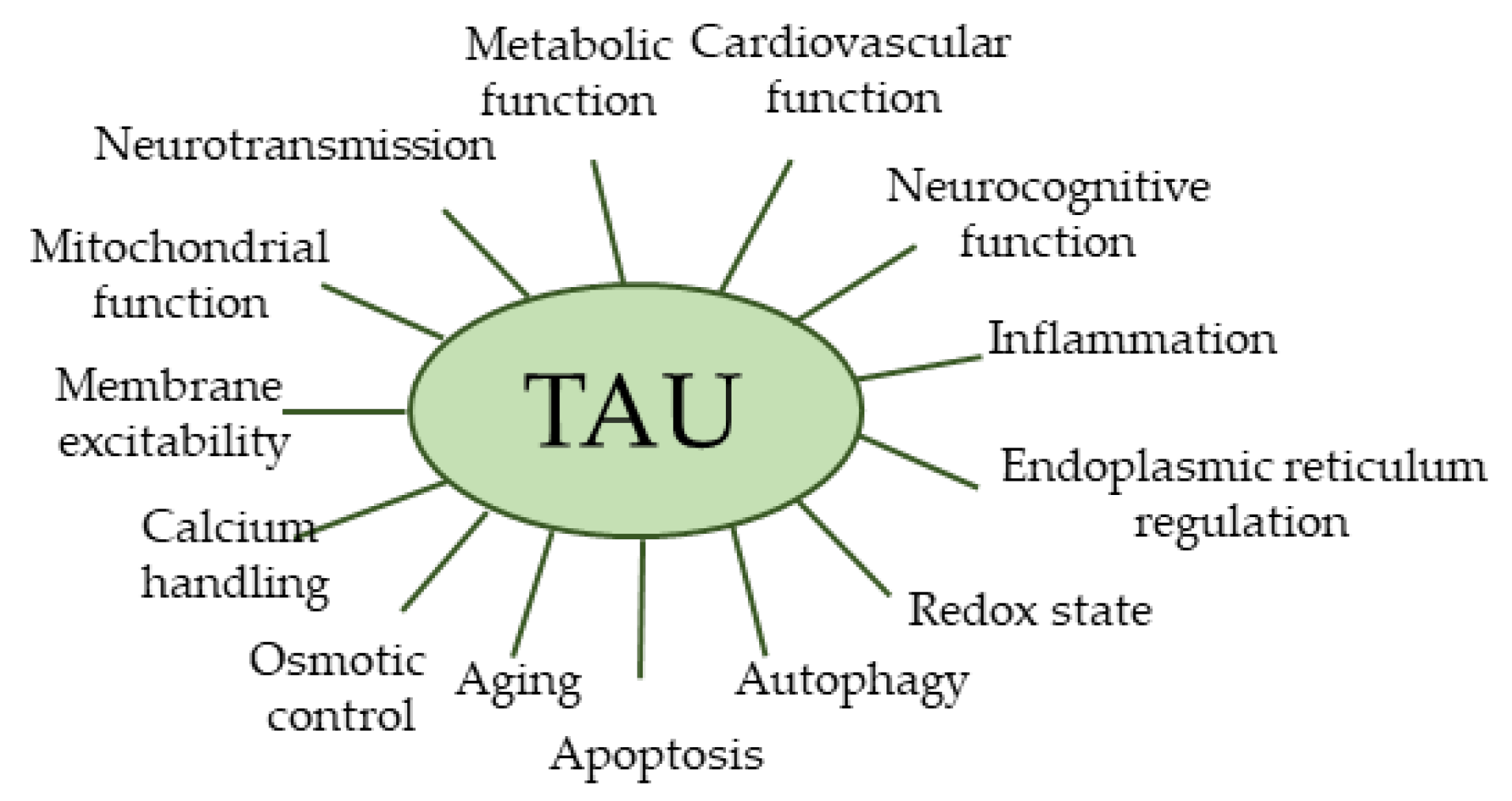
7. Biological Activities of TAU
8. TAU and Cholesterol
9. TAU Supplementation and/or Administration in Some Pathological Conditions
10. Some Genetic Diseases
11. TAU and CVD
12. TAU in DM and Metabolic Syndrome
13. TAU in Fetal and Neonatal Life: Intrauterine Growth Restriction, Pre-Eclampsia, Gestational Diabetes, and Developmental Trajectory
13.1. TAU and Developmental Trajectories
13.2. Intrauterine Growth Restriction (IUGR)
13.3. Pre-Eclampsia
13.4. Gestational Diabetes Mellitus
14. The Safety Profile of TAU in Humans
15. Is TAU Activity Influenced by Sex and Gender?
16. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Santulli, G.; Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Lombardi, A. Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients 2023, 15, 4236. [Google Scholar] [CrossRef]
- Kawasaki, A.; Ono, A.; Mizuta, S.; Kamiya, M.; Takenaga, T.; Murakami, S. The Taurine Content of Japanese Seaweed. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1105–1112. [Google Scholar] [CrossRef]
- Taurine Uses, Benefits & Dosage—Drugs.Com Herbal Database. Available online: https://www.drugs.com/npp/taurine.html (accessed on 22 October 2024).
- Grand View Research. Taurine Supplements Market Size and Share Report, 2030; Grand View Research: California, CA, USA, 2023. [Google Scholar]
- Statista. Adults in the U.S. Taking Dietary Supplements by Gender 2020; Statista: Hamburg, Germany, 2024. [Google Scholar]
- Hansen, S.H. The Role of Taurine in Diabetes and the Development of Diabetic Complications. Diabetes/Metab. Res. Rev. 2001, 17, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Murakami, S. Taurine Deficiency Associated with Dilated Cardiomyopathy and Aging. J. Pharmacol. Sci. 2024, 154, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef]
- Guan, L.; Miao, P. The Effects of Taurine Supplementation on Obesity, Blood Pressure and Lipid Profile: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Pharmacol. 2020, 885, 173533. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Bennardini, F.; Mattana, A.; Miceli, M.; Ciuti, M.; Mian, M.; Gironi, A.; Anichini, R.; Seghieri, G. Plasma and Platelet Taurine Are Reduced in Subjects with Insulin-Dependent Diabetes Mellitus: Effects of Taurine Supplementation. Am. J. Clin. Nutr. 1995, 61, 1115–1119. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Gender Differences in Metabolism; Nutrition and Supplements. J. Sci. Med. Sport 2000, 3, 287–298. [Google Scholar] [CrossRef]
- Marino, M.; Masella, R.; Bulzomi, P.; Campesi, I.; Malorni, W.; Franconi, F. Nutrition and Human Health from a Sex-Gender Perspective. Mol. Asp. Med. 2011, 32, 1–70. [Google Scholar] [CrossRef]
- Minucci, D. Lifelong Gender Health Programming in Fetal Life. Ital. J. Gend. Specif. Med. 2018, 4, 91–100. [Google Scholar]
- Grigore, D.; Ojeda, N.B.; Alexander, B.T. Sex Differences in the Fetal Programming of Hypertension. Gend. Med. 2008, 5 (Suppl. S1), S121–S132. [Google Scholar] [CrossRef]
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G. Arterial Hypertension in the Female World: Pathophysiology and Therapy. J. Cardiovasc. Med. 2016, 17, 229–236. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; Kintscher, U.; et al. Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar]
- Seghieri, G.; Policardo, L.; Anichini, R.; Franconi, F.; Campesi, I.; Cherchi, S.; Tonolo, G. The Effect of Sex and Gender on Diabetic Complications. Curr. Diabetes Rev. 2017, 13, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Dworatzek, E.; Seeland, U.; Kararigas, G.; Arnal, J.F.; Brunelleschi, S.; Carpenter, T.C.; Erdmann, J.; Franconi, F.; Giannetta, E.; et al. Sex in Basic Research: Concepts in the Cardiovascular Field. Cardiovasc. Res. 2017, 113, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T. Identification of a Novel Enzyme and the Regulation of Key Enzymes in Mammalian Taurine Synthesis. J. Pharmacol. Sci. 2024, 154, 9–17. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, J.; Liu, H.; Qiu, G.; Cui, S. Testosterone Enhances Taurine Synthesis by Upregulating Androgen Receptor and Cysteine Sulfinic Acid Decarboxylase Expressions in Male Mouse Liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G295–G304. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, J.; Cao, W.; Liu, J.; Cui, S. Estradiol Decreases Taurine Level by Reducing Cysteine Sulfinic Acid Decarboxylase via the Estrogen Receptor-α in Female Mice Liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G277–G286. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Engelen, M.P.K.J.; Simbo, S.Y.; Ten Have, G.A.M.; Thaden, J.J.; Cynober, L.; Deutz, N.E.P. Metabolic Consequences of Supplemented Methionine in a Clinical Context. J. Nutr. 2020, 150, 2538S–2547S. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Galistu, A.; Carru, C.; Franconi, F.; Fois, M.; Zinellu, A. Glutamyl Cycle in the Rat Liver Appears to Be Sex-Gender Specific. Exp. Toxicol. Pathol. 2013, 65, 585–589. [Google Scholar] [CrossRef]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Bastos, B.L.; Nair, T.; Riermeier, A.; et al. Taurine Deficiency as a Driver of Aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Ahmad, F.; Sharma, K.; Hadley, M. Taurine in Congestive Heart Failure. Int. J. Clin. Cardiol. 2021, 8, 246. [Google Scholar] [CrossRef]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Baliou, S.; Kyriakopoulos, A.M.; Goulielmaki, M.; Panayiotidis, M.I.; Spandidos, D.A.; Zoumpourlis, V. Significance of Taurine Transporter (TauT) in Homeostasis and Its Layers of Regulation. Mol. Med. Rep. 2020, 22, 2163–2173. [Google Scholar] [CrossRef]
- Buisset, A.; Gohier, P.; Leruez, S.; Muller, J.; Amati-Bonneau, P.; Lenaers, G.; Bonneau, D.; Simard, G.; Procaccio, V.; Annweiler, C.; et al. Metabolomic Profiling of Aqueous Humor in Glaucoma Points to Taurine and Spermine Deficiency: Findings from the Eye-D Study. J. Proteome Res. 2019, 18, 1307–1315. [Google Scholar] [CrossRef]
- Liu, F.C.; Cheng, M.L.; Lo, C.J.; Hsu, W.C.; Lin, G.; Lin, H.T. Exploring the Aging Process of Cognitively Healthy Adults by Analyzing Cerebrospinal Fluid Metabolomics Using Liquid Chromatography-Tandem Mass Spectrometry. BMC Geriatr. 2023, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Konz, I.; Mehl, F.; Teav, T.; Oikonomidi, A.; Peyratout, G.; van der Velpen, V.; Popp, J.; Ivanisevic, J. A Global HILIC-MS Approach to Measure Polar Human Cerebrospinal Fluid Metabolome: Exploring Gender-Associated Variation in a Cohort of Elderly Cognitively Healthy Subjects. Anal. Chim. Acta 2018, 1037, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Do, K.Q.; Lauer, C.J.; Schreiber, W.; Zollinger, M.; Gutteck-Amsler, U.; Cuénod, M.; Holsboer, F. Gamma-Glutamylglutamine and Taurine Concentrations Are Decreased in the Cerebrospinal Fluid of Drug-Naive Patients with Schizophrenic Disorders. J. Neurochem. 1995, 65, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Carru, C.; Zinellu, A.; Occhioni, S.; Sanna, M.; Palermo, M.; Tonolo, G.; Mercuro, G.; Franconi, F. Regular Cigarette Smoking Influences the Transsulfuration Pathway, Endothelial Function, and Inflammation Biomarkers in a Sex-Gender Specific Manner in Healthy Young Humans. Am. J. Transl. Res. 2013, 5, 497–509. [Google Scholar]
- Merheb, M.; Daher, R.T.; Nasrallah, M.; Sabra, R.; Ziyadeh, F.N.; Barada, K. Taurine Intestinal Absorption and Renal Excretion Test in Diabetic Patients: A Pilot Study. Diabetes Care 2007, 30, 2652–2654. [Google Scholar] [CrossRef]
- Bianchi, L.; Lari, R.; Anichini, R.; De Bellis, A.; Berti, A.; Napoli, Z.; Seghieri, G.; Franconi, F. Taurine Transporter Gene Expression in Peripheral Mononuclear Blood Cells of Type 2 Diabetic Patients. Amino Acids 2012, 42, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Napoli, Z.; Seghieri, G.; Bianchi, L.; Anichini, R.; De Bellis, A.; Campesi, I.; Carru, C.; Occhioni, S.; Zinellu, A.; Franconi, F. Taurine Transporter Gene Expression in Mononuclear Blood Cells of Type 1 Diabetes Patients. J. Diabetes Res. 2016, 2016, 7313162. [Google Scholar] [CrossRef]
- Sak, D.; Erdenen, F.; Müderrisoglu, C.; Altunoglu, E.; Sozer, V.; Gungel, H.; Guler, P.A.; Sak, T.; Uzun, H. The Relationship between Plasma Taurine Levels and Diabetic Complications in Patients with Type 2 Diabetes Mellitus. Biomolecules 2019, 9, 96. [Google Scholar] [CrossRef]
- Khoramjoo, M.; Wang, K.; Srinivasan, K.; Gheblawi, M.; Mandal, R.; Rousseau, S.; Wishart, D.; Prasad, V.; Richer, L.; Cheung, A.M.; et al. Plasma Taurine Level Is Linked to Symptom Burden and Clinical Outcomes in Post-COVID Condition. PLoS ONE 2024, 19, e0304522. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.R.; Ban, M.S.; Lee, H.; Cho, J.Y.; Jo, S.J. Metabolic Profiling of Psoriasis Vulgaris and Palmoplantar Pustulosis. Exp. Dermatol. 2024, 33, e15159. [Google Scholar] [CrossRef] [PubMed]
- Kouchiwa, T.; Wada, K.; Uchiyama, M.; Kasezawa, N.; Niisato, M.; Murakami, H.; Fukuyama, K.; Yokogoshi, H. Age-Related Changes in Serum Amino Acids Concentrations in Healthy Individuals. Clin. Chem. Lab. Med. 2012, 50, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, J.; Wang, H.; Qiao, Y.; Li, W.; Gao, M.; Liu, E.; Yu, Z.; Hu, G.; Fang, Z.; et al. Serum Sulfur-Containing Amino Acids and Risk of Maternal Gestational Diabetes and Adverse Growth Patterns in Offspring. Nutrients 2023, 15, 4089. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zheng, W.; Yuan, X.; Liu, C.; Zhang, Y.; Song, W.; Wang, X.; Liang, S.; Ma, X.; et al. Dynamic Changes of Serum Taurine and the Association with Gestational Diabetes Mellitus: A Nested Case-Control Study. Front. Endocrinol. 2023, 14, 1116044. [Google Scholar] [CrossRef]
- Seghieri, G.; Tesi, F.; Bianchi, L.; Loizzo, A.; Saccomanni, G.; Ghirlanda, G.; Anichini, R.; Franconi, F. Taurine in Women with a History of Gestational Diabetes. Diabetes Res. Clin. Pract. 2007, 76, 187–192. [Google Scholar] [CrossRef]
- Sun, S.; He, D.; Luo, C.; Lin, X.; Wu, J.; Yin, X.; Jia, C.; Pan, Q.; Dong, X.; Zheng, F.; et al. Metabolic Syndrome and Its Components Are Associated with Altered Amino Acid Profile in Chinese Han Population. Front. Endocrinol. 2022, 12, 795044. [Google Scholar] [CrossRef]
- Spanou, L.; Dimou, A.; Kostara, C.E.; Bairaktari, E.; Anastasiou, E.; Tsimihodimos, V. A Study of the Metabolic Pathways Affected by Gestational Diabetes Mellitus: Comparison with Type 2 Diabetes. Diagnostics 2022, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Martínez-Vacas, A.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P. Taurine: A Promising Nutraceutic in the Prevention of Retinal Degeneration. Neural Regen. Res. 2024, 19, 606–610. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine: New Implications for an Old Amino Acid. FEMS Microbiol. Lett. 2003, 226, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Zhao, P.; Qi, Y.; Zhao, P.; Qi, Y. Taurine: A Comprehensive Review of Its Origin, Pharmacological Properties, Potential Health Benefits, Therapeutic Applications, and Safety Profile. Food Sci. Hum. Wellness 2025, 15. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Wu, M.; Chen, Y.; Wang, X.; Li, X.; Liu, F. The Role of Taurine through Endoplasmic Reticulum in Physiology and Pathology. Biochem. Pharmacol. 2024, 226, 116386. [Google Scholar] [CrossRef]
- Tega, Y.; Kawauchi, Y.; Akanuma, S.; Inagaki, M.; Tachikawa, M.; Hosoya, K. In Vitro Characterization of Taurine Transport Using the Human Brain Microvascular Endothelial Cell Line as a Human Blood-Brain Barrier Model. Drug Metab. Pharmacokinet. 2024, 61, 101040. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.Y.; Lu, Y.F.; Christopher Corton, J.; Klaassen, C.D. Sex-, Age-, and Race/Ethnicity-Dependent Variations in Drug-Processing and NRF2-Regulated Genes in Human Livers. Drug Metab. Dispos. 2021, 49, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshikawa, N.; Ito, H.; Schaffer, S.W. Impact of Taurine Depletion on Glucose Control and Insulin Secretion in Mice. J. Pharmacol. Sci. 2015, 129, 59–64. [Google Scholar] [CrossRef]
- Tochitani, S. Taurine: A Maternally Derived Nutrient Linking Mother and Offspring. Metabolites 2022, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Shennan, D.B.; Thomson, J. Estrogen Regulation and Ion Dependence of Taurine Uptake by MCF-7 Human Breast Cancer Cells. Cell. Mol. Biol. Lett. 2007, 12, 396–406. [Google Scholar] [CrossRef]
- Miyauchi, S.; Abbot, E.L.; Zhuang, L.; Subramanian, R.; Ganapathy, V.; Thwaites, D.T. Isolation and Function of the Amino Acid Transporter PAT1 (Slc36a1) from Rabbit and Discrimination between Transport via PAT1 and System IMINO in Renal Brush-Border Membrane Vesicles. Mol. Membr. Biol. 2005, 22, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.U.; Pedersen, M.; Müller, S.; Kæstel, T.; Bjerg, M.; Ulaganathan, N.; Nielsen, S.; Carlsen, K.L.; Nøhr, M.K.; Holm, R. Inhibitory Effects of 17-α-Ethinyl-Estradiol and 17-β-Estradiol on Transport via the Intestinal Proton-Coupled Amino Acid Transporter (PAT1) Investigated in Vitro and in Vivo. J. Pharm. Sci. 2021, 110, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective Role of Taurine against Oxidative Stress. Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Ulusoy, K.G. Effects of Taurine on Vascular Tone. Amino Acids 2022, 54, 1527–1540. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Novel Taurine-Containing Uridine Derivatives and Mitochondrial Human Diseases. Nucleic Acids Symp. Ser. 2001, 1, 257–258. [Google Scholar] [CrossRef][Green Version]
- Jong, C.J.; Ito, T.; Prentice, H.; Wu, J.Y.; Schaffer, S.W. Role of Mitochondria and Endoplasmic Reticulum in Taurine-Deficiency-Mediated Apoptosis. Nutrients 2017, 9, 795. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Zhao, H.; Fang, L.; Wang, X. Enhancing Healthy Aging with Small Molecules: A Mitochondrial Perspective. Med. Res. Rev. 2024, 44, 1904–1922. [Google Scholar] [CrossRef]
- Olesen, M.A.; Pradenas, E.; Villavicencio-Tejo, F.; Porter, G.A.; Johnson, G.V.W.; Quintanilla, R.A. Mitochondria-Tau Association Promotes Cognitive Decline and Hippocampal Bioenergetic Deficits during the Aging. Free Radic. Biol. Med. 2024, 217, 141–156. [Google Scholar] [CrossRef]
- Jakovljevic, N.K.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Gajovic, J.S.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Shikata, E.; Yamamoto, Y.; Ito, T.; Matsuda, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine Inhibits Apoptosis by Preventing Formation of the Apaf-1/Caspase-9 Apoptosome. Am. J. Physiol. Cell Physiol. 2004, 287, C949–C953. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, X.; Li, H.; Liu, D.; Le, G.; Gan, F.; Pan, C.; Huang, K.; Chen, X. Regulation of Taurine in OTA-Induced Apoptosis and Autophagy. Toxicon 2020, 181, 82–90. [Google Scholar] [CrossRef]
- Faraji, S.; Rajaeinejad, M.; Bagheri, H.; Ardalan, M.A.; Moutabian, H.; Ehsani, F.; Pourarjmand, M.; Mirshafieyan, S.S.; Alazamani, F.; Cheraghi, S. Modulation of Ionizing Radiation-Induced Apoptosis by Taurine in Human Peripheral Blood Lymphocytes: Flow Cytometry-Based Quantification. J. Biomed. Phys. Eng. 2024, 14, 287–298. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Li, S.; Ren, Q.J.; Xie, C.H.; Cui, Y.; Xu, L.T.; Wang, Y.D.; Li, S.; Liang, X.Q.; Wen, B.; Liang, M.K.; et al. Taurine Attenuates Activation of Hepatic Stellate Cells by Inhibiting Autophagy and Inducing Ferroptosis. World J. Gastroenterol. 2024, 30, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kobayashi, M.; Mizunoe, Y.; Yoshida, M.; Yasukawa, H.; Hoshino, S.; Itagawa, R.; Furuichi, T.; Okita, N.; Sudo, Y.; et al. Taurine Is an Amino Acid with the Ability to Activate Autophagy in Adipocytes. Amino Acids 2018, 50, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yahyavy, S.; Valizadeh, A.; Saki, G.; Khorsandi, L. Taurine Induces Autophagy and Inhibits Oxidative Stress in Mice Leydig Cells. JBRA Assist. Reprod. 2020, 24, 250–256. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, S.; Tao, J.; Li, Y.; He, Z.; Liu, Q.; Zhao, J.; Deng, Y.; Kang, J.; Zhang, X.; et al. Taurine Suppresses ROS-Dependent Autophagy via Activating Akt/MTOR Signaling Pathway in Calcium Oxalate Crystals-Induced Renal Tubular Epithelial Cell Injury. Aging 2020, 12, 17353–17366. [Google Scholar] [CrossRef]
- Campesi, I.; Brunetti, A.; Capobianco, G.; Galistu, A.; Montella, A.; Ieri, F.; Franconi, F. Sex Differences in X-Ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine. Antioxidants 2022, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J. VRACs and Other Ion Channels and Transporters in the Regulation of Cell Volume and Beyond. Nat. Rev. Mol. Cell Biol. 2016, 17, 293–307. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Ju Jong, C.; Kc, R.; Azuma, J. Physiological Roles of Taurine in Heart and Muscle. J. Biomed. Sci. 2010, 17 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.S.; Chang, K.J.; Kim, S.H.; Cheong, S.H. Ribose-Taurine Suppresses Inflammation through NF-ΚB Regulation in Activated RAW 264.7 Macrophages. Adv. Exp. Med. Biol. 2019, 1155, 1057–1067. [Google Scholar] [CrossRef]
- Barua, M.; Liu, Y.; Quinn, M.R. Taurine Chloramine Inhibits Inducible Nitric Oxide Synthase and TNF-Alpha Gene Expression in Activated Alveolar Macrophages: Decreased NF-KappaB Activation and IkappaB Kinase Activity. J. Immunol. 2001, 167, 2275–2281. [Google Scholar] [CrossRef]
- Imae, M.; Asano, T.; Murakami, S. Potential Role of Taurine in the Prevention of Diabetes and Metabolic Syndrome. Amino Acids 2014, 46, 81–88. [Google Scholar] [CrossRef]
- Seol, S.I.; Kang, I.S.; Lee, J.S.; Lee, J.K.; Kim, C. Taurine Chloramine-Mediated Nrf2 Activation and HO-1 Induction Confer Protective Effects in Astrocytes. Antioxidants 2024, 13, 169. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Seyyed Shoura, S.M.; Fathollahi, P.; Shadbad, M.A.; Papi, S.; Ostadrahimi, A.; Faghfuri, E. Profiling Inflammatory and Oxidative Stress Biomarkers Following Taurine Supplementation: A Systematic Review and Dose-Response Meta-Analysis of Controlled Trials. Eur. J. Clin. Nutr. 2022, 76, 647–658. [Google Scholar] [CrossRef]
- Barbiera, A.; Sorrentino, S.; Fard, D.; Lepore, E.; Sica, G.; Dobrowolny, G.; Tamagnone, L.; Scicchitano, B.M. Taurine Administration Counteracts Aging-Associated Impingement of Skeletal Muscle Regeneration by Reducing Inflammation and Oxidative Stress. Antioxidants 2022, 11, 1016. [Google Scholar] [CrossRef]
- Flori, L.; Veneziano, S.; Martelli, A.; Piragine, E.; Calderone, V. Transsulfuration Pathway Products and H2S-Donors in Hyperhomocysteinemia: Potential Strategies Beyond Folic Acid. Int. J. Mol. Sci. 2025, 26, 6430. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile Acid Metabolism and Signaling, the Microbiota, and Metabolic Disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, R.; Kobayashi, M.; Sakurai, M.; Yoshida, M.; Kaneko, H.; Mizunoe, Y.; Nozaki, Y.; Okita, N.; Sudo, Y.; Higami, Y. Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids. Int. J. Mol. Sci. 2022, 23, 1793. [Google Scholar] [CrossRef] [PubMed]
- Golonka, R.M.; Yeoh, B.S.; Saha, P.; Tian, Y.; Chiang, J.Y.L.; Patterson, A.D.; Gewirtz, A.T.; Joe, B.; Vijay-Kumar, M. Sex Dimorphic Effects of Bile Acid Metabolism in Liver Cancer in Mice. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 719–735. [Google Scholar] [CrossRef]
- Song, Q.; Kobayashi, S.; Kataoka, Y.; Oda, H. Direct Molecular Action of Taurine on Hepatic Gene Expression Associated with the Amelioration of Hypercholesterolemia in Rats. Antioxidants 2024, 13, 990. [Google Scholar] [CrossRef]
- Simon, F.R.; Fortune, J.; Iwahashi, M.; Bowman, S.; Wolkoff, A.; Sutherland, E. Characterization of the Mechanisms Involved in the Gender Differences in Hepatic Taurocholate Uptake. Am. J. Physiol. 1999, 276, G556–G565. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.R.; Fortune, J.; Iwahashi, M.; Qadri, I.; Sutherland, E. Multihormonal Regulation of Hepatic Sinusoidal Ntcp Gene Expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G782–G794. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Csanaky, I.L.; Klaassen, C.D. Gender-Divergent Profile of Bile Acid Homeostasis during Aging of Mice. PLoS ONE 2012, 7, e32551. [Google Scholar] [CrossRef] [PubMed]
- Slijepcevic, D.; Roscam Abbing, R.L.P.; Katafuchi, T.; Blank, A.; Donkers, J.M.; van Hoppe, S.; de Waart, D.R.; Tolenaars, D.; van der Meer, J.H.M.; Wildenberg, M.; et al. Hepatic Uptake of Conjugated Bile Acids Is Mediated by Both Sodium Taurocholate Cotransporting Polypeptide and Organic Anion Transporting Polypeptides and Modulated by Intestinal Sensing of Plasma Bile Acid Levels in Mice. Hepatology 2017, 66, 1631–1643. [Google Scholar] [CrossRef]
- Campesi, I.; Seghieri, G.; Franconi, F. Type 2 Diabetic Women Are Not Small Type 2 Diabetic Men: Sex-and-Gender Differences in Antidiabetic Drugs. Curr. Opin. Pharmacol. 2021, 60, 40–45. [Google Scholar] [CrossRef]
- Campesi, I.; Montella, A.; Seghieri, G.; Franconi, F. The Person’s Care Requires a Sex and Gender Approach. J. Clin. Med. 2021, 10, 4770. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Ansar, M.; Ranza, E.; Shetty, M.; Paracha, S.A.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine Treatment of Retinal Degeneration and Cardiomyopathy in a Consanguineous Family with SLC6A6 Taurine Transporter Deficiency. Hum. Mol. Genet. 2020, 29, 618–623. [Google Scholar] [CrossRef]
- Ueda, S.; Yagi, M.; Tomoda, E.; Matsumoto, S.; Ueyanagi, Y.; Do, Y.; Setoyama, D.; Matsushima, Y.; Nagao, A.; Suzuki, T.; et al. Mitochondrial Haplotype Mutation Alleviates Respiratory Defect of MELAS by Restoring Taurine Modification in TRNA with 3243A > G Mutation. Nucleic Acids Res. 2023, 51, 7480–7495. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, Y.; Hagiwara, H.; Nishimatsu, S.I.; Hirakawa, A.; Kamimura, N.; Ohtsubo, H.; Fukai, Y.; Murakami, T.; Koga, Y.; Goto, Y.I.; et al. Taurine Supplementation for Prevention of Stroke-like Episodes in MELAS: A Multicentre, Open-Label, 52-Week Phase III Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 529–536. [Google Scholar] [CrossRef]
- Van Hove, J.L.K.; Freehauf, C.L.; Ficicioglu, C.; Pena, L.D.M.; Moreau, K.L.; Henthorn, T.K.; Christians, U.; Jiang, H.; Cowan, T.M.; Young, S.P.; et al. Biomarkers of Oxidative Stress, Inflammation, and Vascular Dysfunction in Inherited Cystathionine β-Synthase Deficient Homocystinuria and the Impact of Taurine Treatment in a Phase 1/2 Human Clinical Trial. J. Inherit. Metab. Dis. 2019, 42, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Tzang, C.C.; Lin, W.C.; Lin, L.H.; Lin, T.Y.; Chang, K.V.; Wu, W.T.; Özçakar, L. Insights into the Cardiovascular Benefits of Taurine: A Systematic Review and Meta-Analysis. Nutr. J. 2024, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, J.; Sakkal, S.; Apostolopoulos, V.; Zulli, A.; Gadanec, L.K. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients 2023, 15, 2562. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of Endothelium-Derived Nitric Oxide Production and Activity by Taurine and Taurine-Conjugated Bile Acids. Nitric Oxide 2020, 94, 48–53. [Google Scholar] [CrossRef]
- Ahn, C.S. Effect of Taurine Supplementation on Plasma Homocysteine Levels of the Middle-Aged Korean Women. Adv. Exp. Med. Biol. 2009, 643, 415–422. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Ikeda, K.; Miura, A.; Mizushima, S.; Miki, T.; Nara, Y. Distribution of Twenty-Four Hour Urinary Taurine Excretion and Association with Ischemic Heart Disease Mortality in 24 Populations of 16 Countries: Results from the WHO-CARDIAC Study. Hypertens. Res. 2001, 24, 453–457. [Google Scholar] [CrossRef]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Pearte, C.; Costa, M.; Chen, Y. Serum Taurine and Risk of Coronary Heart Disease: A Prospective, Nested Case-Control Study. Eur. J. Nutr. 2013, 52, 169–178. [Google Scholar] [CrossRef]
- Sharma, S.; Sahoo, B.M.; Banik, B.K. Biological Effects and Mechanisms of Taurine in Various Therapeutics. Curr. Drug Discov. Technol. 2023, 20, 60–78. [Google Scholar] [CrossRef]
- McGurk, K.A.; Kasapi, M.; Ware, J.S. Effect of Taurine Administration on Symptoms, Severity, or Clinical Outcome of Dilated Cardiomyopathy and Heart Failure in Humans: A Systematic Review. Wellcome Open Res. 2022, 7, 9. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The Effects of Oral Taurine on Resting Blood Pressure in Humans: A Meta-Analysis. Curr. Hypertens. Rep. 2018, 20, 81. [Google Scholar] [CrossRef]
- Lala, A.; TayalL, U.; Hamo, C.E.; Youmans, Q.; Al-Khatib, S.M.; Bozkurt, B.; Davis, M.B.; Januzzi, J.; Mentz, R.; Sauer, A.; et al. Sex Differences in Heart Failure. J. Card. Fail. 2022, 28, 477–498. [Google Scholar] [CrossRef]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Ohmori, H.; Maeda, S. Taurine Supplementation Attenuates Delayed Increase in Exercise-Induced Arterial Stiffness. Appl. Physiol. Nutr. Metab. 2016, 41, 618–623. [Google Scholar] [CrossRef]
- Azuma, J.; Sawamura, A.; Awata, N.; Hasegawa, H.; Ogura, K.; Harada, H.; Ohta, H.; Yamauchi, K.; Kishimoto, S.; Yamagami, T. Double-Blind Randomized Crossover Trial of Taurine in Congestive Heart-Failure. Curr. Ther. Res. Clin. Exp. 1983, 34, 543–547. [Google Scholar]
- Azuma, J.; Sawamura, A.; Awata, N.; Ohta, H.; Hamaouchi, T.; Harada, H.; Takihara, K.; Hasegawa, H.; Yamagami, T.; Ishiyama, T.; et al. Therapeutic Effect of Taurine in Congestive Heart Failure: A Double-Blind Crossover Trial. Clin. Cardiol. 1985, 8, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sedova, E.M.; Magnitskaya, O.V. A Clinical Experience of Taurine and Trimetazidine Use in Premenopausal Women with Chronic Heart Failure. Kardiologiia 2010, 50, 62–63. [Google Scholar]
- Beyranvand, M.R.; Kadkhodai Khalafi, M.; Roshan, V.D.; Choobineh, S.; Parsa, S.A.; Piranfar, M.A. Effect of Taurine Supplementation on Exercise Capacity of Patients with Heart Failure. J. Cardiol. 2011, 57, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Gordeev, I.G.; Pokrovskaya, E.M.; Luchinkina, E.E. Taurine Effects on the Occurrence of Cardiac Arrhythmias and QT Interval Dispersion in Patients with Postinfarction Cardiosclerosis and Chronic Heart Failure: A Comparative Randomised Study. Cardiovasc. Ther. Prev. 2012, 11, 63–68. [Google Scholar] [CrossRef]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of Taurine in Chronic Congestive Heart Failure and Its Prospective Application. Jpn. Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef]
- Fujita, T.; Ando, K.; Noda, H.; Ito, Y.; Sato, Y. Effects of Increased Adrenomedullary Activity and Taurine in Young Patients with Borderline Hypertension. Circulation 1987, 75, 525–532. [Google Scholar] [CrossRef]
- Jeejeebhoy, F.; Keith, M.; Freeman, M.; Barr, A.; McCall, M.; Kurian, R.; Mazer, D.; Errett, L. Nutritional Supplementation with MyoVive Repletes Essential Cardiac Myocyte Nutrients and Reduces Left Ventricular Size in Patients with Left Ventricular Dysfunction. Am. Heart J. 2002, 143, 1092–1100. [Google Scholar] [CrossRef]
- Averin, E. Use of Taurine during Rehabilitation after Cardiac Surgery. Adv. Exp. Med. Biol. 2015, 803, 637–649. [Google Scholar] [CrossRef]
- Ahmadian, M.; Dabidi Roshan, V.; Ashourpore, E. Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. J. Diet. Suppl. 2017, 14, 422–432. [Google Scholar] [CrossRef]
- Adamchik, A.S.; Kryuchkova, I.V.; Ruban, G.M.; Blagodyreva, Y.A. New Potential of Pharmaceutical Therapy in Diastolic Chronic Heart Failure Treatment. Available online: https://www.researchgate.net/publication/292322246_New_potential_of_pharmaceutical_therapy_in_diastolic_chronic_heart_failure_treatment (accessed on 23 October 2024).
- Valiollah Dabidi, R. Effects of taurine supplementation on response of the cardiac injury biomarkers to Bruce diagnostic protocol in patients with heart failure. Koomesh 2011, 13, 41. [Google Scholar]
- Esmaeili, F.; Maleki, V.; Kheirouri, S.; Alizadeh, M. The Effects of Taurine Supplementation on Metabolic Profiles, Pentosidine, Soluble Receptor of Advanced Glycation End Products and Methylglyoxal in Adults with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Can. J. Diabetes 2021, 45, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Qaisar, S.A.; Kadhim, M.M.; Ahmadi, Y.; Davari, M. Protective and Therapeutic Effectiveness of Taurine Supplementation plus Low Calorie Diet on Metabolic Parameters and Endothelial Markers in Patients with Diabetes Mellitus: A Randomized, Clinical Trial. Nutr. Metab. 2022, 19, 49, Erratum in Nutr. Metab. 2022, 19, 62.. [Google Scholar] [CrossRef] [PubMed]
- Spohr, C.; Brøns, C.; Winther, K.; Dyerberg, J.; Vaag, A. No Effect of Taurine on Platelet Aggregation in Men with a Predisposition to Type 2 Diabetes Mellitus. Platelets 2005, 16, 301–305. [Google Scholar] [CrossRef]
- Moloney, M.A.; Casey, R.G.; O’Donnell, D.H.; Fitzgerald, P.; Thompson, C.; Bouchier-Hayes, D.J. Two Weeks Taurine Supplementation Reverses Endothelial Dysfunction in Young Male Type 1 Diabetics. Diabetes Vasc. Dis. Res. 2010, 7, 300–310. [Google Scholar] [CrossRef]
- Schwarzer, R.; Kivaranovic, D.; Mandorfer, M.; Paternostro, R.; Wolrab, D.; Heinisch, B.; Reiberger, T.; Ferlitsch, M.; Gerner, C.; Trauner, M.; et al. Randomised Clinical Study: The Effects of Oral Taurine 6g/Day vs Placebo on Portal Hypertension. Aliment. Pharmacol. Ther. 2018, 47, 86–94. [Google Scholar] [CrossRef]
- Ahmed, K.; Choi, H.N.; Yim, J.E. The Impact of Taurine on Obesity-Induced Diabetes Mellitus: Mechanisms Underlying Its Effect. Endocrinol. Metab. 2023, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhang, Z.; Yang, Z.; Rao, B. The Effects of Taurine Supplementation on Diabetes Mellitus in Humans: A Systematic Review and Meta-Analysis. Food Chem. Mol. Sci. 2022, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Mahdavi, R.; Hajizadeh-Sharafabad, F.; Alizadeh, M. The Effects of Taurine Supplementation on Oxidative Stress Indices and Inflammation Biomarkers in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetol. Metab. Syndr. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Premanath, M.; Mahesh, M.; Babu, M.; Bhanukumar, M.; Devananda, D. Can N Acetyl Cysteine—Taurine Provide Additional Reduction in Microalbuminuria in Type 2 Diabetic Patients Already on Optimum Doses of Angiotensin Converting Enzyme Inhibitors? Int. J. Health Allied Sci. 2019, 8, 236–241. [Google Scholar] [CrossRef]
- Masouleh, S.S.; Bagheri, R.; Ashtary-Larky, D.; Cheraghloo, N.; Wong, A.; Bilesvar, O.Y.; Suzuki, K.; Siahkouhian, M. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients 2021, 13, 3958. [Google Scholar] [CrossRef]
- Li, C.; Gao, L.; Lv, C.; Li, Z.; Fan, S.; Liu, X.; Rong, X.; Huang, Y.; Liu, J. Active Role of Amino Acid Metabolism in Early Diagnosis and Treatment of Diabetic Kidney Disease. Front. Nutr. 2023, 10, 1239838. [Google Scholar] [CrossRef]
- Sarnobat, D.; Moffett, R.C.; Ma, J.; Flatt, P.R.; McClenaghan, N.H.; Tarasov, A.I. Taurine Rescues Pancreatic β-Cell Stress by Stimulating α-Cell Transdifferentiation. BioFactors 2023, 49, 646–662. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Sarkar, P.; Basak, P.; Ghosh, S.; Kundu, M.; Sil, P.C. Prophylactic Role of Taurine and Its Derivatives against Diabetes Mellitus and Its Related Complications. Food Chem. Toxicol. 2017, 110, 109–121. [Google Scholar] [CrossRef]
- Nisoli, E.; Aquilani, R.; D’Antona, G. Amino Acid Supplements and Diabetes. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: Cambridge, MA, USA, 2013; pp. 83–95. [Google Scholar] [CrossRef]
- Murakami, S.; Funahashi, K.; Tamagawa, N.; Ning, M.; Ito, T. Taurine Ameliorates Streptozotocin-Induced Diabetes by Modulating Hepatic Glucose Metabolism and Oxidative Stress in Mice. Metabolites 2022, 12, 524. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Z.; Jia, S.; Yang, R. A Systematic Review of Preclinical Studies on the Taurine Role during Diabetic Nephropathy: Focused on Anti-Oxidative, Anti-Inflammation, and Anti-Apoptotic Effects. Toxicol. Mech. Methods 2022, 32, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, X.; Bian, Y.; Meng, L.; Wu, Y.; Ma, Y.; Li, C.; Wang, J.; Fu, Z.; Dai, J.; et al. Taurine Reduces Apoptosis Mediated by Endoplasmic Reticulum Stress in Islet β-Cells Induced by High-Fat and -Glucose Diets. Food Chem. Toxicol. 2023, 175, 113700. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Calpona, P.R.; Caponetti, A.; Romano, G.; Di Benedetto, A.; Cucinotta, D.; Di Giorgio, R.M. Taurine and Osmoregulation: Platelet Taurine Content, Uptake, and Release in Type 2 Diabetic Patients. Metabolism 2001, 50, 60–64. [Google Scholar] [CrossRef]
- Bocca, C.; Le Paih, V.; Chao De La Barca, J.M.; Kouassy Nzoughet, J.; Amati-Bonneau, P.; Blanchet, O.; Védie, B.; Géromin, D.; Simard, G.; Procaccio, V.; et al. A Plasma Metabolomic Signature of Leber Hereditary Optic Neuropathy Showing Taurine and Nicotinamide Deficiencies. Hum. Mol. Genet. 2021, 30, 21–29. [Google Scholar] [CrossRef]
- Inam-u-llah; Piao, F.; Aadil, R.M.; Suleman, R.; Li, K.; Zhang, M.; Wu, P.; Shahbaz, M.; Ahmed, Z. Ameliorative Effects of Taurine against Diabetes: A Review. Amino Acids 2018, 50, 487–502. [Google Scholar] [CrossRef]
- Güngel, H.; Erdenen, F.; Pasaoglu, I.; Sak, D.; Ogreden, T.; Kilic Muftuoglu, I. New Insights into Diabetic and Vision-Threatening Retinopathy: Importance of Plasma Long Pentraxine 3 and Taurine Levels. Curr. Eye Res. 2021, 46, 818–823. [Google Scholar] [CrossRef]
- Heller-Stilb, B.; van Roeyen, C.; Rascher, K.; Hartwig, H.G.; Huth, A.; Seeliger, M.W.; Warskulat, U.; Häussinger, D. Disruption of the Taurine Transporter Gene (Taut) Leads to Retinal Degeneration in Mice. FASEB J. 2002, 16, 231–233. [Google Scholar] [CrossRef]
- Lee, D.; Smith, L.E.H. Therapeutic Effects of Taurine and Histidine Supplementation in Retinal Diseases. Life 2024, 14, 1566. [Google Scholar] [CrossRef]
- Zeng, K.; Xu, H.; Mi, M.; Chen, K.; Zhu, J.; Yi, L.; Zhang, T.; Zhang, Q.; Yu, X. Effects of Taurine on Glial Cells Apoptosis and Taurine Transporter Expression in Retina under Diabetic Conditions. Neurochem. Res. 2010, 35, 1566–1574. [Google Scholar] [CrossRef]
- Di Leo, M.; Santini, S.; Cercone, S.; Lepore, D.; Gentiloni Silveri, N.; Caputo, S.; Greco, A.; Giardina, B.; Franconi, F.; Ghirlanda, G. Chronic Taurine Supplementation Ameliorates Oxidative Stress and Na+ K+ ATPase Impairment in the Retina of Diabetic Rats. Amino Acids 2002, 23, 401–406. [Google Scholar] [CrossRef]
- Di Leo, M.; Ghirlanda, G.; Gentiloni Silveri, N.; Giardina, B.; Franconi, F.; Santini, S. Potential Therapeutic Effect of Antioxidants in Experimental Diabetic Retina: A Comparison between Chronic Taurine and Vitamin E plus Selenium Supplementations. Free Radic. Res. 2003, 37, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, J.; Yuan, Y.; Wang, L.; Wang, Q.; Yuan, F. Taurine Protects Retinal Cells and Improves Synaptic Connections in Early Diabetic Rats. Curr. Eye Res. 2020, 45, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Home, P. IDF Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes: Recommendations for Standard, Comprehensive, and Minimal Care. Diabet. Med. 2006, 23, 579–593. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas 2021; International Diabetes Federation: Brussels, Belgium, 2021.
- He, J.; Li, X.; Yan, M.; Chen, X.; Sun, C.; Tan, J.; Song, Y.; Xu, H.; Wu, L.; Yang, Z. Inulin Reduces Kidney Damage in Type 2 Diabetic Mice by Decreasing Inflammation and Serum Metabolomics. J. Diabetes Res. 2024, 2024, 1222395. [Google Scholar] [CrossRef]
- Zou, J.; Song, Q.; Shaw, P.C.; Zuo, Z. Dendrobium Officinale Regulate Lipid Metabolism in Diabetic Mouse Liver via PPAR-RXR Signaling Pathway: Evidence from an Integrated Multi-Omics Analysis. Biomed. Pharmacother. 2024, 173, 116395. [Google Scholar] [CrossRef]
- Norman, J.E.; Nuthikattu, S.; Milenkovic, D.; Villablanca, A.C. Sex Modifies the Impact of Type 2 Diabetes Mellitus on the Murine Whole Brain Metabolome. Metabolites 2023, 13, 1012. [Google Scholar] [CrossRef]
- Tkáč, I.; Xie, T.; Shah, N.; Larson, S.; Dubinsky, J.M.; Gomez-Pastor, R.; McLoughlin, H.S.; Orr, H.T.; Eberly, L.E.; Öz, G. Regional Sex Differences in Neurochemical Profiles of Healthy Mice Measured by Magnetic Resonance Spectroscopy at 9.4 Tesla. Front. Neurosci. 2023, 17, 1278828. [Google Scholar] [CrossRef]
- Zheng, S.J.; Luo, Y.; Wang, J.B.; Chen, X.M.; Xu, Y.; Xiao, J.H. Regulated Intestinal Microbiota and Gut Immunity to Ameliorate Type 1 Diabetes Mellitus: A Novel Mechanism for Stem Cell-Based Therapy. Biomed. Pharmacother. 2024, 170, 116033. [Google Scholar] [CrossRef]
- Kwak, H.C.; Kim, Y.M.; Oh, S.J.; Kim, S.K. Sulfur Amino Acid Metabolism in Zucker Diabetic Fatty Rats. Biochem. Pharmacol. 2015, 96, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Merlin, E.; Salio, C.; Ferrini, F. Painful Diabetic Neuropathy: Sex-Specific Mechanisms and Differences from Animal Models to Clinical Outcomes. Cells 2024, 13, 2024. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Wang, N.; Tong, J.; Sun, W.; Yang, J.; Wu, G. Alleviation of Taurine on Liver Injury of Type 2 Diabetic Rats by Improving Antioxidant and Anti-Inflammatory Capacity. Heliyon 2024, 10, e28400. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Simon, Y.; Normand, A.; Jazzar, A.; Najibeddine, H.; Khalil, A.; Jacques, D. Short-Communication: Short-Term Treatment with Taurine Prevents the Development of Cardiac Hypertrophy and Early Death in Hereditary Cardiomyopathy of the Hamster and Is Sex-Dependent. Nutrients 2022, 14, 3287. [Google Scholar] [CrossRef]
- Bkaily, G.; Simon, Y.; Abou Abdallah, J.; Ouertane, C.; Essalhi, A.; Khalil, A.; Jacques, D. Short Communication: Taurine Long-Term Treatment Prevents the Development of Cardiac Hypertrophy, and Premature Death in Hereditary Cardiomyopathy of the Hamster Is Sex-Independent. Nutrients 2024, 16, 946. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J.; Ramírez, B.; Becerril, S.; Rodríguez, A.; Mentxaka, A.; Valentí, V.; Moncada, R.; Reina, G.; Baixauli, J.; et al. Decreased Expression of the NLRP6 Inflammasome Is Associated with Increased Intestinal Permeability and Inflammation in Obesity with Type 2 Diabetes. Cell. Mol. Life Sci. 2024, 81, 77. [Google Scholar] [CrossRef]
- Jung, Y.M.; Choi, M.J. Relation of Taurine Intake during Pregnancy and Newborns’ Growth. Adv. Exp. Med. Biol. 2019, 1155, 283–292. [Google Scholar] [CrossRef]
- Menendez-Castro, C.; Rascher, W.; Hartner, A. Intrauterine Growth Restriction—Impact on Cardiovascular Diseases Later in Life. Mol. Cell. Pediatr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Cederlöf, E.T.; Lindhagen, L.; Lundgren, M.; Lindahl, B.; Christersson, C. Pregnancy Complications and Long-Term Risk of Cardiovascular Events in Women with Structural Heart Disease. Open Heart 2024, 11, e002833. [Google Scholar] [CrossRef]
- Troisi, J.; Symes, S.J.K.; Lombardi, M.; Cavallo, P.; Colucci, A.; Scala, G.; Adair, D.C.; Guida, M.; Richards, S.M. Placental Metabolomics of Fetal Growth Restriction. Metabolites 2023, 13, 235. [Google Scholar] [CrossRef]
- Beggan, L.A.; Mulhern, M.S.; Mæhre, H.K.; McSorley, E.M.; Yeates, A.J.; Zavez, A.; Thurston, S.W.; Shamlaye, C.; van Wijngaarden, E.; Davidson, P.W.; et al. Associations between Serum Taurine Concentrations in Mothers and Neonates and the Children’s Anthropometrics and Early Neurodevelopment: Results from the Seychelles Child Development Study, Nutrition Cohort 2. Neurotoxicology 2023, 99, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A Comprehensive Review on Infant Formula: Nutritional and Functional Constituents, Recent Trends in Processing and Its Impact on Infants’ Gut Microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O. An Overview of Parenteral Nutrition from Birth to Adolescence Based on a Composite Fish Oil Containing Lipid Emulsion and a Pediatric Amino Acid Solution. Nutrients 2024, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Rajindrajith, S. Fetal Programming of Obesity and Type 2 Diabetes. World J. Diabetes 2022, 13, 482–497. [Google Scholar] [CrossRef]
- Gillman, M.W. Developmental Origins of Health and Disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef]
- Hales, C.; Barker, D. The Thrifty Phenotype Hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Martínez-Vacas, A.; Picaud, S.; Villegas-Pérez, M.P.; García-Ayuso, D. Taurine: An Essential Amino Sulfonic Acid for Retinal Health. Neural Regen. Res. 2023, 18, 807–808. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and Its Analogs in Neurological Disorders: Focus on Therapeutic Potential and Molecular Mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 295–305. [Google Scholar] [CrossRef]
- Roysommuti, S.; Malila, P.; Jirakulsomchok, D.; Wyss, J.M. Adult Renal Function Is Modified by Perinatal Taurine Status in Conscious Male Rats. J. Biomed. Sci. 2010, 17, S31. [Google Scholar] [CrossRef]
- Roysommuti, S.; Wyss, J.M. Perinatal Taurine Exposure Affects Adult Arterial Pressure Control. Amino Acids 2014, 46, 57–72. [Google Scholar] [CrossRef]
- Mensegue, M.F.; Burgueño, A.L.; Tellechea, M.L. Perinatal Taurine Exerts a Hypotensive Effect in Male Spontaneously Hypertensive Rats and Down-Regulates Endothelial Oxide Nitric Synthase in the Aortic Arch. Clin. Exp. Pharmacol. Physiol. 2020, 47, 780–789. [Google Scholar] [CrossRef]
- Bustamante, J.; Alonso, F.J.; Lobo, M.V.T.; Giné, E.; Tamarit-Rodriguez, J.; Solís, J.M.; Martín Del Río, R. Taurine Levels and Localization in Pancreatic Islets. Adv. Exp. Med. Biol. 1998, 442, 65–69. [Google Scholar] [CrossRef]
- Samadi, M.; Haghi-Aminjan, H.; Sattari, M.; Hooshangi Shayesteh, M.R.; Bameri, B.; Armandeh, M.; Naddafi, M.; Eghbal, M.A.; Abdollahi, M. The Role of Taurine on Chemotherapy-Induced Cardiotoxicity: A Systematic Review of Non-Clinical Study. Life Sci. 2021, 265, 118813. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, Y.; Zhao, C. Taurine Attenuates Liver Autophagy and Injury of Offspring in Gestational Diabetic Mellitus Rats. Life Sci. 2020, 257, 117889. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, A.; Carta, S.; Bennardini, F.; Coinu, R.; Loizzo, S.; Guarino, I.; Seghieri, G.; Ghirlanda, G.; Franconi, F. Neonatal Taurine Administration Modifies Metabolic Programming in Male Mice. Early Hum. Dev. 2007, 83, 693–696. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Amino Acids during Pregnancy and Offspring Cardiovascular-Kidney-Metabolic Health. Nutrients 2024, 16, 1263. [Google Scholar] [CrossRef]
- Franconi, F.; Diana, G.; Fortuna, A.; Galietta, G.; Trombetta, G.; Valentini, G.; Seghieri, G.; Loizzo, A. Taurine Administration during Lactation Modifies Hippocampal CA1 Neurotransmission and Behavioural Programming in Adult Male Mice. Brain Res. Bull. 2004, 63, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Fukuda, A. Maternal Taurine as a Modulator of Cl- Homeostasis as Well as of Glycine/GABAA Receptors for Neocortical Development. Front. Cell. Neurophysiol. 2023, 17, 1221441. [Google Scholar] [CrossRef] [PubMed]
- Savateeva-Lyubimova, T.N.; Sivak, K.V.; Stosman, K.I.; Aleksandrov, A.G. The Effect of Taurine on State of Experimental Gestosis in Rats. Rev. Clin. Pharmacol. Drug Ther. 2023, 21, 49–56. [Google Scholar] [CrossRef]
- Hirst, C. Placental Taurine Transport in Pre-Eclampsia—Research Explorer. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2015. [Google Scholar]
- Pokrovskaya, T.; Khadieva, T.; Gureev, V.V.; Pokrovskii, V.; Patrachanov, E.; Kovalenko, I.; Shutov, V.; Shabalin, A. Correction of the Endothelial Function and the Hemostasis System Disorders with Ademethionin and Taurine in Model Associated with ADMA-like Preeclampsia. J. Res. Med. Dent. Sci. 2021, 9, 386–393. [Google Scholar]
- Kuc, S.; Koster, M.P.H.; Pennings, J.L.A.; Hankemeier, T.; Berger, R.; Harms, A.C.; Dane, A.D.; Schielen, P.C.J.I.; Visser, G.H.A.; Vreeken, R.J. Metabolomics Profiling for Identification of Novel Potential Markers in Early Prediction of Preeclampsia. PLoS ONE 2014, 9, e98540. [Google Scholar] [CrossRef]
- Metzger, B.; Lowe, L.; Dyer, A.; Trimble, E.; Chaovarindr, U.; Coustan, D.; Hadden, D.; McCance, D.; Hod, M.; McIntyre, H.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Y.; Ma, L.; Liu, L.; Hu, T.; An, Z.; Yao, A.M.; Xia, L.Y. The Relationship between Plasma Taurine Levels in Early Pregnancy and Later Gestational Diabetes Mellitus Risk in Chinese Pregnant Women. Sci. Rep. 2021, 11, 7993. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Fields, J.; Magee, M.K.; Kerksick, C.M.; Jones, M.T. Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating. Nutrients 2022, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Vidot, H.; Cvejic, E.; Carey, S.; Strasser, S.I.; McCaughan, G.W.; Allman-Farinelli, M.; Shackel, N.A. Randomised Clinical Trial: Oral Taurine Supplementation versus Placebo Reduces Muscle Cramps in Patients with Chronic Liver Disease. Aliment. Pharmacol. Ther. 2018, 48, 704–712. [Google Scholar] [CrossRef]
- Rubio, C.; Cámara, M.; Giner, R.M.; González-Muñoz, M.J.; López-García, E.; Morales, F.J.; Moreno-Arribas, M.V.; Portillo, M.P.; Bethencourt, E. Caffeine, D-Glucuronolactone and Taurine Content in Energy Drinks: Exposure and Risk Assessment. Nutrients 2022, 14, 5103. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Hathcock, J.N. Risk Assessment for the Amino Acids Taurine, L-Glutamine and L-Arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety and Efficacy of Taurine as a Feed Additive for All Animal Species. EFSA J. 2012, 10, 2736. [Google Scholar] [CrossRef]
- Sharma, S.; Rodems, B.J.; Baker, C.D.; Kaszuba, C.M.; Franco, E.I.; Smith, B.R.; Ito, T.; Swovick, K.; Welle, K.; Zhang, Y.; et al. Taurine from Tumour Niche Drives Glycolysis to Promote Leukaemogenesis. Nature 2025, 644, 263–272. [Google Scholar] [CrossRef]
- Yao, H.T.; Lin, P.P.; Chang, Y.W.; Chen, C.T.; Chiang, M.T.; Chang, L.; Kuo, Y.C.; Tsai, H.T.; Yeh, T.K. Effect of Taurine Supplementation on Cytochrome P450 2E1 and Oxidative Stress in the Liver and Kidneys of Rats with Streptozotocin-Induced Diabetes. Food Chem. Toxicol. 2009, 47, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.A.; Cruz, G.B.; Cabañas, E.; Joseph, J.N.; Mian, M.; Madhira, S.K.V.; Akintunde, C.A.; Clarke, E.G.; Skeen, J.C.; Bonitto, J.R.; et al. In Vivo Sex-Dependent Effects of Perinatal Pb2+ Exposure on Pilocarpine-Induced Seizure Susceptibility and Taurine Neuropharmacology. Adv. Exp. Med. Biol. 2022, 1370, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, E.M.; Meyer, D.M.; Stecher, L.; Sailer, M.; Daniel, H.; Hauner, H.; Bader, B.L. Fetal Sex Modulates Placental MicroRNA Expression, Potential MicroRNA-MRNA Interactions, and Levels of Amino Acid Transporter Expression and Substrates: INFAT Study Subpopulation Analysis of n-3 LCPUFA Intervention during Pregnancy and Associations with Offspring Body Composition. BMC Mol. Cell Biol. 2021, 22, 15. [Google Scholar] [CrossRef]
- Koza, L.A.; Grossberg, A.N.; Bishop, M.; Prusmack, C.; Linseman, D.A. Sex-Specific Antioxidant Biomarker Depletion in Patients with a History of Mild Traumatic Brain Injury. Adv. Redox Res. 2024, 10, 100097. [Google Scholar] [CrossRef]
- Abud, G.F.; De Carvalho, F.G.; Batitucci, G.; Travieso, S.G.; Bueno Junior, C.R.; Barbosa Junior, F.; Marchini, J.S.; de Freitas, E.C. Taurine as a Possible Antiaging Therapy: A Controlled Clinical Trial on Taurine Antioxidant Activity in Women Ages 55 to 70. Nutrition 2022, 101, 111706. [Google Scholar] [CrossRef]
- Legato, M. Principles of Gender-Specific Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Greaves, L.; Brabete, A.C.; Maximos, M.; Huber, E.; Li, A.; Lê, M.L.; Eltonsy, S.; Boscoe, M. Sex, Gender, and the Regulation of Prescription Drugs: Omissions and Opportunities. Int. J. Environ. Res. Public Health 2023, 20, 2962. [Google Scholar] [CrossRef]
| Number and Sex of Subjects | Age (Years) | Ethnicity | Biological Fluid | Healthy Subjects | Disease | TAU Levels | References |
|---|---|---|---|---|---|---|---|
| 26 cases and 26 controls; 46% women | >60 | Not specified | Aqueous humor | 26 controls | primary open-angle glaucoma | <in glaucoma | [28] |
| 21 men 71 women | 55.9 ± 18.1 44.8 ± 15.9 | Chinese | CSF | cognitively healthy | Not specified | = | [29] |
| 12 men 20 women | 65 | Swiss | CSF | cognitively healthy | No disease | >women | [30] |
| 15 schizophrenic women 11 schizophrenic men 9 healthy women 6 health men | 21–53 | German | CSF | 15 controls | schizophrenic disorders | <patients =in men and women | [31] |
| 83 healthy men 85 healthy women | 18–40 | Italian | Plasma | 85 controls | No disease | = | [32] |
| 16 (6 women) | 18–65 | Lebanese | Plasma and urine | 8 controls | 6 T2D + 2 T1D | Basal TAU = After an oral TAU load, DM patients < plasma TAU levels at peak | [33] |
| 81 cases and 45 controls; 24% women | >40 | Italian | Plasma | 45 controls | T2D | <in T2D =in men and women | [34] |
| 35 cases and 33 controls; 56% women | >40 | Italian | Plasma | 33 controls | T1D | =in cases and controls | [35] |
| 59 cases and 28 controls; 54% women | 37–82 | Turkish | Plasma | 28 controls | T2D | <TAU especially in patients with neuropathy | [36] |
| 39 cases and 34 controls | 35–70 | Italian | Plasma | 34 controls | T1D | <plasma TAU in patients | [10] |
| 89 cases and 28 controls; 44% women | >50 | Multiethnic | Plasma | 28 controls | COVID-19 | >TAU in cases | [37] |
| 93 psoriasis vulgaris; 20 palmoplantar pustulosis; 20 controls; 34% of women | >18 | Korean | Plasma | 20 controls | Psoriasis vulgaris and Palmoplantar pustulosis | >TAU in psoriasis vulgaris =TAU in palmoplantar pustulosis | [38] |
| 23–88 | Japanese | Serum | controls | M < with age F no change with age | [39] | ||
| 486 women 259 with GDM | GDM age 29.2 ± 3.3 Control age 29.2 ± 2.7 | Chinese | Serum | 227 controls | GDM | <TAU is associated with the risk of GDM | [40] |
| 47 cases 47 controls | 33 32 | Chinese | Serum | 47 controls | GMD | TAU < 1 trimester TAU = 2 trimester | [41] |
| 43 cases; 7 IGT; 22 controls; GDM | 32 ± 3 34 ± 3 34 ± 4 | Italian | Serum | 22 controls | GDM | <TAU in cases | [42] |
| 72 cases and 401 controls; 44% women | 40–65 | Chinese | Serum | 401 controls | MetS | <TAU in cases | [43] |
| 62 with GDM 77 controls | 34.27 ± 5.07 32.69 ± 4.72 | Greek | Serum | 77 healthy control | GDM | <TAU in cases | [44] |
| Disease | Number of Patients | Ratio f/m | Dose (g) and Duration | Age (Years) | Geographical Localization | References |
|---|---|---|---|---|---|---|
| Healthy men | 29 | 0/29 | 6/day; 15 days | TAU 25.4 ± 1.0 Placebo 25.2 ± 1 | Japan | [108] |
| Congestive HF | 58 | 30/28 | 6/day; 4 weeks * | 38–89 | Japan | [109] |
| Congestive HF | 14 | 5/9 | 6/day; 4 weeks * | 68.71 ± 9.10 | Japan | [110] |
| Congestive HF Class II–III | 55 | NR | 1/day, 1 month | 45–62 | Russia | [111] |
| HF with LVEF < 50% | 29 | 3/26 | 1.5/day; 2 weeks | 60 ± 6 | Iran | [112] |
| Congestive HF Class II–III | 40 | NR | 1.5/day; 3 months | 40–70 | Russia | [113] |
| Idiopathic dilated cardiomyopathy | 17 | 6/11 | 3/day; 6 weeks | NA | Japan | [114] |
| Prehypertensive individuals | 86 | 44/42 | 1.6/day; 12 weeks | 56.75 ± 8.26 | China | [105] |
| Borderline hypertension | 19 | NR | 6/day; 1 week | 20–25 | Japan | [115] |
| Aorto-coronary bypass | 38 | 2/36 | 3/day; 35 days | TAU 62 ± 11 Placebo 69 ± 5 | Canada | [116] |
| Coronary heart disease/Heart valve defects | 48 | 12/36 | 0.5/day; 3 months | TAU 49.79 ± 1.4 Placebo 48.65 ± 1.5 | Russia | [117] |
| Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity | 16 | NR | 0.5/day; 2 weeks | TAU 62.12 ± 12 Placebo 60.13 ± 8.3 | Iran | [118] |
| MetS and diastolic HF | 78 | 59/19 | 1/day 12 months | 31–66 | Russia | [119] |
| HF | 16 | NR | 1.5/day 2 weeks | TAU 61.7 ± 6.4 Placebo 60.4 ± 6.9 | Iran | [120] |
| T2D | 46 | 32/14 | 3/day | TAU 42.74 ± 7.21 Placebo 43.52 ± 6.94 | Canada | [121] |
| T2D | 120 | 97/23 | 3 g/day; 8 weeks | TAU 52.13 ± 8.1 Placebo 53.13 ± 8.1 | Iran | [122] |
| T2D | 18 | 0/18 | 1.5/day; 8 weeks | 40 ± 8 | Denmark | [123] |
| T1D | 19 | 0/19 | 1.5/day; 2 weeks | 28.0 ± 2 | Ireland | [124] |
| Patients with hepatic venous pressure gradient ≥ 12 mm Hg | 22 | 8/14 | 6/day; 4 weeks | 52 ± 11 | Austria | [125] |
| Animals | DM Models | Sex | Effects | References |
|---|---|---|---|---|
| Mice | High-Fat Diet + STZ | Males | TAU induces inulin metabolism | [152] |
| Mice db/db and C57BL6/KsJ mice (control) | Genetic | Males | Dendrobium officinale ameliorates the liver metabolism pathway of TAU | [153] |
| db/db mice (T2D) compared to wild-type (WT) C57Bl6/J mice | Genetic | Males and Females | TAU > only in the female brain | [154] |
| Wild-type mice with C57BL/6 | Males and Females | Brain TAU > only in males | [155] | |
| C57BL/6 mice | STZ | Males | Human amniotic mesenchymal stem cells promote the increase in TAU in intestinal cells | [156] |
| Zucker rat | Genetic | Males | Plasma TAU< | [157] |
| Sprague Dawley rats | STZ | Males and females | Glycemia, mechanical allodynia, >females, Weight loss > males Aromatase > at 12 weeks in females | [158] |
| Sprague Dawley rats | STZ | Males | In the liver, TAU increases antioxidant biomarkers and reduces inflammation | [159] |
| Sprague Dawley rats | STZ | Sex of animals unreported | TAU protects from diabetic neuropathy | |
| Type 2 diabetic Goto–Kakizaki rats | Genetic | Males and Females | Sexually dimorphism in nerve repair in diabetic rats; axonal outgrowth after transection and activated Schwann cells > in males than in females | [158] |
| Hamster | Hereditary cardiomyopathy females present more lesions | Males and Females | Short-term TAU prevents the development of cardiac hypertrophy only in males | [160] |
| Hamster | Hereditary cardiomyopathy females present more lesions | Males and Females | Long-term TAU prevents the development of hypertrophy in both males and females | [161] |
| Human adipocyte and CCL-241 cells from obese patients | Obesity | Men and Women | NLRP6 is upregulated by TAU in CCL-241 enterocytes | [162] |
| Human | GDM | Women | Low serum level of TAU is a risk factor for GDM | [40] |
| Human | GDM | Women | TAU reduced in women with a history of prior GDM | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seghieri, G.; Campesi, I.; Tonolo, G.; Bennardini, F.; Stendardi, I.; Matucci, R.; Franconi, F. Do Sex and Gender Interact with the Biological Actions of Taurine? A Critical Rereading of the Literature. Int. J. Mol. Sci. 2025, 26, 8097. https://doi.org/10.3390/ijms26168097
Seghieri G, Campesi I, Tonolo G, Bennardini F, Stendardi I, Matucci R, Franconi F. Do Sex and Gender Interact with the Biological Actions of Taurine? A Critical Rereading of the Literature. International Journal of Molecular Sciences. 2025; 26(16):8097. https://doi.org/10.3390/ijms26168097
Chicago/Turabian StyleSeghieri, Giuseppe, Ilaria Campesi, Giancarlo Tonolo, Federico Bennardini, Isabella Stendardi, Rosanna Matucci, and Flavia Franconi. 2025. "Do Sex and Gender Interact with the Biological Actions of Taurine? A Critical Rereading of the Literature" International Journal of Molecular Sciences 26, no. 16: 8097. https://doi.org/10.3390/ijms26168097
APA StyleSeghieri, G., Campesi, I., Tonolo, G., Bennardini, F., Stendardi, I., Matucci, R., & Franconi, F. (2025). Do Sex and Gender Interact with the Biological Actions of Taurine? A Critical Rereading of the Literature. International Journal of Molecular Sciences, 26(16), 8097. https://doi.org/10.3390/ijms26168097










