Cell-Free DNA Based Next-Generation Sequencing Does Not Differentiate Between Oligoprogression and Systemic Progression in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors—An Explorative Study
Abstract
1. Introduction
2. Results
2.1. Patient and Sample Characteristics
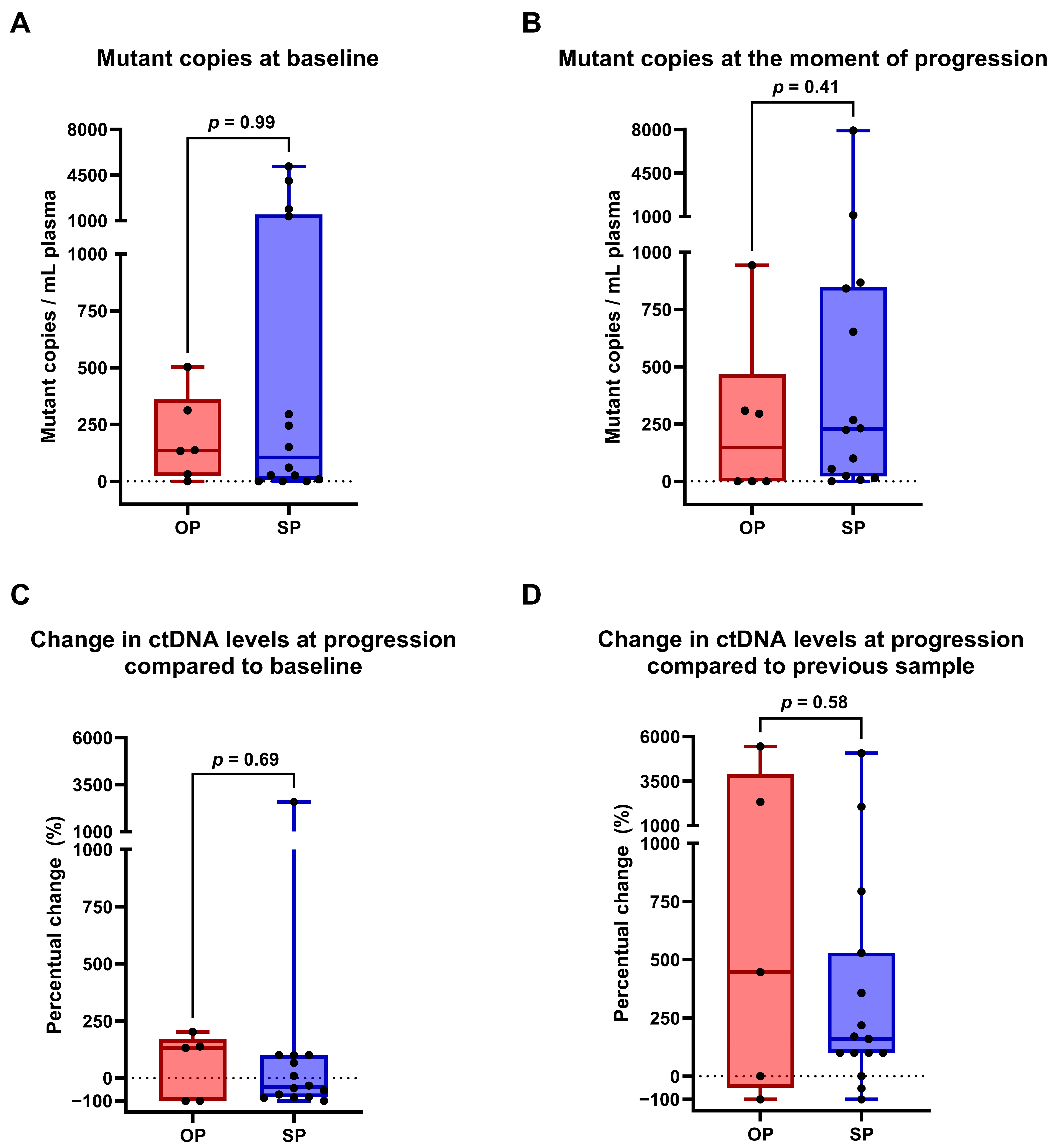
2.2. ctDNA Profiling to Discriminate Between True OP and SP
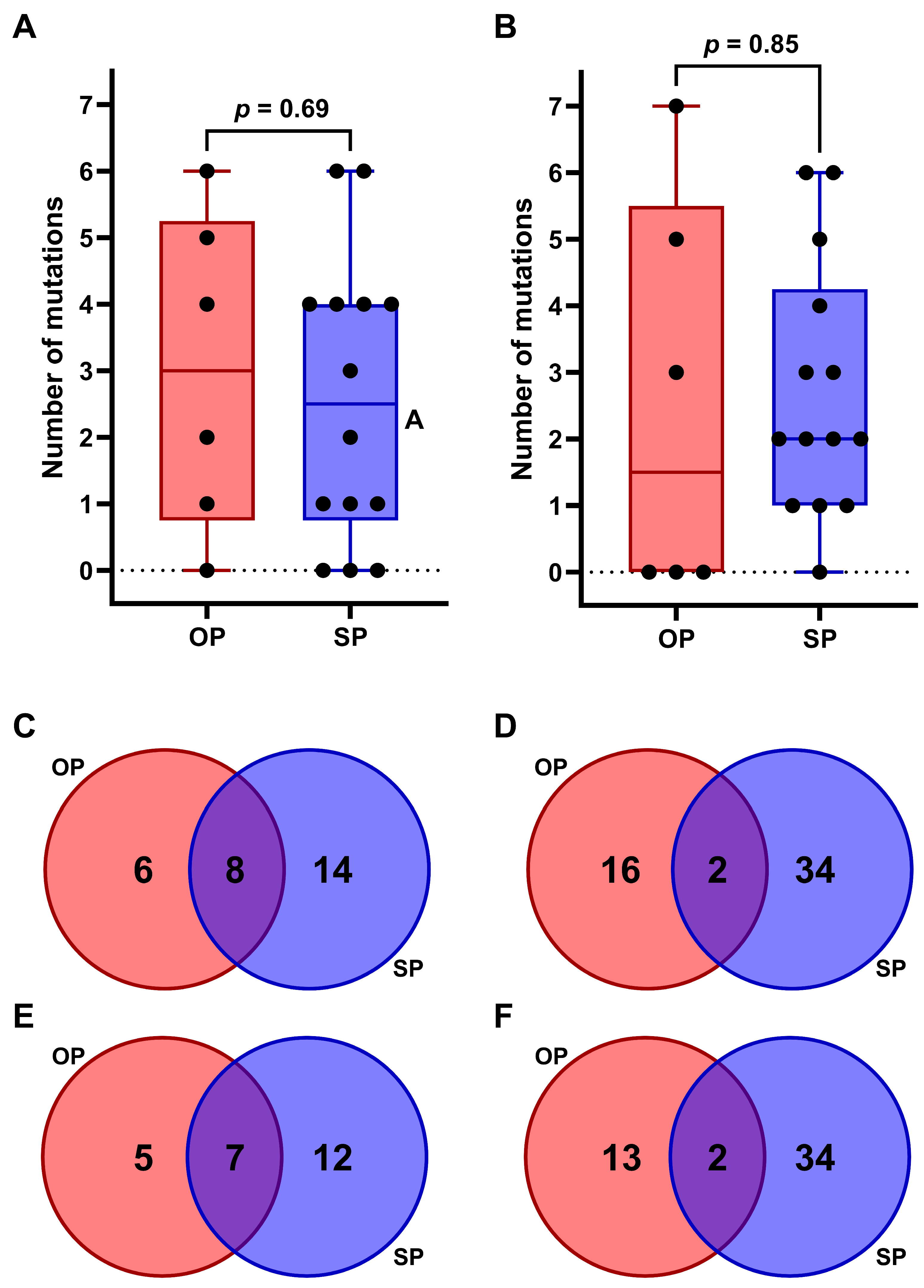
2.3. Comparative Analysis of Somatic Variants in OP Versus SP
3. Discussion
4. Materials and Methods
4.1. Patient Inclusion and Sample Collection
4.2. ccfDNA and gDNA Extraction
4.3. ctDNA NGS Analysis and Interpretation
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Anti-CTLA4 | Anti-Cytotoxic T-lymphocyte-associated protein 4 |
| ccfDNA | Circulating cell-free DNA |
| CH | Clonal haematopoiesis |
| CT | Computed tomography |
| ctDNA | Circulating tumor DNA |
| ECOG | Eastern Cooperative Oncology Group |
| gDNA | Genomic DNA |
| ICI | Immune checkpoint inhibitors |
| InDels | Insertion/deletion mutation |
| MOD | Molecular oligometastatic disease |
| NCCN | National Comprehensive Cancer Network |
| NGS | Next-Generation Sequencing |
| NSCLC | Non-small cell lung cancer |
| OP | Oligoprogression |
| OS | Overall survival |
| RECIST | Response evaluation criteria in solid tumors |
| RT | Radiotherapy |
| PBMC | Peripheral blood mononuclear cells |
| PD-1 | Programmed death 1 |
| PD-L1 | Programmed death ligand 1 |
| PFS | Progression-free survival |
| PR | Partial response |
| SBRT | Stereotactic body radiation therapy |
| SD | Stable disease |
| SNP | Single-nucleotide polymorphism |
| SNV | Single-nucleotide variants |
| SP | Systemic progression |
| TMB | Tumor mutational burden |
| UMCG | University Medical Center Groningen |
| VUmc | location of Amsterdam University Medical Center |
| VUS | Variant of unknown significance |
References
- Zhou, F.; Qiao, M.; Zhou, C. The Cutting-Edge Progress of Immune-Checkpoint Blockade in Lung Cancer. Cell Mol. Immunol. 2021, 18, 279–293. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The Promises and Challenges of Tumor Mutation Burden as an Immunotherapy Biomarker: A Perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Attili, I.; Morganti, S.; Del Signore, E.; Gianoncelli, L.; Spitaleri, G.; Stati, V.; Catania, C.; Curigliano, G.; de Marinis, F. Clinical Features Affecting Survival in Metastatic NSCLC Treated with Immunotherapy: A Critical Review of Published Data. Cancer Treat. Rev. 2020, 89, 102085. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, A.; Rohlin, A.; Raghavan, S. Immune Checkpoint Blockade and Biomarkers of Clinical Response in Non–Small Cell Lung Cancer. Scand. J. Immunol. 2020, 92, e12980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Sakthivel, G.; Milano, M.T.; Qiu, H.; Singh, D.P. Oligoprogression in Non-Small Cell Lung Cancer: A Narrative Review. J. Thorac. Dis. 2022, 14, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; Desouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and Classification of Oligometastatic Disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus Recommendation. Lancet Oncolocy 2020, 21, 18–28. [Google Scholar] [CrossRef]
- Brown, L.J.; Ahn, J.; Gao, B.; Gee, H.; Nagrial, A.; Pires da Silva, I.; Hau, E. Radiotherapy Improves Survival in NSCLC After Oligoprogression on Immunotherapy: A Cohort Study. JTO Clin. Res. Rep. 2024, 5, 100695. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Eng, J.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; et al. Standard-of-Care Systemic Therapy with or without Stereotactic Body Radiotherapy in Patients with Oligoprogressive Breast Cancer or Non-Small-Cell Lung Cancer (Consolidative Use of Radiotherapy to Block [CURB] Oligoprogression): An Open-Label, Randomised, Controlled, Phase 2 Study. Lancet 2024, 403, 171–182. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Guttmann, D.M.; Shaverdian, N.; Shepherd, A.F.; Eng, J.; Gelblum, D.; Xu, A.J.; Namakydoust, A.; Iqbal, A.; et al. Consolidative Use of Radiotherapy to Block (CURB) Oligoprogression—Interim Analysis of the First Randomized Study of Stereotactic Body Radiotherapy in Patients with Oligoprogressive Metastatic Cancers of the Lung and Breast. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1325–1326. [Google Scholar] [CrossRef]
- Tomasik, B.; Skrzypski, M.; Bieńkowski, M.; Dziadziuszko, R.; Jassem, J. Current and Future Applications of Liquid Biopsy in Non-Small-Cell Lung Cancer—A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 594–614. [Google Scholar] [CrossRef]
- van der Leest, P.; Hiddinga, B.; Miedema, A.; Aguirre Azpurua, M.L.; Rifaela, N.; ter Elst, A.; Timens, W.; Groen, H.J.M.; van Kempen, L.C.; Hiltermann, T.J.N.; et al. Circulating Tumor DNA as a Biomarker for Monitoring Early Treatment Responses of Patients with Advanced Lung Adenocarcinoma Receiving Immune Checkpoint Inhibitors. Mol. Oncol. 2021, 15, 2910–2922. [Google Scholar] [CrossRef]
- van der Leest, P.; Janning, M.; Rifaela, N.; Azpurua, M.L.A.; Kropidlowski, J.; Loges, S.; Lozano, N.; Sartori, A.; Irwin, D.; Lamy, P.-J.; et al. Detection and Monitoring of Tumor-Derived Mutations in Circulating Tumor DNA Using the UltraSEEK Lung Panel on the MassARRAY System in Metastatic Non-Small Cell Lung Cancer Patients. Int. J. Mol. Sci. 2023, 24, 13390. [Google Scholar] [CrossRef]
- Stadler, J.C.; Belloum, Y.; Deitert, B.; Sementsov, M.; Heidrich, I.; Gebhardt, C.; Keller, L.; Pantel, K. Current and Future Clinical Applications of CtDNA in Immuno-Oncology. Cancer Res. 2022, 82, 349–358. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Herbretau, G.; Dumenil, C.; Julie, C.; Giraud, V.; Labrune, S.; Dumoulin, J.; Tisserand, J.; Emile, J.F.; Blons, H.; et al. Circulating Tumor DNA Evaluated by Next-Generation Sequencing Is Predictive of Tumor Response and Prolonged Clinical Benefit with Nivolumab in Advanced Non-Small Cell Lung Cancer. Oncoimmunology 2018, 7, e1424675. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Nabet, B.Y.; Rizvi, H.; Chaudhuri, A.A.; Wells, D.K.; Dunphy, M.P.S.; Chabon, J.J.; Liu, C.L.; Hui, A.B.; Arbour, K.C.; et al. Circulating Tumor DNA Analysis to Assess Risk of Progression after Long-Term Response to PD-(L)1 Blockade in NSCLC. Clin. Cancer Res. 2020, 26, 2849–2858. [Google Scholar] [CrossRef]
- Pellini, B.; Madison, R.W.; Childress, M.A.; Miller, S.T.; Gjoerup, O.; Cheng, J.; Huang, R.S.P.; Krainock, M.; Gupta, P.; Zou, W.; et al. Circulating Tumor DNA Monitoring on Chemo-Immunotherapy for Risk Stratification in Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 4596–4605. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, S.; Zeng, Q.; Zhang, M.; Ni, Z.; Li, X.; Xu, X. A Retrospective Study Analyzing Missed Diagnosis of Lung Metastases at Their Early Stages on Computed Tomography. J. Thorac. Dis. 2019, 11, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, N.P.; Badiyan, S.N.; Samson, P.P.; Stowe, H.B.; Wang, Y.E.; Star, R.; Devarakonda, S.; Govindan, R.; Waqar, S.N.; Robinson, C.G.; et al. Pre-Radiotherapy CtDNA Liquid Biopsy for Risk Stratification of Oligometastatic Non-Small Cell Lung Cancer. NPJ Precis. Oncol. 2023, 7, 100. [Google Scholar] [CrossRef]
- Fu, R.; Xiong, Y.; Cai, M.; Chen, R.R.; Wu, Y.L.; Zhong, W.Z. OA05.05 Pre-Local Consolidative Therapy Circulating Tumor DNA Defines Molecular Oligometastatic Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2024, 19, S18–S19. [Google Scholar] [CrossRef]
- Arrieta, O.; Guijosa, A.; Heredia, D.; Dávila-Dupont, D.; Rios-Garcia, E. Stratifying Risk in Oligoprogressive EGFR-Mutated Non-Small Cell Lung Cancer: The Role of CtDNA. J. Thorac. Oncol. 2024, 19, S323–S324. [Google Scholar] [CrossRef]
- Weber, S.; van der Leest, P.; Donker, H.C.; Schlange, T.; Timens, W.; Tamminga, M.; Hasenleithner, S.O.; Graf, R.; Moser, T.; Spiegl, B.; et al. Dynamic Changes of Circulating Tumor DNA Predict Clinical Outcome in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2021, 5, 1540–1553. [Google Scholar] [CrossRef]
- Ding, H.; Yuan, M.; Yang, Y.; Xu, X.S. Identifying Key Circulating Tumor DNA Parameters for Predicting Clinical Outcomes in Metastatic Non-Squamous Non-Small Cell Lung Cancer after First-Line Chemoimmunotherapy. Nat. Commun. 2024, 15, 6862. [Google Scholar] [CrossRef]
- Gray, J.E.; Okamoto, I.; Sriuranpong, V.; Vansteenkiste, J.; Imamura, F.; Lee, J.S.; Pang, Y.K.; Cobo, M.; Kasahara, K.; Cheng, Y.; et al. Tissue and Plasma EGFR Mutation Analysis in the FLAURA Trial: Osimertinib versus Comparator EGFR Tyrosine Kinase Inhibitor as First-Line Treatment in Patients with EGFR-Mutated Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6644–6652. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Z.J.F.; Zou, W.; Fine, A.D.; Socinski, M.A.; Young, A.; Lipson, D.; Freidin, J.F.; Kennedy, M.; Polisecki, E.; Nishio, M.; et al. A Longitudinal Circulating Tumor DNA-Based Model Associated with Survival in Metastatic Non-Small-Cell Lung Cancer. Nat. Med. 2023, 29, 859–868. [Google Scholar] [CrossRef]
- Anagnostou, V.; Ho, C.; Nicholas, G.; Juergens, R.A.; Sacher, A.; Fung, A.S.; Wheatley-Price, P.; Laurie, S.A.; Levy, B.; Brahmer, J.R.; et al. CtDNA Response after Pembrolizumab in Non-Small Cell Lung Cancer: Phase 2 Adaptive Trial Results. Nat. Med. 2023, 29, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lu, W.; Yu, A.; Wu, H.; He, J. Effect of the STK11 Mutation on Therapeutic Efficacy and Prognosis in Patients with Non-Small Cell Lung Cancer: A Comprehensive Study Based on Meta-Analyses and Bioinformatics Analyses. BMC Cancer 2024, 24, 491. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. STK11 and KEAP1 Mutations as Prognostic Biomarkers in an Observational Real-World Lung Adenocarcinoma Cohort. ESMO Open 2020, 5, e000706. [Google Scholar] [CrossRef]
- AVENIO ctDNA Expanded Kit V2. Available online: https://sequencing.roche.com/global/en/products/group/avenio-ctdna-expanded-kits.html (accessed on 17 June 2025).
- Sidorenkov, G.; Nagel, J.; Meijer, C.; Duker, J.J.; Groen, H.J.M.; Halmos, G.B.; Oonk, M.H.M.; Oostergo, R.J.; Van Der Vegt, B.; Witjes, M.J.H.; et al. The OncoLifeS Data-Biobank for Oncology: A Comprehensive Repository of Clinical Data, Biological Samples, and the Patient’s Perspective. J. Transl. Med. 2019, 17, 374. [Google Scholar] [CrossRef]
- van der Leest, P.; Boonstra, P.A.; ter Elst, A.; van Kempen, L.C.; Tibbesma, M.; Koopmans, J.; Miedema, A.; Tamminga, M.; Groen, H.J.M.; Reyners, A.K.L.; et al. Comparison of Circulating Cell-Free DNA Extraction Methods for Downstream Analysis in Cancer Patients. Cancers 2020, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, P.; Ketelaar, E.M.; van Noesel, C.J.M.; van den Broek, D.; van Boerdonk, R.A.A.; Deiman, B.; Rifaela, N.; van der Geize, R.; Huijsmans, C.J.J.; Speel, E.J.M.; et al. Dutch National Round Robin Trial on Plasma-Derived Circulating Cell-Free DNA Extraction Methods Routinely Used in Clinical Pathology for Molecular Tumor Profiling. Clin. Chem. 2022, 68, 963–972. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, P.; Rozendal, P.; Rifaela, N.; van der Wekken, A.J.; Kievit, H.; de Jager, V.D.; Sidorenkov, G.; van Kempen, L.C.; Hiltermann, T.J.N.; Schuuring, E. Detection of Actionable Mutations in Circulating Tumor DNA for Non-Small Cell Lung Cancer Patients. Commun. Med. 2025, 5, 204. [Google Scholar] [CrossRef] [PubMed]
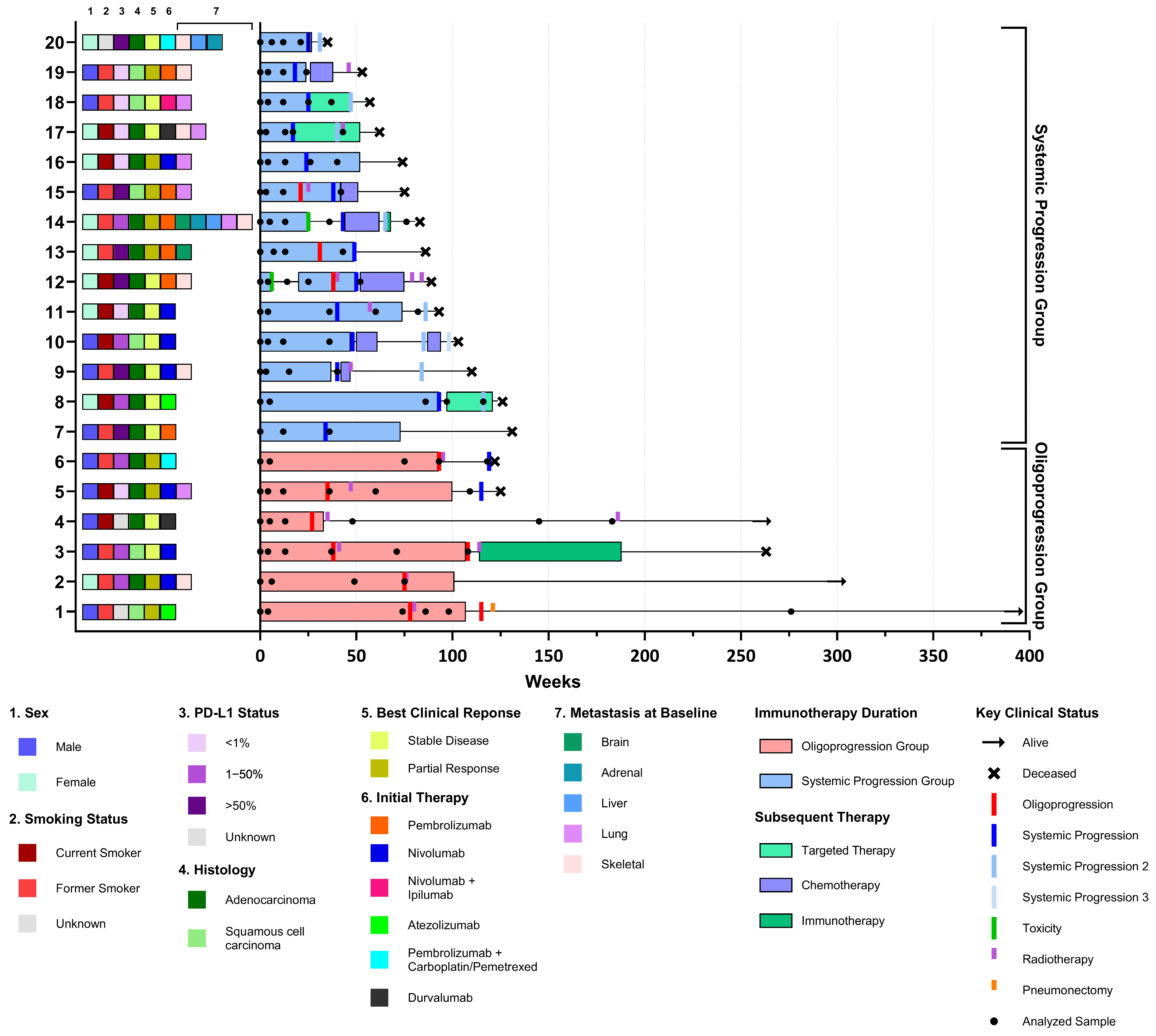
| All Patients (n = 20) | Oligoprogression (n = 6) | Systemic Progression (n = 14) | p-Value | |
|---|---|---|---|---|
| Median age (range) | 67 (44–84) | 70 (60–84) | 64 (44–72) | 0.09 1 |
| Sex | 0.16 2 | |||
| Male | 11 (55%) | 5 (83%) | 6 (43%) | |
| Female | 9 (45%) | 1 (17%) | 8 (57%) | |
| ECOG PS | 0.58 1 | |||
| 0 | 10 (50%) | 2 (33%) | 8 (57%) | |
| 1 | 8 (40%) | 4 (67%) | 4 (29%) | |
| 2 | 1 (5%) | 1 (7%) | ||
| 3 | 1 (5%) | 1 (7%) | ||
| Histology | 1.00 2 | |||
| Adenocarcinoma | 14 (70%) | 4 (67%) | 10 (71%) | |
| Squamous cell carcinoma | 6 (30%) | 2 (33%) | 4 (29%) | |
| Smoking status | 1.00 2 | |||
| Active smoker | 8 (40%) | 2 (33%) | 6 (43%) | |
| Former smoker | 11 (55%) | 4 (67%) | 7 (50%) | |
| Never smoker | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 1 ((5%) | 0 (07%) | 1 (7%) | |
| Current treatment | Not applicable | |||
| Atezolizumab | 2 (10%) | 0 (0%) | 2 (14%) | |
| Durvalumab | 2 (10%) | 1 (17%) | 1 (7%) | |
| Nivolumab | 7 (35%) | 4 (67%) | 3 (21%) | |
| Nivolumab/ipilimumab | 1 (5%) | 0 (0%) | 1 (7%) | |
| Pembrolizumab | 6 (30%) | 0 (0%) | 6 (43%) | |
| Pembrolizumab/carboplatin–pemetrexed | 2 (10%) | 1 (17%) | 1 (7%) | |
| Previous lines of therapies | 0.42 1 | |||
| 0 | 10 (50%) | 2 (33%) | 8 (57%) | |
| 1 | 5 (25%) | 2 (33%) | 3 (21%) | |
| 2 | 4 (20% | 2 (33%) | 2 (14%) | |
| ≥3 | 1 (5%) | 0 (0%) | 1 (7%) | |
| PD-L1 | 0.50 1 | |||
| <1% | 6 (30%) | 1 (17%) | 5 (36%) | |
| 1–50% | 6 (30%) | 3 (50%) | 3 (21%) | |
| >50% | 6 (30%) | 0 (0%) | 6 (43%) | |
| Missing | 2 (10%) | 2 (33%) | 0 (0%) | |
| Number of metastatic sites at start ICI treatment ° | 0.19 1 | |||
| 0 | 1 (5%) | 0 (0%) | 1 (7%) | |
| 1 | 6 (30%) | 3 (50%) | 3 (21%) | |
| 2 | 7 (35%) | 2 (33%) | 5 (36%) | |
| 3 | 3 (15%) | 1 (17%) | 2 (14%) | |
| ≥4 | 3 (15%) | 0 (0%) | 3 (21%) | |
| Total number of metastases at start ICI treatment | 0.09 1 | |||
| <5 | 8 (40%) | 4 (67%) | 4 (29%) | |
| 5–20 | 9 (45%) | 2 (33%) | 7 (50%) | |
| >20 | 3 (15%) | 0 (0%) | 3 (21%) | |
| Metastases associated with poor survival before start ICI treatment | ||||
| Brain | 2 (10%) | 0 (0%) | 2 (14%) | 0.34 1 |
| Liver | 2 (10%) | 0 (0%) | 2 (14%) | 0.34 1 |
| Bone | 7 (35%) | 1 (17%) | 6 (43%) | 0.27 1 |
| Survival in weeks (range) | ||||
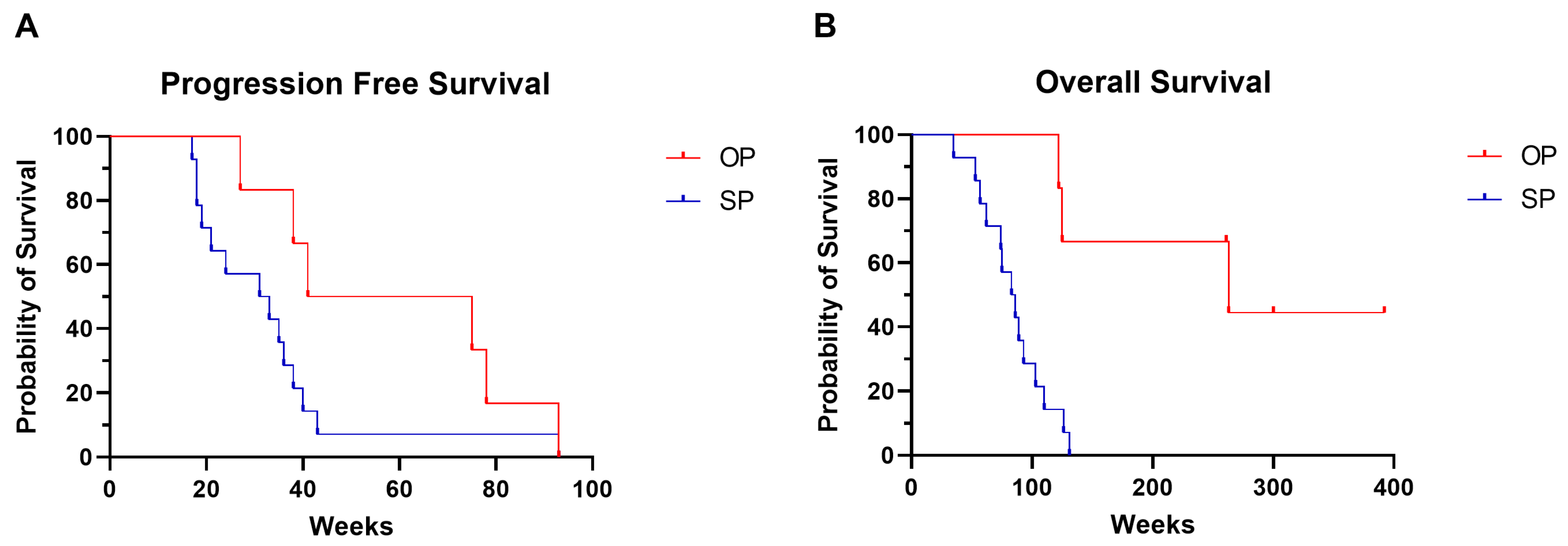
| Median PFS | 37 (17–93) | 58 (27–93) | 32 (17–93) | 0.04 1 |
| Median OS | 103 (35–NR) | 262 (122–370) | 85 (35–131) | 0.002 1 |
| Median duration of ICI treatment | 46 (15–105) | 99 (31–105) | 42 (15–90) | 0.007 1 |
| Local ablative (radio)therapy during ICI treatment * | 10 (50%) | 6 (100%) | 4 (29%) | Not applicable |
| Primary tumor | 4 (20%) | 3 (50%) | 1 (7%) | |
| Metastasis | 6 (30%) | 3 (50%) | 3 (21%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozendal, P.; Kievit, H.; van der Leest, P.; Bahce, I.; Pegtel, M.; Groen, H.J.M.; van Kempen, L.C.; Hiltermann, T.J.N.; Schuuring, E. Cell-Free DNA Based Next-Generation Sequencing Does Not Differentiate Between Oligoprogression and Systemic Progression in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors—An Explorative Study. Int. J. Mol. Sci. 2025, 26, 8087. https://doi.org/10.3390/ijms26168087
Rozendal P, Kievit H, van der Leest P, Bahce I, Pegtel M, Groen HJM, van Kempen LC, Hiltermann TJN, Schuuring E. Cell-Free DNA Based Next-Generation Sequencing Does Not Differentiate Between Oligoprogression and Systemic Progression in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors—An Explorative Study. International Journal of Molecular Sciences. 2025; 26(16):8087. https://doi.org/10.3390/ijms26168087
Chicago/Turabian StyleRozendal, Pim, Hanneke Kievit, Paul van der Leest, Idris Bahce, Michiel Pegtel, Harry J. M. Groen, Léon C. van Kempen, T. Jeroen N. Hiltermann, and Ed Schuuring. 2025. "Cell-Free DNA Based Next-Generation Sequencing Does Not Differentiate Between Oligoprogression and Systemic Progression in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors—An Explorative Study" International Journal of Molecular Sciences 26, no. 16: 8087. https://doi.org/10.3390/ijms26168087
APA StyleRozendal, P., Kievit, H., van der Leest, P., Bahce, I., Pegtel, M., Groen, H. J. M., van Kempen, L. C., Hiltermann, T. J. N., & Schuuring, E. (2025). Cell-Free DNA Based Next-Generation Sequencing Does Not Differentiate Between Oligoprogression and Systemic Progression in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors—An Explorative Study. International Journal of Molecular Sciences, 26(16), 8087. https://doi.org/10.3390/ijms26168087










