Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications
Abstract
1. Introduction
2. Pain Classification
3. Fundamentals of the Trigeminal Nociceptive Pathway
4. Understanding the Peripheral and Central Transmission of Nociceptive Pain
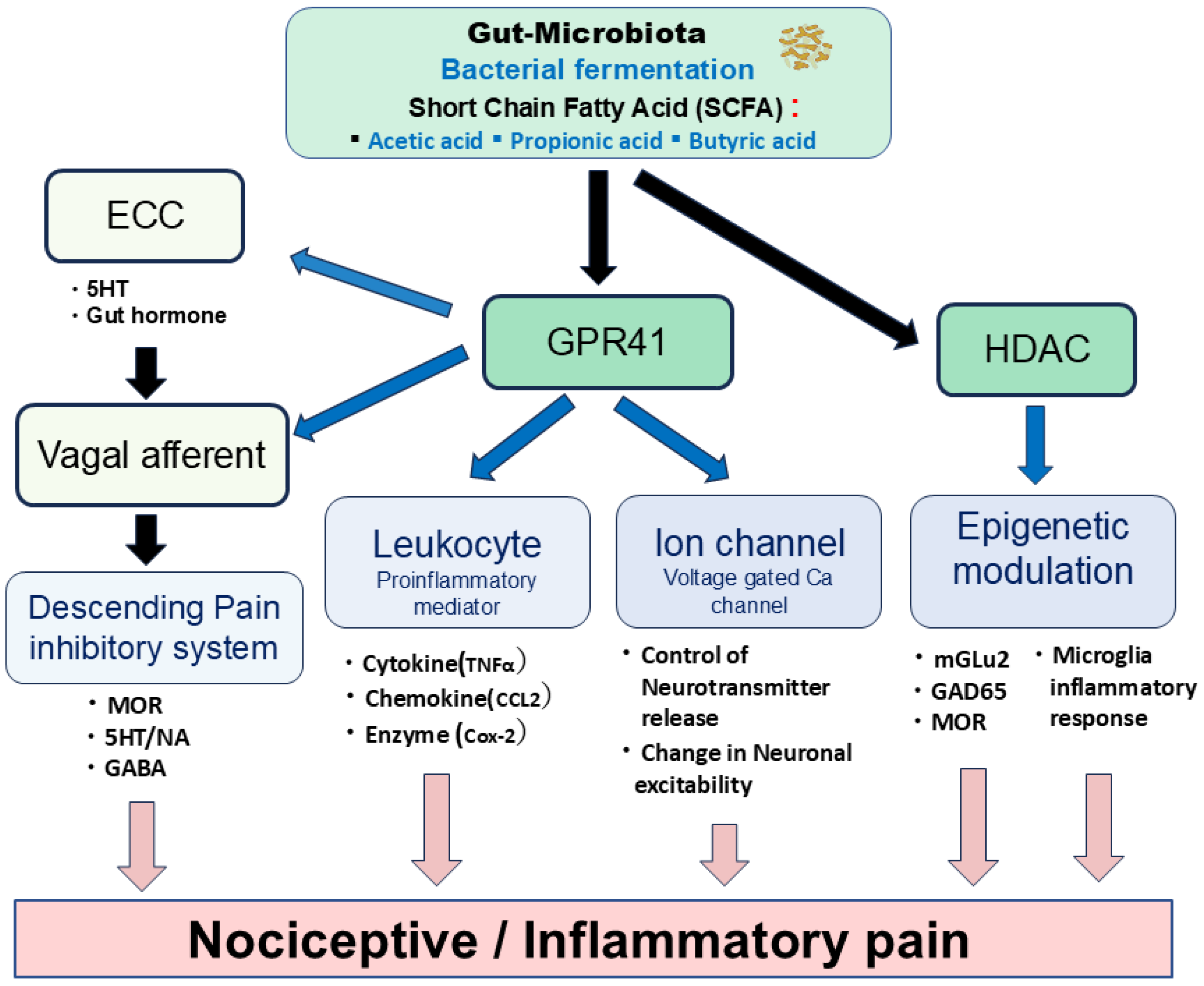
5. Possible Mechanisms Underlying SCFAs’ Modulation of Pain
6. Potential Modulatory Mechanisms of Nociceptive Pain by SCFAs
7. Potential Modulatory Mechanisms of Inflammatory Pain by SCFAs
8. Functional Role of SCFAs in Pain Modulation and Future Direction
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| SCFA | Short-chain fatty acid |
| GPR | G-protein-coupled receptor |
| FFAR | Free fatty acid receptor |
| HCAR | Hydroxy-carboxylic acid receptor |
| SPF | Specific pathogen-free |
| GF | Germ-free |
| NTG | Nitroglycerin |
| FMT | Fecal microbiota transplantation |
| IBS | Irritable bowel syndrome |
| TG | Trigeminal ganglion |
| C1-C2 | Upper cervical dorsal horn |
| SpVc | Trigeminal spinal nucleus caudalis |
| SpVc | Trigeminal spinal nucleus oralis |
| WDR | Wide-dynamic range |
| Nav | Voltage-gated Na channel |
| Kv | Voltage-gated K channel |
| Cav | Voltage-gated Ca channel |
| EPSP | Excitatory post synaptic potential |
| ASIC | Acid sensing channel |
| TRPA1 | Transient receptor ankyrin 1 |
| TTX | Tetrodotoxin |
| HDAC | Histone deacetylase |
| MOR | μ-opioid receptor |
| 5HT | Serotonin |
| NA | Noradrenaline |
| GABA | γ-aminobutylic acid |
| TNFα | Tumor necrosis factorα |
| CCL2 | Chemokine c-c ligand 2 |
| IL | Interleukin |
| CFA | Complete Freund’s adjuvant |
| COX-2 | Cyclooxygenase 2 |
| MGLU2 | Metabotropic glutamate receptor 2 |
| GAD65 | Glutamic acid decarboxylase 65 |
| PKA | Protein kinase A |
| PKC | Protein Kinase C |
| EP | E-type prostanoid |
| PGE2 | Prostaglandin E2 |
| Glu | Glutamate |
| NF-κB | Nuclear factor-kappa B |
| BBB | Blood–brain barrier |
References
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, X.; Han, B.; Tang, X.; Liu, R.; Ji, Q.; Zhiu, Z.; Zhang, L. Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization. Mol. Pain 2021, 17, 1744806921996520. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ishimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Dang, G.; Wu, W.; Zhang, H.; Everaert, N. A new paradigm for a new simple chemical: Butyrate & immune regulation. Food Func. 2021, 12, 12181–12193. [Google Scholar]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.F.; Huang, L.; et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut dysbiosis in patients with anorexia nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol alleviates temporomandibular joint inflammatory pain by recovering disturbed gut microbiota. Brain Behav. Immun. 2020, 87, 455–464. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, M.-Y.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. eLife 2017, 6, e25887. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, S.; Shu, H.; Yanagisawa, L.; Tao, F. Gut microbiota dysbiosis enhances migraine-like pain via TNFα upregulation. Mol. Neurobiol. 2020, 57, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Zhu, S.; Fang, Y.; Wang, B.; Jia, Q.; Hao, H.; Kao, J.Y.; He, Q.; Song, L.; et al. Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation. Acta Pharmacol. Sin. 2021, 42, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutvin, S.A.L.W.; Troost, F.J.; Kilkens, T.O.C.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.A.E.; Venema, K.; Brummer, R.-J.M. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Tang, Y.; Du, J.; Wu, H.; Wang, M.; Liu, S.; Tao, F. Potential therapeutic effect of short-chain fatty acids on chronic pain. Curr. Pharmacol. 2024, 22, 191–203. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Y.-Q. Spinal glial activation contributes to pathological pain states. Neurosci. Biobehav. Rev. 2008, 32, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F. Pain Theories. In Science of Pain; Basbaum, A.I., Bushnell, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 5–10. [Google Scholar]
- Iwata, K.; Takeda, M.; Oh, S.B.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Balasubramaniam, R., McCullough, M.J., Eds.; Springer International: Zurich, Switzerland, 2017; pp. 1749–1773. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5 (Suppl. 11), 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J. Chronic Orofacial Pain: Models, Mechanisms, and Genetic and Related Environmental Influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Shinoda, M.; Suzuro, H.; Iwata, K.; Hayashi, Y. Plastic changes in nociceptive pathways contributing to persistent orofacial pain. J. Oral Biosci. 2022, 64, 263–270. [Google Scholar] [CrossRef]
- Sessle, B.J. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J. Oral Biosci. 2011, 53, 318–329. [Google Scholar] [CrossRef]
- Al-Khater, K.M.; Todd, A.J. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J. Comp. Neurol. 2009, 515, 629–646. [Google Scholar] [CrossRef]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.H.; Price, M.P.; Gautam, M.; Benson, C.J.; Gong, H.; Welsh, M.J.; Brennan, T.J. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS ONE 2012, 7, e35225. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef]
- Borzan, J.; Zhao, C.; Mayer, R.A.; Raja, S.N. A role for acid-sensing ion channel 3, but not acid-sensing ion channels 2, in sensing dynamic mechanical stimuli. Anesthesiology 2010, 113, 647–654. [Google Scholar] [CrossRef]
- Price, M.P.; McIlwrath, S.L.; Xie, J.; Cheng, C.; Qiao, J.; Tarr, D.E.; Sluka, K.A.; Brennan, T.L.; Lewin, G.R.; Welsh, M.J. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neurons 2001, 32, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Boje, K.M.K. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood–brain barrier. J. Pharmacol. Exp. Ther. 2004, 311, 92–98. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Cousens, L.S.; Gallwitz, D.; Alberts, B.M. Different accessibilities in chromatin to histone acetylase. J. Biol. Chem. 1979, 254, 1716–1723. [Google Scholar] [CrossRef]
- Khan, O.; Fotheringham, S.; Wood, V.; Stimson, L.; Zhang, C.; Pezzella, F.; Duvic, M.; Kerr, D.J.; La Thangue, N.B. HR23B is a biomarker for tumor sensitivity to HDAC inhibitor-based therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 6532–6537. [Google Scholar] [CrossRef]
- Borgonetti, V.; Pressi, G.; Bertaiola, O.; Guarnerio, C.; Mandrone, M.; Chiocchio, I.; Galeotti, N. Attenuation of neuroinflammation in microglia cells by extracts with high content of rosmarinic acid from in vitro cultured officinalis cels. J. Pharmacol. Biomed. Anal. 2022, 220, 114969. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, Y.Q.; Zou, F.; Bie, B.; Pan, Z.Z. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat. Med. 2011, 17, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Weng, Y.; Ouyang, B.; Ding, Z.; Song, Z.; Zou, W.; Huang, C.; Guo, Q. HDAC inhibitor TSA ameliorates mechanical hypersensitivity and potentiates analgesic effect of morphine in a rat model of bone cancer pain by restoring μ-opioid receptor in spinal cord. Brain Res. 2017, 1669, 97–105. [Google Scholar] [CrossRef]
- Zammataro, M.; Sortino, M.A.; Parenti, C.; Gereau, R.W., IV; Chiechio, S. HDAC and HAT inhibitors differently affect analgesia mediated by group II metabotropic glutamate receptors. Mol. Pain 2014, 10, 1744–8069-10-68. [Google Scholar] [CrossRef]
- Nohr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Won, Y.J.; Lu, V.B.; Puhl, H.L., 3rd; Ikeda, S.R. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodriguees, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Zhu, J.; Owyang, C. Serotonin released from intestinal enterochromaffin cells mediates luminal non–cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 2000, 118, 1197–1207. [Google Scholar] [CrossRef]
- Strader, A.D.; Woods, S.C. Gastrointestinal hormones and food intake. Gastroenterology 2005, 128, 175–191. [Google Scholar] [CrossRef]
- Randich, A.; Gebhart, G. Vagal afferent modulation of nociception. Brain Res. Rev. 1992, 17, 77–99. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Randich, A. Brain stem modulation of nociception. In Brain Stem Mechanisms of Behavior; Klem, W.R., Vertes, R.P., Eds.; Wiley-Interscience: New York, NY, USA, 1990; pp. 315–352. [Google Scholar]
- Takeda, M.; Tanimoto, T.; Ojima, K.; Matsumoto, S. Suppressive effect of vagal afferents on the activity of trigeminal neurons related to jaw-opening reflex in rats: Involvement of endogenous opioid system. Brain Res. Bull. 1998, 47, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanimoto, T.; Matsumoto, S. Volume expansion suppresses the tooth-pulp evoked jaw-opening reflex related activity of trigeminal neurons in rats. Brain Res. Bull. 2002, 58, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Sashide, Y.; Takeda, M. Gut microbiota-derived short-chain fatty acid suppresses the excitability of rat nociceptive secondary neurons via G-protein-coupled receptor 41 signaling. Mol. Pain 2025, 21, 1–10. [Google Scholar] [CrossRef]
- Jaccus, M.O.; Ubele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Regulation of T-type calcium channels in the peripheral pain pathway. Channels 2007, 1, 238–245. [Google Scholar] [CrossRef]
- Gambeta, E.; Chichorro, J.G.; Zamponi, G.W. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Mol. Pain 2020, 16, 1–18. [Google Scholar] [CrossRef]
- Gambeta, E.; Gandini, M.A.; Souza, I.A.; Zamponi, G.W. Cav3.2 calcium channels contribute to trigeminal neuralgia. Pain 2022, 163, 2315–2325. [Google Scholar] [CrossRef]
- Waise, T.M.Z.; Dranse, H.J.; Lam, T.K.T. The metabolic role of vagal afferent innervation. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 625–636. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Takeda, M.; Tanimoto, T.; Katsuumi, I.; Matsumoto, S. Tooth-pulp-evoked rostral spinal trigeminal nucleus neuron activity is suppressed by conditioning sciatic nerve stimulation in the rats: Possible role of 5-HT3 receptor mediated GABAergic inhibition. Brain Res. Bull. 2005, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Takeda, M.; Nishikawa, T.; Matsumoto, S. The role of 5-HT3 receptors in the vagal afferent activation-induced of C1 spinal neurons projected from tooth-pulp in the rat. J. Pharmacol. Exp. Ther. 2004, 311, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Iwasaki, Y.; Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate activity. J. Nutr. 2003, 133, 2485–2493. [Google Scholar] [CrossRef]
- Luhrs, H.; Gerke, T.; Muller, J.G.; Melcher, R.; Schauber, J.; Boxberge, F.; Scheppach, W.; Merzel, T. Butyrate inhibits NF-kappa B activation in lamina propria macrophage of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002, 37, 456–458. [Google Scholar] [CrossRef]
- Russo, R.; De Carto, C.; Avagliano, C.; Cristiano, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 2016, 103, 279–291. [Google Scholar] [CrossRef]
- Kukkar, A.; Sigh, N.; Jaggi, A.S. Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J. Formos. Med. Assoc. 2014, 113, 921–928. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Takehana, S.; Shibuya, E.; Matsuzawa, N.; Hidaka, H.; Kanai, Y.; Inoue, M.; Kubota, Y.; Shimazu, Y.; Takeda, M. Resveratrol attenuates inflammation-induced hyperexcitability of trigeminal spinal nucleus caudalis neurons associated with hyperalgesia in rats. Mol. Pain 2016, 12, 1744806916643082. [Google Scholar] [CrossRef]
- Murakami, N.; Yoshikawa, K.; Tsukada, K.; Kamino, N.; Hayashi, Y.; Hitomi, S.; Kimura, Y.; Shibuta, I.; Osada, A.; Sato, S.; et al. Butyric acid modulates periodontal nociception in Porphyromonas gingivalis-induced periodontitis. J. Oral Sci. 2022, 64, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Sashide, Y.; Utugi, S.; Takeda, M. A short chain fatty acid, butyrate suppresses the hyperexcitability of rat nociceptive primary neurons involved in inflammatory hyperalgesia. Molecules 2025, 30, 2407. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, L.S.; Wallace, M.S. Acute pain mechanisms. Surg. Clin. N. Am. 1999, 79, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Roch, M.; Messlinger, K.; Kulchitsky, V.; Tichonovich, O.; Azev, O.; Koulchitsky, S. Ongoing activity in trigeminal wide-dynamic range neurons is driven from the periphery. Neuroscience 2007, 150, 681–691. [Google Scholar] [CrossRef]
- Diener, M.; Scharrer, E. The effect of short-chain fatty acids on Cl- and K+ conductance in rat colonic crypts. Pflugers Arch. 1994, 426, 472–480. [Google Scholar] [CrossRef]
- Diener, M.; Scharrer, E. Effects of short-chain fatty acids on cell volume regulation and chloride transport in the rat distal colon. Comp. Biochem. Physiol. A Physiol. 1997, 118, 375–379. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, J.; Hankir, M.; Zhang, S.; et al. The short chain fatty acids acetate reduces food intake via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Takeda, M.; Sashide, Y. Pain management with natural products: Neurophysiological insights. Int. J. Mol. Sci. 2025, 26, 6305. [Google Scholar] [CrossRef]
- LaCroix-Fralish, M.L.; Ledoux, J.B.; Mogil, J.S. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain 2007, 131, 3e1–3e4. [Google Scholar] [CrossRef]
- Geranton, S.M.; Morenilla-Palao, C.; Hunt, S.P. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticityrelated gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J. Neurosci. 2007, 27, 6163–6173. [Google Scholar] [CrossRef]
- Griffin, R.S.; Costigan, M.; Brenner, G.J.; Ma, C.H.; Scholz, J.; Moss, A.; Allchorne, A.J.; Stahl, G.L.; Woolf, C.J. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J. Neurosci. 2007, 27, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Wei, D.; Zou, S.; Ren, K.; Dubner, R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol. Pain 2010, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Ishikawa, H.; Sano, R.; Shimazu, Y.; Takeda, M. Effect of resveratrol on the hyperexcitability of nociceptive neurons associated with ectopic hyperalgesia induced by experimental tooth movement. Eur. J. Oral Biosci. 2020, 128, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Svensson, C.I.; Koehrn, F.J.; Bhuskute, A.; Sorkin, L.S. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 2010, 149, 243–253. [Google Scholar] [CrossRef]
- Zhang, L.; Berta, T.; Xu, Z.Z.; Liu, T.; Park, J.Y.; Ji, R.R. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011, 152, 419–427. [Google Scholar] [CrossRef]
- Pane, K.; Boccella, S.; Guida, F.; Franzese, M.; Maione, S.; Salvatore, M. Role of gut microbiota in neuropathy and neuropathic pain states: A systemic preclinical review. Neurobiol. Dis. 2022, 170, 105773. [Google Scholar] [CrossRef]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immun. 2020, 85, 128–141. [Google Scholar] [CrossRef]
- Boccella, S.; Guida, F.; De Logu, F.; De Gregorio, D.; Mazzitelli, M.; Belardo, C.; Iannotta, M.; Serra, N.; Nassini, R.; De Novellis, V.; et al. Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J. 2019, 33, 1062–1073. [Google Scholar] [CrossRef]
- Offermanns, S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metab. 2017, 28, 227–236. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Hauser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Yoo, Y.-M.; Kim, K.-H. Current understanding of nociplastic pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, M.; Sashide, Y.; Utugi, S. Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 8082. https://doi.org/10.3390/ijms26168082
Takeda M, Sashide Y, Utugi S. Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(16):8082. https://doi.org/10.3390/ijms26168082
Chicago/Turabian StyleTakeda, Mamoru, Yukito Sashide, and Syogo Utugi. 2025. "Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications" International Journal of Molecular Sciences 26, no. 16: 8082. https://doi.org/10.3390/ijms26168082
APA StyleTakeda, M., Sashide, Y., & Utugi, S. (2025). Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications. International Journal of Molecular Sciences, 26(16), 8082. https://doi.org/10.3390/ijms26168082





