The Influence of Insulin Resistance and Type 2 Diabetes on Cognitive Decline and Dementia in Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Search String
2.2.1. MeSH
2.2.2. Non-MeSH
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Characteristics of Selected Studies
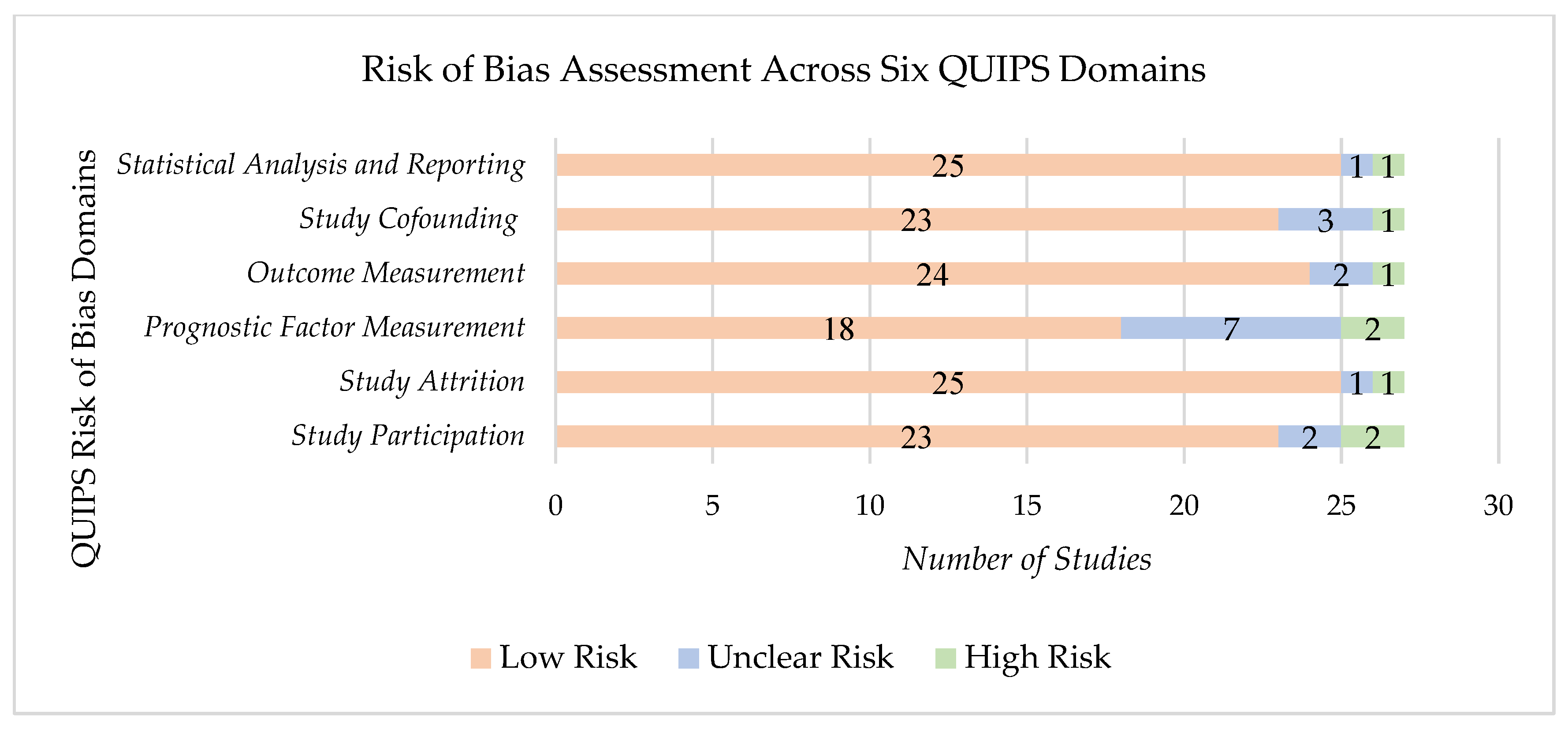
3.2. Study Quality and Potential Sources
3.3. Effect of Exposure on Outcome
3.3.1. T2DM and IR
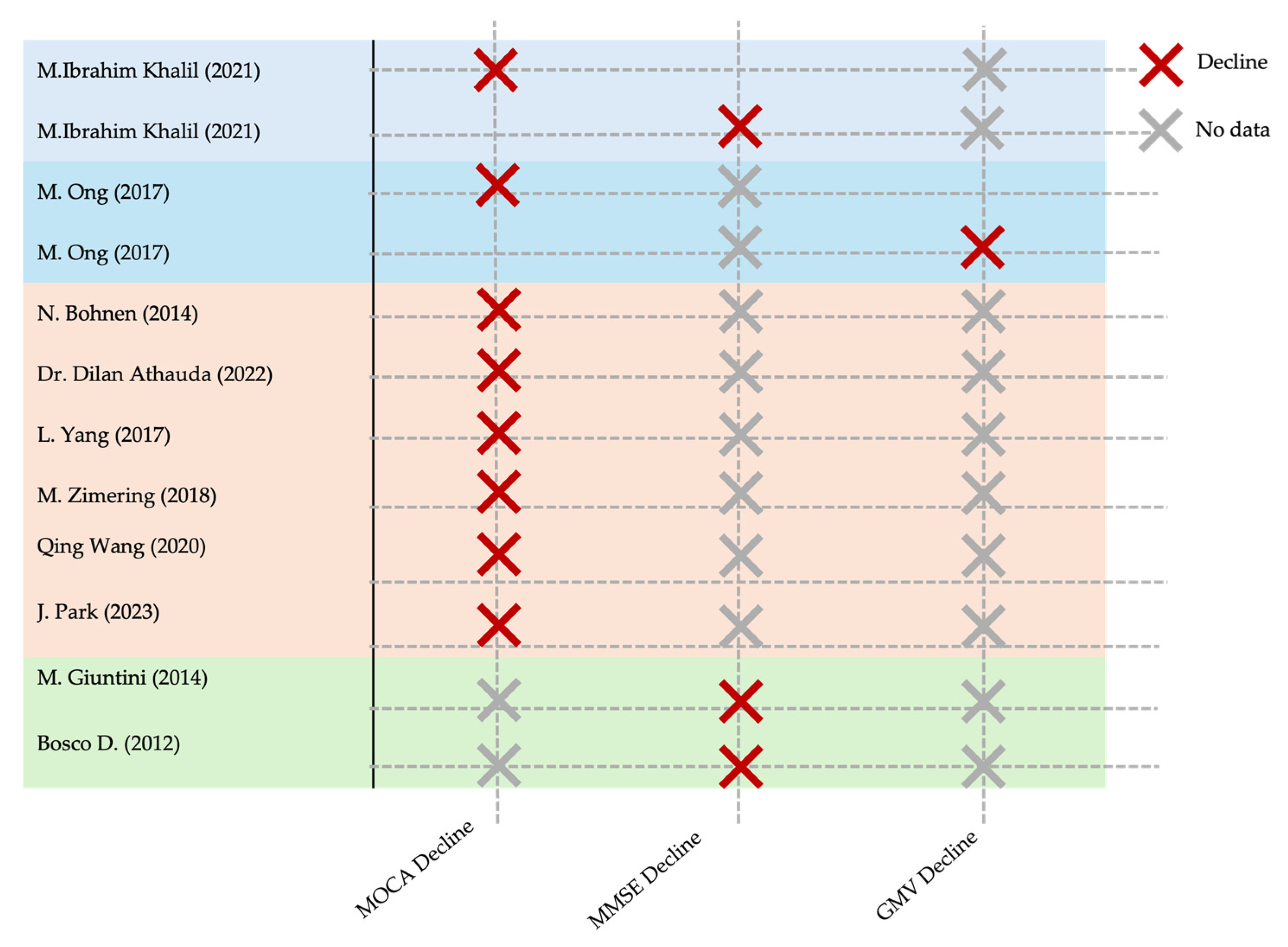
3.3.2. Cognitive Decline (CD) and Parkinson’s Disease Dementia (PDD)
4. Discussion
4.1. Major Findings
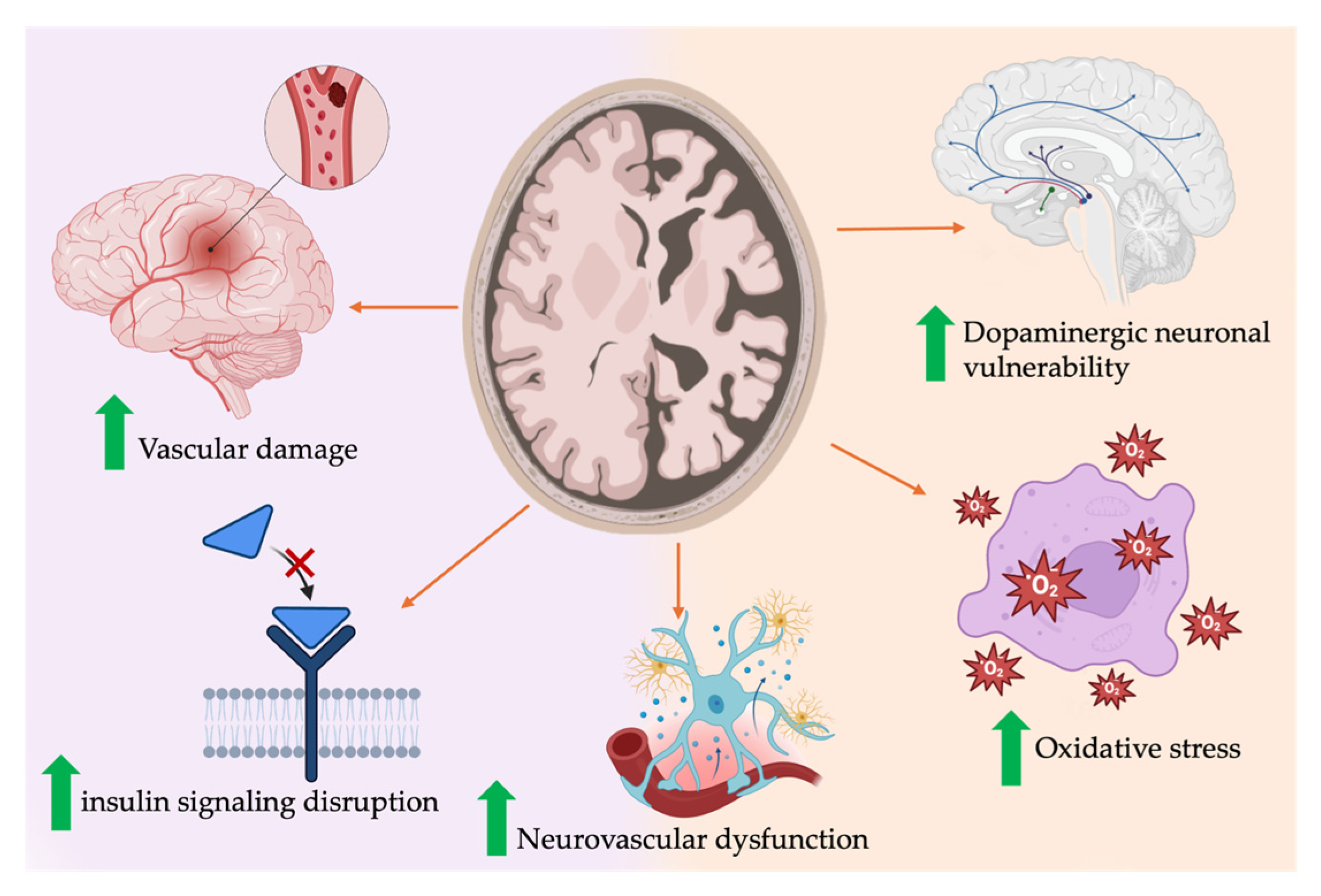
4.2. Mechanisms and Pathophysiology Linking T2DM/IR to Cognitive Decline
4.2.1. Insulin Resistance and Brain Function
4.2.2. T2DM
4.2.3. Vascular Contributions
4.3. Limitations and Bias
4.4. Comparison with the Literature and Clinical Implications
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Kim, W.S.; Michaelian, J.C.; Lewis, S.J.G.; Phillips, C.L.; D’Rozario, A.L.; Chatterjee, P.; Martins, R.N.; Grunstein, R.; Halliday, G.M.; et al. Predicting neurodegeneration from sleep related biofluid changes. Neurobiol. Dis. 2024, 190, 106369. [Google Scholar] [CrossRef]
- Tong, J. The pathogenesis and treatment of Parkinson’s disease. Theor. Nat. Sci. 2024, 47, 15–19. [Google Scholar] [CrossRef]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition deficits in Parkinson’s disease: Mechanisms and treatment. Park. Dis. 2020, 2020, 2076942. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Aarsland, D.; Marsh, L.; Schrag, A. Neuropsychiatric symptoms in Parkinson’s disease. Mov. Disord. 2009, 24, 2175–2186. [Google Scholar] [CrossRef]
- Hanagasi, H.A.; Tufekcioglu, Z.; Emre, M. Dementia in Parkinson’s disease. J. Neurol. Sci. 2017, 374, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Teli, S.; Shalavadi, M.; Chandrashekhar, V.M.; Anawal, L. Type 2 Diabetes Mellitus and Parkinson’s Disease: An Emerging Link. Curr. Signal Transduct. Ther. 2025, 20, E15743624334102. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Koros, C.; Stefanis, L.; Scarmeas, N. Parkinsonism and dementia. J. Neurol. Sci. 2022, 433, 120015. [Google Scholar] [CrossRef]
- Dintica, C.S.; Yaffe, K. Epidemiology and Risk Factors for Dementia. Psychiatr. Clin. 2022, 45, 677–689. [Google Scholar] [CrossRef]
- Cui, Y.; Tang, T.Y.; Lu, C.Q.; Ju, S. Insulin resistance and cognitive impairment: Evidence from neuroimaging. J. Magn. Reson. Imaging 2022, 56, 1621–1649. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, W.; Liu, F.T.; Li, J.Q.; Cao, X.P.; Tan, L.; Wang, J.; Yu, J. Modifiable risk factors for cognitive impairment in Parkinson’s disease: A systematic review and meta-analysis of prospective cohort studies. Mov. Disord. 2019, 34, 876–883. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, Correction in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef]
- Kandiah, N.; Zhang, A.; Cenina, A.R.; Au, W.L.; Nadkarni, N.; Tan, L.C. Montreal Cognitive Assessment for the screening and prediction of cognitive decline in early Parkinson’s disease. Park. Relat. Disord. 2014, 20, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Zimering, M.B. Circulating Neurotoxic 5-HT2A receptor agonist autoantibodies in adult type 2 diabetes with Parkinson’s disease. J. Endocrinol. Diabetes 2018, 5, 10. [Google Scholar] [CrossRef][Green Version]
- Petrou, M.; Davatzikos, C.; Hsieh, M.; Albin, R.; Kotagal, V.; Müller, M.L.; Koeppe, R.A.; Herman, W.H.; Frey, K.A.; Bohnen, N.I. Diabetes, gray matter loss, and cognition in the setting of Parkinson disease. Acad. Radiol. 2016, 23, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Gao, X.; McCullough, M.L.; Jacobs, E.J.; Patel, A.V.; Mayo, T.; Schwarzschild, M.A.; Ascherio, A. Obesity, diabetes, and risk of Parkinson’s disease. Mov. Disord. 2011, 26, 2253–2259. [Google Scholar] [CrossRef]
- Alsharif, A.A.; Wei, L.; Ma, T.; Man, K.K.; Lau, W.C.; Brauer, R.; Almetwazi, M.; Howard, R.; Wong, I.C. Prevalence and incidence of dementia in people with diabetes mellitus. J. Alzheimer’s Dis. 2020, 75, 607–615. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Kim, R. Association between prediabetes and cognitive function in Parkinson’s disease. Brain Behav. 2023, 13, e2838. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J. Neurol. Sci. 2010, 293, 82–86. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, Y.; Chung, S.J.; Moon, S.-J.; Kim, C.K.; Kim, J.H.; Oh, K.; Yoon, J.S.; Seo, S.W.; Cho, G.J.; et al. Fasting glucose variability and risk of dementia in Parkinson’s disease: A 9-year longitudinal follow-up study of a nationwide cohort. Front. Aging Neurosci. 2024, 15, 1292524. [Google Scholar] [CrossRef]
- Khalil, M.I.; Kundu, N.C.; Munira, S.; Rahman, M.R. Predictors of Parkinson’s disease Dementia in a Sample of Bangladeshi Patients: Parkinson’s disease dementia. Bangladesh Med. Res. Counc. Bull. 2021, 47, 192–198. [Google Scholar] [CrossRef]
- Hogg, E.; Athreya, K.; Basile, C.; Tan, E.E.; Kaminski, J.; Tagliati, M. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease. J. Park. Dis. 2018, 8, 259–265. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kotagal, V.; Müller, M.L.; Koeppe, R.A.; Scott, P.J.; Albin, R.L.; Frey, K.A.; Petrou, M. Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Park. Relat. Disord. 2014, 20, 1394–1398. [Google Scholar] [CrossRef]
- Bosco, D.; Plastino, M.; Cristiano, D.; Colica, C.; Ermio, C.; De Bartolo, M.; Mungari, P.; Fonte, G.; Consoli, D.; Consoli, A.; et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J. Neurol. Sci. 2012, 315, 39–43. [Google Scholar] [CrossRef]
- Markaki, I.; Ntetsika, T.; Sorjonen, K.; Svenningsson, P.; Group, B.S. Euglycemia indicates favorable motor outcome in Parkinson’s disease. Mov. Disord. 2021, 36, 1430–1434. [Google Scholar] [CrossRef]
- Ong, M.; Foo, H.; Chander, R.J.; Wen, M.-C.; Au, W.L.; Sitoh, Y.Y.; Tan, L.; Kandiah, N. Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson’s disease. J. Neurol. Sci. 2017, 377, 122–126. [Google Scholar] [CrossRef]
- Giuntini, M.; Baldacci, F.; Del Prete, E.; Bonuccelli, U.; Ceravolo, R. Diabetes is associated with postural and cognitive domains in Parkinson’s disease. Results from a single-center study. Park. Relat. Disord. 2014, 20, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jin, Z.; Zhen, Q.; Qi, L.; Liu, C.; Wang, P.; Liu, Y.; Fang, J.; Liu, Y.; Su, Y.; et al. Hyperglycemia affects axial signs in patients with Parkinson’s disease through mechanisms of insulin resistance or non-insulin resistance. Neurol. Sci. 2024, 45, 2011–2019. [Google Scholar] [CrossRef]
- Barter, J.D.; Thomas, D.; Ni, L.; Bay, A.A.; Johnson, T.M.; Prusin, T.; Hackney, M.E. Parkinson’s disease and diabetes mellitus: Individual and combined effects on motor, cognitive, and psychosocial functions. Healthcare 2023, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Evans, J.; Wernick, A.; Virdi, G.; Choi, M.L.; Lawton, M.; Vijiaratnam, N.; Girges, C.; Ben-Shlomo, Y.; Ismail, K.; et al. The impact of type 2 diabetes in Parkinson’s disease. Mov. Disord. 2022, 37, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yuan, F.; Chen, Z.; Zhu, S.; Chang, Z.; Yang, W.; Deng, B.; Que, R.; Cao, P.; Chao, Y.; et al. Vascular, inflammatory and metabolic risk factors in relation to dementia in Parkinson’s disease patients with type 2 diabetes mellitus. Aging 2020, 12, 15682–15704. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Horta, S.; Bejr-Kasem, H.; Horta-Barba, A.; Pascual-Sedano, B.; Santos-García, D.; de Deus-Fonticoba, T.; Jesús, S.; Aguilar, M.; Planellas, L.; García-Caldentey, J.; et al. Identifying comorbidities and lifestyle factors contributing to the cognitive profile of early Parkinson’s disease. BMC Neurol. 2021, 21, 477. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Li, B.; Wang, M.; Yu, L.; Wan, Y.; Gan, J.; Zhang, Y.; Liu, Z.; Wang, X. Multiple evidences for association between cognitive impairment and dysglycemia in Parkinson’s disease: Implications for clinical practice. Front. Aging Neurosci. 2017, 9, 355. [Google Scholar] [CrossRef]
- Schelp, A.O.; Mendes-Chiloff, C.L.; Paduan, V.C.; Corrente, J.E.; Vieira, A.; Marchette, J.C.N.; De Souza, J.T.; Luvizuto, G.J.; Nogueira, C.R.; Bazan, R. Amnestic dementia impairment in Parkinson’s disease: The role of body composition, ageing and insulin resistance. Clin. Nutr. ESPEN 2017, 20, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Zimmermann, J.; Sixel-Döring, F.; Focke, N.K.; Wicke, T.; Ebentheuer, J.; Schaumburg, M.; Lang, E.; Friede, T.; Trenkwalder, C.; et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov. Disord. 2019, 34, 67–77. [Google Scholar] [CrossRef]
- de Pablo-Fernández, E.; Courtney, R.; Rockliffe, A.; Gentleman, S.; Holton, J.L.; Warner, T.T. Faster disease progression in Parkinson’s disease with type 2 diabetes is not associated with increased α-synuclein, tau, amyloid-β or vascular pathology. Neuropathol. Appl. Neurobiol. 2021, 47, 1080–1091. [Google Scholar] [CrossRef]
- Zhang, B.; Song, C.; Tang, X.; Tian, M.; Liu, Y.; Yan, Z.; Duan, R.; Liu, Y. Type 2 diabetes microenvironment promotes the development of Parkinson’s disease by activating microglial cell inflammation. Front. Cell Dev. Biol. 2024, 12, 1422746. [Google Scholar] [CrossRef]
- Hong, C.-T.; Chen, K.-Y.; Wang, W.; Chiu, J.-Y.; Wu, D.; Chao, T.-Y.; Hu, C.-J.; Chau, K.-Y.D.; Bamodu, O.A. Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction, and deregulation of the polo-like kinase 2 signaling. Cells 2020, 9, 740. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, Y.-Q.; Xu, L.-L.; Qiao, C.; Sun, X.-L.; Ding, J.-H.; Lu, M.; Hu, G. Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice. Exp. Neurol. 2014, 251, 22–29. [Google Scholar] [CrossRef]
- Morris, J.K.; Bomhoff, G.L.; Stanford, J.A.; Geiger, P.C. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R1082–R1090. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Klein, S. Alpha-synuclein promotes dopaminergic neuron death in Parkinson’s disease through microglial and NLRP3 activation. USURJ Univ. Sask. Undergrad. Res. J. 2020, 6. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin resistance and oxidative stress in the brain: What’s new? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed]
- Cholerton, B.; Baker, L.D.; Craft, S. Insulin resistance and pathological brain ageing. Diabet. Med. 2011, 28, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Feng, J.; Liu, R.; Dong, Y.; Chen, H.; Xu, J.; Jiang, X.; Li, R.; Lv, P. Cerebral small vessel disease is associated with mild cognitive impairment in type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1985–1994. [Google Scholar] [CrossRef]
- Yaffe, K.; Falvey, C.; Hamilton, N.; Schwartz, A.V.; Simonsick, E.M.; Satterfield, S.; Cauley, J.A.; Rosano, C.; Launer, L.J.; Strotmeyer, E.S.; et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch. Neurol. 2012, 69, 1170–1175. [Google Scholar] [CrossRef]
- Zagare, A.; Kurlovics, J.; Almeida, C.; Ferrante, D.; Frangenberg, D.; Vitali, A.; Gomez-Giro, G.; Jäger, C.; Antony, P.; Halder, R.; et al. Insulin resistance compromises midbrain organoid neuronal activity and metabolic efficiency predisposing to Parkinson’s disease pathology. J. Tissue Eng. 2025, 16, 20417314241295928. [Google Scholar] [CrossRef]
- Moran, C.; Phan, T.G.; Chen, J.; Blizzard, L.; Beare, R.; Venn, A.; Münch, G.; Wood, A.G.; Forbes, J.; Greenaway, T.M.; et al. Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care 2013, 36, 4036–4042. [Google Scholar] [CrossRef]
- Sato, N.; Morishita, R. Brain alterations and clinical symptoms of dementia in diabetes: Aβ/tau-dependent and independent mechanisms. Front. Endocrinol. 2014, 5, 143. [Google Scholar] [CrossRef]
- Shams, R.; Banik, N.L.; Haque, A. Calpain in the cleavage of alpha-synuclein and the pathogenesis of Parkinson’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 167, 107–124. [Google Scholar]
- Barisano, G.; Montagne, A.; Kisler, K.; Schneider, J.A.; Wardlaw, J.M.; Zlokovic, B.V. Blood-brain barrier link to human cognitive impairment and Alzheimer’s disease. Nat. Cardiovasc. Res. 2022, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 2018, 19, 283–301. [Google Scholar] [CrossRef]
- Paul, J.; Strickland, S.; Melchor, J.P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J. Exp. Med. 2007, 204, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3. [Google Scholar] [CrossRef]
- Aguirre-Vidal, Y.; Montes, S.; Mota-López, A.C.; Navarrete-Vázquez, G. Antidiabetic drugs in Parkinson’s disease. Clinical Park. Relat. Disord. 2024, 11, 100265. [Google Scholar] [CrossRef]
- Yu, H.; Sun, T.; He, X.; Wang, Z.; Zhao, K.; An, J.; Wen, L.; Li, J.-Y.; Li, W.; Feng, J. Association between Parkinson’s disease and diabetes mellitus: From epidemiology, pathophysiology and prevention to treatment. Aging Dis. 2022, 13, 1591. [Google Scholar] [CrossRef]
- Curry, D.W.; Stutz, B.; Andrews, Z.B.; Elsworth, J.D. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J. Park. Dis. 2018, 8, 161–181. [Google Scholar] [CrossRef]
- Ou, R.; Wei, Q.; Hou, Y.; Zhang, L.; Liu, K.; Lin, J.; Jiang, Z.; Song, W.; Cao, B.; Shang, H. Effect of diabetes control status on the progression of Parkinson’s disease: A prospective study. Ann. Clin. Transl. Neurol. 2021, 8, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.; Linden, A.B.; Fernandes, M.; Molero, Y.; Winchester, L.; Sproviero, W.; Ghose, U.; Li, Q.S.; Launer, L.J.; van Duijn, C.M.; et al. Comparative effect of metformin versus sulfonylureas with dementia and Parkinson’s disease risk in US patients over 50 with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2022, 10, e003036. [Google Scholar] [CrossRef]
- Kalinderi, K.; Papaliagkas, V.; Fidani, L. GLP-1 receptor agonists: A new treatment in Parkinson’s disease. Int. J. Mol. Sci. 2024, 25, 3812. [Google Scholar] [CrossRef]
- Malatt, C.; Wu, T.; Bresee, C.; Hogg, E.; Wertheimer, J.C.; Tan, E.; Pomeroy, H.; Obialisi, G.; Tagliati, M. Liraglutide improves non-motor function and activities of daily living in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial (P9-11.005). Neurology 2022, 98 (Suppl. S18), 3068. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Zhao, L.; Han, C.; Zheng, Z.; Xiu, S.L.; Chan, P. Risk of mini-mental state examination (MMSE) decline in the elderly with type 2 diabetes: A Chinese community-based cohort study. BMC Endocr. Disord. 2020, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Gruber-Baldini, A.L.; Rainer von Coelln, F.; Savitt, J.M.; Reich, S.G.; Armstrong, M.J.; Shulman, L.M. Comparison of mini-mental state examination and Montreal cognitive assessment ratings across levels of Parkinson’s disease severity. J. Park. Dis. 2021, 11, 1995–2003. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Bostantjopoulou, S.; Stefanova, E.; Falup-Pecurariu, C.; Kramberger, M.G.; Geurtsen, G.J.; Antonini, A.; Weintraub, D.; Aarsland, D. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: A multicenter 1-year follow-up study. J. Neural Transm. 2016, 123, 431–438. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Cauzzo, S.; Weis, L.; Garon, M.; Pistonesi, F.; Cianci, V.; Nasi, M.L.; Vianello, F.; Zecchinelli, A.L.; Pezzoli, G.; et al. Optimal MMSE and MoCA cutoffs for cognitive diagnoses in Parkinson’s disease: A data-driven decision tree model. J. Neurol. Sci. 2024, 466, 123283. [Google Scholar] [CrossRef] [PubMed]
- Uyar, M.; Lezius, S.; Buhmann, C.; Pötter-Nerger, M.; Schulz, R.; Meier, S.; Gerloff, C.; Kuhle, J.; Choe, C.-U. Diabetes, Glycated Hemoglobin (HbA1c), and Neuroaxonal Damage in Parkinson’s Disease (MARK-PD Study). Mov. Disord. 2022, 37, 1299–1304. [Google Scholar] [CrossRef] [PubMed]


| PECO Component | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population (P) | 1—Adults (>18 years) diagnosed with PD, with or without a documented diagnosis of T2DM or IR. 2—Studies assessing cognitive outcomes specifically in PD patients with known T2DM or IR. | Studies on populations without a PD diagnosis or focusing on other neurodegenerative disorders. |
| Exposure (E) | 1—Studies examining the impact of DM and IR on cognitive function in PD patients. 2—Observational studies that specifically measure IR (using, e.g., HOMA-IR, HbA1c) and non-human populations. | 1—Studies not specifically analyzing T2DM or IR in the context of PD. 2—Research focused exclusively on other metabolic disorders without any connection to T2DM or IR. |
| Comparison (C) | 1—Comparisons between PD patients with T2DM or IR versus those without these metabolic conditions. 2—Comparative studies examining cognitive outcomes between PD populations stratified by T2DM status, IR levels, or glycemic control. | 1—Studies lacking a comparison group related to metabolic conditions or without cognitive assessment data. |
| Outcome (O) | 1—Cognitive outcomes measured by validated cognitive scales (e.g., MMSE, MOCA) in PD patients. 2—Diagnosis or evidence of dementia, or CD specifically attributed to PD. | 1—Studies that do not measure cognitive outcomes. 2—Outcomes unrelated to CD or dementia. |
| Study Characteristics | 1—Primary articles. 2—Case–control studies. 3—Original works written exclusively in English. 4—Studies published in the last 20 years (>2005). 5—Preclinical/clinical studies. | 1—Editorials. 2—Letters. 3—Expert opinion. |
| Characteristics | Details |
|---|---|
| Study Designs |
|
| Sample Size |
|
| Age Range |
|
| Gender Distribution |
|
| Ethnicity |
|
| Clinical Studies | |||||
|---|---|---|---|---|---|
| Author, Year, Country | Population | Exposure | Measurement Tool | Main Cognitive Outcome | Key Findings |
| Alaa A., 2020, (UK) [18] | - Total N = 544,162 - Age: 73.1 - Male 41.9%, Female 58.1% | T2DM | N/A | 139/544,162 PDD | Increase in the prevalence and incidence of dementia in diabetic individuals from 2000 to 2016. |
| J. Park, 2023 [19] | - Total N = 262 - Age: 71.1 - 57% male, 43% Female | T2DM and Prediabetes | Diabetes (HbA1c ≥ 6.5); prediabetes (HbA1c 5.7–6.4%) | CD | Prediabetes and diabetes were associated with worse MoCA scores in PD patients (p = 0.002). |
| Miyake Y., 2010, Japan [20] | - N = PD 249, non-PD 368 - Age: 69.1 ± 8.4 - Gender: N/A | T2DM | Structured questionnaire | These vascular risk factors had a significantly lower risk of developing PD | The study found inverse associations between vascular/metabolic diseases and PD. |
| Seong-Beom Koh, 2024, South Korea [21] | - Total N = 9264 - Age: 71.3 ± 8.45 - Gender: N/A | T2DM | HBA1c (%) | 1757/9264 PDD | Compared to consistent hyperglycemia, glucose variability is noted to have more deleterious effects on inflammation. |
| M. Ibrahim Khalil, 2021, Bangladesh [22] | - Total N = 131 - Age: 73.32 ± 8.86 - Gender: N/A | T2DM | FBG ≥ 7.0 mmol/L and/or 2 h post | 17/29 PDD | Diabetes was identified as a significant risk factor for the exacerbation of cognitive decline. |
| E. Hogg, 2018, USA [23] | - Total N = 160 - Age: 67.7 ± 10.5 - Males: 109, Females: 51 | IR | HOMA-IR | 90/160 PDD | No significant correlation was found between HOMA-IR and MoCA, MDS-UPDRS. |
| N. Bohnen, 2014, USA [24] | - Total N = 148 - Age: 67.3 ± 6.1 - 109 Males, 39 Females | T2DM | Self-reported | CD | The study observed that PD patients with DM showed the greatest impairments in attentional function. |
| Bosco D., 2012, Italy [25] | - Total N = 110 - Age: 65 ± 6.2 - 72 Males, 38 Females | IR | 2-h OGTT, HOMA-index, MMSE, UPDRS, MADRS | IR is strongly associated with dementia in PD | IR correlated with lower MMSE scores. |
| I. Markaki, 2021, Sweden [26] | - Total N = 244 - Age: 64 - 64% Male, 36% Female | T2DM | HBA1c (%) | N/A | Cognitive decline was observed with imbalances in HbA1c levels, but associations were not statistically significant. |
| M. Ong, 2017, Singapore [27] | - Total N = 77 - Age: 64.4 ± 7.62 - 76.9% Males, 23.1% Females | T2DM | HBA1c or FBG ≥ 7 | 12/77 PDD | The study provides evidence that PD patients with DM exhibit lower gray matter volume |
| M. Giuntini, 2014 [28] | - Total N = 100 - Age: 64.46 ± 6.72 - 56% Male, 44% Female | T2DM | American Diabetes Association criteria for DM | CD | The presence of DM in PD has a negative impact on the progression of PD. |
| R. Wang, 2024, China [29] | - Total N = 73 - Age: 61.68 ± 7.45 - 61.3% Female, 38.7% Male | IR | HOMA-IR: Median: 2.68 (1.56, 3.41) | N/A | The study found no significant change in cognitive function. |
| Jolie D. Barter, 2023 [30] | - Total N = 424 - Age: 69.94 ± 7.6 - 83.3% Male, 16.7% Female | T2DM | Self-report + fasting glucose | CD | Significant interaction effects (p < 0.01) for Stroop Interference and logical memory recall. |
| N. Palacios, 2011, USA [17] | - Total N = 147,096 - Age = 63.6, - Gender: N/A | T2DM | Self-reported | N/A | Contrasts with some prior studies reporting increased risk. |
| Dr. Dilan Athauda, 2022, UK [31] | - Total N = 1930 - Age: 71.1 (0.7) - 72.5% Male, 27.5% Female | T2DM | Self-reported | CD | After controlling for confounders, findings indicated that PD patients with T2DM had more severe symptoms. |
| Qing Wang, 2020, China [32] | - Total N = 928 - Age: 79.0 - Gender: N/A | T2DM | HBA1c (%) | 31/215 PDD | The study identified that lower levels of LDL-C and higher levels of fibrinogen were associated with more severe CD in PD patients with T2DM. |
| Saul Martínez-Horta, 2021, Spain [33] | - Total N = 533 - Age: 67.1 ± 6.4 - Gender: Male % higher | T2DM | N/A | PDD | The study found that IL-2 and IL-6 levels were higher in patients with PDD and DM. |
| L. Yang, 2017, China [34] | - Total N = 282 - Age: 70.79 ± 7.63 - 51.8% males, 48.2% Females | T2DM | HBA1c (%) | CD | Regression analysis showed a significant negative correlation between MoCA scores and both HbA1c and IR. |
| Arthur Oscar Schelp, 2017, Brazil [35] | - Total N = 142 - Age: 73.85 ± 6.62 - 64.4% Male, 35.6% Female | IR | HOMA-IR | CD + PDD | IR, older age, and lower education levels correlated with poorer memory performance. |
| Brit Mollenhaur, 2019, Germany [36] | - Total N = 135 - Age: 64.55 ± 9.84 - Gender: N/A | T2DM | HBA1c (%) | CD | Specific biomarkers, such as elevated periodic limb movement index during sleep, led to cognitive decline. |
| Eduardo de Pablo-Fernández, 2021, UK [37] | - Total N = 132 - Age: 70.4 ± 8.1 - 45% Male, 55% Female | T2DM | FPG ≥ 126 mg/dL | N/A | The study provides evidence that PD with T2DM shows faster disease progression. |
| M. Petrou, 2016, USA [16] | - Total N = 36 - Age: 66.0 ± 5.2 - 83.3% Male, 16.7% Female | T2DM | N/A | CD | The study provides evidence that diabetes is associated with greater gray matter loss in PD patients. |
| M. Zimering, 2018, USA [15] | - Total N = 23 - Age: 70.8 ± 5.6 - Gender: older adult males | T2DM | HBA1c (%) | 1/10 PDD | The study showed that mean accelerated neuroblastoma cell loss was induced by diabetic Parkinson’s disease. |
| Preclinical Studies | |||||
| Zhang et al., 2024, China [38] | - Total N = N/A - Age: 4-week-old mice - Gender: 100% Male | T2DM | Blood glucose levels | T2DM exacerbated the motor and cognitive symptoms in PD mice | T2DM microenvironment significantly exacerbates PD pathology, primarily through mitochondrial dysfunction. |
| Hong et al., 2020, China [39] | - Total N = 12 - Age: N/A - Gender: Male rats | IR + MPTP | HOMA-IR | Dopaminergic neuron loss and oxidative stress (exacerbated) | IR promotes PD pathology by disrupting PLK2 signaling and mitochondrial function. |
| Wang et al., 2014, China [40] | - Total N = 40 mice - Age: 10–12 weeks - Gender: Male | T2DM (HFD + low-dose STZ) | Fasting glucose, insulin levels, inflammatory markers | Dopaminergic neuronal degeneration (exacerbated) | T2DM-induced metabolic inflammation amplifies susceptibility to dopaminergic neuron loss. |
| Morris et al., 2010, USA [41] | - N = 4 - Age: N/A - Gender: Male mice | HFD (IR model) | Dopaminergic neuron count (SNpc), tyrosine hydroxylase staining | CD | HFD-induced IR worsens dopaminergic neuron vulnerability in PD models. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeidan, O.; Jaragh, N.; Tama, M.; Alkhalifa, M.; Alqayem, M.; Butler, A.E. The Influence of Insulin Resistance and Type 2 Diabetes on Cognitive Decline and Dementia in Parkinson’s Disease: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8078. https://doi.org/10.3390/ijms26168078
Zeidan O, Jaragh N, Tama M, Alkhalifa M, Alqayem M, Butler AE. The Influence of Insulin Resistance and Type 2 Diabetes on Cognitive Decline and Dementia in Parkinson’s Disease: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(16):8078. https://doi.org/10.3390/ijms26168078
Chicago/Turabian StyleZeidan, Osama, Noor Jaragh, Maya Tama, Maryam Alkhalifa, Maryam Alqayem, and Alexandra E. Butler. 2025. "The Influence of Insulin Resistance and Type 2 Diabetes on Cognitive Decline and Dementia in Parkinson’s Disease: A Systematic Review" International Journal of Molecular Sciences 26, no. 16: 8078. https://doi.org/10.3390/ijms26168078
APA StyleZeidan, O., Jaragh, N., Tama, M., Alkhalifa, M., Alqayem, M., & Butler, A. E. (2025). The Influence of Insulin Resistance and Type 2 Diabetes on Cognitive Decline and Dementia in Parkinson’s Disease: A Systematic Review. International Journal of Molecular Sciences, 26(16), 8078. https://doi.org/10.3390/ijms26168078






