Common SNCA Genetic Variants and Parkinson’s Disease Risk: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. SNP rs356165
3.2. SNP rs2583988
3.3. SNP rs356219
3.4. SNP rs11931074
4. Discussion
4.1. SNP rs356165
4.2. SNP rs2583988
4.3. SNP rs356219
4.4. SNP rs11931074
5. Strengths and Limitations
6. Implications and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| SNP | Single-nucleotide polymorphisms |
| PD-MCI | Parkinson’s disease-mild cognitive impairment |
| GCTA | Genome-wide complex trait analysis |
| GWAS | Genome-wide association studies |
| OR | Odds ratios |
| CI | Confidence interval |
| NOS | Newcastle-Ottawa Scale |
| PDD | Parkinson’s disease dementia |
| AAO | Earlier age at onset |
| 3′ UTR | 3′ untranslated region |
| iRBD | Idiopathic REM sleep behaviour disorder |
References
- Maraganore, D.M.; de Andrade, M.; Elbaz, A.; Farrer, M.J.; Ioannidis, J.P.; Krüger, R.; Rocca, W.A.; Schneider, N.K.; Lesnick, T.G.; Lincoln, S.J.; et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA 2006, 296, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.F.; Li, K.; Yu, R.L.; Sun, Q.Y.; Wang, L.; Yao, L.Y.; Hu, Y.C.; Lv, Z.Y.; Luo, L.Z.; Shen, L.; et al. Polygenic determinants of Parkinson’s disease in a Chinese population. Neurobiol. Aging 2015, 36, 1765.e1–1765.e6. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.C.; Fuchs, J.; Hofer, A.; Zimprich, A.; Lichtner, P.; Illig, T.; Berg, D.; Wüllner, U.; Meitinger, T.; Gasser, T. Multiple regions of α-synuclein are associated with Parkinson’s disease. Ann. Neurol. 2005, 57, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Simón-Sánchez, J.; Schulte, C.; Bras, J.M.; Sharma, M.; Gibbs, J.R.; Berg, D.; Paisan-Ruiz, C.; Lichtner, P.; Scholz, S.W.; Hernandez, D.G.; et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009, 41, 1308–1312. [Google Scholar] [CrossRef]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M.; Kawaguchi, T.; Tsunoda, T.; Watanabe, M.; Takeda, A.; et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009, 41, 1303–1307. [Google Scholar] [CrossRef]
- Kruger, R. Ala 30 Pro mutation in the gene encoding α synuclein in Parkinson disease. Nat. Genet. 1998, 18, 106–108. [Google Scholar] [CrossRef]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B. The new mutation, E46K, of α-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Hadjigeorgiou, G.M.; Xiromerisiou, G.; Gourbali, V.; Aggelakis, K.; Scarmeas, N.; Papadimitriou, A.; Singleton, A. Association of alpha-synuclein Rep1 polymorphism and Parkinson’s disease: Influence of Rep1 on age at onset. Mov. Disord. 2006, 21, 534–539. [Google Scholar] [CrossRef]
- Trotta, L.; Guella, I.; Soldà, G.; Sironi, F.; Tesei, S.; Canesi, M.; Pezzoli, G.; Goldwurm, S.; Duga, S.; Asselta, R. SNCA and MAPT genes: Independent and joint effects in Parkinson disease in the Italian population. Park. Relat. Disord. 2012, 18, 257–262. [Google Scholar] [CrossRef]
- Krüger, R.; Vieira-Saecker, A.M.; Kuhn, W.; Berg, D.; Müller, T.; Kühnl, N.; Fuchs, G.A.; Storch, A.; Hungs, M.; Woitalla, D.; et al. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann. Neurol. 1999, 45, 611–617. [Google Scholar] [CrossRef]
- Lesage, S.; Brice, A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009, 18, R48–R59. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zang, Q.; Hu, F.; Wei, H.; Ma, J.; Xu, Y. Alpha-synuclein gene polymorphism affects risk of dementia in Han Chinese with Parkinson’s disease. Neurosci. Lett. 2019, 706, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ning, Y.; Saffari, S.E.; Xiao, B.; Niu, C.; Ng, S.Y.E.; Chia, N.; Choi, X.; Heng, D.L.; Tan, Y.J.; et al. Identifying clinical features and blood biomarkers associated with mild cognitive impairment in Parkinson disease using machine learning. Eur. J. Neurol. 2023, 30, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Ross, O.A.; Ioannidis, J.P.A.; Soto-Ortolaza, A.I.; Moisan, F.; Aasly, J.; Annesi, G.; Bozi, M.; Brighina, L.; Chartier-Harlin, M.C. Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann. Neurol. 2011, 69, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Scott, W.K.; Wang, G.; Mayhew, G.; Li, Y.J.; Vance, J.M.; Martin, E.R. Gene-gene interaction between FGF20 and MAOB in Parkinson disease. Ann. Hum. Genet. 2008, 72, 157–162. [Google Scholar] [CrossRef]
- Wider, C.; Vilariño-Güell, C.; Heckman, M.G.; Jasinska-Myga, B.; Ortolaza-Soto, A.I.; Diehl, N.N.; Crook, J.E.; Cobb, S.A.; Bacon, J.A.; Aasly, J.O.; et al. SNCA, MAPT, and GSK3B in Parkinson disease: A gene-gene interaction study. Eur. J. Neurol. 2011, 18, 876–881. [Google Scholar] [CrossRef]
- Keller, M.F.; Saad, M.; Bras, J.; Bettella, F.; Nicolaou, N.; Simón-Sánchez, J.; Mittag, F.; Büchel, F.; Sharma, M.; Gibbs, J.R. Using genome-wide complex trait analysis to quantify ‘missing heritability’in Parkinson’s disease. Hum. Mol. Genet. 2012, 21, 4996–5009. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 9 April 2025).
- Winkler, S.; Hagenah, J.; Lincoln, S.; Heckman, M.; Haugarvoll, K.; Lohmann-Hedrich, K.; Kostic, V.; Farrer, M.; Klein, C. alpha-Synuclein and Parkinson disease susceptibility. Neurology 2007, 69, 1745–1750. [Google Scholar] [CrossRef]
- Myhre, R.; Toft, M.; Kachergus, J.; Hulihan, M.M.; Aasly, J.O.; Klungland, H.; Farrer, M.J. Multiple alpha-synuclein gene polymorphisms are associated with Parkinson’s disease in a Norwegian population. Acta Neurol. Scand. 2008, 118, 320–327. [Google Scholar] [CrossRef]
- Davis, A.A.; Andruska, K.M.; Benitez, B.A.; Racette, B.A.; Perlmutter, J.S.; Cruchaga, C. Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol. Aging 2016, 37, 209.e1–209.e7. [Google Scholar] [CrossRef]
- Cardo, L.F.; Coto, E.; de Mena, L.; Ribacoba, R.; Mata, I.F.; Menéndez, M.; Moris, G.; Alvarez, V. Alpha-synuclein transcript isoforms in three different brain regions from Parkinson’s disease and healthy subjects in relation to the SNCA rs356165/rs11931074 polymorphisms. Neurosci. Lett. 2014, 562, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ross, O.A.; Gosal, D.; Stone, J.T.; Lincoln, S.J.; Heckman, M.G.; Irvine, G.B.; Johnston, J.A.; Gibson, J.M.; Farrer, M.J.; Lynch, T. Familial genes in sporadic disease: Common variants of alpha-synuclein gene associate with Parkinson’s disease. Mech. Ageing Dev. 2007, 128, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wu-Chou, Y.H.; Chen, Y.T.; Yeh, T.H.; Chang, H.C.; Weng, Y.H.; Lai, S.C.; Huang, C.L.; Chen, R.S.; Huang, Y.Z.; Chen, C.C.; et al. Genetic variants of SNCA and LRRK2 genes are associated with sporadic PD susceptibility: A replication study in a Taiwanese cohort. Park. Relat. Disord. 2013, 19, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Campêlo, C.L.C.; Cagni, F.C.; de Siqueira Figueredo, D.; Oliveira, L.G., Jr.; Silva-Neto, A.B.; Macêdo, P.T.; Santos, J.R.; Izídio, G.S.; Ribeiro, A.M.; de Andrade, T.G.; et al. Variants in SNCA Gene Are Associated with Parkinson’s Disease Risk and Cognitive Symptoms in a Brazilian Sample. Front. Aging Neurosci. 2017, 9, 198. [Google Scholar] [CrossRef]
- Emelyanov, A.; Kulabukhova, D.; Garaeva, L.; Senkevich, K.; Verbitskaya, E.; Nikolaev, M.; Andoskin, P.; Kopytova, A.; Milyukhina, I.; Yakimovskii, A.; et al. SNCA variants and alpha-synuclein level in CD45+ blood cells in Parkinson’s disease. J. Neurol. Sci. 2018, 395, 135–140. [Google Scholar] [CrossRef]
- Heckman, M.G.; Soto-Ortolaza, A.I.; Diehl, N.N.; Carrasquillo, M.M.; Uitti, R.J.; Wszolek, Z.K.; Graff-Radford, N.R.; Ross, O.A. Evaluation of the role of SNCA variants in survival without neurological disease. PLoS ONE 2012, 7, e42877. [Google Scholar] [CrossRef]
- Szwedo, A.A.; Pedersen, C.C.; Ushakova, A.; Forsgren, L.; Tysnes, O.B.; Counsell, C.E.; Alves, G.; Lange, J.; Macleod, A.D.; Maple-Grødem, J. Association of SNCA Parkinson’s Disease Risk Polymorphisms With Disease Progression in Newly Diagnosed Patients. Front. Neurol. 2020, 11, 620585. [Google Scholar] [CrossRef]
- Fernández-Santiago, R.; Martín-Flores, N.; Antonelli, F.; Cerquera, C.; Moreno, V.; Bandres-Ciga, S.; Manduchi, E.; Tolosa, E.; Singleton, A.B.; Moore, J.H.; et al. SNCA and mTOR Pathway Single Nucleotide Polymorphisms Interact to Modulate the Age at Onset of Parkinson’s Disease. Mov. Disord. 2019, 34, 1333–1344. [Google Scholar] [CrossRef]
- Pan, F.; Dong, H.; Ding, H.; Ye, M.; Liu, W.; Wu, Y.; Zhang, X.; Chen, Z.; Luo, Y.; Ding, X. SNP rs356219 of the α-synuclein (SNCA) gene is associated with Parkinson’s disease in a Chinese Han population. Park. Relat. Disord. 2012, 18, 632–634. [Google Scholar] [CrossRef]
- Salas-Leal, A.C.; Salas-Pacheco, S.M.; Gavilán-Ceniceros, J.A.P.; Castellanos-Juárez, F.X.; Méndez-Hernández, E.M.; La Llave-León, O.; Camacho-Luis, A.; Quiñones-Canales, G.; Romero-Gutiérrez, E.; Arias-Carrión, O.; et al. α-syn and SNP rs356219 as a potential biomarker in blood for Parkinson’s disease in Mexican Mestizos. Neurosci. Lett. 2021, 754, 135901. [Google Scholar] [CrossRef]
- Goris, A.; Williams-Gray, C.H.; Clark, G.R.; Foltynie, T.; Lewis, S.J.; Brown, J.; Ban, M.; Spillantini, M.G.; Compston, A.; Burn, D.J.; et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann. Neurol. 2007, 62, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Li, N.N.; Mao, X.Y.; Chang, X.L.; Zhao, D.M.; Zhang, J.H.; Liao, Q.; Yu, W.J.; Tan, E.K.; Peng, R. SNCA rs356219 variant increases risk of sporadic Parkinson’s disease in ethnic Chinese. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162b, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Sasaki, S.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Shimada, H.; Kawamura, N.; et al. SNCA polymorphisms, smoking, and sporadic Parkinson’s disease in Japanese. Park. Relat. Disord. 2012, 18, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.; Cheng, L.; Li, N.N.; Wang, L.; Tan, E.K.; Peng, R. Interaction between SNCA, LRRK2 and GAK increases susceptibility to Parkinson’s disease in a Chinese population. eNeurologicalSci 2015, 1, 3–6. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, H.; Gen, S.; Zhang, H.; Wang, S.; Hua, L.; Yang, X.; Zhang, S.; Li, J.; Wang, Y. Interaction between SNCA gene polymorphisms and T2DM with Parkinson’s disease. Acta Neurol. Scand. 2020, 142, 443–448. [Google Scholar] [CrossRef]
- Shahmohammadibeni, N.; Rahimi-Aliabadi, S.; Jamshidi, J.; Emamalizadeh, B.; Shahmohammadibeni, H.A.; Zare Bidoki, A.; Akhavan-Niaki, H.; Eftekhari, H.; Abdollahi, S.; Shekari Khaniani, M.; et al. The analysis of association between SNCA, HUSEYO and CSMD1 gene variants and Parkinson’s disease in Iranian population. Neurol. Sci. 2016, 37, 731–736. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Q.Q.; Ou, R.; Cao, B.; Chen, X.; Zhao, B.; Guo, X.; Yang, Y.; Chen, K.; Wu, Y.; et al. Genetic Variants of SNCA Are Associated with Susceptibility to Parkinson’s Disease but Not Amyotrophic Lateral Sclerosis or Multiple System Atrophy in a Chinese Population. PLoS ONE 2015, 10, e0133776. [Google Scholar] [CrossRef]
- Tan, E.K.; Kwok, H.H.; Tan, L.C.; Zhao, W.T.; Prakash, K.M.; Au, W.L.; Pavanni, R.; Ng, Y.Y.; Satake, W.; Zhao, Y.; et al. Analysis of GWAS-linked loci in Parkinson disease reaffirms PARK16 as a susceptibility locus. Neurology 2010, 75, 508–512. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, B.; Guo, J.; Wu, X.; Sun, Q.; Shi, C.; Hu, L.; Wang, C.; Wang, L.; Tan, L.; et al. Variant in the 3’ region of SNCA associated with Parkinson’s disease and serum α-synuclein levels. J. Neurol. 2012, 259, 497–504. [Google Scholar] [CrossRef]
- Rahimi, M.; Akbari, M.; Jamshidi, J.; Tafakhori, A.; Emamalizadeh, B.; Darvish, H. Genetic analysis of SNCA gene polymorphisms in Parkinson’s disease in an Iranian population. Basal Ganglia 2017, 10, 4–7. [Google Scholar] [CrossRef]
- Blažeković, A.; Jerčić, K.G.; Borovečki, F. SNCA 3’ UTR Genetic Variants in Patients with Parkinson’s Disease. Biomolecules 2021, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Tichopad, A.; Golub, Y.; Munz, M.; Schweitzer, K.J.; Wolf, B.; Berg, D.; Mueller, J.C.; Gasser, T. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008, 22, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Heilbron, K.; Vallerga, C.L.; Bandres-Ciga, S.; von Coelln, R.; Pihlstrøm, L.; Simón-Sánchez, J.; Schulte, C.; Sharma, M.; Krohn, L.; et al. Parkinson’s disease age at onset genome-wide association study: Defining heritability, genetic loci, and α-synuclein mechanisms. Mov. Disord. 2019, 34, 866–875. [Google Scholar] [CrossRef]
- Ng, A.S.L.; Tan, Y.J.; Zhao, Y.; Saffari, S.E.; Lu, Z.; Ng, E.Y.L.; Ng, S.Y.E.; Chia, N.S.Y.; Setiawan, F.; Xu, Z. SNCA Rep1 promoter variability influences cognition in Parkinson’s disease. Mov. Disord. 2019, 34, 1232–1236. [Google Scholar] [CrossRef]
- Ng, A.S.L.; Tan, Y.J.; Lu, Z.; Ng, E.Y.L.; Ng, S.Y.E.; Chia, N.S.Y.; Setiawan, F.; Xu, Z.; Tay, K.Y.; Prakash, K.M. Plasma alpha-synuclein detected by single molecule array is increased in PD. Ann. Clin. Transl. Neurol. 2019, 6, 615–619. [Google Scholar] [CrossRef]
- Edwards, T.L.; Scott, W.K.; Almonte, C.; Burt, A.; Powell, E.H.; Beecham, G.W.; Wang, L.; Züchner, S.; Konidari, I.; Wang, G.; et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 2010, 74, 97–109. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Makarious, M.B.; Leonard, H.L.; Bandres-Ciga, S.; Iwaki, H.; Nalls, M.A.; Noyce, A.J.; Singleton, A.B. A population scale analysis of rare SNCA variation in the UK Biobank. Neurobiol. Dis. 2021, 148, 105182. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, L.; Sun, Q.; Pan, H.; Guo, J.; Tang, B. A comprehensive analysis of the association between SNCA polymorphisms and the risk of Parkinson’s disease. Front. Mol. Neurosci. 2018, 11, 391. [Google Scholar] [CrossRef]
- Ghanbari, M.; Darweesh, S.K.; de Looper, H.W.; van Luijn, M.M.; Hofman, A.; Ikram, M.A.; Franco, O.H.; Erkeland, S.J.; Dehghan, A. Genetic Variants in MicroRNAs and Their Binding Sites Are Associated with the Risk of Parkinson Disease. Hum. Mutat. 2016, 37, 292–300. [Google Scholar] [CrossRef]
- Cardo, L.F.; Coto, E.; De Mena, L.; Ribacoba, R.; Lorenzo-Betancor, O.; Pastor, P.; Samaranch, L.; Mata, I.F.; Díaz, M.; Moris, G.; et al. A search for SNCA 3′ UTR variants identified SNP rs356165 as a determinant of disease risk and onset age in Parkinson’s disease. J. Mol. Neurosci. 2012, 47, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Y.; Hu, W.B.; Liu, L.; Yu, L.H.; Xi, J.; He, X.H.; Zhu, M.R.; Liu, Z.L.; Xu, Y.M. Lack of replication of a previously reported association between polymorphism in the 3’UTR of the alpha-synuclein gene and Parkinson’s disease in Chinese subjects. Neurosci. Lett. 2010, 479, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Vilariño-Güell, C.; Rajput, M.L.; Ross, O.A.; Soto-Ortolaza, A.I.; Lincoln, S.J.; Cobb, S.A.; Heckman, M.G.; Farrer, M.J.; Rajput, A. Alpha-synuclein polymorphisms are associated with Parkinson’s disease in a Saskatchewan population. Mov. Disord. 2009, 24, 2411–2414. [Google Scholar] [CrossRef]
- Lee, P.C.; Bordelon, Y.; Bronstein, J.; Sinsheimer, J.S.; Farrer, M.; Ritz, B. Head injury, α-synuclein genetic variability and Parkinson’s disease. Eur. J. Neurol. 2015, 22, 874–878. [Google Scholar] [CrossRef]
- Toffoli, M.; Dreussi, E.; Cecchin, E.; Valente, M.; Sanvilli, N.; Montico, M.; Gagno, S.; Garziera, M.; Polano, M.; Savarese, M.; et al. SNCA 3′UTR genetic variants in patients with Parkinson’s disease and REM sleep behavior disorder. Neurol. Sci. 2017, 38, 1233–1240. [Google Scholar] [CrossRef]
- Ritz, B.; Rhodes, S.L.; Bordelon, Y.; Bronstein, J. α-Synuclein Genetic Variants Predict Faster Motor Symptom Progression in Idiopathic Parkinson Disease. PLoS ONE 2012, 7, e36199. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, X.; Liu, Z.; Wang, J.; Xie, A. Association of rs356219 and rs3822086 polymorphisms with the risk of Parkinson’s disease: A meta-analysis. Neurosci. Lett. 2019, 709, 134380. [Google Scholar] [CrossRef]
- Tan, E.K.; Chai, A.; Teo, Y.Y.; Zhao, Y.; Tan, C.; Shen, H.; Chandran, V.R.; Teoh, M.L.; Yih, Y.; Pavanni, R.; et al. Alpha-synuclein haplotypes implicated in risk of Parkinson’s disease. Neurology 2004, 62, 128–131. [Google Scholar] [CrossRef]
- Refenes, N.; Kreutz, R.; Bolbrinker, J.; Tagaris, G.; Orlacchio, A.; Drakoulis, N. Non-replication of Association between MAPT-SNCA Synergistical Interaction and Susceptibility to Parkinson’s Disease in a Southern European population. Rev. Clin. Pharmacol. Pharmacokinet. Int. 2010, 24, 205–207. [Google Scholar]
- Fernández-Santiago, R.; Garrido, A.; Infante, J.; González-Aramburu, I.; Sierra, M.; Fernández, M.; Valldeoriola, F.; Muñoz, E.; Compta, Y.; Martí, M.J.; et al. α-synuclein (SNCA) but not dynamin 3 (DNM3) influences age at onset of leucine-rich repeat kinase 2 (LRRK2) Parkinson’s disease in Spain. Mov. Disord. 2018, 33, 637–641. [Google Scholar] [CrossRef]
- Brockmann, K.; Schulte, C.; Hauser, A.K.; Lichtner, P.; Huber, H.; Maetzler, W.; Berg, D.; Gasser, T. SNCA: Major genetic modifier of age at onset of Parkinson’s disease. Mov. Disord. 2013, 28, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Botta-Orfila, T.; Ezquerra, M.; Pastor, P.; Fernández-Santiago, R.; Pont-Sunyer, C.; Compta, Y.; Lorenzo-Betancor, O.; Samaranch, L.; Martí, M.J.; Valldeoriola, F. Age at onset in LRRK2-associated PD is modified by SNCA variants. J. Mol. Neurosci. 2012, 48, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Li, Y.; Niu, M.; Zhou, L.; Yao, M.; Zhu, L.; Ye, G.; Kang, W.; Liu, J. Variants in the SNCA Locus Are Associated with the Progression of Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.F.; Shi, M.; Agarwal, P.; Chung, K.A.; Edwards, K.L.; Factor, S.A.; Galasko, D.R.; Ginghina, C.; Griffith, A.; Higgins, D.S.; et al. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch. Neurol. 2010, 67, 1350–1356. [Google Scholar] [CrossRef]
- Mata, I.F.; Yearout, D.; Alvarez, V.; Coto, E.; de Mena, L.; Ribacoba, R.; Lorenzo-Betancor, O.; Samaranch, L.; Pastor, P.; Cervantes, S.; et al. Replication of MAPT and SNCA, but not PARK16-18, as susceptibility genes for Parkinson’s disease. Mov. Disord. 2011, 26, 819–823. [Google Scholar] [CrossRef]
- Westerlund, M.; Belin, A.C.; Anvret, A.; Håkansson, A.; Nissbrandt, H.; Lind, C.; Sydow, O.; Olson, L.; Galter, D. Cerebellar alpha-synuclein levels are decreased in Parkinson’s disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material. FASEB J. 2008, 22, 3509–3514. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Guazzetti, S.; Renzetti, S.; Broberg, K.; Caci, M.; Covolo, L.; Crippa, P.; Gelatti, U.; Hashim, D.; Oppini, M.; et al. Metal Exposure and SNCA rs356219 Polymorphism Associated with Parkinson Disease and Parkinsonism. Front. Neurol. 2020, 11, 556337. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Dürr, A.; Wood, N.W.; Parkinson, M.H.; Camuzat, A.; Hulot, J.S.; Morrison, K.E.; Renton, A.; Sussmuth, S.D.; Landwehrmeyer, B.G.; et al. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS ONE 2009, 4, e7114. [Google Scholar] [CrossRef]
- Jo, S.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Ryu, H.S.; Chung, S.J. Microarray Genotyping Identifies New Loci Associated with Dementia in Parkinson’s Disease. Genes 2021, 12, 1975. [Google Scholar] [CrossRef]
- Chung, S.J.; Jung, Y.; Hong, M.; Kim, M.J.; You, S.; Kim, Y.J.; Kim, J.; Song, K. Alzheimer’s disease and Parkinson’s disease genome-wide association study top hits and risk of Parkinson’s disease in Korean population. Neurobiol. Aging 2013, 34, 2695.e1–2695.e7. [Google Scholar] [CrossRef]
- Chen, W.; Kang, W.Y.; Chen, S.; Wang, Y.; Xiao, Q.; Wang, G.; Liu, J.; Chen, S.D. Hyposmia correlates with SNCA variant and non-motor symptoms in Chinese patients with Parkinson’s disease. Park. Relat. Disord. 2015, 21, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, Y.; Chen, W.; Chen, S.; Wang, Y.; Xiao, Q.; Liu, J.; Fung, V.S.; Halliday, G.; Chen, S. Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson’s disease. Park. Relat. Disord. 2016, 24, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Liang, D.; Pan, H.; Xu, Q.; Guo, J.; Sun, Q.; Tang, B.; Yan, X. Genetic Impact on Clinical Features in Parkinson’s Disease: A Study on SNCA -rs11931074. Park. Dis. 2018, 2018, 2754541. [Google Scholar] [CrossRef]
- Kang, W.; Chen, W.; Yang, Q.; Zhang, L.; Zhang, L.; Wang, X.; Dong, F.; Zhao, Y.; Chen, S.; Quinn, T.J.; et al. Salivary total α-synuclein, oligomeric α-synuclein and SNCA variants in Parkinson’s disease patients. Sci. Rep. 2016, 6, 28143. [Google Scholar] [CrossRef]
- Si, Q.Q.; Yuan, Y.S.; Zhi, Y.; Wang, M.; Wang, J.W.; Shen, Y.T.; Wang, L.N.; Li, J.Y.; Wang, X.X.; Zhang, K.Z. SNCA rs11931074 polymorphism correlates with spontaneous brain activity and motor symptoms in Chinese patients with Parkinson’s disease. J. Neural Transm. 2019, 126, 1037–1045. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Wang, Y.; Xu, Z.M.; Wang, Y.; Yang, Q.; Wang, G.; Tan, Y.Y.; Ma, J.F.; Zhang, J.; et al. Analysis of genome-wide association study-linked loci in Parkinson’s disease of Mainland China. Mov. Disord. 2013, 28, 1892–1895. [Google Scholar] [CrossRef]
- Du, B.; Xue, Q.; Liang, C.; Fan, C.; Liang, M.; Zhang, Y.; Bi, X.; Hou, L. Association between alpha-synuclein (SNCA) rs11931074 variability and susceptibility to Parkinson’s disease: An updated meta-analysis of 41,811 patients. Neurol. Sci. 2020, 41, 271–280. [Google Scholar] [CrossRef]
- Chung, S.J.; König, I.R.; Lohmann, K.; Hinrichs, F.; Kim, J.; Ryu, H.S.; Lee, H.J.; Kim, K.; Lee, J.H.; Jung, K.W.; et al. Association of SNCA variants with α-synuclein of gastric and colonic mucosa in Parkinson’s disease. Park. Relat. Disord. 2019, 61, 151–155. [Google Scholar] [CrossRef]

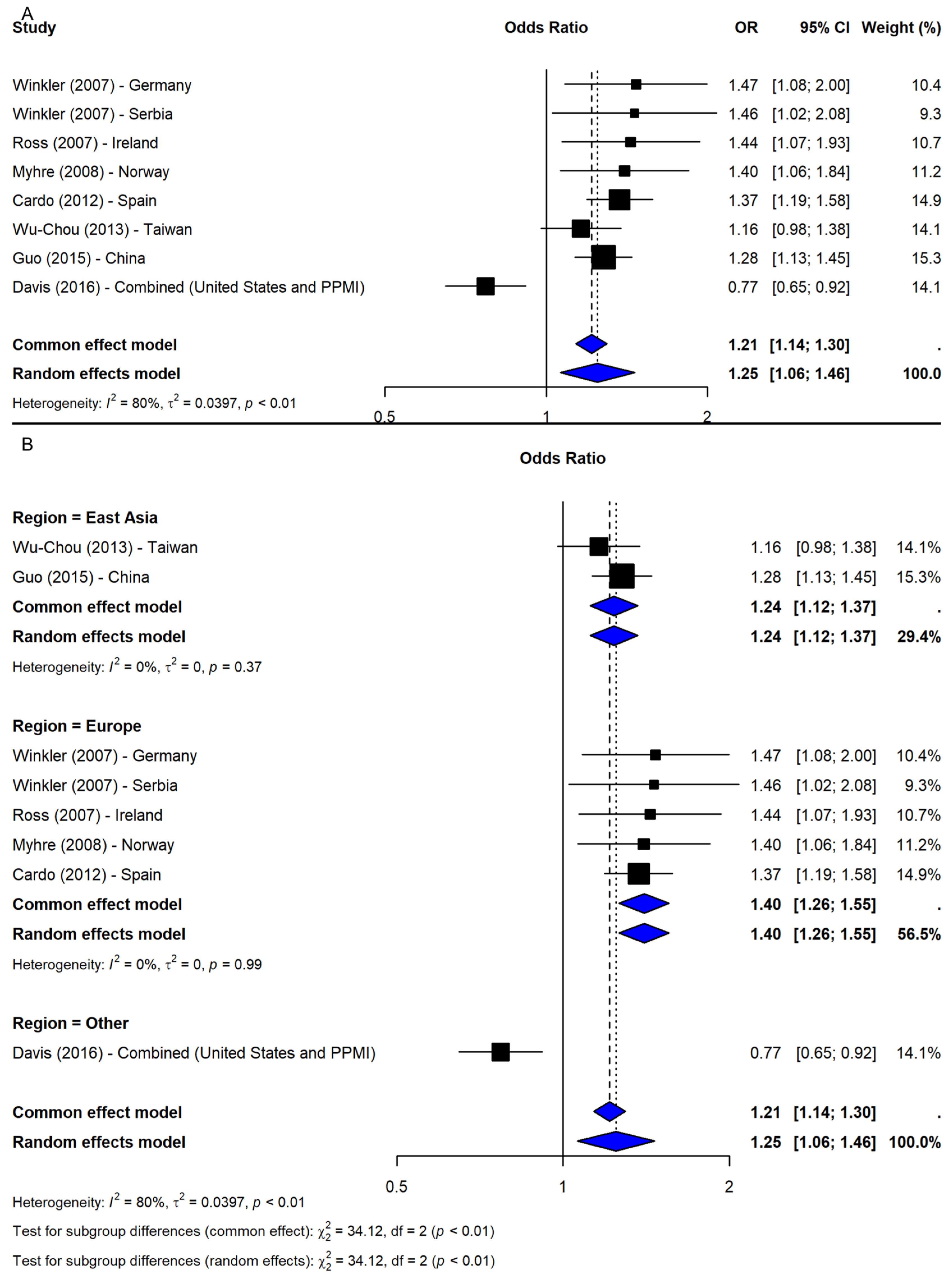
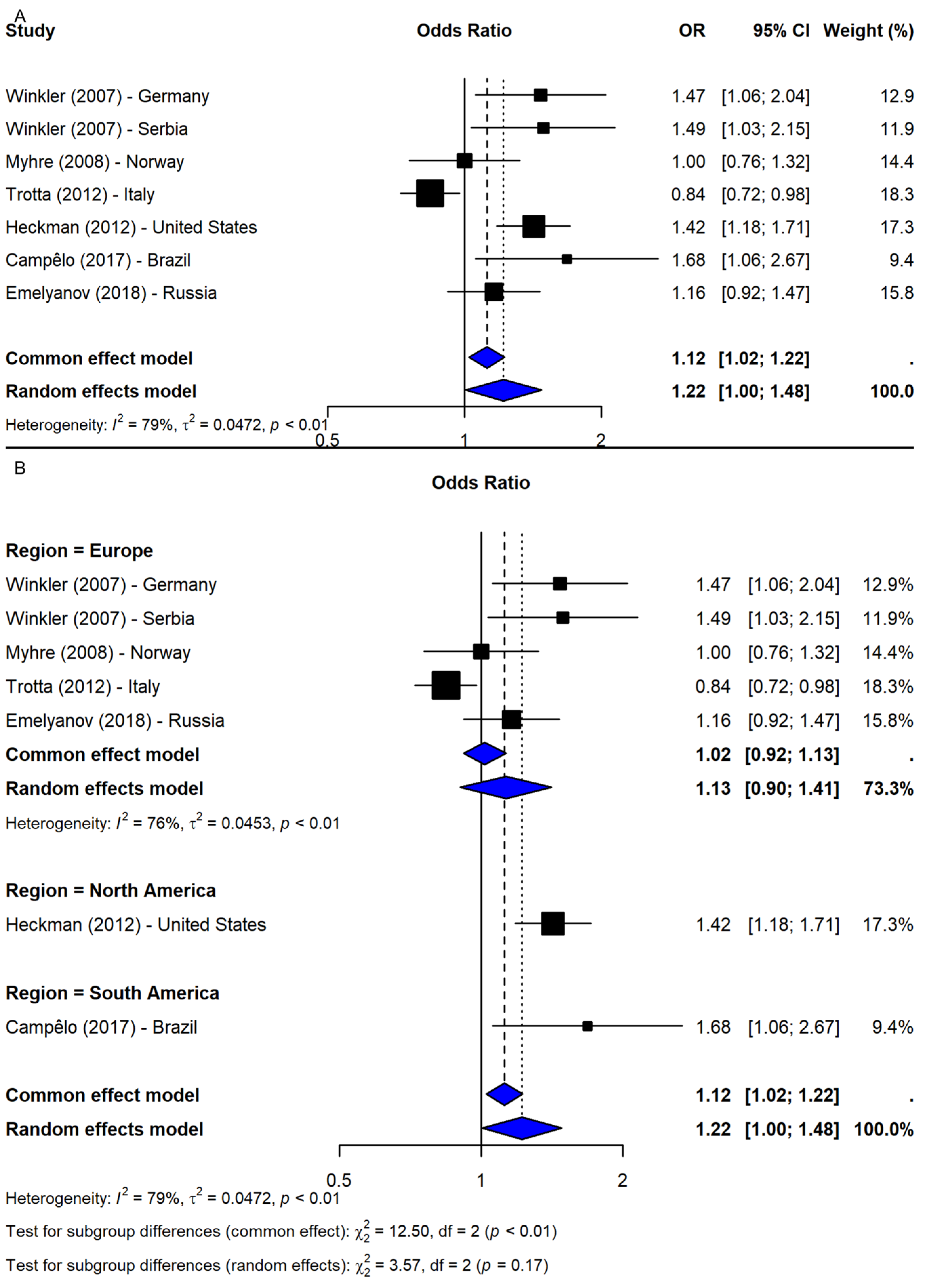

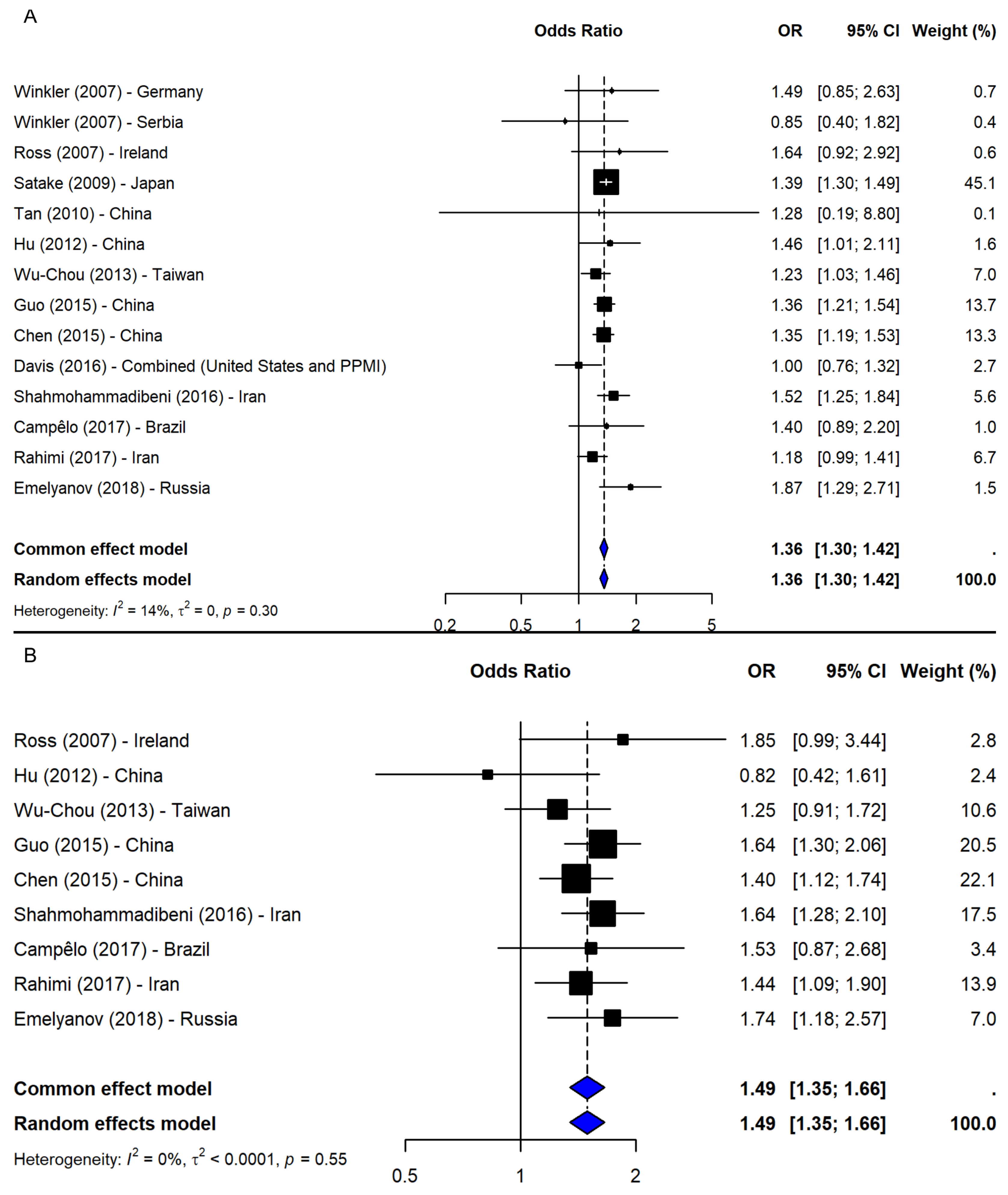
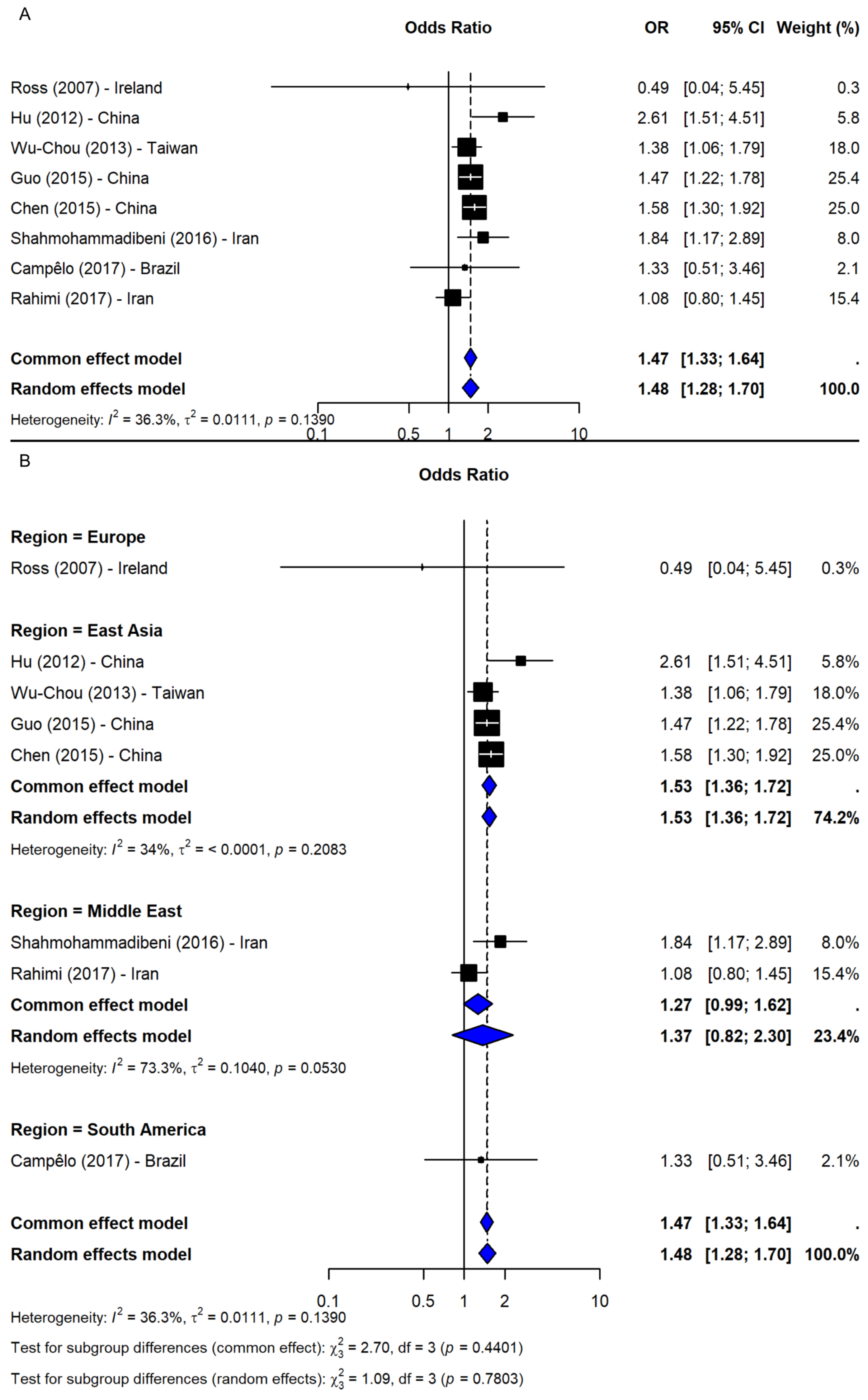
| SNP ID | Comparison | N | OR (95% CI) | p-Value 1 | Z | I-Square | p-Value 2 | Model |
|---|---|---|---|---|---|---|---|---|
| Allelic G vs. A | 8 | 1.25 (1.06, 1.46) | 0.0071 | 2.69 | 80.1% | <0.0001 | R | |
| rs356165 | Dominant AG + GG vs. AA | 5 | 1.37 (1.21, 1.54) | <0.0001 | 5.17 | 7.8% | 0.3620 | F |
| Recessive GG vs. AG + AA | 5 | 1.50 (1.31, 1.71) | <0.0001 | 5.85 | 0.0% | 0.8834 | F | |
| Allelic: T vs. C | 7 | 1.22 (1.00, 1.48) | 0.0446 | 2.01 | 79.2% | <0.0001 | R | |
| rs2583988 | Dominant TT + TC vs. CC | 3 | 1.14 (0.93, 1.40) | 0.2076 | 1.26 | 0.0% | 0.4163 | F |
| Recessive TT vs. TC + CC | 3 | 1.31 (0.86, 2.01) | 0.2057 | 1.27 | 55.2% | 0.1070 | R | |
| Allelic: G vs. A | 16 | 1.35 (1.22; 1.50) | <0.0001 | 5.74 | 80.7% | <0.0001 | R | |
| rs356219 | Dominant: AG + GG vs. AA | 14 | 1.46 (1.29, 1.65) | <0.0001 | 6.10 | 51.8% | 0.0125 | R |
| Recessive: GG vs. AG + AA | 14 | 1.69 (1.49, 1.91) | <0.0001 | 8.12 | 50.2% | 0.0164 | R | |
| Allelic: T vs. G | 14 | 1.36 (1.30, 1.42) | <0.0001 | 13.03 | 13.7% | 0.3029 | F | |
| rs11931074 | Dominant: TG + TT vs. GG | 9 | 1.49 (1.35, 1.66) | <0.0001 | 7.61 | 0.0% | 0.5477 | F |
| Recessive: TT vs. TG + GG | 8 | 1.48 (1.28,1.70) | <0.0001 | 5.43 | 36.3% | 0.1390 | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, R.; Shirazi, M.; Sadat-Madani, S.F.; Yeo Cheng Long, M.Z.; Singh, C.L.; Tan, J.Y.; Deng, X.; Hashemi Fard, S.M.; Ng, S.Y.E.; Ng, A.S.L.; et al. Common SNCA Genetic Variants and Parkinson’s Disease Risk: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 6001. https://doi.org/10.3390/ijms26136001
Mohammadi R, Shirazi M, Sadat-Madani SF, Yeo Cheng Long MZ, Singh CL, Tan JY, Deng X, Hashemi Fard SM, Ng SYE, Ng ASL, et al. Common SNCA Genetic Variants and Parkinson’s Disease Risk: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(13):6001. https://doi.org/10.3390/ijms26136001
Chicago/Turabian StyleMohammadi, Raziyeh, Mahdi Shirazi, Sayedeh Fatemeh Sadat-Madani, Matthew Zachary Yeo Cheng Long, Corrine Lee Singh, Jayne Y. Tan, Xiao Deng, Seyed Majid Hashemi Fard, Samuel Y. E. Ng, Adeline S. L. Ng, and et al. 2025. "Common SNCA Genetic Variants and Parkinson’s Disease Risk: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 13: 6001. https://doi.org/10.3390/ijms26136001
APA StyleMohammadi, R., Shirazi, M., Sadat-Madani, S. F., Yeo Cheng Long, M. Z., Singh, C. L., Tan, J. Y., Deng, X., Hashemi Fard, S. M., Ng, S. Y. E., Ng, A. S. L., Tan, L. C. S., & Saffari, S. E. (2025). Common SNCA Genetic Variants and Parkinson’s Disease Risk: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(13), 6001. https://doi.org/10.3390/ijms26136001








