BRD4 Mediates Transforming Growth Factor-β-Induced Smooth Muscle Cell Differentiation from Mesenchymal Progenitor Cells
Abstract
1. Introduction
2. Results
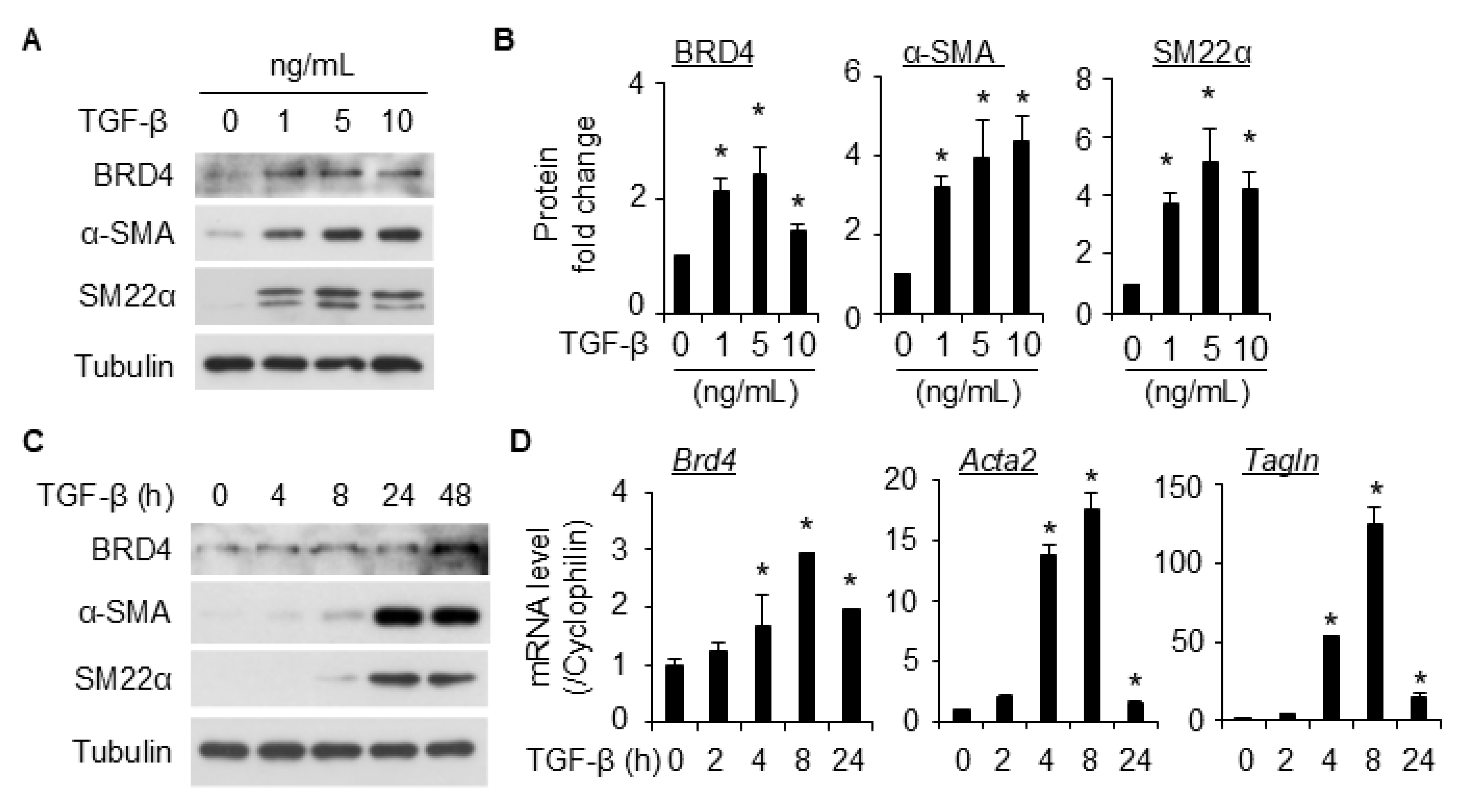
2.1. BRD4 Expression Is Up-Regulated in TGF-β-Induced SMC Differentiation
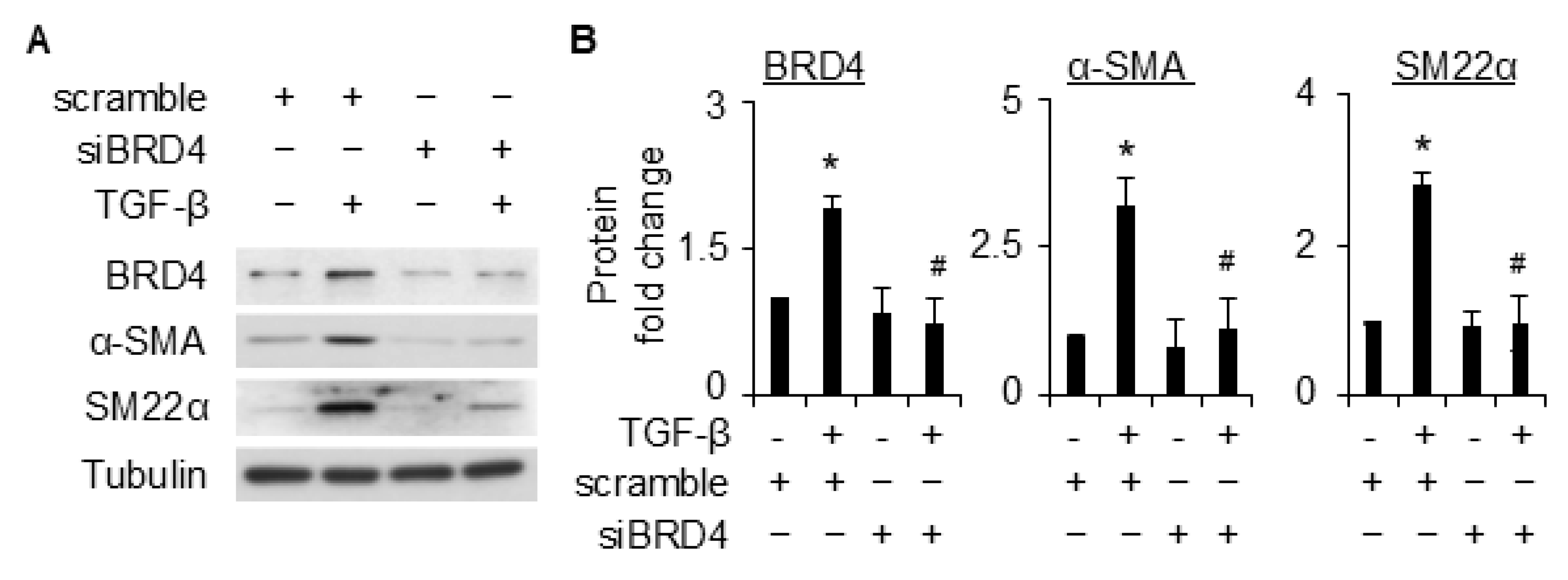
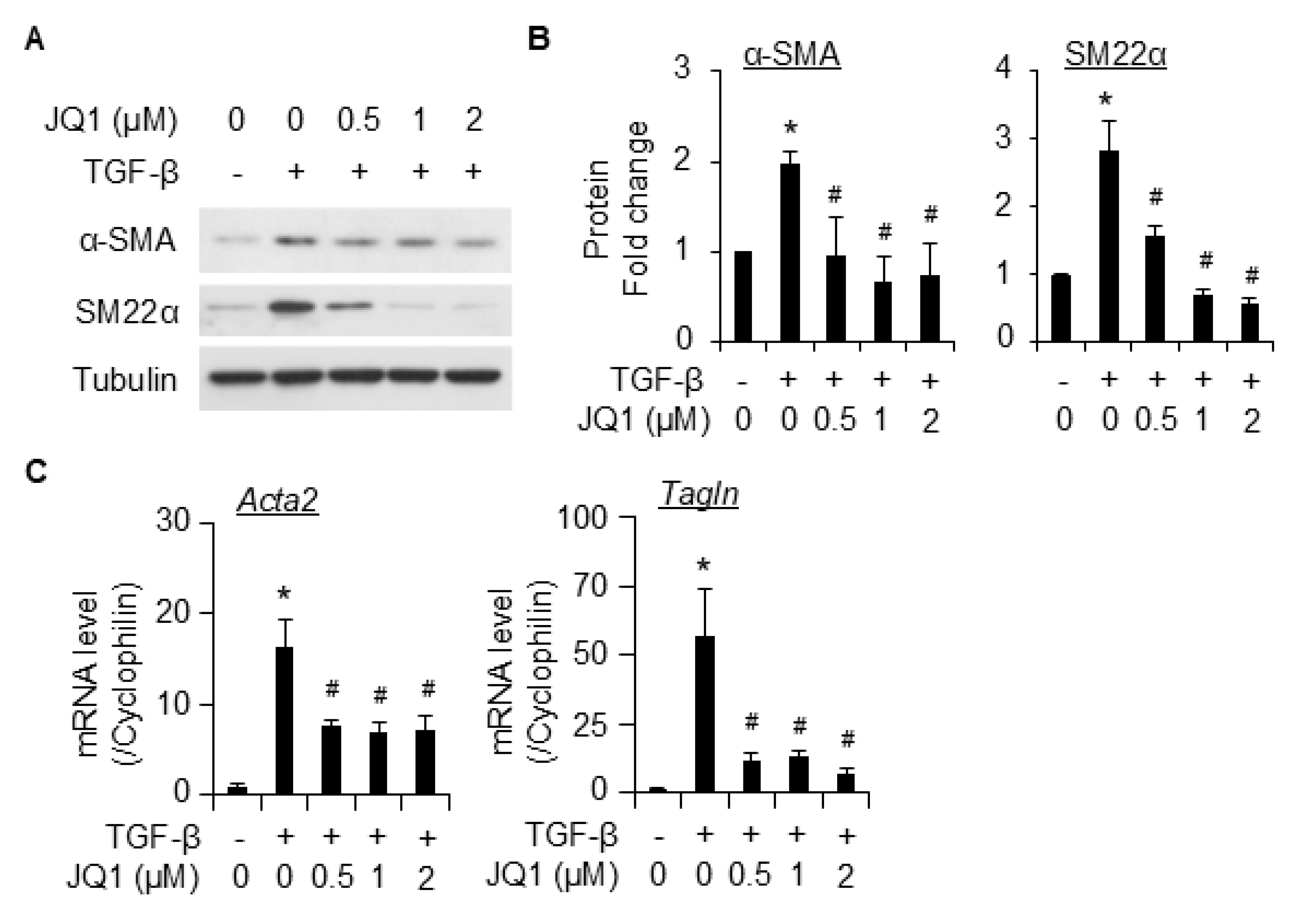
2.2. BRD4 Is Involved in TGF-β-Induced SMC Differentiation
2.3. BRD4 Mediated SMC Maker Expression Appears to Be Independent of Smad3 Phosphorylation
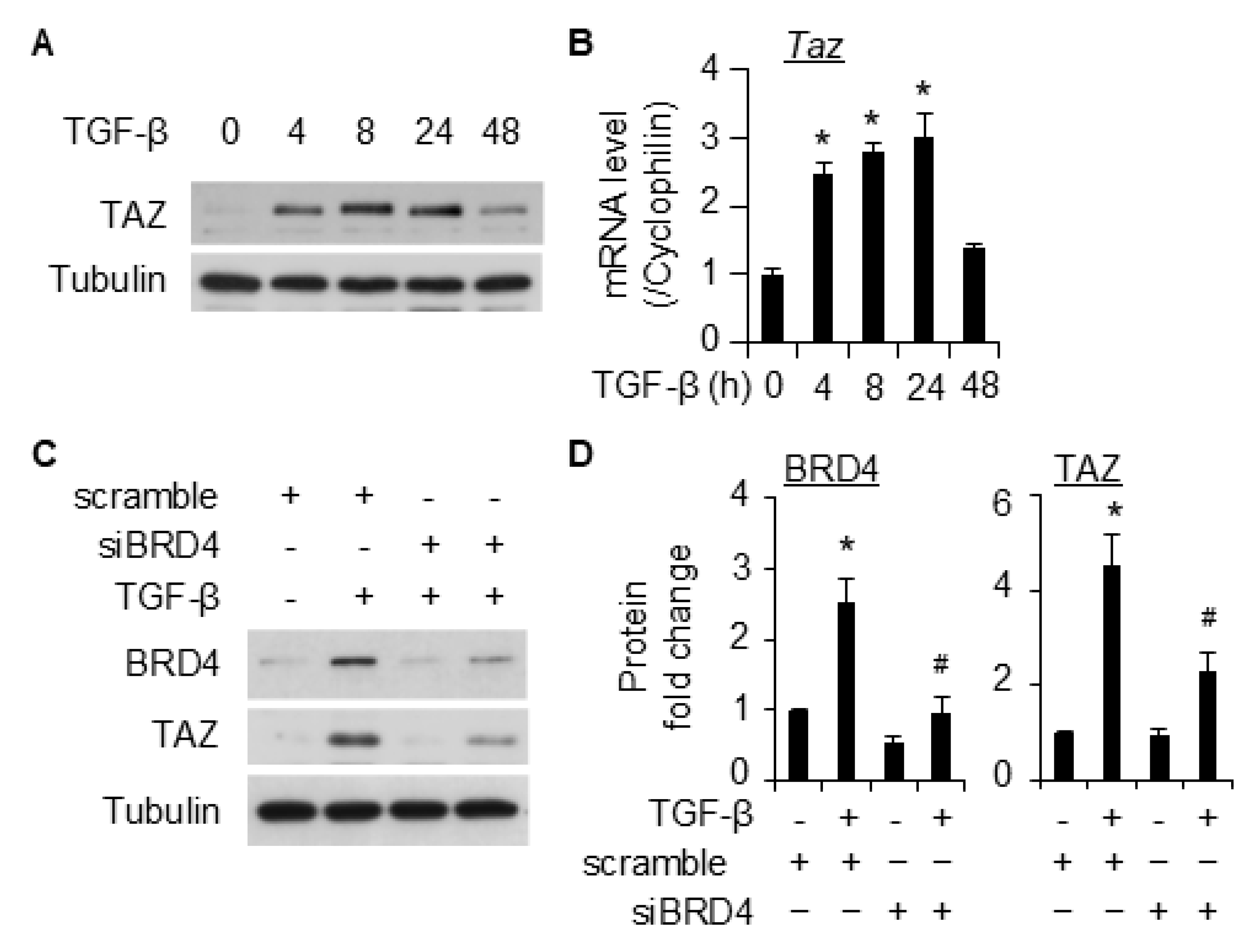
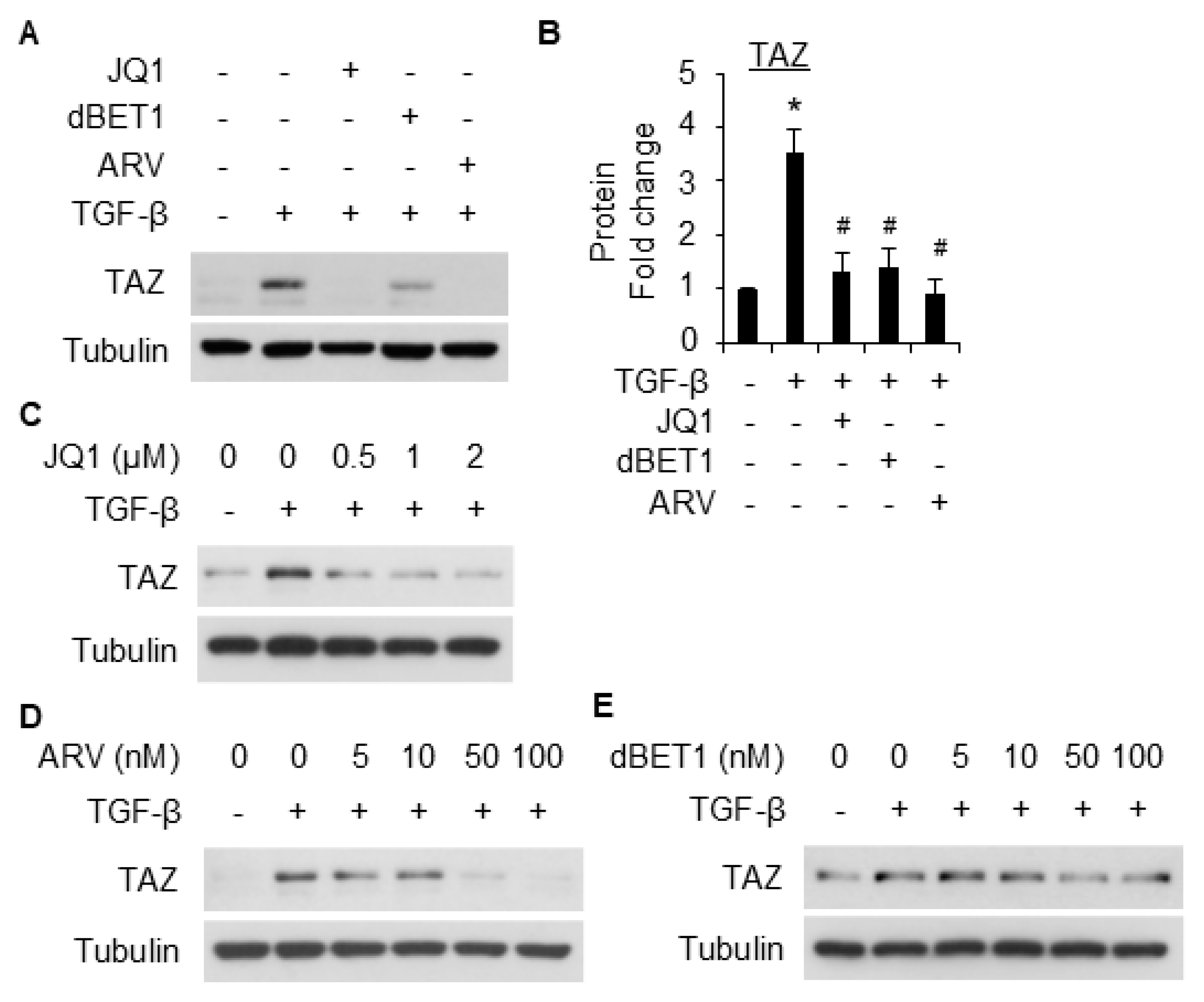
2.4. BRD4 Mediates SMC Marker Gene Expression Through TAZ
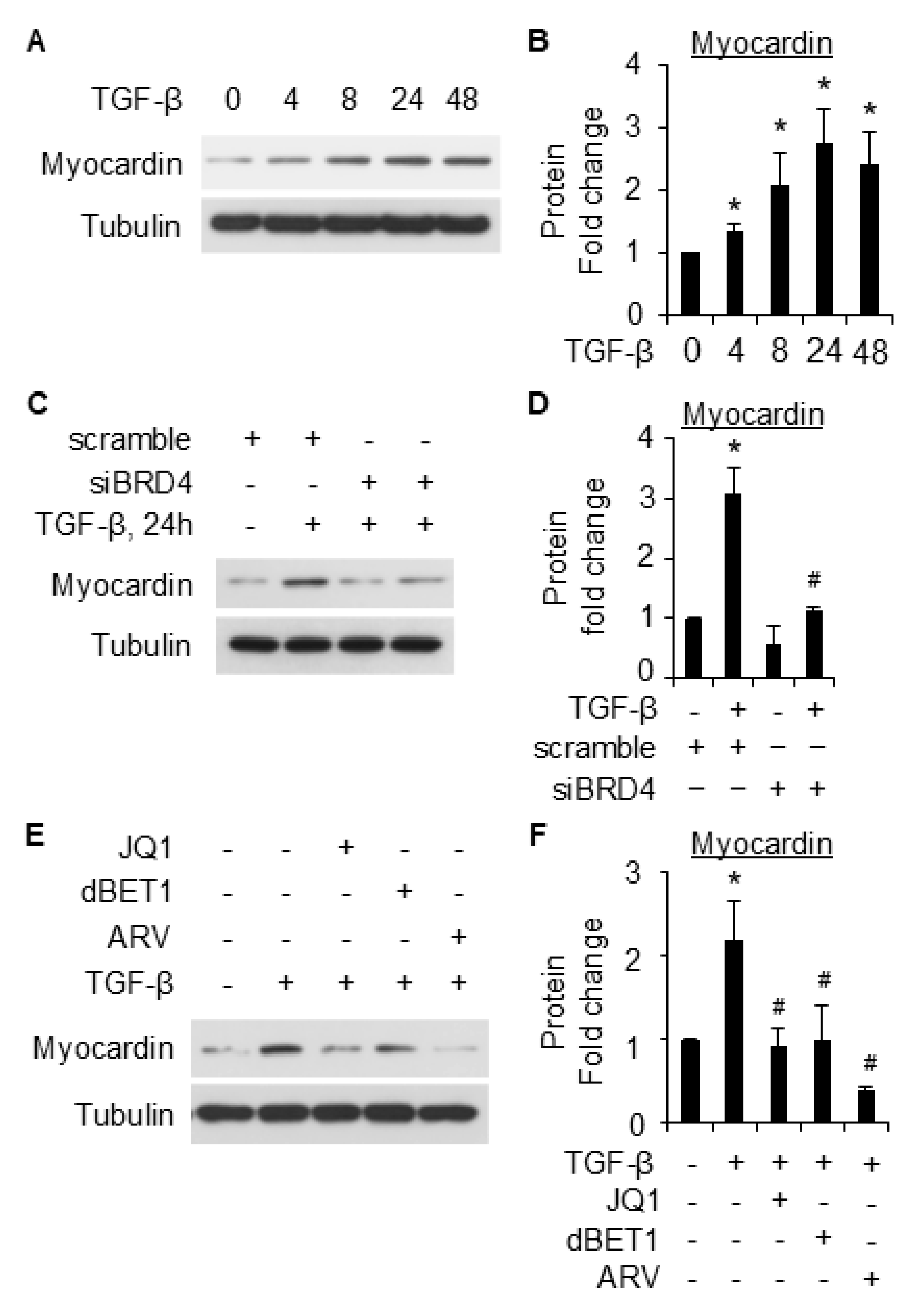
2.5. Myocardin Is Involved in BRD4-Mediated SMC Marker Gene Expression
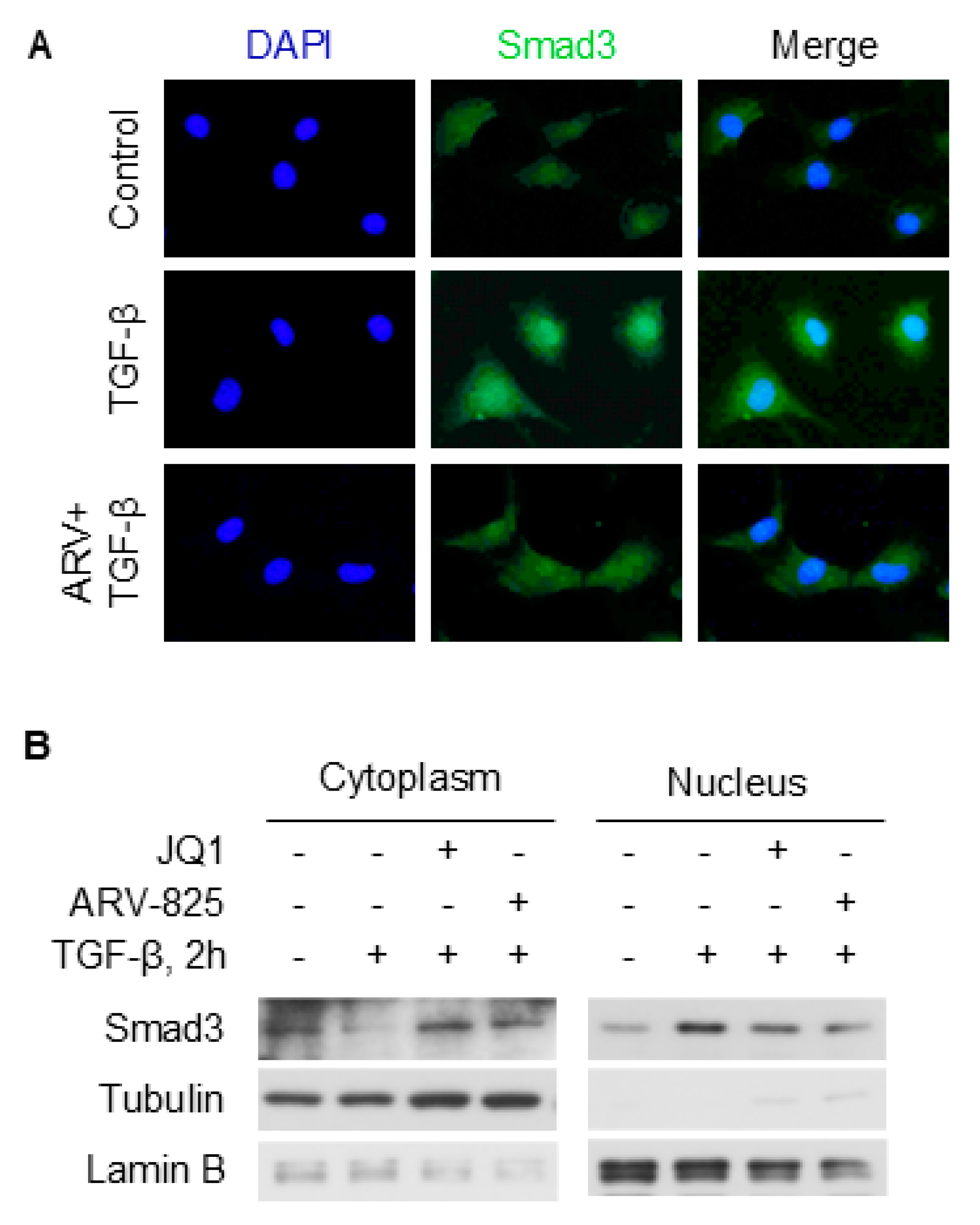
2.6. BRD4 Enhances Smad3 Nuclear Retention Likely Involving TAZ
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Treatments
4.3. Western Blotting (WB) and Fractionation WB Assays
4.4. RNA Extraction and Quantitative Real-Time PCR (qPCR) Analysis
4.5. Small Interfering RNA (siRNA) Transfection
4.6. Immunofluorescence Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRD4 | bromodomain-containing protein 4 |
| BCA | bicinchoninic acid |
| IF | immunofluorescence |
| RIPA | radioimmunoprecipitation assay |
| TGF-β | transforming growth factor-β |
| SMC | smooth muscle cell |
| TAZ | transcriptional coactivator with PDZ-binding motif |
References
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Chakraborty, R.; Chatterjee, P.; Dave, J.M.; Ostriker, A.C.; Greif, D.M.; Rzucidlo, E.M.; Martin, K.A. Targeting smooth muscle cell phenotypic switching in vascular disease. JVS-Vasc. Sci. 2021, 2, 79–94. [Google Scholar] [CrossRef]
- Xie, C.; Ritchie, R.P.; Huang, H.; Zhang, J.; Chen, Y.E. Smooth muscle cell differentiation in vitro: Models and underlying molecular mechanisms. Arter. Thromb. Vasc. Biol. 2011, 31, 1485–1494. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, S.Y. Transforming growth factor-beta and smooth muscle differentiation. World J. Biol. Chem. 2012, 3, 41–52. [Google Scholar] [CrossRef]
- Dong, K.; Guo, X.; Chen, W.; Hsu, A.C.; Shao, Q.; Chen, J.F.; Chen, S.Y. Mesenchyme homeobox 1 mediates transforming growth factor-beta (TGF-beta)-induced smooth muscle cell differentiation from mouse mesenchymal progenitors. J. Biol. Chem. 2018, 293, 8712–8719. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.P.; Somlyo, A.V.; Hautmann, M.; Somlyo, A.P.; Owens, G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001, 276, 341–347. [Google Scholar] [CrossRef]
- Xie, W.B.; Li, Z.; Miano, J.M.; Long, X.; Chen, S.Y. Smad3-mediated myocardin silencing: A novel mechanism governing the initiation of smooth muscle differentiation. J. Biol. Chem. 2011, 286, 15050–15057. [Google Scholar] [CrossRef]
- Guo, X.; Olajuyin, A.; Tucker, T.A.; Idell, S.; Qian, G. BRD4 as a Therapeutic Target in Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 13231. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pan, D.; Li, G.; Chen, K.; Hu, X. Regulation of programmed cell death by Brd4. Cell Death Dis. 2022, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; de Soysa, T.Y.; Pelonero, A.; Sapp, V.; Shah, P.P.; Wang, Q.; Li, L.; Lee, C.Y.; Sadagopan, N.; Nishino, T.; et al. A genome-wide CRISPR screen identifies BRD4 as a regulator of cardiomyocyte differentiation. Nat. Cardiovasc. Res. 2024, 3, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Guo, H.; Ma, M.; Zha, Z. BRD4 facilitates osteogenic differentiation of human bone marrow mesenchymal stem cells through WNT4/NF-kappaB pathway. J. Orthop. Surg. Res. 2023, 18, 876. [Google Scholar] [CrossRef]
- Rodriguez, R.M.; Suarez-Alvarez, B.; Salvanes, R.; Huidobro, C.; Torano, E.G.; Garcia-Perez, J.L.; Lopez-Larrea, C.; Fernandez, A.F.; Bueno, C.; Menendez, P.; et al. Role of BRD4 in hematopoietic differentiation of embryonic stem cells. Epigenetics 2014, 9, 566–578. [Google Scholar] [CrossRef]
- Linares-Saldana, R.; Kim, W.; Bolar, N.A.; Zhang, H.; Koch-Bojalad, B.A.; Yoon, S.; Shah, P.P.; Karnay, A.; Park, D.S.; Luppino, J.M.; et al. Publisher Correction: BRD4 orchestrates genome folding to promote neural crest differentiation. Nat. Genet. 2021, 53, 1480–1492, Erratum in Nat. Genet. 2021, 53, 1723. [Google Scholar] [CrossRef]
- Song, S.; Liu, L.; Yu, Y.; Zhang, R.; Li, Y.; Cao, W.; Xiao, Y.; Fang, G.; Li, Z.; Wang, X.; et al. Inhibition of BRD4 attenuates transverse aortic constriction- and TGF-beta-induced endothelial-mesenchymal transition and cardiac fibrosis. J. Mol. Cell. Cardiol. 2019, 127, 83–96. [Google Scholar] [CrossRef]
- Stratton, M.S.; Bagchi, R.A.; Felisbino, M.B.; Hirsch, R.A.; Smith, H.E.; Riching, A.S.; Enyart, B.Y.; Koch, K.A.; Cavasin, M.A.; Alexanian, M.; et al. Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation. Circ. Res. 2019, 125, 662–677. [Google Scholar] [CrossRef]
- Sanders, Y.Y.; Lyv, X.; Zhou, Q.J.; Xiang, Z.; Stanford, D.; Bodduluri, S.; Rowe, S.M.; Thannickal, V.J. Brd4-p300 inhibition downregulates Nox4 and accelerates lung fibrosis resolution in aged mice. JCI Insight 2020, 5, e137127. [Google Scholar] [CrossRef]
- Pinney, D.F.; Emerson, C.P., Jr. 10T1/2 cells: An in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ. Health Perspect. 1989, 80, 221–227. [Google Scholar] [CrossRef]
- Kurpinski, K.; Lam, H.; Chu, J.; Wang, A.; Kim, A.; Tsay, E.; Agrawal, S.; Schaffer, D.V.; Li, S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010, 28, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oparil, S.; Novak, L.; Cao, X.; Shi, W.; Lucas, J.; Chen, Y.F. ANP signaling inhibits TGF-beta-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. 2007, 102, 390–398. [Google Scholar] [CrossRef]
- Varelas, X.; Sakuma, R.; Samavarchi-Tehrani, P.; Peerani, R.; Rao, B.M.; Dembowy, J.; Yaffe, M.B.; Zandstra, P.W.; Wrana, J.L. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 2008, 10, 837–848. [Google Scholar] [CrossRef]
- Pagiatakis, C.; Sun, D.; Tobin, S.W.; Miyake, T.; McDermott, J.C. TGFbeta-TAZ/SRF signalling regulates vascular smooth muscle cell differentiation. FEBS J. 2017, 284, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.Z.; Bialik, J.F.; Speight, P.; Dan, Q.; Yeung, T.; Szaszi, K.; Pedersen, S.F.; Kapus, A. TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J. Biol. Chem. 2017, 292, 14902–14920. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.P.; Hinson, J.S. Regulation of smooth muscle differentiation by the myocardin family of serum response factor co-factors. J. Thromb. Haemost. 2005, 3, 1976–1984. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, Y.; Sun, H.; Zhang, Y.; Yang, J.; Brasier, A.R. BRD4 mediates NF-kappaB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L1183–L1201. [Google Scholar] [CrossRef]
- Alexanian, M.; Przytycki, P.F.; Micheletti, R.; Padmanabhan, A.; Ye, L.; Travers, J.G.; Gonzalez-Teran, B.; Silva, A.C.; Duan, Q.; Ranade, S.S.; et al. A transcriptional switch governs fibroblast activation in heart disease. Nature 2021, 595, 438–443. [Google Scholar] [CrossRef]
- Zhou, B.; Mu, J.; Gong, Y.; Lu, C.; Zhao, Y.; He, T.; Qin, Z. Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-beta-mediated Nox4 expression. Redox Biol. 2017, 11, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Shafran, J.S.; Jafari, N.; Casey, A.N.; Gyorffy, B.; Denis, G.V. BRD4 regulates key transcription factors that drive epithelial-mesenchymal transition in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 268–277. [Google Scholar] [CrossRef]
- Rios-Lopez, D.G.; Tecalco-Cruz, A.C.; Martinez-Pastor, D.; Sosa-Garrocho, M.; Tapia-Urzua, G.; Aranda-Lopez, Y.; Ortega-Dominguez, B.; Recillas-Targa, F.; Vazquez-Victorio, G.; Macias-Silva, M. TGF-beta/SMAD canonical pathway induces the expression of transcriptional cofactor TAZ in liver cancer cells. Heliyon 2023, 9, e21519. [Google Scholar] [CrossRef]
- Gallardo, F.S.; Cruz-Soca, M.; Bock-Pereda, A.; Faundez-Contreras, J.; Gutierrez-Rojas, C.; Gandin, A.; Torresan, V.; Casar, J.C.; Ravasio, A.; Brandan, E. Role of TGF-beta/SMAD/YAP/TAZ signaling in skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol. 2025, 328, C1015–C1028. [Google Scholar] [CrossRef]
- Wang, D.; Chang, P.S.; Wang, Z.; Sutherland, L.; Richardson, J.A.; Small, E.; Krieg, P.A.; Olson, E.N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 2001, 105, 851–862. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Lai, L.; Belaguli, N.S.; Dean, D.A.; Schwartz, R.J.; Zimmer, W.E. Transforming growth factor-beta induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J. Biol. Chem. 2002, 277, 6287–6295. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Li, J.; Urabe, G.; Huang, Y.; Zhang, M.; Wang, B.; Marcho, L.; Shen, H.; Kent, K.C.; Guo, L.W. A Role for Polo-Like Kinase 4 in Vascular Fibroblast Cell-Type Transition. JACC Basic Transl. Sci. 2021, 6, 257–283. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Leung, J.Y.; Balamurugan, S.; Tergaonkar, V.; Loh, A.H.P.; Chiang, C.M.; Taneja, R. BRD4 isoforms have distinct roles in tumour progression and metastasis in rhabdomyosarcoma. EMBO Rep. 2024, 25, 832–852. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lee, C.F.; Lai, H.T.; Yu, C.T.; Lee, J.E.; Zuo, H.; Tsai, S.Y.; Tsai, M.J.; Ge, K.; Wan, Y.; et al. Opposing Functions of BRD4 Isoforms in Breast Cancer. Mol. Cell 2020, 78, 1114–1132 e10. [Google Scholar] [CrossRef]
- Qian, G.; Adeyanju, O.; Roy, S.; Sunil, C.; Jeffers, A.; Guo, X.; Ikebe, M.; Idell, S.; Tucker, T.A. DOCK2 Promotes Pleural Fibrosis by Modulating Mesothelial to Mesenchymal Transition. Am. J. Respir. Cell Mol. Biol. 2022, 66, 171–182. [Google Scholar] [CrossRef]
- Qian, G.; Adeyanju, O.; Sunil, C.; Huang, S.K.; Chen, S.Y.; Tucker, T.A.; Idell, S.; Guo, X. Dedicator of Cytokinesis 2 (DOCK2) Deficiency Attenuates Lung Injury Associated with Chronic High-Fat and High-Fructose Diet-Induced Obesity. Am. J. Pathol. 2022, 192, 226–238. [Google Scholar] [CrossRef]
- Guo, X.; Sunil, C.; Adeyanju, O.; Parker, A.; Huang, S.; Ikebe, M.; Tucker, T.A.; Idell, S.; Qian, G. PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and beta-catenin signaling pathways. Sci. Rep. 2022, 12, 3053. [Google Scholar]
- Qian, G.; Adeyanju, O.; Cai, D.; Tucker, T.A.; Idell, S.; Chen, S.Y.; Guo, X. DOCK2 Promotes Atherosclerosis by Mediating the Endothelial Cell Inflammatory Response. Am. J. Pathol. 2024, 194, 599–611. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olajuyin, A.; Mandlem, V.; Sunil, C.; Hou, Y.; Adeyanju, O.; Petkar, S.; Li, Q.; Tucker, T.A.; Idell, S.; Chen, S.-Y.; et al. BRD4 Mediates Transforming Growth Factor-β-Induced Smooth Muscle Cell Differentiation from Mesenchymal Progenitor Cells. Int. J. Mol. Sci. 2025, 26, 8074. https://doi.org/10.3390/ijms26168074
Olajuyin A, Mandlem V, Sunil C, Hou Y, Adeyanju O, Petkar S, Li Q, Tucker TA, Idell S, Chen S-Y, et al. BRD4 Mediates Transforming Growth Factor-β-Induced Smooth Muscle Cell Differentiation from Mesenchymal Progenitor Cells. International Journal of Molecular Sciences. 2025; 26(16):8074. https://doi.org/10.3390/ijms26168074
Chicago/Turabian StyleOlajuyin, Ayobami, Venkatakirankumar Mandlem, Christudas Sunil, Yunzhuan Hou, Oluwaseun Adeyanju, Sana Petkar, Qinying Li, Torry A. Tucker, Steven Idell, Shi-You Chen, and et al. 2025. "BRD4 Mediates Transforming Growth Factor-β-Induced Smooth Muscle Cell Differentiation from Mesenchymal Progenitor Cells" International Journal of Molecular Sciences 26, no. 16: 8074. https://doi.org/10.3390/ijms26168074
APA StyleOlajuyin, A., Mandlem, V., Sunil, C., Hou, Y., Adeyanju, O., Petkar, S., Li, Q., Tucker, T. A., Idell, S., Chen, S.-Y., Guo, X., & Qian, G. (2025). BRD4 Mediates Transforming Growth Factor-β-Induced Smooth Muscle Cell Differentiation from Mesenchymal Progenitor Cells. International Journal of Molecular Sciences, 26(16), 8074. https://doi.org/10.3390/ijms26168074





