Hyaluronan-Related Granulomatous Synovitis, Adipositis, and Osteomyelitis in the Osteoarthritic Knee: A Morphological Case Series of Three Patients
Abstract
1. Introduction
- Provide the first detailed morphological description of e-HA-triggered foreign body reactions across synovial, adipose, and osseous tissues.
- Identify potential pathways of e-HA penetration into different joint compartments.
- Emphasize the underreported conflict between e-HA and host tissues, which perceive it as a foreign material.
2. Results
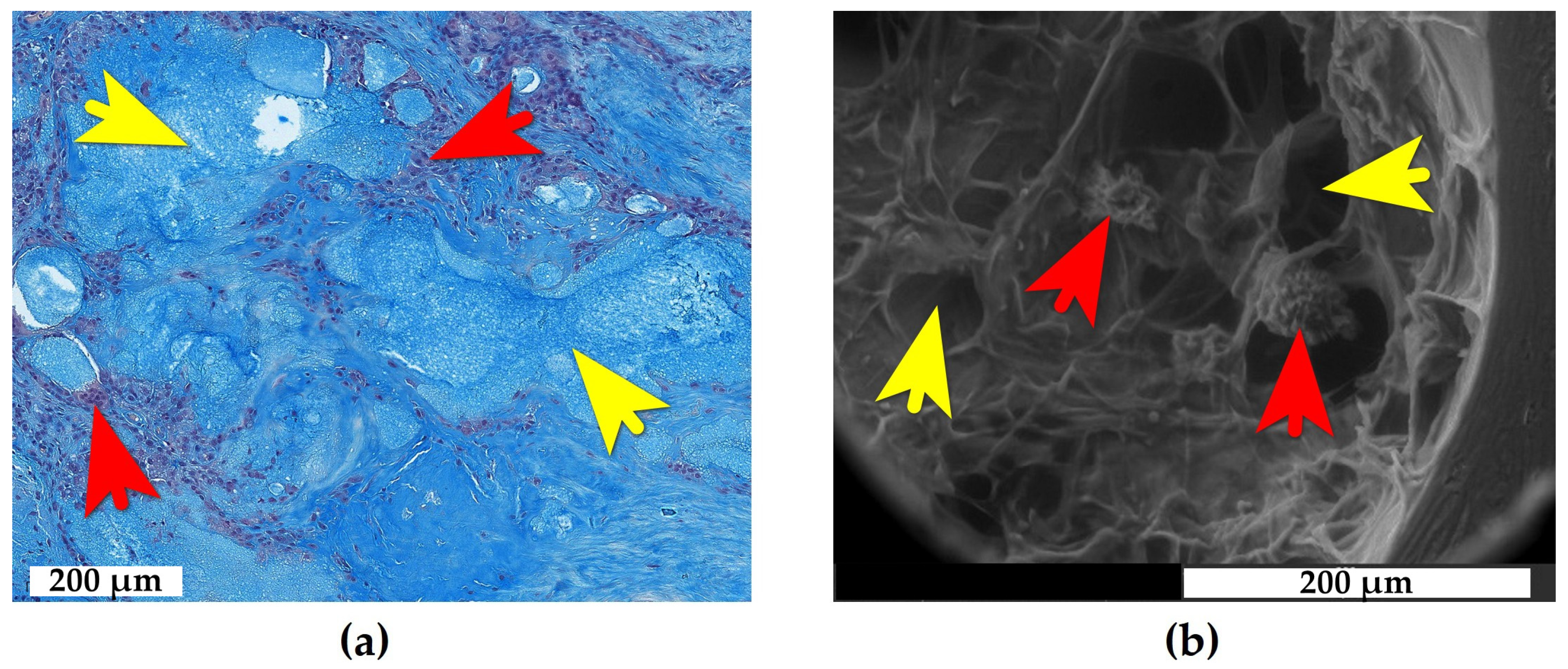
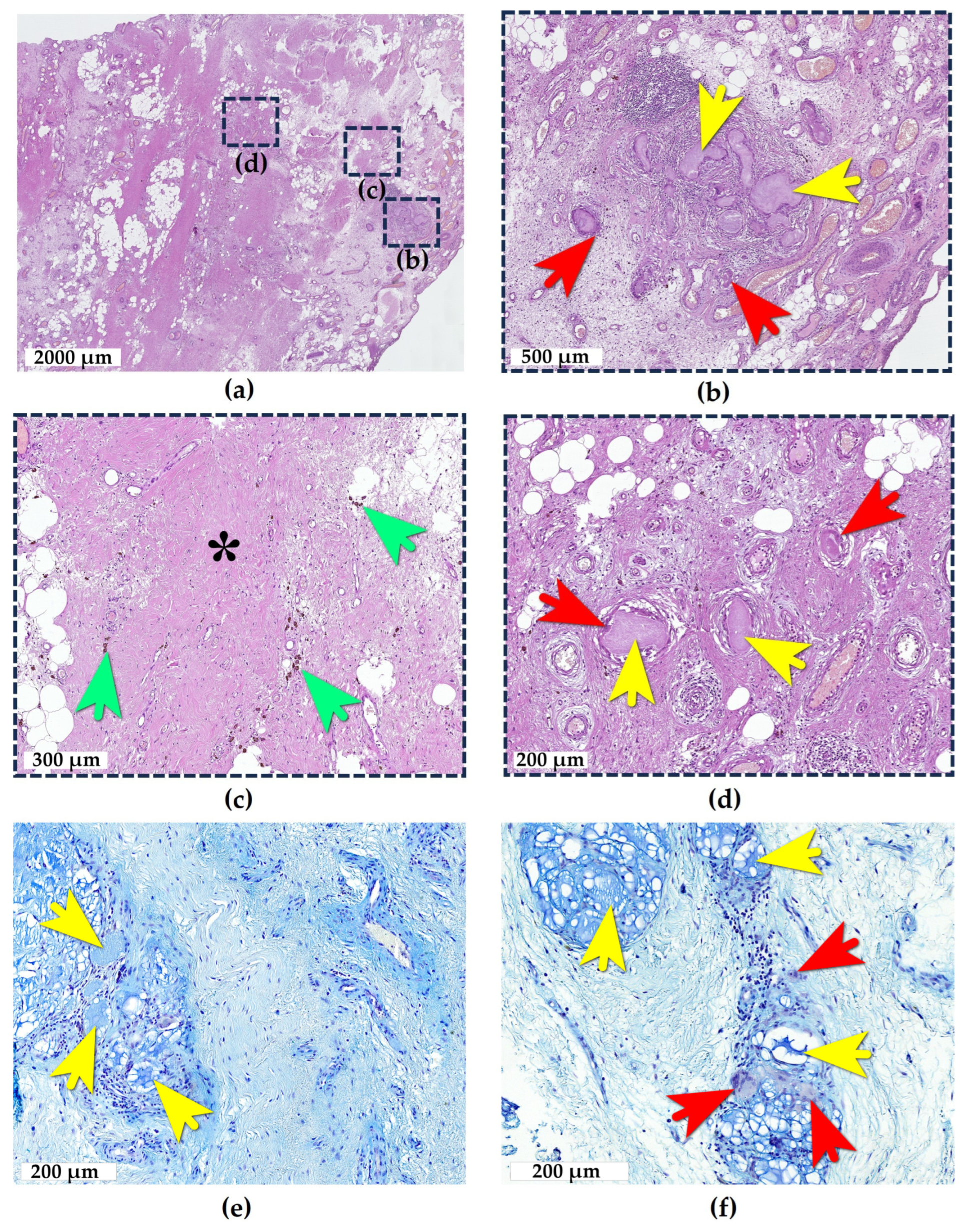
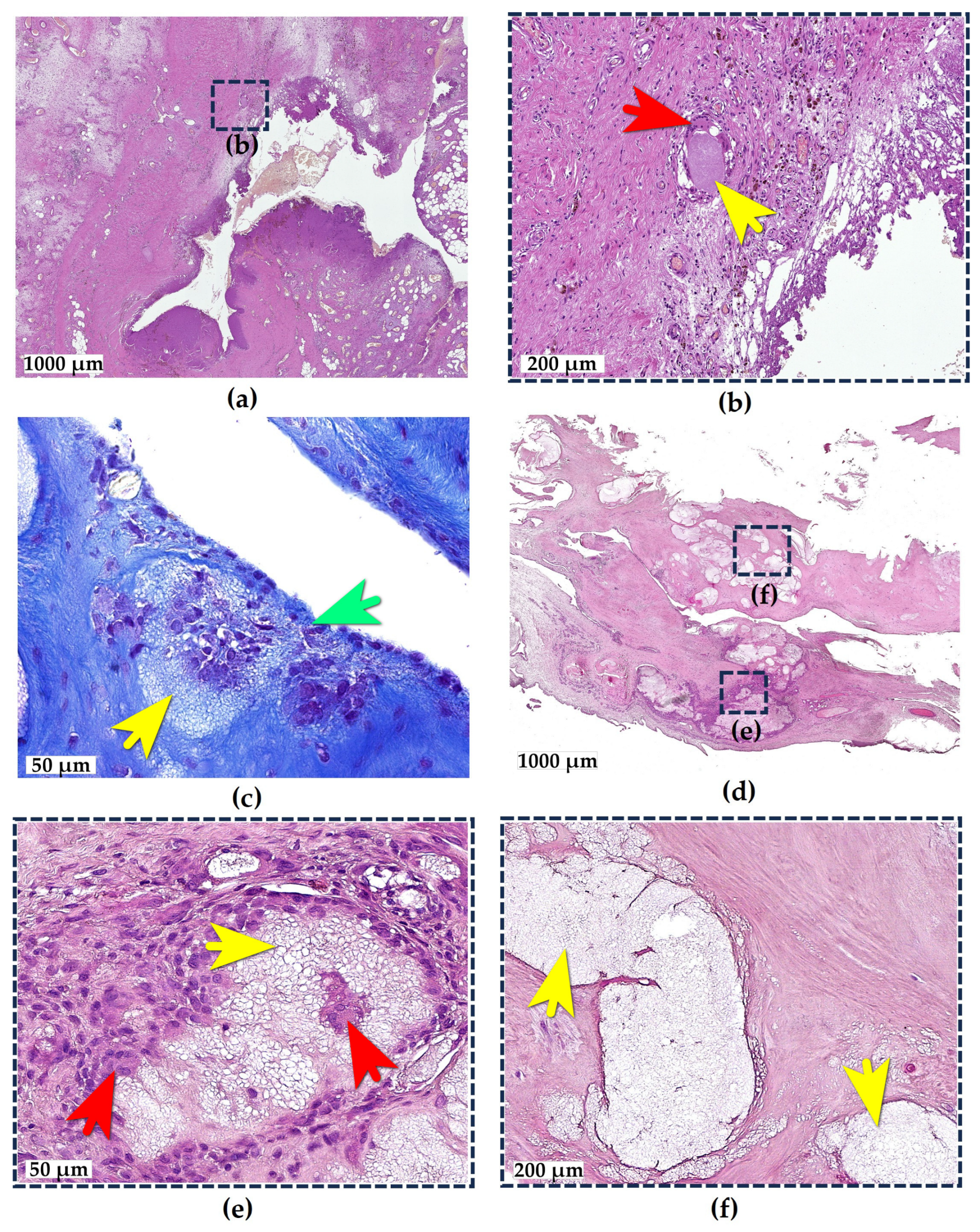
2.1. Adipose Tissue Response to e-HA
2.2. Synovial Tissue Response to e-HA
2.3. Bone Tissue Response to e-HA
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Technique of Surgery and Sampling of Histological Material
4.3. Morphological Analysis
4.3.1. Light Microscopy
4.3.2. Scanning Electron Microscopy (SEM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| e-HA | Exogenous hyaluronan |
| OA | Osteoarthritis |
| SEM | Scanning electron microscopy |
| FBGCs | Foreign body giant cells |
References
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic acid: A key ingredient in the therapy of inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- Legre-Boyer, V. Viscosupplémentation: Techniques, indications, résultats: Viscosupplementation: Techniques, indications, results. In Conférences d’enseignement 2014; Elsevier: Amsterdam, The Netherlands, 2014; pp. 141–153. [Google Scholar]
- Bellamy, N.; Campbell, J.; Welch, V.; Gee, T.L.; Bourne, R.; Wells, G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2006, CD005321. [Google Scholar] [CrossRef]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthop. Rev. 2021, 13, 25549. [Google Scholar] [CrossRef]
- Leighton, R.; Fitzpatrick, J.; Smith, H.; Crandall, D.; Flannery, C.R.; Conrozier, T. Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Access Rheumatol. Res. Rev. 2018, 10, 43–54. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 458280. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The role of hyaluronic acid in cartilage boundary lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Concoff, A.; Niazi, F.; Farrokhyar, F.; Alyass, A.; Rosen, J.; Nicholls, M. Delay to TKA and costs associated with knee osteoarthritis care using intra-articular hyaluronic acid: Analysis of an administrative database. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2021, 14, 1179544121994092. [Google Scholar] [CrossRef]
- Phillips, M.; Bhandari, M.; Grant, J.; Bedi, A.; Trojian, T.; Johnson, A.; Schemitsch, E. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: An international perspective. Orthop. J. Sports Med. 2021, 9, 23259671211030272. [Google Scholar] [CrossRef]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef]
- DeMoya, C.D.; Joenathan, A.; Lawson, T.B.; Felson, D.T.; Schaer, T.P.; Bais, M.; Albro, M.B.; Mäkelä, J.; Snyder, B.D.; Grinstaff, M.W. Advances in viscosupplementation and tribosupplementation for early-stage osteoarthritis therapy. Nat. Rev. Rheumatol. 2024, 20, 432–451. [Google Scholar] [CrossRef]
- Strand, V.; McIntyre, L.F.; Beach, W.R.; Miller, L.E.; Block, J.E. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: A systematic review and meta-analysis of randomized, saline-controlled trials. J. Pain Res. 2015, 8, 217–228. [Google Scholar]
- Maheu, E.; Rannou, F.; Reginster, J.-Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef]
- Ong, K.L.; Runa, M.; Xiao, Z.; Ngai, W.; Lau, E.; Altman, R.D. Severe acute localized reactions following intra-articular hyaluronic acid injections in knee osteoarthritis. Cartilage 2021, 13, 1474S–1486S. [Google Scholar] [CrossRef]
- Humphries, D.; Baria, M.; Fitzpatrick, J. Severe acute localized reactions after intra-articular hyaluronic acid injections: A narrative review and physician’s guide to incidence, prevention, and management of these adverse reactions. J. Cartil. Jt. Preserv. 2025, 5, 100187. [Google Scholar] [CrossRef]
- Puttick, M.; Wade, J.; Chalmers, A.; Connell, D.; Rangno, K. Acute local reactions after intraarticular hylan for osteoarthritis of the knee. J. Rheumatol. 1995, 22, 1311–1314. [Google Scholar]
- Goldberg, V.M.; Coutts, R.D. Pseudoseptic reactions to hylan viscosupplementation: Diagnosis and treatment. Clin. Orthop. Relat. Res. 2004, 419, 130–137. [Google Scholar] [CrossRef]
- Fang, W.H.; Chen, X.T.; Vangsness, C.T., Jr. Ultrasound-guided knee injections are more accurate than blind injections: A systematic review of randomized controlled trials. Arthrosc. Sports Med. Rehabil. 2021, 3, e1177–e1187. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Wu, Y.-T.; Reeves, K.D.; Hadzic, A.; Perez, M.F.; Fu, S.N. A novel infrapatellar approach of ultrasound-guided intra-articular injection of the knee from both lateral and medial side: A case series. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X221149954. [Google Scholar] [CrossRef]
- Zardawi, I.M.; Chan, I. Synvisc perisynovitis. Pathology 2001, 33, 519–520. [Google Scholar] [CrossRef]
- Chen, A.L.; Desai, P.; Adler, E.M.; Di Cesare, P.E. Granulomatous inflammation after Hylan GF 20 viscosupplementation of the knee: A report of six cases. J. Bone Jt. Surg. 2002, 84, 1142–1147. [Google Scholar] [CrossRef]
- Michou, L.; Job-Deslandre, C.; de Pinieux, G.; Kahan, A. Granulomatous synovitis after intraarticular Hylan GF-20. A report of two cases. Jt. Bone Spine 2004, 71, 438–440. [Google Scholar] [CrossRef]
- Weinrauch, P.; Trigger, R.; Tsikleas, G. Bilateral Hip Joint Hylan G-F 20 Granulomatous Synovitis due to Viscosupplementation Injections. Case Rep. Orthop. 2014, 2014, 494073. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Garcia-Gimenez, V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: Clinical findings, long-term follow-up and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 150–161. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Lee, S.-K.; Kim, S.M.; Cho, S.H.; Lee, J.D.; Kim, H.S. Adverse reactions to injectable soft tissue fillers: Memorable cases and their clinico-pathological overview. J. Cosmet. Laser Ther. 2015, 17, 102–108. [Google Scholar] [CrossRef]
- Alcântara, C.E.P.; Noronha, M.S.; Cunha, J.F.; Flores, I.L.; Mesquita, R.A. Granulomatous reaction to hyaluronic acid filler material in oral and perioral region: A case report and review of literature. J. Cosmet. Dermatol. 2018, 17, 578–583. [Google Scholar] [CrossRef]
- Rosendy, G.; Cavalcante, I.L.; Barros, C.C.d.S.; López-Labady, J.; González, N.; Pérez-Alfonzo, R.; González, M.G.; Freire, C.H.; de Arruda, J.A.A.; de Andrade, B.A.B. Adverse Reactions Associated with Dermal Fillers in the Oral and Maxillofacial Region: A Venezuelan Experience. Head Neck Pathol. 2023, 17, 631–637. [Google Scholar] [CrossRef]
- Santos, L.G.; Jardim, L.C.; Schuch, L.F.; Silveira, F.M.; Wagner, V.P.; Pires, F.R.; Santos, J.N.D.; Martins, M.D. Foreign body reactions related to orofacial esthetic fillers: A systematic review. Oral Dis. 2024, 30, 855–864. [Google Scholar] [CrossRef]
- Chiang, Y.; Pierone, G.; Al-Niaimi, F. Dermal fillers: Pathophysiology, prevention and treatment of complications. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.A.; de Oliveira, L.Q.R.; Martelli-Júnior, H.; Pires, F.R.; Carvas, J.B.; Rogerio, V.E.; de Azevedo Rabelo, V.; Coletta, R.D. Adverse reactions to the injection of face and neck aesthetic filling materials: A systematic review. Med. Oral Patol. Oral Y Cir. Bucal 2022, 28, e278. [Google Scholar] [CrossRef]
- Schumacher, H.; Paul, C.; Hitchon, C.; El-Gabalawy, H.; Zonay, L.; Clayburne, G.; Sieck, M.; Schwab, E. Hyaluronate effects on synovium and synovial fluid. A prospective blinded study in patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2006, 14, 501–503. [Google Scholar] [CrossRef]
- Doddridge, J.R.; Banner, K.A.; Hunter, N.B.; Beckmann, N.M. Foreign body giant cell reaction due to Durolane (hyaluronic acid derivative) injection—A case report. Skelet. Radiol. 2025, 54, 1359–1364. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Muneta, T.; Ozeki, N.; Nakagawa, Y.; Udo, M.; Saito, R.; Koga, H.; Tsuji, K.; Sekiya, I. Weekly injections of Hylan GF 20 delay cartilage degeneration in partial meniscectomized rat knees. BMC Musculoskelet. Disord. 2016, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Esenyel, C.; Demirhan, M.; Esenyel, M.; Sonmez, M.; Kahraman, S.; Senel, B.; Ozdes, T. Comparison of four different intra-articular injection sites in the knee: A cadaver study. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 573–577. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.H.; Ki, M.; Kim, M.J.; Kang, S.; Lee, J.; Lee, J.-R.; Han, Y.-J.; Son, J.-S. The comparison of two different intraarticular injections using a sonographic anterolateral approach in patients with osteoarthritic knee. Korean J. Pain 2018, 31, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Haslock, I.; MacFarlane, D.; Speed, C. Intra-articular and soft tissue injections: A survey of current practice. Rheumatology 1995, 34, 449–452. [Google Scholar] [CrossRef]
- Jackson, D.W.; Evans, N.A.; Thomas, B.M. Accuracy of needle placement into the intra-articular space of the knee. J. Bone Jt. Surg. 2002, 84, 1522–1527. [Google Scholar] [CrossRef]
- Toda, Y.; Tsukimura, N. A comparison of intra-articular hyaluronan injection accuracy rates between three approaches based on radiographic severity of knee osteoarthritis. Osteoarthr. Cartil. 2008, 16, 980–985. [Google Scholar] [CrossRef]
- Telikicherla, M.; Kamath, S.U. Accuracy of needle placement into the intra-articular space of the knee in osteoarthritis patients for viscosupplementation. J. Clin. Diagn. Res. 2016, 10, RC15–RC17. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Santavirta, S.; Konttinen, Y.; Bergroth, V.; Eskola, A.; Tallroth, K.; Lindholm, T. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J. Bone Jt. Surg. 1990, 72, 252–258. [Google Scholar] [CrossRef]
- Willert, H.-G.; Bertram, H.; Buchhorn, G.H. Osteolysis in alloarthroplasty of the hip: The role of bone cement fragmentation. Clin. Orthop. Relat. Res. 1990, 258, 108–121. [Google Scholar] [CrossRef]
- Goodman, S.; Davidson, J.; Song, Y.; Martial, N.; Fornasier, V. Histomorphological reaction of bone to different concentrations of phagocytosable particles of high-density polyethylene and Ti-6Al-4V alloy in vivo. Biomaterials 1996, 17, 1943–1947. [Google Scholar] [CrossRef]
- Myers, S.; Flusser, D.; Brandt, K.; Heck, D. Prevalence of cartilage shards in synovium and their association with synovitis in patients with early and endstage osteoarthritis. J. Rheumatol. 1992, 19, 1247–1251. [Google Scholar]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Terkawi, M.A.; Onodera, T.; Homan, K.; Iwasaki, N. A novel cartilage fragments stimulation model revealed that macrophage inflammatory response causes an upregulation of catabolic factors of chondrocytes in vitro. Cartilage 2021, 12, 354–361. [Google Scholar] [CrossRef]
- Arenas, A.; Lopez-Blasco, J.J.; Fernandez, P. Intraosseous pseudotumor after knee viscosupplementation: A case report. JBJS Case Connect. 2015, 5, e110. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, Y.; Long, X.; Wang, X. Mandibular osteomyelitis after hyaluronic acid injection. J. Cosmet. Dermatol. 2021, 20, 457–459. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Martin, K.L. Handbook of Histology Methods for Bone and Cartilage; Springer: New York, NY, USA, 2003. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: London, UK, 2008. [Google Scholar]
- Jimenez-Palomar, I.; Shipov, A.; Shahar, R.; Barber, A.H. Influence of SEM vacuum on bone micromechanics using in situ AFM. J. Mech. Behav. Biomed. Mater. 2012, 5, 149–155. [Google Scholar] [CrossRef] [PubMed]

| Patients | Tissue Samples | e-HA-Induced Pathology |
|---|---|---|
| D., 73 y.o., female | Areolar synovial membrane | Synovitis |
| O., 58 y.o., male | Areolar synovial membrane and Hoffa fat pad | Synovitis and adipositis |
| S., 59 y.o., female | Areolar and fibrous synovial membrane and subchondral bone | Synovitis and osteomyelitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyalina, V.; Eshmotova, G.; Karavan, A.; Korshunov, A.; Zarov, A.; Bonartsev, A.; Serejnikova, N.; Prizov, A.; Airapetov, G.; Volkov, A. Hyaluronan-Related Granulomatous Synovitis, Adipositis, and Osteomyelitis in the Osteoarthritic Knee: A Morphological Case Series of Three Patients. Int. J. Mol. Sci. 2025, 26, 8073. https://doi.org/10.3390/ijms26168073
Lyalina V, Eshmotova G, Karavan A, Korshunov A, Zarov A, Bonartsev A, Serejnikova N, Prizov A, Airapetov G, Volkov A. Hyaluronan-Related Granulomatous Synovitis, Adipositis, and Osteomyelitis in the Osteoarthritic Knee: A Morphological Case Series of Three Patients. International Journal of Molecular Sciences. 2025; 26(16):8073. https://doi.org/10.3390/ijms26168073
Chicago/Turabian StyleLyalina, Vera, Gulnara Eshmotova, Alexandra Karavan, Andrey Korshunov, Alexey Zarov, Anton Bonartsev, Natalia Serejnikova, Alexey Prizov, George Airapetov, and Alexey Volkov. 2025. "Hyaluronan-Related Granulomatous Synovitis, Adipositis, and Osteomyelitis in the Osteoarthritic Knee: A Morphological Case Series of Three Patients" International Journal of Molecular Sciences 26, no. 16: 8073. https://doi.org/10.3390/ijms26168073
APA StyleLyalina, V., Eshmotova, G., Karavan, A., Korshunov, A., Zarov, A., Bonartsev, A., Serejnikova, N., Prizov, A., Airapetov, G., & Volkov, A. (2025). Hyaluronan-Related Granulomatous Synovitis, Adipositis, and Osteomyelitis in the Osteoarthritic Knee: A Morphological Case Series of Three Patients. International Journal of Molecular Sciences, 26(16), 8073. https://doi.org/10.3390/ijms26168073





