Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS
Abstract
1. Introduction
1.1. Unraveling ALS: A Race Against Time in Fast Progressors
1.2. Defining Fast Progression: Where Every Month Counts
1.3. Why Identifying Fast Progressors Is a Game-Changer
1.4. Bridging the Knowledge Gap: A Call for Aggressive Scientific Action
2. Key Characteristics of Fast Progressors in ALS
2.1. Rapid Symptom Onset and Accelerated Progression
2.2. Shorter Survival Time
2.3. Early Bulbar or Respiratory Involvement
2.4. Genetic and Molecular Factors Driving Fast Progression
2.5. Younger Age at Onset in Certain Cases
2.6. Environmental and Lifestyle Factors Contributing to Rapid Progression
2.7. Poor Response to Standard ALS Treatments
3. Biomarkers Associated with Fast ALS Progression
3.1. Blood and CSF Biomarkers: Indicators of Axonal Damage, Proteinopathy, and Systemic Stress
3.1.1. Axonal Damage: NfL and pNfH as Gold-Standard Biomarkers
3.1.2. Neuroinflammation and Astrocytic Activation: GFAP, IL-6, and Peripheral Cytokines
3.1.3. Oxidative Stress and Metabolic Dysfunction: 8-OHdG, 4-HNE, and Emerging Metabolomic Biomarkers
3.1.4. Biomarkers of Autophagy and Lysosomal Dysfunction
3.1.5. AI-Powered Multibiomarker Integration and Liquid Biopsies
3.2. Neuroimaging Biomarkers: Structural, Functional, and Molecular Imaging in Fast ALS Progressors
3.2.1. Structural Integrity and White Matter Damage: DTI
3.2.2. Cortical Atrophy and Microstructural Changes: Structural MRI and Ultra-High-Field 7T MRI
3.2.3. Functional Disruption of Motor Networks: fMRI, MEG, and Motor Network Connectivity
3.2.4. Neuroinflammation and Microglial Activation: TSPO-PET Imaging
3.2.5. Emerging Imaging Techniques: Magnetic Resonance Spectroscopy, Susceptibility-Weighted Imaging, and Optical Coherence Tomography (OCT)
3.2.6. AI-Driven Imaging Analysis: Personalized Predictions and Biomarker Integration
3.3. Electrophysiological Biomarkers: Dynamic Markers of Motor Neuron Function and Neuromuscular Integrity
3.3.1. Motor Unit Number Estimation: A Key Biomarker of Motor Neuron Reserve and Disease Progression
3.3.2. Compound Muscle Action Potential: Tracking Functional Motor Unit Output and Neuromuscular Transmission
3.3.3. High-Density Surface Electromyography: Mapping Motor Unit Firing Patterns and Neuromuscular Instability
3.3.4. Single-Fiber Electromyography (SF-EMG) and Neuromuscular Jitter: Early Markers of Synaptic Failure
3.3.5. Peripheral Nerve Excitability Tests: Monitoring Axonal Membrane Function and Ion Channel Activity
3.3.6. Emerging Techniques: Microelectrode Arrays and Neuromuscular Ultrasound
3.3.7. AI-Powered Electrophysiological Analysis and Multimodal Integration
4. Genetic and Molecular Factors Driving Fast ALS Progression
4.1. SOD1 Variants: Oxidative Stress, Protein Misfolding, and Mitochondrial Dysfunction in Fast ALS Progressors
4.1.1. Toxic Gain-of-Function Mechanisms in SOD1 Variants: Protein Misfolding and Aggregation
4.1.2. Oxidative Stress and Reactive Oxygen Species Accumulation
4.1.3. RNA-Binding Protein Mislocalization and Splicing Errors
4.1.4. Gene–Environment Interactions: Amplifying Motor Neuron Vulnerability
4.1.5. Liquid Biopsies and Circulating SOD1 Aggregates for Therapeutic Monitoring
4.2. C9orf72 Expansions: Toxic RNA, Dipeptide Repeat Proteins, and Nucleocytoplasmic Transport Dysfunction
4.2.1. RNA Toxicity: Formation of RNA Foci and Sequestration of RNA-Binding Proteins
4.2.2. Dipeptide Repeat Proteins: Aggregation, Stress Granule Disruption, and Cellular Toxicity
4.2.3. Nucleocytoplasmic Transport Dysfunction: Disruption of Nuclear Pore Complex (NPC) Integrity
4.2.4. Loss of C9orf72 Function: Autophagy Failure and Immune Dysregulation
4.2.5. Gene–Environment Interactions: Accelerating Neurodegeneration in C9orf72 Expansion Carriers
4.2.6. Liquid Biopsies and Circulating Biomarkers: Non-Invasive Monitoring of Disease Progression
4.2.7. Therapeutic Approaches: Targeting Multiple Mechanisms for Maximum Efficacy
- Target sense and antisense G4C2 transcription with ASOs to reduce RNA foci and toxic DPRs following transcription [165].
- DPR-targeting antibodies or small molecules to inhibit RAN translation or neutralize toxic DPRs [166].
- Chaperone-based therapy or small molecules to facilitate restoration of nucleocytoplasmic transport associated with stabilization of the NPC [167].
- Autophagy inducers and lysosomal stabilizers to enhance clearance of cytotoxic protein aggregates and damaged organelles [168].
4.3. FUS Variants: Early-Onset ALS, RNA Dysregulation, and Proteinopathy in Fast Progressors
4.3.1. Cytoplasmic Mislocalization, Aggregation, and Toxic Gain-of-Function Mechanisms
4.3.2. Posttranslational Modifications: Regulators of FUS Aggregation and Toxicity
4.3.3. RNA Dysregulation and Missplicing Events in FUS-Related ALS
4.3.4. Defective DNA Damage Response and Genome Instability
4.3.5. Stress Granule Dysregulation and Persistent Aggregation
4.3.6. Cross-Talk Between FUS, TDP-43, and C9orf72 Pathology
4.3.7. Emerging Biomarkers and Therapeutic Approaches for FUS-Related ALS
4.4. TARDBP Variants: TDP-43 Proteinopathy, RNA Dysregulation, and Neurodegeneration in Fast ALS Progressors
4.4.1. Proteins Shifting into the Cytoplasm, Clumping Together, and Acquiring Harmful New Functions
4.4.2. Loss of Nuclear TDP-43 Function: RNA Dysregulation and Splicing Defects
4.4.3. Posttranslational Modifications and the Amplification of TDP-43 Toxicity
4.4.4. Mitochondrial Dysfunction and Oxidative Stress
4.4.5. Cross-Talk with Other ALS-Related Proteins (FUS and C9orf72 DPRs)
4.4.6. Emerging Biomarkers and Therapeutic Approaches
4.5. ATXN2 Expansions: Intermediate PolyQ Expansions and Increased ALS Risk
4.5.1. Intermediate PolyQ Expansions: A Genetic Modifier of ALS Risk
4.5.2. Phase Separation and Aberrant Liquid-to-Solid Transitions in Stress Granules
4.5.3. Cross-Talk Between ATXN2 Expansions and TDP-43 Proteinopathy
4.5.4. Non-Canonical Roles of Ataxin-2: Cytoskeletal Regulation, Calcium Homeostasis, and Mitochondrial RNA Stability
4.5.5. Autophagy Failure and Impaired Proteostasis
4.5.6. Emerging Biomarkers and Therapeutic Strategies
5. Potential Therapeutic Approaches for Fast Progressors in ALS
5.1. FDA-Approved and Off-Label Drug Treatments: Foundation of Neuroprotection
5.1.1. Emerging Combination Therapies and Synergistic Effects
5.1.2. Biomarker-Guided Personalized Treatment Regimens
5.1.3. Experimental and Off-Label Drug Applications: Metformin, Tamoxifen, and Ceftriaxone
5.2. Gene Therapy and RNA-Based Approaches: Precision Treatments for Targeted ALS Subtypes
5.2.1. Tofersen (SOD1-ALS Antisense Therapy): A Groundbreaking Milestone in RNA-Based ALS Treatment
5.2.2. C9orf72-Targeted Therapies: ASOs and CRISPR-Based Approaches to Eliminate Toxic RNA and DPRs
5.2.3. FUS-ALS Gene Therapy: AAV9-Based Trials for Rapidly Progressing ALS
- AAV9 delivery of ASOs or siRNAs to silence mutant FUS and reduce cytoplasmic aggregate burden [273].
- AAV9 delivery of CRISPR-Cas9 for permanent correction of FUS variants, especially gain-of-function variants [274].
- AAV9 delivery of neuroprotective factors (e.g., VEGF and GDNF) to protect motor neurons from cell death while tackling FUS toxicity [275].
5.2.4. Innovations in Delivery Systems: AAV Engineering, Lipid Nanoparticles, and Immune Evasion
5.2.5. Biomarker-Guided Optimization: Personalizing Gene Therapy for Fast Progressors
5.3. Stem Cell and Regenerative Therapies: Restoring and Protecting Motor Neurons in Fast Progressors
5.3.1. Mechanisms of Action: Beyond Neurotrophic Support
5.3.2. NurOwn (Mesenchymal Stem Cells—MSCs): Secreting Neurotrophic and Anti-Inflammatory Factors
5.3.3. Neural Stem Cell Therapy: Replacing Lost Motor Neurons and Rebuilding Neural Circuits
5.3.4. Exosome-Based Stem Cell Therapies: A Cell-Free Alternative
5.3.5. Biomaterials and Scaffolds to Improve Stem Cell Integration
5.3.6. Biomarker-Guided Monitoring of Stem Cell Therapies
5.4. Neuroprotective and Anti-Inflammatory Therapies: Targeting Cellular Stress and Chronic Inflammation in Fast ALS Progressors
5.4.1. IL-6 and TNF-α Blockers: Suppressing Cytokine Cascades and Protecting Motor Neurons
5.4.2. Rapamycin and mTOR Modulators: Enhancing Autophagy and Mitigating Proteotoxic Stress
5.4.3. Arimoclomol: Heat Shock Protein Modulation to Stabilize Misfolded Proteins
5.4.4. Targeting ER Stress and the Unfolded Protein Response
5.4.5. Biomarker-Guided Optimization of Anti-Inflammatory and Neuroprotective Therapies
5.5. Metabolic and Nutritional Strategies: Enhancing Energy Balance and Mitochondrial Function in Fast ALS Progressors
5.5.1. High-Calorie, High-Fat Diet: Countering Hypermetabolism and Extending Survival
5.5.2. The Ketogenic Diet and Exogenous Ketone Supplements: Neuroprotection Through Alternative Energy Sources
5.5.3. The Gut–Brain Axis: A Novel Metabolic Target in ALS Therapy
5.5.4. Brown Adipose Tissue (BAT) Activation: A Novel Energy-Regulating Mechanism
5.5.5. Biomarker-Guided Personalized Metabolic Interventions
- Monitoring REE and body composition helps recalibrate caloric intake for weight loss and maintaining energy and avoiding muscle loss and negative energy status [327].
- Plasma ketones evaluate the effectiveness a ketogenic diet or supplemental exogenous ketone in hypermetabolic patients with low energy status and maintenance of energy balance [322].
- Mitochondrial biomarkers (i.e., ATP production rate or levels of reactive oxygen species, mitochondrial membrane potential) can help assess the impact of the amended mitochondrial targeted therapy [328].
- Gut microbiome profiling can guide our choice in prebiotic and probiotic supplements after identifying the microbial imbalances contributing to metabolic deficits which may impact ALS and mitochondrial function [329].
5.6. Symptom Management for Fast Progressors: Optimizing Quality of Life and Functional Independence
5.6.1. Respiratory Support: Expanding Options Beyond Non-Invasive Ventilation
5.6.2. Nutritional Support: Gastrostomy Tube Placement and Biomarker-Guided Nutrition
5.6.3. Spasticity and Cramps: Combining Pharmacological and Non-Pharmacological Interventions
5.6.4. Sialorrhea (Drooling): Advanced Pharmacological and Minimally Invasive Interventions
5.6.5. Assistive Communication: Eye-Tracking Systems and Brain–Computer Interfaces (BCIs)
5.6.6. Emerging Innovations: Personalized and Adaptive Care
6. Emerging and Experimental Therapies in Clinical Trials: Pioneering Advances to Combat Fast ALS Progression
6.1. CNM-Au8: A Bioenergetic Catalyst for Restoring Mitochondrial Function and Cellular Energy
6.2. Verdiperstat: Targeting Microglial Overactivation and Neuroinflammation
6.3. Pridopidine: Sigma-1 Receptor (S1R) Modulation for Neuroprotection and Motor Function Recovery
6.4. Wave ASOs: RNA-Based Therapeutics Targeting C9orf72-Related ALS
6.5. ALS iPSC-Based Therapy: Personalized Stem Cell Replacement and Genetic Correction
7. Clinical Trials for Fast Progressors: Precision Research in the Era of Personalized ALS Therapies
7.1. TRICALS: A European Powerhouse for ALS Clinical Trials and Innovation
7.2. European Union Clinical Trials Register: Comprehensive Access to ALS-Specific Trials
7.3. ALS Therapy Development Institute (ALS TDI) and the ALS Trial Navigator
7.4. Global Trials: Asia-Pacific and Australia’s Contributions to ALS Research
7.5. Tofersen for Presymptomatic SOD1 ALS (ATLAS Trial): Pre-Emptive Neuroprotection
7.6. AI-Powered Adaptive Trial Designs: Revolutionizing ALS Research
8. Conclusions: A Paradigm Shift in the Treatment of Fast ALS Progressors
8.1. Summary of Key Findings: Addressing the Complex Pathophysiology of Fast Progressors
8.2. The Critical Role of Early Identification and Biomarker-Driven Classification
8.3. Future Research Directions: The Road Ahead in Gene Therapy, Neuroprotection, and Clinical Innovation
8.4. A Call for Personalized Medicine and Global Collaboration
8.5. The Urgency to Act: A Window We Cannot Afford to Miss
8.6. Final Words: Transforming ALS from Fatal to Treatable
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucchi, C.; Simonini, C.; Rustichelli, C.; Avallone, R.; Zucchi, E.; Martinelli, I.; Biagini, G.; Mandrioli, J. Reduced Levels of Neurosteroids in Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Biomolecules 2024, 14, 1076. [Google Scholar] [CrossRef]
- Ortiz-Corredor, F.; Correa-Arrieta, C.; Forero Diaz, J.J.; Castellar-Leones, S.; Gil-Salcedo, A. Profiles of disease progression and predictors of mortality in Colombian patients with amyotrophic lateral sclerosis: A comprehensive longitudinal study. Amyotroph. Lateral Scler. Front. Degener. 2025, 26, 141–148. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Benetton, C.; Brunet, A.; Ye, X.; Logunov, E.; Douchamps, V.; Megat, S.; Andry, V.; Kan, V.W.Y.; Stuart-Lopez, G.; et al. Cortical hyperexcitability in mouse models and patients with amyotrophic lateral sclerosis is linked to noradrenaline deficiency. Sci. Transl. Med. 2024, 16, eadg3665. [Google Scholar] [CrossRef]
- Ohnari, K.; Mafune, K.; Adachi, H. Fasciculation potentials are related to the prognosis of amyotrophic lateral sclerosis. PLoS ONE 2024, 19, e0313307. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, J.; Dutta, R.; Trapp, B.; Pieper, A.A.; Cheng, F. Network medicine informed multiomics integration identifies drug targets and repurposable medicines for Amyotrophic Lateral Sclerosis. Npj Syst. Biol. Appl. 2024, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; Canna, A.; Sharbafshaaer, M.; di Nardo, F.; Canale, F.; Passaniti, C.; Pirozzi, M.A.; Silvestro, M.; Orologio, I.; Russo, A.; et al. Brain neurovascular coupling in amyotrophic lateral sclerosis: Correlations with disease progression and cognitive impairment. Eur. J. Neurol. 2025, 32, e16540. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Hamatani, T.; Atsuta, N.; Sano, F.; Nakamura, R.; Hayashi, Y.; Sobue, G. ALSFRS-R decline rate prior to baseline is not useful for stratifying subsequent progression of functional decline. Amyotroph. Lateral Scler. Front. Degener. 2024, 25, 388–399. [Google Scholar] [CrossRef]
- Shen, T.; Vogel, J.W.; Duda, J.; Phillips, J.S.; Cook, P.A.; Gee, J.; Elman, L.; Quinn, C.; Amado, D.A.; Baer, M.; et al. Novel data-driven subtypes and stages of brain atrophy in the ALS–FTD spectrum. Transl. Neurodegener. 2023, 12, 57. [Google Scholar] [CrossRef]
- Psychogios, I.; Hu, Y.; Seitz, C.; Joyce, E.E.; Lovik, A.; Ingre, C.; Fang, F. Exploring clinical chemistry markers in amyotrophic lateral sclerosis: Insights into survival and disease trajectories. J. Neurol. 2025, 272, 7. [Google Scholar] [CrossRef]
- Liu, C.-B.; Yang, D.-G.; Li, J.; Qin, C.; Zhang, X.; Liu, J.; Li, D.-P.; Li, J.-J. Diffusion tensor imaging reveals brain structure changes in dogs after spinal cord injury. Neural Regen. Res. 2023, 18, 176. [Google Scholar] [CrossRef]
- van Unnik, J.W.J.; Meyjes, M.; Janse van Mantgem, M.R.; van den Berg, L.H.; van Eijk, R.P.A. Remote monitoring of amyotrophic lateral sclerosis using wearable sensors detects differences in disease progression and survival: A prospective cohort study. eBioMedicine 2024, 103, 105104. [Google Scholar] [CrossRef]
- Yilmaz, R.; Grehl, T.; Eckrich, L.; Marschalkowski, I.; Weishaupt, K.; Valkadinov, I.; Simic, M.; Brenner, D.; Andersen, P.M.; Wolf, J.; et al. Frequency of C9orf72 and SOD1 mutations in 302 sporadic ALS patients from three German ALS centers. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 414–419. [Google Scholar] [CrossRef]
- Ziff, O.J.; Neeves, J.; Mitchell, J.; Tyzack, G.; Martinez-Ruiz, C.; Luisier, R.; Chakrabarti, A.M.; McGranahan, N.; Litchfield, K.; Boulton, S.J.; et al. Integrated transcriptome landscape of ALS identifies genome instability linked to TDP-43 pathology. Nat. Commun. 2023, 14, 2176. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, M.; Akrani, I.; Tsoka, A.; Myrianthopoulos, V.; Mikros, E.; Vorgias, C.; Hatzinikolaou, D.G. Discovery of Novel Inhibitors against ALS-Related SOD1(A4V) Aggregation through the Screening of a Chemical Library Using Differential Scanning Fluorimetry (DSF). Pharmaceuticals 2024, 17, 1286. [Google Scholar] [CrossRef] [PubMed]
- Pioro, E.P.; Brooks, B.R.; Liu, Y.; Zhang, J.; Apple, S. Efficacy of Radicava® IV (intravenous edaravone) in subjects with differing trajectories of disease progression in amyotrophic lateral sclerosis: Use of a novel statistical approach for post hoc analysis of a pivotal phase 3 clinical trial. J. Neurol. Sci. 2024, 467, 123290. [Google Scholar] [CrossRef]
- Wiesenfarth, M.; Dorst, J.; Brenner, D.; Elmas, Z.; Parlak, Ö.; Uzelac, Z.; Kandler, K.; Mayer, K.; Weiland, U.; Herrmann, C.; et al. Effects of tofersen treatment in patients with SOD1-ALS in a “real-world” setting—A 12-month multicenter cohort study from the German early access program. eClinicalMedicine 2024, 69, 102495. [Google Scholar] [CrossRef]
- Din Abdul Jabbar, M.A.; Guo, L.; Nag, S.; Guo, Y.; Simmons, Z.; Pioro, E.P.; Ramasamy, S.; Yeo, C.J.J. Predicting amyotrophic lateral sclerosis (ALS) progression with machine learning. Amyotroph. Lateral Scler. Front. Degener. 2024, 25, 242–255. [Google Scholar] [CrossRef]
- Weiss, A.; Gilbert, J.W.; Flores, I.V.R.; Belgrad, J.; Ferguson, C.; Dogan, E.O.; Wightman, N.; Mocarski, K.; Echeverria, D.; Summers, A.; et al. RNAi-mediated silencing of SOD1 profoundly extends survival and functional outcomes in ALS mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Meijboom, K.E.; Abdallah, A.; Fordham, N.P.; Nagase, H.; Rodriguez, T.; Kraus, C.; Gendron, T.F.; Krishnan, G.; Esanov, R.; Andrade, N.S.; et al. CRISPR/Cas9-mediated excision of ALS/FTD-causing hexanucleotide repeat expansion in C9ORF72 rescues major disease mechanisms in vivo and in vitro. Nat. Commun. 2022, 13, 6286. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Deng, M. New developments and opportunities in drugs being trialed for amyotrophic lateral sclerosis from 2020 to 2022. Front. Pharmacol. 2022, 13, 1054006. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Turano, E.; Scambi, I.; Virla, F.; Nodari, A.; Pezzini, F.; Galiè, M.; Bonetti, B.; Mariotti, R. A Cellular Model of Amyotrophic Lateral Sclerosis to Study the Therapeutic Effects of Extracellular Vesicles from Adipose Mesenchymal Stem Cells on Microglial Activation. Int. J. Mol. Sci. 2024, 25, 5707. [Google Scholar] [CrossRef] [PubMed]
- Amylyx Pharmaceuticals Inc. AMX0035 for Amyotrophic Lateral Sclerosis—Compassionate Use Protocol, clinicaltri-als.gov, Clinical Trial Registration NCT04516096, April 2023. Available online: https://clinicaltrials.gov/study/NCT04516096 (accessed on 8 February 2025).
- Oiwa, K.; Watanabe, S.; Onodera, K.; Iguchi, Y.; Kinoshita, Y.; Komine, O.; Sobue, A.; Okada, Y.; Katsuno, M.; Yamanaka, K. Monomerization of TDP-43 is a key determinant for inducing TDP-43 pathology in amyotrophic lateral sclerosis. Sci. Adv. 2023, 9, eadf6895. [Google Scholar] [CrossRef]
- François-Moutal, L.; Scott, D.D.; Ambrose, A.J.; Zerio, C.J.; Rodriguez-Sanchez, M.; Dissanayake, K.; May, D.G.; Carlson, J.M.; Barbieri, E.; Moutal, A.; et al. Heat shock protein Grp78/BiP/HspA5 binds directly to TDP-43 and mitigates toxicity associated with disease pathology. Sci. Rep. 2022, 12, 8140. [Google Scholar] [CrossRef]
- McFarlane, R.; Galvin, M.; Heverin, M.; Mac Domhnaill, É.; Murray, D.; Meldrum, D.; Bede, P.; Bolger, A.; Hederman, L.; Impey, S.; et al. PRECISION ALS—An integrated pan European patient data platform for ALS. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 389–393. [Google Scholar] [CrossRef]
- Parnianpour, P.; Steinbach, R.; Buchholz, I.J.; Grosskreutz, J.; Kalra, S. T1-weighted MRI texture analysis in amyotrophic lateral sclerosis patients stratified by the D50 progression model. Brain Commun. 2024, 6, fcae389. [Google Scholar] [CrossRef]
- Qin, J.; Wang, X.; Fan, G.; Zhang, W.; Wu, X.; Wang, B.; Liu, Y. Identifying amyotrophic lateral sclerosis using diffusion tensor imaging, and correlation with neurofilament markers. Sci. Rep. 2024, 14, 28110. [Google Scholar] [CrossRef]
- Acien, A.; Calcagno, N.; Burke, K.M.; Mondesire-Crump, I.; Holmes, A.A.; Mruthik, S.; Goldy, B.; Syrotenko, J.E.; Scheier, Z.; Iyer, A.; et al. A novel digital tool for detection and monitoring of amyotrophic lateral sclerosis motor impairment and progression via keystroke dynamics. Sci. Rep. 2024, 14, 16851. [Google Scholar] [CrossRef]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Masullo, M.; Quadalti, C.; Avoni, P.; Polischi, B.; Cherici, A.; Capellari, S.; Salvi, F.; et al. Plasma and CSF Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: A Cross-Sectional and Longitudinal Study. Front. Aging Neurosci. 2021, 13, 753242. [Google Scholar] [CrossRef]
- Thompson, A.G.; Marsden, R.; Talbot, K.; Turner, M.R. Primary care blood tests show lipid profile changes in pre-symptomatic amyotrophic lateral sclerosis. Brain Commun. 2023, 5, fcad211. [Google Scholar] [CrossRef]
- Zimyanin, V.L.; Pielka, A.-M.; Glaß, H.; Japtok, J.; Großmann, D.; Martin, M.; Deussen, A.; Szewczyk, B.; Deppmann, C.; Zunder, E.; et al. Live Cell Imaging of ATP Levels Reveals Metabolic Compartmentalization within Motoneurons and Early Metabolic Changes in FUS ALS Motoneurons. Cells 2023, 12, 1352. [Google Scholar] [CrossRef]
- Femiano, C.; Bruno, A.; Gilio, L.; Buttari, F.; Dolcetti, E.; Galifi, G.; Azzolini, F.; Borrelli, A.; Furlan, R.; Finardi, A.; et al. Inflammatory signature in amyotrophic lateral sclerosis predicting disease progression. Sci. Rep. 2024, 14, 19796. [Google Scholar] [CrossRef]
- Correia, J.P.; Gromicho, M.; Pronto-Laborinho, A.C.; Oliveira Santos, M.; de Carvalho, M. Creatine Kinase and Respiratory Decline in Amyotrophic Lateral Sclerosis. Brain Sci. 2024, 14, 661. [Google Scholar] [CrossRef]
- Barbato, F.; Bombaci, A.; Colacicco, G.; Bruno, G.; Ippolito, D.; Pota, V.; Dongiovanni, S.; Sica, G.; Bocchini, G.; Valente, T.; et al. Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study. J. Clin. Med. 2024, 13, 3103. [Google Scholar] [CrossRef]
- Tsoneva, E.; Dimitrova, P.; Metodiev, M.; Shivarov, V.; Vasileva-Slaveva, M.; Yordanov, A.; Kostov, S. Utility of expression of 4-hydroxynonenal tested by immunohistochemistry for cervical cancer. Prz. Menopauzalny 2024, 23, 6–13. [Google Scholar] [CrossRef]
- Çeli K, H.E.A.; Tuna, G.; Ceylan, D.; Küçükgöncü, S. A comparative meta-analysis of peripheral 8-hydroxy-2′-deoxyguanosine (8-OHdG) or 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) levels across mood episodes in bipolar disorder. Psychoneuroendocrinology 2023, 151, 106078. [Google Scholar] [CrossRef]
- Günther, R.; Pal, A.; Williams, C.; Zimyanin, V.L.; Liehr, M.; von Neubeck, C.; Krause, M.; Parab, M.G.; Petri, S.; Kalmbach, N.; et al. Alteration of Mitochondrial Integrity as Upstream Event in the Pathophysiology of SOD1-ALS. Cells 2022, 11, 1246. [Google Scholar] [CrossRef]
- Sarmet, M.; Santos, D.B.; Mangilli, L.D.; Million, J.L.; Maldaner, V.; Zeredo, J.L. Chronic respiratory failure negatively affects speech function in patients with bulbar and spinal onset amyotrophic lateral sclerosis: Retrospective data from a tertiary referral center. Logoped. Phoniatr. Vocol. 2024, 49, 17–26. [Google Scholar] [CrossRef]
- Apreleva Kolomeytseva, A.T.; Brylev, L.; Eshghi, M.; Bottaeva, Z.; Zhang, J.; Fachner, J.C.; Street, A.J. Home-Based Music Therapy to Support Bulbar and Respiratory Functions of Persons with Early and Mid-Stage Amyotrophic Lateral Sclerosis—Protocol and Results from a Feasibility Study. Brain Sci. 2022, 12, 494. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, H.; Cai, L.; Ye, Q.; Wang, H.; Liu, B.; Zhang, W.; Li, J. Inflammatory Signaling Induces Mitochondrial Dysfunction and Neuronal Death in Traumatic Brain Injury via Downregulation of OXPHOS Genes. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Fu, C.; Wang, L.; Cai, W. IL6 receptor inhibitors: Exploring the therapeutic potential across multiple diseases through drug target Mendelian randomization. Front. Immunol. 2024, 15, 1452849. [Google Scholar] [CrossRef]
- Babu, M.; Favretto, F.; de Opakua, A.I.; Rankovic, M.; Becker, S.; Zweckstetter, M. Proline/arginine dipeptide repeat polymers derail protein folding in amyotrophic lateral sclerosis. Nat. Commun. 2021, 12, 3396. [Google Scholar] [CrossRef]
- Jafarinia, H.; van der Giessen, E.; Onck, P.R. C9orf72 polyPR directly binds to various nuclear transport components. Elife 2023, 12, RP89694. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Cai, Y.; Wen, H.; Wang, L.; Zhu, M.; Chen, Y.; Yu, Y.; Lu, X.; Zhou, M.; et al. FUS P525L mutation causing amyotrophic lateral sclerosis and movement disorders. Brain Behav. 2020, 10, e01625. [Google Scholar] [CrossRef]
- Alirzayeva, H.; Loureiro, R.; Koyuncu, S.; Hommen, F.; Nabawi, Y.; Zhang, W.H.; Dao, T.T.P.; Wehrmann, M.; Lee, H.J.; Vilchez, D. ALS-FUS mutations cause abnormal PARylation and histone H1.2 interaction, leading to pathological changes. Cell Rep. 2024, 43, 114626. [Google Scholar] [CrossRef]
- Wen, J.; Ou, S.-J.; Liu, J.-B.; Zeng, W.; Yang, R.; Qu, Y.-D.; Li, J.-X.; Xia, C.-L.; Yang, Y.; Zhang, W.; et al. Single-cell RNA sequencing reveals the role of immune-related autophagy in aseptic loosening of biomaterials bone-implant. Biomater. Adv. 2025, 169, 214190. [Google Scholar] [CrossRef]
- Tzeplaeff, L.; Jürs, A.V.; Wohnrade, C.; Demleitner, A.F. Unraveling the Heterogeneity of ALS—A Call to Redefine Patient Stratification for Better Outcomes in Clinical Trials. Cells 2024, 13, 452. [Google Scholar] [CrossRef]
- Reed, K.K.; Silverman, A.E.; Abbaspour, A.; Burger, K.S.; Bulik, C.M.; Carroll, I.M. Energy expenditure during nutritional rehabilitation: A scoping review to investigate hypermetabolism in individuals with anorexia nervosa. J. Eat. Disord. 2024, 12, 63. [Google Scholar] [CrossRef]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Alshehri, R.S.; Abuzinadah, A.R.; Alrawaili, M.S.; Alotaibi, M.K.; Alsufyani, H.A.; Alshanketi, R.M.; AlShareef, A.A. A Review of Biomarkers of Amyotrophic Lateral Sclerosis: A Pathophysiologic Approach. Int. J. Mol. Sci. 2024, 25, 10900. [Google Scholar] [CrossRef]
- Benatar, M.; Macklin, E.A.; Malaspina, A.; Rogers, M.-L.; Hornstein, E.; Lombardi, V.; Renfrey, D.; Shepheard, S.; Magen, I.; Cohen, Y.; et al. Prognostic clinical and biological markers for amyotrophic lateral sclerosis disease progression: Validation and implications for clinical trial design and analysis. eBioMedicine 2024, 108, 105323. [Google Scholar] [CrossRef]
- Huber, R.G.; Pandey, S.; Chhangani, D.; Rincon-Limas, D.E.; Staff, N.P.; Yeo, C.J.J. Identification of potential pathways and biomarkers linked to progression in ALS. Ann. Clin. Transl. Neurol. 2022, 10, 150–165. [Google Scholar] [CrossRef]
- Gagliardi, D.; Rizzuti, M.; Masrori, P.; Saccomanno, D.; Del Bo, R.; Sali, L.; Meneri, M.; Scarcella, S.; Milone, I.; Hersmus, N.; et al. Exploiting the role of CSF NfL, CHIT1, and miR-181b as potential diagnostic and prognostic biomarkers for ALS. J. Neurol. 2024, 271, 7557–7571. [Google Scholar] [CrossRef]
- Banack, S.A.; Dunlop, R.A.; Mehta, P.; Mitsumoto, H.; Wood, S.P.; Han, M.; Cox, P.A. A microRNA diagnostic biomarker for amyotrophic lateral sclerosis. Brain Commun. 2024, 6, fcae268. [Google Scholar] [CrossRef]
- Meyer, T.; Schumann, P.; Weydt, P.; Petri, S.; Koc, Y.; Spittel, S.; Bernsen, S.; Günther, R.; Weishaupt, J.H.; Dreger, M.; et al. Neurofilament light-chain response during therapy with antisense oligonucleotide tofersen in SOD1-related ALS: Treatment experience in clinical practice. Muscle Nerve 2023, 67, 515–521. [Google Scholar] [CrossRef]
- Brkušanin, M.; Kosać, A.; Branković-Srećković, V.; Jovanović, K.; Perić, S.; Karanović, J.; Matijašević Joković, S.; Garai, N.; Pešović, J.; Nikolić, D.; et al. Phosphorylated neurofilament heavy chain in cerebrospinal fluid and plasma as a Nusinersen treatment response marker in childhood-onset SMA individuals from Serbia. Front. Neurol. 2024, 15, 1394001. [Google Scholar] [CrossRef]
- Orlova, A.; Malygin, Y.; Gofman, A.; Sotulenko, S.; Gandalian, V.; Kartashov, I.; Brylev, L.; Bolevich, S.; Nikolic Turnic, T.; Jakovljevic, V. Survival Prognostic Factors of Non-Invasive Ventilation in Amyotrophic Lateral Sclerosis: A Systematic Review. Life 2024, 14, 1664. [Google Scholar] [CrossRef]
- Paez-Colasante, X.; Figueroa-Romero, C.; Rumora, A.E.; Hur, J.; Mendelson, F.E.; Hayes, J.M.; Backus, C.; Taubman, G.F.; Heinicke, L.; Walter, N.G.; et al. Cytoplasmic TDP43 Binds microRNAs: New Disease Targets in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2020, 14, 117. [Google Scholar] [CrossRef]
- Baxi, E.G.; Thompson, T.; Li, J.; Kaye, J.A.; Lim, R.G.; Wu, J.; Ramamoorthy, D.; Lima, L.; Vaibhav, V.; Matlock, A.; et al. Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat. Neurosci. 2022, 25, 226–237. [Google Scholar] [CrossRef]
- Jiang, Q.; Guo, Y.; Yang, T.; Li, S.; Hou, Y.; Lin, J.; Xiao, Y.; Ou, R.; Wei, Q.; Shang, H. Cystatin C is associated with poor survival in amyotrophic lateral sclerosis patients. Front. Neurosci. 2024, 17, 1309568. [Google Scholar] [CrossRef]
- Lee, A.; Henderson, R.; Arachchige, B.J.; Robertson, T.; McCombe, P.A. Proteomic investigation of ALS motor cortex identifies known and novel pathogenetic mechanisms. J. Neurol. Sci. 2023, 452, 120753. [Google Scholar] [CrossRef]
- Lin, J.; Ou, R.; Li, C.; Hou, Y.; Zhang, L.; Wei, Q.; Pang, D.; Liu, K.; Jiang, Q.; Yang, T.; et al. Plasma glial fibrillary acidic protein as a biomarker of disease progression in Parkinson’s disease: A prospective cohort study. BMC Med. 2023, 21, 420. [Google Scholar] [CrossRef]
- Brodovitch, A.; Boucraut, J.; Delmont, E.; Parlanti, A.; Grapperon, A.-M.; Attarian, S.; Verschueren, A. Combination of serum and CSF neurofilament-light and neuroinflammatory biomarkers to evaluate ALS. Sci. Rep. 2021, 11, 703. [Google Scholar] [CrossRef]
- Poutoglidou, F.; Pourzitaki, C.; Manthou, M.E.; Saitis, A.; Malliou, F.; Kouvelas, D. Infliximab and tocilizumab reduce anxiety-like behavior, improve cognitive performance and reverse neuropathological alterations in juvenile rats with severe autoimmune arthritis. Int. Immunopharmacol. 2021, 99, 107917. [Google Scholar] [CrossRef]
- Hosaka, T.; Tsuji, H.; Tamaoka, A. Biomolecular Modifications Linked to Oxidative Stress in Amyotrophic Lateral Sclerosis: Determining Promising Biomarkers Related to Oxidative Stress. Processes 2021, 9, 1667. [Google Scholar] [CrossRef]
- Parvanovova, P.; Evinova, A.; Grofik, M.; Hnilicova, P.; Tatarkova, Z.; Turcanova-Koprusakova, M. Mitochondrial Dysfunction in Sporadic Amyotrophic Lateral Sclerosis Patients: Insights from High-Resolution Respirometry. Biomedicines 2024, 12, 1294. [Google Scholar] [CrossRef]
- Cieminski, K.; Flis, D.J.; Dzik, K.P.; Kaczor, J.J.; Wieckowski, M.R.; Antosiewicz, J.; Ziolkowski, W. Swim Training Affects on Muscle Lactate Metabolism, Nicotinamide Adenine Dinucleotides Concentration, and the Activity of NADH Shuttle Enzymes in a Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 11504. [Google Scholar] [CrossRef]
- Beckers, J.; Tharkeshwar, A.K.; Fumagalli, L.; Contardo, M.; Van Schoor, E.; Fazal, R.; Thal, D.R.; Chandran, S.; Mancuso, R.; Van Den Bosch, L.; et al. A toxic gain-of-function mechanism in C9orf72 ALS impairs the autophagy-lysosome pathway in neurons. Acta Neuropathol. Commun. 2023, 11, 151. [Google Scholar] [CrossRef]
- Trist, B.G.; Fifita, J.A.; Hogan, A.; Grima, N.; Smith, B.; Troakes, C.; Vance, C.; Shaw, C.; Al-Sarraj, S.; Blair, I.P.; et al. Co-deposition of SOD1, TDP-43 and p62 proteinopathies in ALS: Evidence for multifaceted pathways underlying neurodegeneration. Acta Neuropathol. Commun. 2022, 10, 122. [Google Scholar] [CrossRef]
- Lachance, V.; Wang, Q.; Sweet, E.; Choi, I.; Cai, C.-Z.; Zhuang, X.-X.; Zhang, Y.; Jiang, J.L.; Blitzer, R.D.; Bozdagi-Gunal, O.; et al. Autophagy protein NRBF2 has reduced expression in Alzheimer’s brains and modulates memory and amyloid-beta homeostasis in mice. Mol. Neurodegener. 2019, 14, 43. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Uchihashi, T.; Kawata, S.; Nishiura, A.; Yamamoto, T.; Hiraoka, S.; Yokota, Y.; Isomura, E.T.; Kogo, M.; Tanaka, S.; et al. Role and Potential of Artificial Intelligence in Biomarker Discovery and Development of Treatment Strategies for Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2025, 26, 4346. [Google Scholar] [CrossRef]
- Zeng, X.; Lafferty, T.K.; Sehrawat, A.; Chen, Y.; Ferreira, P.C.L.; Bellaver, B.; Povala, G.; Kamboh, M.I.; Klunk, W.E.; Cohen, A.D.; et al. Multi-analyte proteomic analysis identifies blood-based neuroinflammation, cerebrovascular and synaptic biomarkers in preclinical Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 68. [Google Scholar] [CrossRef]
- Denaro, C.; Stephenson, D.; Müller, M.L.T.M.; Piccoli, B.; Azer, K. Advancing precision medicine therapeutics for Parkinson’s utilizing a shared quantitative systems pharmacology model and framework. Front. Syst. Biol. 2024, 4, 1351555. [Google Scholar] [CrossRef]
- Barry, R.L.; Babu, S.; Anteraper, S.A.; Triantafyllou, C.; Keil, B.; Rowe, O.E.; Rangaprakash, D.; Paganoni, S.; Lawson, R.; Dheel, C.; et al. Ultra-high field (7T) functional magnetic resonance imaging in amyotrophic lateral sclerosis: A pilot study. NeuroImage Clin. 2021, 30, 102648. [Google Scholar] [CrossRef]
- Cumbers, G.A.; Harvey-Latham, E.D.; Kassiou, M.; Werry, E.L.; Danon, J.J. Emerging TSPO-PET Radiotracers for Imaging Neuroinflammation: A Critical Analysis. Semin. Nucl. Med. 2024, 54, 856–874. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujita, K.; Suzuki, M.; Kunugi, A.; Hirozane, Y.; Kunikata, T.; Takahashi, B.; Narazaki, G.; Kondo, H.; Haji, S.; et al. Neuroinflammation and glycosylation-related cerebrospinal fluid proteins for predicting functional decline in amyotrophic lateral sclerosis: A proteomic study. Front. Neurol. 2024, 15, 1418320. [Google Scholar] [CrossRef]
- Müller, H.-P.; Abrahao, A.; Beaulieu, C.; Benatar, M.; Dionne, A.; Genge, A.; Frayne, R.; Graham, S.J.; Gibson, S.; Korngut, L.; et al. Temporal and spatial progression of microstructural cerebral degeneration in ALS: A multicentre longitudinal diffusion tensor imaging study. NeuroImage Clin. 2024, 43, 103633. [Google Scholar] [CrossRef]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 2021, 12, 6914. [Google Scholar] [CrossRef]
- Tondo, G.; Mazzini, L.; Caminiti, S.P.; Sarnelli, M.F.; Corrado, L.; Matheoud, R.; D’Alfonso, S.; Cantello, R.; Sacchetti, G.M.; Perani, D.; et al. Clinical relevance of single-subject brain metabolism patterns in amyotrophic lateral sclerosis mutation carriers. NeuroImage Clin. 2022, 36, 103222. [Google Scholar] [CrossRef]
- Milella, G.; Introna, A.; Mezzapesa, D.M.; D’Errico, E.; Fraddosio, A.; Ucci, M.; Zoccolella, S.; Simone, I.L. Clinical Profiles and Patterns of Neurodegeneration in Amyotrophic Lateral Sclerosis: A Cluster-Based Approach Based on MR Imaging Metrics. Am. J. Neuroradiol. 2023, 44, 403–409. [Google Scholar] [CrossRef]
- Sneha, N.P.; Dharshini, S.A.P.; Taguchi, Y.-h.; Gromiha, M.M. Tracing ALS Degeneration: Insights from Spinal Cord and Cortex Transcriptomes. Genes 2024, 15, 1431. [Google Scholar] [CrossRef]
- Harrison, D.M.; Sati, P.; Klawiter, E.C.; Narayanan, S.; Bagnato, F.; Beck, E.S.; Barker, P.; Calvi, A.; Cagol, A.; Donadieu, M.; et al. The use of 7T MRI in multiple sclerosis: Review and consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Brain Commun. 2024, 6, fcae359. [Google Scholar] [CrossRef]
- Wu, X.; Auerbach, E.J.; Vu, A.T.; Moeller, S.; Lenglet, C.; Schmitter, S.; Van de Moortele, P.-F.; Yacoub, E.; Uğurbil, K. High-resolution whole-brain diffusion MRI at 7T using radiofrequency parallel transmission. Magn. Reson. Med. 2018, 80, 1857–1870. [Google Scholar] [CrossRef]
- Dey, A.; Luk, C.C.; Ishaque, A.; Ta, D.; Srivastava, O.; Krebs, D.; Seres, P.; Hanstock, C.; Beaulieu, C.; Korngut, L.; et al. Motor cortex functional connectivity is associated with underlying neurochemistry in ALS. J. Neurol. Neurosurg. Psychiatry 2023, 94, 193–200. [Google Scholar] [CrossRef]
- Temp, A.G.M.; Dyrba, M.; Büttner, C.; Kasper, E.; Machts, J.; Kaufmann, J.; Vielhaber, S.; Teipel, S.; Prudlo, J. Cognitive Profiles of Amyotrophic Lateral Sclerosis Differ in Resting-State Functional Connectivity: An fMRI Study. Front. Neurosci. 2021, 15, 682100. [Google Scholar] [CrossRef]
- Dyer, M.S.; Odierna, G.L.; Clark, R.M.; Woodhouse, A.; Blizzard, C.A. Synaptic remodeling follows upper motor neuron hyperexcitability in a rodent model of TDP-43. Front. Cell. Neurosci. 2023, 17, 1274979. [Google Scholar] [CrossRef]
- Boon, L.I.; Hillebrand, A.; Schoonheim, M.M.; Twisk, J.W.; Stam, C.J.; Berendse, H.W. Cortical and Subcortical Changes in MEG Activity Reflect Parkinson’s Progression over a Period of 7 Years. Brain Topogr. 2023, 36, 566–580. [Google Scholar] [CrossRef]
- Fairley, L.H.; Sahara, N.; Aoki, I.; Ji, B.; Suhara, T.; Higuchi, M.; Barron, A.M. Neuroprotective effect of mitochondrial translocator protein ligand in a mouse model of tauopathy. J. Neuroinflamm. 2021, 18, 76. [Google Scholar] [CrossRef]
- Garland, E.F.; Antony, H.; Kulagowska, L.; Scott, T.; Rogien, C.; Bottlaender, M.; Nicoll, J.A.R.; Boche, D. The microglial translocator protein (TSPO) in Alzheimer’s disease reflects a phagocytic phenotype. Acta Neuropathol. 2024, 148, 62. [Google Scholar] [CrossRef]
- Nutma, E.; Fancy, N.; Weinert, M.; Tsartsalis, S.; Marzin, M.C.; Muirhead, R.C.J.; Falk, I.; Breur, M.; de Bruin, J.; Hollaus, D.; et al. Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nat. Commun. 2023, 14, 5247. [Google Scholar] [CrossRef] [PubMed]
- Salerno, S.; Viviano, M.; Baglini, E.; Poggetti, V.; Giorgini, D.; Castagnoli, J.; Barresi, E.; Castellano, S.; Da Settimo, F.; Taliani, S. TSPO Radioligands for Neuroinflammation: An Overview. Molecules 2024, 29, 4212. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Dogan, I.; Reetz, K.; Romanzetti, S. Neurochemical changes in the progression of Huntington’s disease: A meta-analysis of in vivo 1H-MRS studies. Neurobiol. Dis. 2024, 199, 106574. [Google Scholar] [CrossRef]
- Maier, S.; Nickel, K.; Lange, T.; Oeltzschner, G.; Dacko, M.; Endres, D.; Runge, K.; Schumann, A.; Domschke, K.; Rousos, M.; et al. Increased cerebral lactate levels in adults with autism spectrum disorders compared to non-autistic controls: A magnetic resonance spectroscopy study. Mol. Autism 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gverović Antunica, A.; Puzović, V.; Didović Pavičić, A.; Čanović, S.; Kovačević, P.; Vučemilović, P.A.F.; Konjevoda, S. Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer’s Disease. Biomedicines 2025, 13, 283. [Google Scholar] [CrossRef]
- Onciul, R.; Tataru, C.-I.; Dumitru, A.V.; Crivoi, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Toader, C. Artificial Intelligence and Neuroscience: Transformative Synergies in Brain Research and Clinical Applications. J. Clin. Med. 2025, 14, 550. [Google Scholar] [CrossRef]
- Behler, A.; Lulé, D.; Ludolph, A.C.; Kassubek, J.; Müller, H.-P. Longitudinal monitoring of amyotrophic lateral sclerosis by diffusion tensor imaging: Power calculations for group studies. Front. Neurosci. 2022, 16, 929151. [Google Scholar] [CrossRef]
- Behler, A.; Müller, H.-P.; Ludolph, A.C.; Kassubek, J. Diffusion Tensor Imaging in Amyotrophic Lateral Sclerosis: Machine Learning for Biomarker Development. Int. J. Mol. Sci. 2023, 24, 1911. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Cano, L.A.; Pizá, A.G.; Farfán, F.D. Electrophysiological Biomarkers for the Assessment of Motor Efficiency in Sport. J. Sports Res. 2022, 9, 10–25. [Google Scholar] [CrossRef]
- Campanini, I.; Merlo, A.; Disselhorst-Klug, C.; Mesin, L.; Muceli, S.; Merletti, R. Fundamental Concepts of Bipolar and High-Density Surface EMG Understanding and Teaching for Clinical, Occupational, and Sport Applications: Origin, Detection, and Main Errors. Sensors 2022, 22, 4150. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, A.K.; Al-Mahdawi, A.M.; Hamdan, F.B. Motor unit number estimation versus compound muscle action potential in the evaluation of motor unit loss in amyotrophic lateral sclerosis. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 45. [Google Scholar] [CrossRef]
- Sancho, J.; Martínez, D.; Bures, E.; Díaz, J.L.; Ponz, A.; Servera, E. Bulbar impairment score and survival of stable amyotrophic lateral sclerosis patients after noninvasive ventilation initiation. ERJ Open Res. 2018, 4, 00159–02017. [Google Scholar] [CrossRef]
- Thelen, M.P.; Wirth, B.; Kye, M.J. Mitochondrial defects in the respiratory complex I contribute to impaired translational initiation via ROS and energy homeostasis in SMA motor neurons. Acta Neuropathol. Commun. 2020, 8, 223. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Zaczek, Z.; Panczyk, M.; Osowska, S.; Kowalczyk, P.; Kramkowski, K.; Sobocki, J. Molecular Oxygen Levels and Percentages of DNA Damage in TPN Patients. Nutrients 2023, 15, 2206. [Google Scholar] [CrossRef]
- Teran-Tinedo, J.R.; Gonzalez-Rubio, J.; Najera, A.; Lorente-Gonzalez, M.; Cano-Sanz, E.; De La Calle-Gil, I.; Ortega-Fraile, M.Á.; Carballo-López, D.; Hernández-Nuñez, J.; Churruca-Arróspide, M.; et al. Effect of the Early Combination of Continuous Positive Airway Pressure and High-Flow Nasal Cannula on Mortality and Intubation Rates in Patients with COVID-19 and Acute Respiratory Distress Syndrome. The DUOCOVID Study. Arch. Bronconeumol. 2023, 59, 288–294. [Google Scholar] [CrossRef]
- Arnold, F.J.; Putka, A.F.; Raychaudhuri, U.; Hsu, S.; Bedlack, R.S.; Bennett, C.L.; La Spada, A.R. Revisiting Glutamate Excitotoxicity in Amyotrophic Lateral Sclerosis and Age-Related Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 5587. [Google Scholar] [CrossRef]
- Shum, C.; Hedges, E.C.; Allison, J.; Lee, Y.; Arias, N.; Cocks, G.; Chandran, S.; Ruepp, M.-D.; Shaw, C.E.; Nishimura, A.L. Mutations in FUS lead to synaptic dysregulation in ALS-iPSC derived neurons. Stem Cell Rep. 2024, 19, 187–195. [Google Scholar] [CrossRef]
- Barrois, R.; Barnerias, C.; Deladrière, E.; Leloup-Germa, V.; Tervil, B.; Audic, F.; Boulay, C.; Cances, C.; Cintas, P.; Davion, J.B.; et al. A new score combining compound muscle action potential (CMAP) amplitudes and motor score is predictive of motor outcome after AVXS-101 (Onasemnogene Abeparvovec) SMA therapy. Neuromuscul. Disord. NMD 2023, 33, 309–314. [Google Scholar] [CrossRef]
- Gamucci, F.; Pallante, M.; Molle, S.; Merlo, E.; Bertuglia, A. A Preliminary Study on the Use of HD-sEMG for the Functional Imaging of Equine Superficial Muscle Activation during Dynamic Mobilization Exercises. Animals 2022, 12, 785. [Google Scholar] [CrossRef]
- Noto, Y.-I.; Kitaoji, T.; Watanabe, K.; Mizuno, T. Assessment of motor unit firing by high-density surface electromyography detects motor neuronal hyperexcitable state in amyotrophic lateral sclerosis. Muscle Nerve 2023, 68, 149–156. [Google Scholar] [CrossRef]
- Pikor, D.; Hurła, M.; Słowikowski, B.; Szymanowicz, O.; Poszwa, J.; Banaszek, N.; Drelichowska, A.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Calcium Ions in the Physiology and Pathology of the Central Nervous System. Int. J. Mol. Sci. 2024, 25, 13133. [Google Scholar] [CrossRef]
- Bashford, J.; Mills, K.; Shaw, C. The evolving role of surface electromyography in amyotrophic lateral sclerosis: A systematic review. Clin. Neurophysiol. 2020, 131, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Ketabforoush, A.; Wang, M.; Arnold, W.D. Stimulated Single Fiber Electromyography (SFEMG) for Assessing Neuromuscular Junction Transmission in Rodent Models. J. Vis. Exp. 2024. [Google Scholar] [CrossRef] [PubMed]
- Stikvoort García, D.J.L.; Goedee, H.S.; van Eijk, R.P.A.; van Schelven, L.J.; van den Berg, L.H.; Sleutjes, B.T.H.M. Revisiting distinct nerve excitability patterns in patients with amyotrophic lateral sclerosis. Brain 2024, 147, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shakkottai, V.G. Targeting Ion Channels and Purkinje Neuron Intrinsic Membrane Excitability as a Therapeutic Strategy for Cerebellar Ataxia. Life 2023, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Krsek, A.; Jagodic, A.; Baticic, L. Nanomedicine in Neuroprotection, Neuroregeneration, and Blood–Brain Barrier Modulation: A Narrative Review. Medicina 2024, 60, 1384. [Google Scholar] [CrossRef]
- González-González, M.A.; Conde, S.V.; Latorre, R.; Thébault, S.C.; Pratelli, M.; Spitzer, N.C.; Verkhratsky, A.; Tremblay, M.-È.; Akcora, C.G.; Hernández-Reynoso, A.G.; et al. Bioelectronic Medicine: A multidisciplinary roadmap from biophysics to precision therapies. Front. Integr. Neurosci. 2024, 18, 1321872. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Y.; Wei, C.; Xia, Y.; Zhou, P.; Zhang, X. Online prediction of sustained muscle force from individual motor unit activities using adaptive surface EMG decomposition. J. Neuroeng. Rehabil. 2024, 21, 47. [Google Scholar] [CrossRef]
- Donaghy, R.; Pioro, E.P. Neurophysiologic Innovations in ALS: Enhancing Diagnosis, Monitoring, and Treatment Evaluation. Brain Sci. 2024, 14, 1251. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Pearce, T.; Baysal, M.A.; Chakraborty, A.; Tsimberidou, A.M. Convergence of evolving artificial intelligence and machine learning techniques in precision oncology. NPJ Digit. Med. 2025, 8, 75. [Google Scholar] [CrossRef]
- Witzel, S.; Wagner, M.; Zhao, C.; Kandler, K.; Graf, E.; Berutti, R.; Oexle, K.; Brenner, D.; Winkelmann, J.; Ludolph, A.C. Fast versus slow disease progression in amyotrophic lateral sclerosis-clinical and genetic factors at the edges of the survival spectrum. Neurobiol. Aging 2022, 119, 117–126. [Google Scholar] [CrossRef]
- Edgar, S.; Ellis, M.; Abdul-Aziz, N.A.; Goh, K.-J.; Shahrizaila, N.; Kennerson, M.L.; Ahmad-Annuar, A. Mutation analysis of SOD1, C9orf72, TARDBP and FUS genes in ethnically-diverse Malaysian patients with amyotrophic lateral sclerosis (ALS). Neurobiol. Aging 2021, 108, 200–206. [Google Scholar] [CrossRef]
- Virolainen, S.J.; VonHandorf, A.; Viel, K.C.M.F.; Weirauch, M.T.; Kottyan, L.C. Gene–environment interactions and their impact on human health. Genes Immun. 2023, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leykam, L.; Forsberg, K.M.E.; Nordström, U.; Hjertkvist, K.; Öberg, A.; Jonsson, E.; Andersen, P.M.; Marklund, S.L.; Zetterström, P. Specific analysis of SOD1 enzymatic activity in CSF from ALS patients with and without SOD1 mutations. Neurobiol. Dis. 2024, 202, 106718. [Google Scholar] [CrossRef]
- Farrawell, N.E.; Yerbury, J.J. Mutant Cu/Zn Superoxide Dismutase (A4V) Turnover Is Altered in Cells Containing Inclusions. Front. Mol. Neurosci. 2021, 14, 771911. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Tsekrekou, M.; Giannakou, M.; Papanikolopoulou, K.; Skretas, G. Protein aggregation and therapeutic strategies in SOD1- and TDP-43- linked ALS. Front. Mol. Biosci. 2024, 11, 1383453. [Google Scholar] [CrossRef] [PubMed]
- Belosludtseva, N.V.; Matveeva, L.A.; Belosludtsev, K.N. Mitochondrial Dyshomeostasis as an Early Hallmark and a Therapeutic Target in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 16833. [Google Scholar] [CrossRef]
- Sun, J.; Gao, G.; Wang, S.; Liu, H.; Tang, T.-S. Decoding the influence of mitochondrial Ca2+ regulation on neurodegenerative disease progression. Mitochondrial Commun. 2025, 3, 1–15. [Google Scholar] [CrossRef]
- Liu, B.-H.; Xu, C.-Z.; Liu, Y.; Lu, Z.-L.; Fu, T.-L.; Li, G.-R.; Deng, Y.; Luo, G.-Q.; Ding, S.; Li, N.; et al. Mitochondrial quality control in human health and disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Gunes, A.E.; Yılmaz, O.; Erbas, C.; Dagli, S.N.; Celik, H. High serum 8-hydroxy-2′-deoxyguanosine levels predict DNA damage and aging in professional divers. Rev. Assoc. Medica Bras. 1992 2021, 67, 1701–1705. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Wang, Y.; Meng, J.; Tan, G.; Zhao, Q.; Feng, S.; Xu, L.; Pei, Q. Oxidative stress disrupts the cytoskeleton of spinal motor neurons. Brain Behav. 2023, 13, e2870. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zheng, N.; Ding, X. Mitochondrial abnormalities: A hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail. Rev. 2022, 27, 1387–1394. [Google Scholar] [CrossRef]
- Yamashita, T.; Abe, K. Update on Antioxidant Therapy with Edaravone: Expanding Applications in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2945. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Franjkic, T.; Schito, P.; Russo, T.; Nimac, J.; Chami, A.A.; Mele, A.; Vidatic, L.; Kriz, J.; Julien, J.-P.; et al. Emerging Trends in the Field of Inflammation and Proteinopathy in ALS/FTD Spectrum Disorder. Biomedicines 2023, 11, 1599. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.U.; Yao, X.; Qin, D.; Su, H. Variability in SOD1-associated amyotrophic lateral sclerosis: Geographic patterns, clinical heterogeneity, molecular alterations, and therapeutic implications. Transl. Neurodegener. 2024, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-H.; Sarlus, H.; Harris, R.A. Copper toxicity and deficiency: The vicious cycle at the core of protein aggregation in ALS. Front. Mol. Neurosci. 2024, 17, 1408159. [Google Scholar] [CrossRef]
- Landolfo, E.; Cutuli, D.; Decandia, D.; Balsamo, F.; Petrosini, L.; Gelfo, F. Environmental Enrichment Protects against Neurotoxic Effects of Lipopolysaccharide: A Comprehensive Overview. Int. J. Mol. Sci. 2023, 24, 5404. [Google Scholar] [CrossRef] [PubMed]
- Carata, E.; Muci, M.; Di Giulio, S.; Di Giulio, T.; Mariano, S.; Panzarini, E. The Neuromuscular Disorder Mediated by Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Curr. Issues Mol. Biol. 2024, 46, 5999–6017. [Google Scholar] [CrossRef]
- Everett, W.H.; Bucelli, R.C. Tofersen for SOD1 ALS. Neurodegener. Dis. Manag. 2024, 14, 149–160. [Google Scholar] [CrossRef]
- Cantara, S.; Simoncelli, G.; Ricci, C. Antisense Oligonucleotides (ASOs) in Motor Neuron Diseases: A Road to Cure in Light and Shade. Int. J. Mol. Sci. 2024, 25, 4809. [Google Scholar] [CrossRef]
- Nikafshan Rad, H.; Su, Z.; Trinh, A.; Hakim Newton, M.A.; Shamsani, J.; NYGC ALS Consortium; Karim, A.; Sattar, A. Amyotrophic lateral sclerosis diagnosis using machine learning and multi-omic data integration. Heliyon 2024, 10, e38583. [Google Scholar] [CrossRef]
- Kortazar-Zubizarreta, I.; Manero-Azua, A.; Afonso-Agüera, J.; Perez de Nanclares, G. C9ORF72 Gene GGGGCC Hexanucleotide Expansion: A High Clinical Variability from Amyotrophic Lateral Sclerosis to Frontotemporal Dementia. J. Pers. Med. 2023, 13, 1396. [Google Scholar] [CrossRef]
- Kinger, S.; Dubey, A.R.; Kumar, P.; Jagtap, Y.A.; Choudhary, A.; Kumar, A.; Prajapati, V.K.; Dhiman, R.; Mishra, A. Molecular Chaperones’ Potential against Defective Proteostasis of Amyotrophic Lateral Sclerosis. Cells 2023, 12, 1302. [Google Scholar] [CrossRef]
- Ramesh, N.; Daley, E.L.; Gleixner, A.M.; Mann, J.R.; Kour, S.; Mawrie, D.; Anderson, E.N.; Kofler, J.; Donnelly, C.J.; Kiskinis, E.; et al. RNA dependent suppression of C9orf72 ALS/FTD associated neurodegeneration by Matrin-3. Acta Neuropathol. Commun. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Keyhanian, K.; Douthwright, C. Glial Cell Dysfunction in C9orf72-Related Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Cells 2021, 10, 249. [Google Scholar] [CrossRef]
- Templeton, C.W.; Laimins, L.A. HPV induced R-loop formation represses innate immune gene expression while activating DNA damage repair pathways. PLoS Pathog. 2024, 20, e1012454. [Google Scholar] [CrossRef]
- Lampasona, A.; Almeida, S.; Gao, F.-B. Translation of the poly(GR) frame in C9ORF72-ALS/FTD is regulated by cis-elements involved in alternative splicing. Neurobiol. Aging 2021, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Quaegebeur, A.; Glaria, I.; Lashley, T.; Isaacs, A.M. Soluble and insoluble dipeptide repeat protein measurements in C9orf72-frontotemporal dementia brains show regional differential solubility and correlation of poly-GR with clinical severity. Acta Neuropathol. Commun. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- White, M.R.; Mitrea, D.M.; Zhang, P.; Stanley, C.B.; Cassidy, D.E.; Nourse, A.; Phillips, A.H.; Tolbert, M.; Taylor, J.P.; Kriwacki, R.W. C9orf72 Poly(PR) Dipeptide Repeats Disturb Biomolecular Phase Separation and Disrupt Nucleolar Function. Mol. Cell 2019, 74, 713–728.e6. [Google Scholar] [CrossRef]
- Taha, M.S.; Ahmadian, M.R. Nucleophosmin: A Nucleolar Phosphoprotein Orchestrating Cellular Stress Responses. Cells 2024, 13, 1266. [Google Scholar] [CrossRef]
- Ryan, S.; Rollinson, S.; Hobbs, E.; Pickering-Brown, S. C9orf72 dipeptides disrupt the nucleocytoplasmic transport machinery and cause TDP-43 mislocalisation to the cytoplasm. Sci. Rep. 2022, 12, 4799. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, P.; Lau, A.; You, Z.; Durcan, T.M.; Robertson, J. Loss of C9orf72 perturbs the Ran-GTPase gradient and nucleocytoplasmic transport, generating compositionally diverse Importin β-1 granules. Cell Rep. 2023, 42, 112134. [Google Scholar] [CrossRef]
- Lall, D.; Lorenzini, I.; Mota, T.A.; Bell, S.; Mahan, T.E.; Ulrich, J.D.; Davtyan, H.; Rexach, J.E.; Muhammad, A.K.M.G.; Shelest, O.; et al. C9orf72 deficiency promotes microglial mediated synaptic loss in aging and amyloid accumulation. Neuron 2021, 109, 2275–2291.e8. [Google Scholar] [CrossRef]
- Green, E.H.; Kikis, E.A. Determining the effects of nanoparticulate air pollution on proteostasis in Caenorhabditis elegans. PLoS ONE 2020, 15, e0243419. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Vasta, R.; Chia, R.; Traynor, B.J.; Chiò, A. Unraveling the complex interplay between genes, environment, and climate in ALS. eBioMedicine 2021, 75, 103795. [Google Scholar] [CrossRef]
- Querin, G.; Biferi, M.G.; Pradat, P.-F. Biomarkers for C9orf7-ALS in Symptomatic and Pre-symptomatic Patients: State-of-the-art in the New Era of Clinical Trials. J. Neuromuscul. Dis. 2022, 9, 25–37. [Google Scholar] [CrossRef]
- Kempthorne, L.; Vaizoglu, D.; Cammack, A.J.; Carcolé, M.; Roberts, M.J.; Mikheenko, A.; Fisher, A.; Suklai, P.; Muralidharan, B.; Kroll, F.; et al. Dual-targeting CRISPR-CasRx reduces C9orf72 ALS/FTD sense and antisense repeat RNAs in vitro and in vivo. Nat. Commun. 2025, 16, 459. [Google Scholar] [CrossRef]
- Nguyen, L.; Montrasio, F.; Pattamatta, A.; Tusi, S.K.; Bardhi, O.; Meyer, K.D.; Hayes, L.; Nakamura, K.; Banez-Coronel, M.; Coyne, A.; et al. Antibody therapy targeting RAN proteins rescues C9 ALS/FTD phenotypes in C9orf72 mouse model. Neuron 2020, 105, 645–662.e11. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-T.; Liévens, J.-C.; Wang, S.-M.; Chuang, J.-Y.; Khalil, B.; Wu, H.; Chang, W.-C.; Maurice, T.; Su, T.-P. Sigma-1 receptor chaperones rescue nucleocytoplasmic transport deficit seen in cellular and Drosophila ALS/FTD models. Nat. Commun. 2020, 11, 5580. [Google Scholar] [CrossRef]
- Ryter, S.W.; Bhatia, D.; Choi, M.E. Autophagy: A Lysosome-Dependent Process with Implications in Cellular Redox Homeostasis and Human Disease. Antioxid. Redox Signal. 2019, 30, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Hideyama, T.; Yamashita, T.; Kimura, T.; Suzuki, N.; Aoki, M.; Kwak, S. Deficient RNA-editing enzyme ADAR2 in an amyotrophic lateral sclerosis patient with a FUS(P525L) mutation. J. Clin. Neurosci. 2016, 32, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takeuchi, T.; Fujikake, N.; Suzuki, M.; Minakawa, E.N.; Ueyama, M.; Fujino, Y.; Kimura, N.; Nagano, S.; Yokoseki, A.; et al. Dysregulation of stress granule dynamics by DCTN1 deficiency exacerbates TDP-43 pathology in Drosophila models of ALS/FTD. Acta Neuropathol. Commun. 2024, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Rezvykh, A.P.; Ustyugov, A.A.; Chaprov, K.D.; Teterina, E.V.; Nebogatikov, V.O.; Spasskaya, D.S.; Evgen’ev, M.B.; Morozov, A.V.; Funikov, S.Y. Cytoplasmic aggregation of mutant FUS causes multistep RNA splicing perturbations in the course of motor neuron pathology. Nucleic Acids Res. 2023, 51, 5810–5830. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Günther, R.; Japtok, J.; Frech, M.J.; Naumann, M.; Lee, H.O.; Hermann, A. FUS ALS neurons activate major stress pathways and reduce translation as an early protective mechanism against neurodegeneration. Cell Rep. 2023, 42, 112025. [Google Scholar] [CrossRef]
- Li, Q.; Babinchak, W.M.; Surewicz, W.K. Cryo-EM structure of amyloid fibrils formed by the entire low complexity domain of TDP-43. Nat. Commun. 2021, 12, 1620. [Google Scholar] [CrossRef] [PubMed]
- Ripin, N.; Parker, R. Formation, function, and pathology of RNP granules. Cell 2023, 186, 4737–4756. [Google Scholar] [CrossRef]
- Keyzor, I.; Shohet, S.; Castelli, J.; Sitaraman, S.; Veleva-Rotse, B.; Weimer, J.M.; Fox, B.; Willer, T.; Tuske, S.; Crathorne, L.; et al. Therapeutic Role of Pharmacological Chaperones in Lysosomal Storage Disorders: A Review of the Evidence and Informed Approach to Reclassification. Biomolecules 2023, 13, 1227. [Google Scholar] [CrossRef]
- Bedja-Iacona, L.; Richard, E.; Marouillat, S.; Brulard, C.; Alouane, T.; Beltran, S.; Andres, C.R.; Blasco, H.; Corcia, P.; Veyrat-Durebex, C.; et al. Post-Translational Variants of Major Proteins in Amyotrophic Lateral Sclerosis Provide New Insights into the Pathophysiology of the Disease. Int. J. Mol. Sci. 2024, 25, 8664. [Google Scholar] [CrossRef]
- Lenard, A.J.; Hutten, S.; Zhou, Q.; Usluer, S.; Zhang, F.; Bourgeois, B.M.R.; Dormann, D.; Madl, T. Phosphorylation Regulates CIRBP Arginine Methylation, Transportin-1 Binding and Liquid-Liquid Phase Separation. Front. Mol. Biosci. 2021, 8, 689687. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.A.; Cobos, S.N.; Fisher, R.M.A.; Son, E.; Frederic, R.; Segal, R.; Yousuf, H.; Chan, K.; Dansu, D.K.; Torrente, M.P. Direct and Indirect Protein Interactions Link FUS Aggregation to Histone Post-Translational Modification Dysregulation and Growth Suppression in an ALS/FTD Yeast Model. J. Fungi 2025, 11, 58. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Sanjuan-Ruiz, I.; Kan, V.; Megat, S.; De Rossi, P.; Dieterlé, S.; Cassel, R.; Jamet, M.; Kessler, P.; Wiesner, D.; et al. Cytoplasmic FUS triggers early behavioral alterations linked to cortical neuronal hyperactivity and inhibitory synaptic defects. Nat. Commun. 2021, 12, 3028. [Google Scholar] [CrossRef] [PubMed]
- Guerra San Juan, I.; Brunner, J.; Eggan, K.; Toonen, R.F.; Verhage, M. KIF5A regulates axonal repair and time-dependent axonal transport of SFPQ granules and mitochondria in human motor neurons. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kodavati, M.; Wang, H.; Guo, W.; Mitra, J.; Hegde, P.M.; Provasek, V.; Rao, V.H.M.; Vedula, I.; Zhang, A.; Mitra, S.; et al. FUS unveiled in mitochondrial DNA repair and targeted ligase-1 expression rescues repair-defects in FUS-linked motor neuron disease. Nat. Commun. 2024, 15, 2156. [Google Scholar] [CrossRef]
- Niu, Y.; Pal, A.; Szewczyk, B.; Japtok, J.; Naumann, M.; Glaß, H.; Hermann, A. Cell-Type-Dependent Recruitment Dynamics of FUS Protein at Laser-Induced DNA Damage Sites. Int. J. Mol. Sci. 2024, 25, 3526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Q.; Zheng, Y.-H.; Zhang, C.-T.; Liang, W.-W.; Wang, S.-Y.; Wang, X.-D.; Wang, Y.; Wang, T.-H.; Jiang, H.-Q.; Feng, H.-L. Wild-type p53-induced phosphatase 1 down-regulation promotes apoptosis by activating the DNA damage-response pathway in amyotrophic lateral sclerosis. Neurobiol. Dis. 2020, 134, 104648. [Google Scholar] [CrossRef]
- Mariani, D.; Setti, A.; Castagnetti, F.; Vitiello, E.; Stufera Mecarelli, L.; Di Timoteo, G.; Giuliani, A.; D’Angelo, A.; Santini, T.; Perego, E.; et al. ALS-associated FUS mutation reshapes the RNA and protein composition of stress granules. Nucleic Acids Res. 2024, 52, 13269–13289. [Google Scholar] [CrossRef]
- Marcelo, A.; Koppenol, R.; de Almeida, L.P.; Matos, C.A.; Nóbrega, C. Stress granules, RNA-binding proteins and polyglutamine diseases: Too much aggregation? Cell Death Dis. 2021, 12, 592. [Google Scholar] [CrossRef]
- Cozzi, M.; Ferrari, V. Autophagy Dysfunction in ALS: From Transport to Protein Degradation. J. Mol. Neurosci. 2022, 72, 1456–1481. [Google Scholar] [CrossRef]
- Driver, M.D.; Postema, J.; Onck, P.R. The Effect of Dipeptide Repeat Proteins on FUS/TDP43-RNA Condensation in C9orf72 ALS/FTD. J. Phys. Chem. B 2024, 128, 9405–9417. [Google Scholar] [CrossRef]
- Demongin, C.; Tranier, S.; Joshi, V.; Ceschi, L.; Desforges, B.; Pastré, D.; Hamon, L. RNA and the RNA-binding protein FUS act in concert to prevent TDP-43 spatial segregation. J. Biol. Chem. 2024, 300, 105716. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan-Ruiz, I.; Govea-Perez, N.; McAlonis-Downes, M.; Dieterle, S.; Megat, S.; Dirrig-Grosch, S.; Picchiarelli, G.; Piol, D.; Zhu, Q.; Myers, B.; et al. Wild-type FUS corrects ALS-like disease induced by cytoplasmic mutant FUS through autoregulation. Mol. Neurodegener. 2021, 16, 61. [Google Scholar] [CrossRef]
- Tamaki, Y.; Urushitani, M. Molecular Dissection of TDP-43 as a Leading Cause of ALS/FTLD. Int. J. Mol. Sci. 2022, 23, 12508. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Luo, C.; Jiang, Y.; Hu, T.; Lin, B.; Xie, Y.; Lan, J.; Miao, J. Decoding TDP-43: The molecular chameleon of neurodegenerative diseases. Acta Neuropathol. Commun. 2024, 12, 205. [Google Scholar] [CrossRef]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 Pathology in Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, H.; Guo, Q.; Bader, J.; Frottin, F.; Farny, D.; Kleinberger, G.; Haass, C.; Mann, M.; Hartl, F.U.; Baumeister, W.; et al. Gel-like inclusions of C-terminal fragments of TDP-43 sequester stalled proteasomes in neurons. EMBO Rep. 2022, 23, e53890, Erratum in EMBO Rep. 2024, 25, 3161. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Zhang, X.-L.; Hu, H.-Y. Co-Aggregation of TDP-43 with Other Pathogenic Proteins and Their Co-Pathologies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 12380. [Google Scholar] [CrossRef]
- Halim, D.; Gao, F.-B. RNA targets of TDP-43: Which one is more important in neurodegeneration? Transl. Neurodegener. 2022, 11, 12. [Google Scholar] [CrossRef]
- Zafar, S.; Fatima, S.I.; Schmitz, M.; Zerr, I. Current Technologies Unraveling the Significance of Post-Translational Modifications (PTMs) as Crucial Players in Neurodegeneration. Biomolecules 2024, 14, 118. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Tseng, Y.-L.; Lu, P.-C.; Lee, C.-C.; He, R.-Y.; Huang, Y.-A.; Tseng, Y.-C.; Cheng, T.-J.R.; Huang, J.J.-T.; Fang, J.-M. Degradation of neurodegenerative disease-associated TDP-43 aggregates and oligomers via a proteolysis-targeting chimera. J. Biomed. Sci. 2023, 30, 27. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Yan, T.; Perry, G.; Wang, X. TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration. Mol. Cell. Neurosci. 2019, 100, 103396. [Google Scholar] [CrossRef]
- Meng, K.; Jia, H.; Hou, X.; Zhu, Z.; Lu, Y.; Feng, Y.; Feng, J.; Xia, Y.; Tan, R.; Cui, F.; et al. Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines 2025, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.N.; Wu, Y.; Odeh, H.M.; Gendron, T.F.; Jansen-West, K.; Del Rosso, G.; Yue, M.; Jiang, P.; Gomes, E.; Tong, J.; et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci. Transl. Med. 2020, 12, eabb3774. [Google Scholar] [CrossRef]
- Ren, Y.; Li, S.; Chen, S.; Sun, X.; Yang, F.; Wang, H.; Li, M.; Cui, F.; Huang, X. TDP-43 and Phosphorylated TDP-43 Levels in Paired Plasma and CSF Samples in Amyotrophic Lateral Sclerosis. Front. Neurol. 2021, 12, 663637. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.; Huang, L.; Zheng, X.; Wang, L.; Miao, H. Ataxin-2: A powerful RNA-binding protein. Discov. Oncol. 2024, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Sá, R.; Sudria-Lopez, E.; Cañizares Luna, M.; Harschnitz, O.; van den Heuvel, D.M.A.; Kling, S.; Vonk, D.; Westeneng, H.-J.; Karst, H.; Bloemenkamp, L.; et al. ATAXIN-2 intermediate-length polyglutamine expansions elicit ALS-associated metabolic and immune phenotypes. Nat. Commun. 2024, 15, 7484. [Google Scholar] [CrossRef]
- Tavares de Andrade, H.M.; Cintra, V.P.; de Albuquerque, M.; Piccinin, C.C.; Bonadia, L.C.; Duarte Couteiro, R.E.; Sabino de Oliveira, D.; Claudino, R.; Magno Gonçalves, M.V.; Dourado, M.E.T.; et al. Intermediate-length CAG repeat in ATXN2 is associated with increased risk for amyotrophic lateral sclerosis in Brazilian patients. Neurobiol. Aging 2018, 69, e15–e292. [Google Scholar] [CrossRef]
- Watanabe, R.; Higashi, S.; Nonaka, T.; Kawakami, I.; Oshima, K.; Niizato, K.; Akiyama, H.; Yoshida, M.; Hasegawa, M.; Arai, T. Intracellular dynamics of Ataxin-2 in the human brains with normal and frontotemporal lobar degeneration with TDP-43 inclusions. Acta Neuropathol. Commun. 2020, 8, 176. [Google Scholar] [CrossRef]
- Boeynaems, S.; Dorone, Y.; Zhuang, Y.; Shabardina, V.; Huang, G.; Marian, A.; Kim, G.; Sanyal, A.; Şen, N.-E.; Griffith, D.; et al. Poly(A)-binding protein is an ataxin-2 chaperone that regulates biomolecular condensates. Mol. Cell 2023, 83, 2020–2034.e6. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Li, D.; Zhang, Y.; Zhang, S.; Kang, W.; Liu, C.; Le, W.; Wang, L.; Li, D.; et al. Halogen doped graphene quantum dots modulate TDP-43 phase separation and aggregation in the nucleus. Nat. Commun. 2024, 15, 2980. [Google Scholar] [CrossRef]
- Naskar, A.; Nayak, A.; Salaikumaran, M.R.; Vishal, S.S.; Gopal, P.P. Phase separation and pathologic transitions of RNP condensates in neurons: Implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders. Front. Mol. Neurosci. 2023, 16, 1242925. [Google Scholar] [CrossRef]
- Fittschen, M.; Lastres-Becker, I.; Halbach, M.V.; Damrath, E.; Gispert, S.; Azizov, M.; Walter, M.; Müller, S.; Auburger, G. Genetic ablation of ataxin-2 increases several global translation factors in their transcript abundance but decreases translation rate. Neurogenetics 2015, 16, 181–192. [Google Scholar] [CrossRef]
- Alessandrini, F.; Wright, M.; Kurosaki, T.; Maquat, L.E.; Kiskinis, E. ALS-Associated TDP-43 Dysfunction Compromises UPF1-Dependent mRNA Metabolism Pathways Including Alternative Polyadenylation and 3′UTR Length. bioRxiv 2024. [Google Scholar] [CrossRef]
- Becker, L.A.; Huang, B.; Bieri, G.; Ma, R.; Knowles, D.A.; Jafar-Nejad, P.; Messing, J.; Kim, H.J.; Soriano, A.; Auburger, G.; et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 2017, 544, 367–371. [Google Scholar] [CrossRef]
- Parc de Salut Mar Modulation of the Motor Pathway by Transcranial Pulse Stimulation in People with ALS: A Pilot Randomized Trial. clinicaltrials.gov, 2024.
- Modesti, L.; Danese, A.; Angela Maria Vitto, V.; Ramaccini, D.; Aguiari, G.; Gafà, R.; Lanza, G.; Giorgi, C.; Pinton, P. Mitochondrial Ca2+ Signaling in Health, Disease and Therapy. Cells 2021, 10, 1317. [Google Scholar] [CrossRef]
- Canet-Pons, J.; Sen, N.-E.; Arsović, A.; Almaguer-Mederos, L.-E.; Halbach, M.V.; Key, J.; Döring, C.; Kerksiek, A.; Picchiarelli, G.; Cassel, R.; et al. Atxn2-CAG100-KnockIn mouse spinal cord shows progressive TDP43 pathology associated with cholesterol biosynthesis suppression. Neurobiol. Dis. 2021, 152, 105289. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Liu, Y.-J.; Zhang, X.-L.; Liu, Y.-H.; Jiang, L.-L.; Hu, H.-Y. PolyQ-expanded ataxin-2 aggregation impairs cellular processing-body homeostasis via sequestering the RNA helicase DDX6. J. Biol. Chem. 2024, 300, 107413. [Google Scholar] [CrossRef]
- Yao, H.; Cai, C.; Huang, W.; Zhong, C.; Zhao, T.; Di, J.; Tang, J.; Wu, D.; Pang, M.; He, L.; et al. Enhancing mitophagy by ligustilide through BNIP3-LC3 interaction attenuates oxidative stress-induced neuronal apoptosis in spinal cord injury. Int. J. Biol. Sci. 2024, 20, 4382–4406. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Nonis, D.; Nowock, J.; Auburger, G. New alternative splicing variants of the ATXN2 transcript. Neurol. Res. Pract. 2019, 1, 22. [Google Scholar] [CrossRef]
- Shao, M.; Rodrigues, J.; Sousa-Oliveira, I.; Moradialvand, M.; Asadollahi, P.; Veiga, F.; Hameed, H.; Jha, N.K.; Sillanpää, M.; Sethi, G.; et al. Revolutionizing cancer treatment via bioengineered extracellular vesicles: Exploring nanovesicles to fully synthetic solutions. Appl. Mater. Today 2024, 40, 102395. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Ravindran, M.; Banadka, A.; Vandana, C.D.; Priya, K.; Nagella, P.; Kukkemane, K. Amyotrophic Lateral Sclerosis: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Therapies. Pharmaceuticals 2024, 17, 1391. [Google Scholar] [CrossRef]
- Tzeplaeff, L.; Wilfling, S.; Requardt, M.V.; Herdick, M. Current State and Future Directions in the Therapy of ALS. Cells 2023, 12, 1523. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, L.; Sun, Z.; Zhan, X. Personalized Drug Therapy: Innovative Concept Guided with Proteoformics. Mol. Cell. Proteom. MCP 2024, 23, 100737. [Google Scholar] [CrossRef]
- Sanofi. Care (Canadian ALS Riluzole Evaluation) Multicentre Phase IV Comparative Study of the Effects of Riluzole 50mg Bid on the Survival of ALS Subjects Compared to Historical Controls, clinicaltrials.gov, Clinical Trial Registration NCT00542412, October 2007. Available online: https://clinicaltrials.gov/study/NCT00542412 (accessed on 12 February 2025).
- AB Science. Multicenter, Randomised, Double-blind, Placebo-controlled, Parallel Group, Phase 2/3 Study to Compare the Efficacy and Safety of Masitinib, clinicaltrials.gov, Clinical Trial Registration NCT02588677, September 2023. Available online: https://clinicaltrials.gov/study/NCT02588677 (accessed on 12 February 2025).
- Shandiz, E.; Fernandes, G.L.; Henkin, J.S.; McCombe, P.A.; Trajano, G.S.; Henderson, R.D. Assessing the Effect of Riluzole on Motor Unit Discharge Properties. Brain Sci. 2024, 14, 1053. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, K. Effects of the Edaravone, a Drug Approved for the Treatment of Amyotrophic Lateral Sclerosis, on Mitochondrial Function and Neuroprotection. Antioxidants 2022, 11, 195. [Google Scholar] [CrossRef]
- Lynch, D. A Pilot Investigator Initiated Study to Evaluate the Safety, Tolerability and Efficacy of Elamipretide in the Treatment of Advanced Symptoms of Friedreich Ataxia (FRDA), clinicaltrials.gov, Clinical Trial Registration NCT05168774, August 2024. Available online: https://clinicaltrials.gov/study/NCT05168774 (accessed on 1 February 2025).
- Mitsubishi Tanabe Pharma America Inc. Safety Study of Oral Edaravone Administered in Subjects with ALS, clinical-trials.gov, Clinical Trial Registration NCT04165824, May 2023. Available online: https://clinicaltrials.gov/study/NCT04165824 (accessed on 12 February 2025).
- Firdaus, Z.; Li, X. Epigenetic Explorations of Neurological Disorders, the Identification Methods, and Therapeutic Avenues. Int. J. Mol. Sci. 2024, 25, 11658. [Google Scholar] [CrossRef]
- Paganoni, S.; Quintana, M.; Sherman, A.V.; Vestrucci, M.; Wu, Y.; Timmons, J.; Cudkowicz, M. Pooled Resource Open-Access ALS Clinical Trials Consortium Analysis of sodium phenylbutyrate and taurursodiol survival effect in ALS using external controls. Ann. Clin. Transl. Neurol. 2023, 10, 2297–2304. [Google Scholar] [CrossRef]
- Sivakumar, S.; Ghasemi, M.; Schachter, S.C. Targeting NMDA Receptor Complex in Management of Epilepsy. Pharmaceuticals 2022, 15, 1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-X.; Qiu, Q.; Yan, F.; Feng, Y.-C.; Wei, H.-H.; Li, X. Memantine-Assisted Treatment of N-Methyl-D-Aspartate Receptor Antibody Encephalitis: A Mini Review. Neuropsychiatr. Dis. Treat. 2024, 20, 2457–2464. [Google Scholar] [CrossRef]
- Wilkie, C.M.; Barron, J.C.; Brymer, K.J.; Barnes, J.R.; Nafar, F.; Parsons, M.P. The Effect of GLT-1 Upregulation on Extracellular Glutamate Dynamics. Front. Cell. Neurosci. 2021, 15, 661412. [Google Scholar] [CrossRef] [PubMed]
- Kyllo, T.; Allocco, D.; Hei, L.V.; Wulff, H.; Erickson, J.D. Riluzole attenuates acute neural injury and reactive gliosis, hippocampal-dependent cognitive impairments and spontaneous recurrent generalized seizures in a rat model of temporal lobe epilepsy. Front. Pharmacol. 2024, 15, 1466953, Erratum in Front. Pharmacol. 2025, 16, 1592653. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.S.; Newbery, M.; Touffu, A.; Maksour, S.; Chung, J.; Carroll, L.; Zaw, T.; Wu, Y.; Ooi, L. Edaravone and mitochondrial transfer as potential therapeutics for vanishing white matter disease astrocyte dysfunction. CNS Neurosci. Ther. 2023, 29, 2481–2497. [Google Scholar] [CrossRef]
- Riva, N.; Domi, T.; Pozzi, L.; Lunetta, C.; Schito, P.; Spinelli, E.G.; Cabras, S.; Matteoni, E.; Consonni, M.; Bella, E.D.; et al. Update on recent advances in amyotrophic lateral sclerosis. J. Neurol. 2024, 271, 4693–4723. [Google Scholar] [CrossRef]
- Tang, T.Z.; Zhao, Y.; Agarwal, D.; Tharzeen, A.; Patrikeev, I.; Zhang, Y.; DeJesus, J.; Bossmann, S.H.; Natarajan, B.; Motamedi, M.; et al. Serum amyloid A and mitochondrial DNA in extracellular vesicles are novel markers for detecting traumatic brain injury in a mouse model. Iscience 2024, 27, 108932. [Google Scholar] [CrossRef]
- Yip, J.M.X.; Chiang, G.S.H.; Lee, I.C.J.; Lehming-Teo, R.; Dai, K.; Dongol, L.; Wang, L.Y.-T.; Teo, D.; Seah, G.T.; Lehming, N. Mitochondria and the Repurposing of Diabetes Drugs for Off-Label Health Benefits. Int. J. Mol. Sci. 2025, 26, 364. [Google Scholar] [CrossRef]
- Liu, J.; Ting, J.P.; Al-Azzam, S.; Ding, Y.; Afshar, S. Therapeutic Advances in Diabetes, Autoimmune, and Neurological Diseases. Int. J. Mol. Sci. 2021, 22, 2805. [Google Scholar] [CrossRef]
- Diab, R.; Pilotto, F.; Saxena, S. Autophagy and neurodegeneration: Unraveling the role of C9ORF72 in the regulation of autophagy and its relationship to ALS-FTD pathology. Front. Cell. Neurosci. 2023, 17, 1086895, Erratum in Front. Cell. Neurosci. 2023, 17, 1225439. [Google Scholar] [CrossRef] [PubMed]
- Abulseoud, O.A.; Alasmari, F.; Hussein, A.M.; Sari, Y. Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation. Front. Neurosci. 2022, 16, 841036. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Troya, M.; Mohamed-Mohamed, H.; Pardo-Moreno, T.; González-Díaz, A.; Ruger-Navarrete, A.; de la Mata Fernández, M.; Tovar-Gálvez, M.I.; Ramos-Rodríguez, J.J.; García-Morales, V. Advancements in Pharmacological Interventions and Novel Therapeutic Approaches for Amyotrophic Lateral Sclerosis. Biomedicines 2024, 12, 2200. [Google Scholar] [CrossRef]
- Fang, T.; Je, G.; Pacut, P.; Keyhanian, K.; Gao, J.; Ghasemi, M. Gene Therapy in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2066. [Google Scholar] [CrossRef]
- Liscic, R.M.; Alberici, A.; Cairns, N.J.; Romano, M.; Buratti, E. From basic research to the clinic: Innovative therapies for ALS and FTD in the pipeline. Mol. Neurodegener. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Damme, P.V.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef]
- Vinceti, G.; Gallingani, C.; Zucchi, E.; Martinelli, I.; Gianferrari, G.; Simonini, C.; Bedin, R.; Chiari, A.; Zamboni, G.; Mandrioli, J. Young Onset Alzheimer’s Disease Associated with C9ORF72 Hexanucleotide Expansion: Further Evidence for a Still Unsolved Association. Genes 2023, 14, 930. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Aleff, R.A.; Jackson, J.L.; Finch, N.A.; Baker, M.C.; Gendron, T.F.; Murray, M.E.; McLaughlin, I.J.; Harting, J.R.; Graff-Radford, N.R.; et al. Long-read targeted sequencing uncovers clinicopathological associations for C9orf72-linked diseases. Brain 2021, 144, 1082–1088. [Google Scholar] [CrossRef]
- Udine, E.; Finch, N.A.; DeJesus-Hernandez, M.; Jackson, J.L.; Baker, M.C.; Saravanaperumal, S.A.; Wieben, E.; Ebbert, M.T.W.; Shah, J.; Petrucelli, L.; et al. Targeted long-read sequencing to quantify methylation of the C9orf72 repeat expansion. Mol. Neurodegener. 2024, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Petrozziello, T.; Motlagh, N.J.; Monsanto, R.Z.B.; Lei, D.; Murcar, M.G.; Penney, E.B.; Bragg, D.C.; Fernandez-Cerado, C.; Legarda, G.P.; Sy, M.; et al. Targeting Myeloperoxidase to Reduce Neuroinflammation in X-Linked Dystonia Parkinsonism. CNS Neurosci. Ther. 2024, 30, e70109. [Google Scholar] [CrossRef] [PubMed]
- Ashkarran, A.A.; Tadjiki, S.; Lin, Z.; Hilsen, K.; Ghazali, N.; Krikor, S.; Sharifi, S.; Asgari, M.; Hotchkin, M.; Dorfman, A.; et al. Protein Corona Composition of Gold Nanocatalysts. ACS Pharmacol. Transl. Sci. 2024, 7, 1169–1177. [Google Scholar] [CrossRef]
- Wang, Z.; Henriques, A.; Rouvière, L.; Callizot, N.; Tan, L.; Hotchkin, M.T.; Rossignol, R.; Mortenson, M.G.; Dorfman, A.R.; Ho, K.S.; et al. A Mechanism Underpinning the Bioenergetic Metabolism-Regulating Function of Gold Nanocatalysts. Small 2024, 20, 2304082. [Google Scholar] [CrossRef]
- Vucic, S.; Menon, P.; Huynh, W.; Mahoney, C.; Ho, K.S.; Hartford, A.; Rynders, A.; Evan, J.; Evan, J.; Ligozio, S.; et al. Efficacy and safety of CNM-Au8 in amyotrophic lateral sclerosis (RESCUE-ALS study): A phase 2, randomised, double-blind, placebo-controlled trial and open label extension. eClinicalMedicine 2023, 60, 102036. [Google Scholar] [CrossRef]
- Yang, Q.; Li, C.; Chen, Q. SS31 Ameliorates Oxidative Stress via the Restoration of Autophagic Flux to Protect Aged Mice From Hind Limb Ischemia. Front. Cardiovasc. Med. 2022, 9, 789331. [Google Scholar] [CrossRef]
- Paganoni, S.; Hendrix, S.; Dickson, S.P.; Knowlton, N.; Macklin, E.A.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve 2021, 63, 31–39. [Google Scholar] [CrossRef]
- Nikitin, D.; Makam, A.N.; Suh, K.; McKenna, A.; Carlson, J.J.; Richardson, M.; Rind, D.M.; Pearson, S.D. The effectiveness and value of AMX0035 and oral edaravone for amyotrophic lateral sclerosis: A summary from the Institute for Clinical and Economic Review’s Midwest Comparative Effectiveness Public Advisory Council. J. Manag. Care Spec. Pharm. 2023, 29, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, Y.S.; Choi, Y.; Lee, D.H.; Han, S.; Kwon, J.; Noda, T.; Ikawa, M.; Kim, D.; Kim, H.; et al. USF2 and TFEB compete in regulating lysosomal and autophagy genes. Nat. Commun. 2024, 15, 8334. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar] [CrossRef]
- Maruf, A.; Milewska, M.; Kovács, T.; Varga, M.; Vellai, T.; Lalik, A.; Student, S.; Borges, O.; Wandzik, I. Trehalose-releasing nanogels: A step toward a trehalose delivery vehicle for autophagy stimulation. Biomater. Adv. 2022, 138, 212969. [Google Scholar] [CrossRef]
- Bäckström, E.; Bonetti, A.; Johnsson, P.; Öhlin, S.; Dahlén, A.; Andersson, P.; Andersson, S.; Gennemark, P. Tissue pharmacokinetics of antisense oligonucleotides. Mol. Ther. Nucleic Acids 2024, 35, 102133. [Google Scholar] [CrossRef]
- Ngernsombat, C.; Suriya, U.; Prattapong, P.; Verma, K.; Rungrotmongkol, T.; Soonkum, T.; Kuhaudomlarp, S.; Janvilisri, T. Repurposing FDA-approved drugs targeting FZD10 in nasopharyngeal carcinoma: Insights from molecular dynamics simulations and experimental validation. Sci. Rep. 2024, 14, 31461. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, J.D.; Lipari, S.; Mathieu, B.; Beckers, C.; Van de Velde, J.A.; Mignion, L.; Da Silva Morais, M.; Kreuzer, M.; Colauzzi, I.; Capeloa, T.; et al. Mitochondria-targeted antioxidant MitoQ radiosensitizes tumors by decreasing mitochondrial oxygen consumption. Cell Death Discov. 2024, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, M.; Goto, S.; Moritake, K.; Shimada, Y.; Tsuchida, T. Antioxidant capacity of edaravone, quercetin, and myricetin involving probabilistic fluctuations using eosin-Y and eosin-B as fluorescent probes in the ORAC assay. J. Photochem. Photobiol. Chem. 2025, 460, 116142. [Google Scholar] [CrossRef]
- Biogen. Global Early Access Program to Provide Tofersen To Patients with Amyotrophic Lateral Sclerosis (ALS) Associated with a Mutation in the Superoxide Dismutase 1 (SOD1) Gene, clinicaltrials.gov, Clinical Trial Registration NCT04972487, June 2024. Available online: https://clinicaltrials.gov/study/NCT04972487 (accessed on 2 February 2025).
- Biogen. An Extension Study to Assess the Long-Term Safety, Tolerability, Pharmacokinetics, and Effect on Disease Progression of BIIB067 Administered to Previously Treated Adults with Amyotrophic Lateral Sclerosis Caused by Superoxide Dismutase 1 Mutation, clinicaltrials.gov, Clinical Trial Registration NCT03070119, August 2024. Available online: https://clinicaltrials.gov/study/NCT03070119 (accessed on 12 February 2025).
- Chen, S.; Heendeniya, S.N.; Le, B.T.; Rahimizadeh, K.; Rabiee, N.; Zahra, Q.U.A.; Veedu, R.N. Splice-Modulating Antisense Oligonucleotides as Therapeutics for Inherited Metabolic Diseases. BioDrugs 2024, 38, 177–203. [Google Scholar] [CrossRef]
- Sellier, C.; Corcia, P.; Vourc’h, P.; Dupuis, L. C9ORF72 hexanucleotide repeat expansion: From ALS and FTD to a broader pathogenic role? Rev. Neurol. 2024, 180, 417–428. [Google Scholar] [CrossRef]
- Mori, K.; Gotoh, S.; Ikeda, M. Aspects of degradation and translation of the expanded C9orf72 hexanucleotide repeat RNA. J. Neurochem. 2023, 166, 156–171. [Google Scholar] [CrossRef]
- Hautbergue, G.M.; Cleary, J.D.; Guo, S.; Ranum, L.P.W. Therapeutic strategies for C9orf72 amyotrophic lateral sclerosis and frontotemporal dementia. Curr. Opin. Neurol. 2021, 34, 748–755. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Y.; Lu, L.; Gao, Q.; Yu, D.; Sun, M. CRISPR/Cas9: Implication for modeling and therapy of amyotrophic lateral sclerosis. Front. Neurosci. 2023, 17, 1223777. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Drechsler, H.; Japtok, J.; Korten, T.; Diez, S.; Hermann, A. The ALS-Associated FUS (P525L) Variant Does Not Directly Interfere with Microtubule-Dependent Kinesin-1 Motility. Int. J. Mol. Sci. 2021, 22, 2422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Kankel, M.W.; Hana, S.; Lau, S.K.; Zavodszky, M.I.; McKissick, O.; Mastrangelo, N.; Dion, J.; Wang, B.; Ferretti, D.; et al. In vivo genome editing using novel AAV-PHP variants rescues motor function deficits and extends survival in a SOD1-ALS mouse model. Gene Ther. 2023, 30, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Amanat, M.; Nemeth, C.L.; Fine, A.S.; Leung, D.G.; Fatemi, A. Antisense Oligonucleotide Therapy for the Nervous System: From Bench to Bedside with Emphasis on Pediatric Neurology. Pharmaceutics 2022, 14, 2389. [Google Scholar] [CrossRef]
- Dubey, A.K.; Mostafavi, E. Biomaterials-mediated CRISPR/Cas9 delivery: Recent challenges and opportunities in gene therapy. Front. Chem. 2023, 11, 1259435. [Google Scholar] [CrossRef]
- Stansberry, W.M.; Pierchala, B.A. Neurotrophic factors in the physiology of motor neurons and their role in the pathobiology and therapeutic approach to amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2023, 16, 1238453. [Google Scholar] [CrossRef]
- Mandalawatta, H.P.; Rajendra, K.C.; Fairfax, K.; Hewitt, A.W. Emerging trends in virus and virus-like particle gene therapy delivery to the brain. Mol. Ther. Nucleic Acids 2024, 35, 102280. [Google Scholar] [CrossRef]
- Campos, L.J.; Arokiaraj, C.M.; Chuapoco, M.R.; Chen, X.; Goeden, N.; Gradinaru, V.; Fox, A.S. Advances in AAV technology for delivering genetically encoded cargo to the nonhuman primate nervous system. Curr. Res. Neurobiol. 2023, 4, 100086. [Google Scholar] [CrossRef]
- Reddy, D.S.; Abeygunaratne, H.N. Experimental and Clinical Biomarkers for Progressive Evaluation of Neuropathology and Therapeutic Interventions for Acute and Chronic Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 11734. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics 2023, 13, 4138. [Google Scholar] [CrossRef]
- Berry, J.D.; Cudkowicz, M.E.; Windebank, A.J.; Staff, N.P.; Owegi, M.; Nicholson, K.; McKenna-Yasek, D.; Levy, Y.S.; Abramov, N.; Kaspi, H.; et al. NurOwn, phase 2, randomized, clinical trial in patients with ALS. Neurology 2019, 93, e2294–e2305. [Google Scholar] [CrossRef]
- Fan, Y.; Goh, E.L.K.; Chan, J.K.Y. Neural Cells for Neurodegenerative Diseases in Clinical Trials. Stem Cells Transl. Med. 2023, 12, 510–526. [Google Scholar] [CrossRef]
- Hachimi-Idrissi, S. Stem cell therapy in neurological disorders: Promises and concerns. Explor. Neuroprot. Ther. 2023, 3, 346–362. [Google Scholar] [CrossRef]
- Ore, A.; Angelastro, J.M.; Giulivi, C. Integrating Mitochondrial Biology into Innovative Cell Therapies for Neurodegenerative Diseases. Brain Sci. 2024, 14, 899. [Google Scholar] [CrossRef] [PubMed]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J.; et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 2022, 65, 291–302, Erratum in Muscle Nerve 2022, 66, E26–E27. [Google Scholar] [CrossRef] [PubMed]
- Brainstorm-Cell Therapeutics. A Phase 3, Randomized Double-Blind, Placebo-Controlled Multicenter Study to Evaluate Efficacy and Safety of Repeated Administration of NurOwn® (Autologous Mesenchymal Stem Cells Secreting Neurotrophic Factors) in Participants with ALS, clinicaltrials.gov, Clinical Trial Registration NCT03280056, February 2024. Available online: https://clinicaltrials.gov/study/NCT03280056 (accessed on 12 February 2025).
- Maragakis, N.J.; de Carvalho, M.; Weiss, M.D. Therapeutic targeting of ALS pathways: Refocusing an incomplete picture. Ann. Clin. Transl. Neurol. 2023, 10, 1948–1971. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Radoszkiewicz, K.; Rybkowska, P.; Wedzinska, A.; Sarnowska, A. Interaction of Neural Stem Cells (NSCs) and Mesenchymal Stem Cells (MSCs) as a Promising Approach in Brain Study and Nerve Regeneration. Cells 2022, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Feng, B.; Amponsah, A.E.; He, J.; Guo, R.; Liu, B.; Du, X.; Liu, X.; Zhang, S.; Lv, F.; et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res. Ther. 2021, 12, 172. [Google Scholar] [CrossRef]
- Yu, F.; Witman, N.; Yan, D.; Zhang, S.; Zhou, M.; Yan, Y.; Yao, Q.; Ding, F.; Yan, B.; Wang, H.; et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res. Ther. 2020, 11, 490. [Google Scholar] [CrossRef]
- Pinjala, P.; Tryphena, K.P.; Prasad, R.; Khatri, D.K.; Sun, W.; Singh, S.B.; Gugulothu, D.; Srivastava, S.; Vora, L. CRISPR/Cas9 assisted stem cell therapy in Parkinson’s disease. Biomater. Res. 2023, 27, 46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-G.; Wang, J.-L.; Zhang, Y.-X.; Li, L.; Reza, A.M.M.T.; Gurunathan, S. Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases. Int. J. Nanomed. 2023, 18, 3177–3210. [Google Scholar] [CrossRef]
- Putthanbut, N.; Lee, J.Y.; Borlongan, C.V. Extracellular vesicle therapy in neurological disorders. J. Biomed. Sci. 2024, 31, 85. [Google Scholar] [CrossRef]
- Nassar, W.F. Phase 1 Study of The Effect of Cell-Free Cord Blood Derived Microvesicles On β-cell Mass in Type 1 Diabetes Mellitus (T1DM) Patients, clinicaltrials.gov, Clinical Trial Registration NCT02138331, May 2014. Available online: https://clinicaltrials.gov/study/NCT02138331 (accessed on 12 February 2025).
- Rust, R.; Nih, L.R.; Liberale, L.; Yin, H.; El Amki, M.; Ong, L.K.; Zlokovic, B.V. Brain repair mechanisms after cell therapy for stroke. Brain 2024, 147, 3286–3305. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Nathan, N.H.; Ghosh, N.; Pernia, C.D.; Law, J.; Nuryyev, R.; Plaia, A.; Yusof, A.; Tone, B.; Dulcich, M.; et al. A Biomarker for Predicting Responsiveness to Stem Cell Therapy Based on Mechanism-of-Action: Evidence from Cerebral Injury. Cell Rep. 2020, 31, 107622. [Google Scholar] [CrossRef]
- Rahimi Darehbagh, R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem cell therapies for neurological disorders: Current progress, challenges, and future perspectives. Eur. J. Med. Res. 2024, 29, 386. [Google Scholar] [CrossRef]
- Song, J.; Zhou, D.; Cui, L.; Wu, C.; Jia, L.; Wang, M.; Li, J.; Ya, J.; Ji, X.; Meng, R. Advancing stroke therapy: Innovative approaches with stem cell-derived extracellular vesicles. Cell Commun. Signal. 2024, 22, 369. [Google Scholar] [CrossRef]
- Christoforidou, E.; Joilin, G.; Hafezparast, M. Potential of activated microglia as a source of dysregulated extracellular microRNAs contributing to neurodegeneration in amyotrophic lateral sclerosis. J. Neuroinflamm. 2020, 17, 135. [Google Scholar] [CrossRef]
- You, J.; Youssef, M.M.M.; Santos, J.R.; Lee, J.; Park, J. Microglia and Astrocytes in Amyotrophic Lateral Sclerosis: Disease-Associated States, Pathological Roles, and Therapeutic Potential. Biology 2023, 12, 1307. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Migliorini, P.; Bossù, P. Cause or consequence? The role of IL-1 family cytokines and receptors in neuroinflammatory and neurodegenerative diseases. Front. Immunol. 2023, 14, 1128190. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, R.; Zecca, C.; Piccininni, M.; Benmahamed, S.; Dell’Abate, M.T.; Barulli, M.R.; Capozzo, R.; Battista, P.; Logroscino, G. Plasma Inflammatory Cytokines Are Elevated in ALS. Front. Neurol. 2020, 11, 552295. [Google Scholar] [CrossRef]
- Lokau, J.; Kleinegger, F.; Garbers, Y.; Waetzig, G.H.; Grötzinger, J.; Rose-John, S.; Haybaeck, J.; Garbers, C. Tocilizumab does not block interleukin-6 (IL-6) signaling in murine cells. PLoS ONE 2020, 15, e0232612. [Google Scholar] [CrossRef]
- Randhi, R.; Damon, M.; Dixon, K.J. Selective inhibition of soluble TNF using XPro1595 relieves pain and attenuates cerulein-induced pathology in mice. BMC Gastroenterol. 2021, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Gui, L.; Viviani, C.; Armato, U.; Dal Prà, I. NLRP3 Inflammasome’s Activation in Acute and Chronic Brain Diseases-An Update on Pathogenetic Mechanisms and Therapeutic Perspectives with Respect to Other Inflammasomes. Biomedicines 2023, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Qu, T.; Luo, J.; An, P.; Ren, F.; Luo, Y.; Li, Y. mTORC2: A multifaceted regulator of autophagy. Cell Commun. Signal. 2023, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Fernández Comaduran, M.; Minotti, S.; Jacob-Tomas, S.; Rizwan, J.; Larochelle, N.; Robitaille, R.; Sephton, C.F.; Vera, M.; Nalbantoglu, J.N.; Durham, H.D. Impact of histone deacetylase inhibition and arimoclomol on heat shock protein expression and disease biomarkers in primary culture models of familial ALS. Cell Stress Chaperones 2024, 29, 359–380. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Atassi, N.; David, W.; Cudkowicz, M.; Schoenfeld, D. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology 2018, 90, e565–e574. [Google Scholar] [CrossRef]
- Shah, S.; Dooms, M.M.; Amaral-Garcia, S.; Igoillo-Esteve, M. Current Drug Repurposing Strategies for Rare Neurodegenerative Disorders. Front. Pharmacol. 2021, 12, 768023. [Google Scholar] [CrossRef]
- Hasan, S.-A.-M.; James, A.W.; Fazili, F.M.; Tarabishi, S.; Sheikh, N.M.; Shah, Z.A. Endoplasmic Reticulum Stress in Neurodegenerative Diseases. J. Dement. Alzheimers Dis. 2024, 1, 87–97. [Google Scholar] [CrossRef]
- Gebert, M.; Sławski, J.; Kalinowski, L.; Collawn, J.F.; Bartoszewski, R. The Unfolded Protein Response: A Double-Edged Sword for Brain Health. Antioxidants 2023, 12, 1648. [Google Scholar] [CrossRef]
- Kurumi, H.; Yokoyama, Y.; Hirano, T.; Akita, K.; Hayashi, Y.; Kazama, T.; Isomoto, H.; Nakase, H. Cytokine Profile in Predicting the Effectiveness of Advanced Therapy for Ulcerative Colitis: A Narrative Review. Biomedicines 2024, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Moțățăianu, A.; Andone, S.; Stoian, A.; Bălașa, R.; Huțanu, A.; Sărmășan, E. A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 3782. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Oliveira, T.; Montezinho, L.; Simões, R.F.; Carvalho, M.; Ferreiro, E.; Silva, F.S.G. Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis. Cells 2024, 13, 248. [Google Scholar] [CrossRef]
- Burg, T.; Van Den Bosch, L. Abnormal energy metabolism in ALS: A key player? Curr. Opin. Neurol. 2023, 36, 338–345. [Google Scholar] [CrossRef]
- Steyn, F.J.; Ioannides, Z.A.; van Eijk, R.P.A.; Heggie, S.; Thorpe, K.A.; Ceslis, A.; Heshmat, S.; Henders, A.K.; Wray, N.R.; van den Berg, L.H.; et al. Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1016–1023. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Y.; Zhu, Y.; Jin, Q. Association between dietary patterns and the prognosis of amyotrophic lateral sclerosis in China: A cross-sectional study. Front. Nutr. 2024, 11, 1437521. [Google Scholar] [CrossRef]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Dickerson, B.; Maury, J.; Jenkins, V.; Nottingham, K.; Xing, D.; Gonzalez, D.E.; Leonard, M.; Kendra, J.; Ko, J.; Yoo, C.; et al. Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients 2024, 16, 990. [Google Scholar] [CrossRef]
- Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Johnston, S.E.; Simpson, P.; Chang, D.K.; Mather, D.; Dick, R.J. Time-restricted ketogenic diet in amyotrophic lateral sclerosis: A case study. Front. Neurol. 2024, 14, 1329541. [Google Scholar] [CrossRef] [PubMed]
- Saris, C.G.J.; Timmers, S. Ketogenic diets and Ketone suplementation: A strategy for therapeutic intervention. Front. Nutr. 2022, 9, 947567. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef] [PubMed]
- Hachemi, I.; U-Din, M. Brown Adipose Tissue: Activation and Metabolism in Humans. Endocrinol. Metab. 2023, 38, 214–222. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, C.; Liang, Z.; Liang, Y.; Li, Y.; Qiu, J. Exercise Combined with a Low-Calorie Diet Improves Body Composition, Attenuates Muscle Mass Loss, and Regulates Appetite in Adult Women with High Body Fat Percentage but Normal BMI. Sports 2024, 12, 91. [Google Scholar] [CrossRef]
- Gałęska, E.; Kowalczyk, A.; Wrzecińska, M.; García, M.C.; Czerniawska-Piątkowska, E.; Gwoździewicz, S.; Witkiewicz, W.; Dobrzański, Z. The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture. Int. J. Mol. Sci. 2025, 26, 4098. [Google Scholar] [CrossRef]
- Basnet, J.; Eissa, M.A.; Yanes Cardozo, L.L.; Romero, D.G.; Rezq, S. Impact of Probiotics and Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. 2024, 6, 801–815. [Google Scholar] [CrossRef]
- Radunovic, A.; Annane, D.; Rafiq, M.K.; Brassington, R.; Mustfa, N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2017, 2017, CD004427. [Google Scholar] [CrossRef]
- Keogh, C.; Saavedra, F.; Dubo, S.; Aqueveque, P.; Ortega, P.; Gomez, B.; Germany, E.; Pinto, D.; Osorio, R.; Pastene, F.; et al. Non-invasive phrenic nerve stimulation to avoid ventilator-induced diaphragm dysfunction in critical care. Artif. Organs 2022, 46, 1988–1997. [Google Scholar] [CrossRef]
- Sulistyo, A.; Abrahao, A.; Freitas, M.E.; Ritsma, B.; Zinman, L. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2023, 2023, CD004030. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, D.; Huang, H.; Li, D.; Yang, D.; Zhou, J.; Zhao, Y. The safety and effectiveness of high-calorie therapy for treating amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2023, 270, 4729–4743. [Google Scholar] [CrossRef] [PubMed]
- UCB Pharma. A Multicenter, Open-label Randomized Crossover Trial to Assess Subject Preference for KemstroTM Com-pared to Conventional Baclofen Tablets in Subjects with Stable Multiple Sclerosis, clinicaltrials.gov, Clinical Trial Registration NCT00139789, August 2013. Available online: https://clinicaltrials.gov/study/NCT00139789 (accessed on 12 February 2025).
- Teva GTC. A Double-Blind, Randomized, Two-Way Crossover, Comparative Study to Evaluate the Clinical Efficacy and Safety of Novel Sublingual Tizanidine HCl Versus Placebo in Children with Chronic Traumatic Brain Injury, clinicaltrials.gov, Clinical Trial Regis Tration NCT00287157, January 2009. Available online: https://clinicaltrials.gov/study/NCT00287157 (accessed on 12 February 2025).
- Reebye, R.; Jacinto, L.J.; Balbert, A.; Biering-Sørensen, B.; Carda, S.; Draulans, N.; Molteni, F.; O’Dell, M.W.; Picelli, A.; Santamato, A.; et al. Multimodal therapy and use of adjunctive therapies to BoNT-A in spasticity management: Defining terminology to help enhance spasticity treatment. Front. Neurol. 2024, 15, 1432330. [Google Scholar] [CrossRef]
- Garuti, G.; Rao, F.; Ribuffo, V.; Sansone, V.A. Sialorrhea in patients with ALS: Current treatment options. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-A.; Liao, C.-L.; Lin, W.-P.; Hsu, J.C.; Guo, Y.-H.; Lin, Y.-C. Botulinum Toxin Injections for Treatment of Drooling in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Children 2021, 8, 1089. [Google Scholar] [CrossRef]
- Linse, K.; Aust, E.; Joos, M.; Hermann, A. Communication Matters—Pitfalls and Promise of Hightech Communication Devices in Palliative Care of Severely Physically Disabled Patients with Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Awuah, W.A.; Ahluwalia, A.; Darko, K.; Sanker, V.; Tan, J.K.; Tenkorang, P.O.; Ben-Jaafar, A.; Ranganathan, S.; Aderinto, N.; Mehta, A.; et al. Bridging Minds and Machines: The Recent Advances of Brain-Computer Interfaces in Neurological and Neurosurgical Applications. World Neurosurg. 2024, 189, 138–153. [Google Scholar] [CrossRef]
- Filipe, C.B.; Carreira, N.R.; Reis-Pina, P. Optimizing breathlessness management in amyotrophic lateral sclerosis: Insights from a comprehensive systematic review. BMC Palliat. Care 2024, 23, 100. [Google Scholar] [CrossRef]
- Candelise, N.; Salvatori, I.; Scaricamazza, S.; Nesci, V.; Zenuni, H.; Ferri, A.; Valle, C. Mechanistic Insights of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis: An Update on a Lasting Relationship. Metabolites 2022, 12, 233. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef]
- Sighencea, M.G.; Popescu, R.Ș.; Trifu, S.C. From Fundamentals to Innovation in Alzheimer’s Disease: Molecular Findings and Revolutionary Therapies. Int. J. Mol. Sci. 2024, 25, 12311. [Google Scholar] [CrossRef]
- Cihankaya, H.; Theiss, C.; Matschke, V. Little Helpers or Mean Rogue—Role of Microglia in Animal Models of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2021, 22, 993. [Google Scholar] [CrossRef]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef]
- Melchiorri, D.; Merlo, S.; Micallef, B.; Borg, J.-J.; Dráfi, F. Alzheimer’s disease and neuroinflammation: Will new drugs in clinical trials pave the way to a multi-target therapy? Front. Pharmacol. 2023, 14, 1196413. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflamm. 2024, 21, 187. [Google Scholar] [CrossRef]
- Vidovic, M.; Müschen, L.H.; Brakemeier, S.; Machetanz, G.; Naumann, M.; Castro-Gomez, S. Current State and Future Directions in the Diagnosis of Amyotrophic Lateral Sclerosis. Cells 2023, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- López-Carbonero, J.I.; García-Toledo, I.; Fernández-Hernández, L.; Bascuñana, P.; Gil-Moreno, M.J.; Matías-Guiu, J.A.; Corrochano, S. In vivo diagnosis of TDP-43 proteinopathies: In search of biomarkers of clinical use. Transl. Neurodegener. 2024, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Weerawarna, P.M.; Schiefer, I.T.; Soares, P.; Fox, S.; Morimoto, R.I.; Melani, R.D.; Kelleher, N.L.; Luan, C.-H.; Silverman, R.B. Target Identification of a Class of Pyrazolone Protein Aggregation Inhibitor Therapeutics for Amyotrophic Lateral Sclerosis. ACS Cent. Sci. 2024, 10, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.N.; Giudice, C.L.; Lavecchia, A.; Poeta, M.L.; Chiara, M.; Picardi, E.; Pesole, G. Mitochondrial and Nuclear DNA Variants in Amyotrophic Lateral Sclerosis: Enrichment in the Mitochondrial Control Region and Sirtuin Pathway Genes in Spinal Cord Tissue. Biomolecules 2024, 14, 411. [Google Scholar] [CrossRef]
- Nguyen, L. Updates on Disease Mechanisms and Therapeutics for Amyotrophic Lateral Sclerosis. Cells 2024, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, F.; Flynn, L.L.; Anderton, R.S.; Akkari, P.A. Short structural variants as informative genetic markers for ALS disease risk and progression. BMC Med. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Robles, E.; de Celis Alonso, B.; Cantillo-Negrete, J.; Carino-Escobar, R.I.; Arias-Carrión, O. Advanced Magnetic Resonance Imaging for Early Diagnosis and Monitoring of Movement Disorders. Brain Sci. 2025, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.; Keslake, A.; Payne, T.; Onwuegbuzie, A.; Harding, J.; Baster, K.; Hoggard, N.; Shaw, P.J.; Wilkinson, I.D.; Jenkins, T.M. Analysis of brain and spinal MRI measures in a common domain to investigate directional neurodegeneration in motor neuron disease. J. Neurol. 2023, 270, 1682–1690. [Google Scholar] [CrossRef]
- Comley, L.H.; Kline, R.A.; Thomson, A.K.; Woschitz, V.; Landeros, E.V.; Osman, E.Y.; Lorson, C.L.; Murray, L.M. Motor unit recovery following Smn restoration in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 2022, 31, 3107–3119. [Google Scholar] [CrossRef]
- Risi, B.; Cotti Piccinelli, S.; Gazzina, S.; Labella, B.; Caria, F.; Damioli, S.; Poli, L.; Padovani, A.; Filosto, M. Prognostic Usefulness of Motor Unit Number Index (MUNIX) in Patients Newly Diagnosed with Amyotrophic Lateral Sclerosis. J. Clin. Med. 2023, 12, 5036. [Google Scholar] [CrossRef]
- Mehta, K.; Hegde, M.; Girisa, S.; Vishwa, R.; Alqahtani, M.S.; Abbas, M.; Shakibaei, M.; Sethi, G.; Kunnumakkara, A.B. Targeting RTKs/nRTKs as promising therapeutic strategies for the treatment of triple-negative breast cancer: Evidence from clinical trials. Mil. Med. Res. 2024, 11, 76. [Google Scholar] [CrossRef]
- Crouzier, L.; Denus, M.; Richard, E.M.; Tavernier, A.; Diez, C.; Cubedo, N.; Maurice, T.; Delprat, B. Sigma-1 Receptor Is Critical for Mitochondrial Activity and Unfolded Protein Response in Larval Zebrafish. Int. J. Mol. Sci. 2021, 22, 11049. [Google Scholar] [CrossRef]
- Geva, M.; Kusko, R.; Soares, H.; Fowler, K.D.; Birnberg, T.; Barash, S.; Wagner, A.M.; Fine, T.; Lysaght, A.; Weiner, B.; et al. Pridopidine activates neuroprotective pathways impaired in Huntington Disease. Hum. Mol. Genet. 2016, 25, 3975–3987. [Google Scholar] [CrossRef]
- HEALEY ALS. Platform Trial—Regimen F ABBV-CLS-7262, clinicaltrials.gov, Clinical Trial Registration NCT05740813, January 2025. Available online: https://clinicaltrials.gov/study/NCT05740813 (accessed on 12 February 2025).
- Speidell, A.; Bin Abid, N.; Yano, H. Brain-Derived Neurotrophic Factor Dysregulation as an Essential Pathological Feature in Huntington’s Disease: Mechanisms and Potential Therapeutics. Biomedicines 2023, 11, 2275. [Google Scholar] [CrossRef]
- Leskelä, S.; Huber, N.; Hoffmann, D.; Rostalski, H.; Remes, A.M.; Takalo, M.; Hiltunen, M.; Haapasalo, A. Expression of C9orf72 hexanucleotide repeat expansion leads to formation of RNA foci and dipeptide repeat proteins but does not influence autophagy or proteasomal function in neuronal cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119021. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S. RNA dysregulation in neurodegenerative diseases. EMBO J. 2025, 44, 613–638. [Google Scholar] [CrossRef]
- Pupyshev, A.B.; Tikhonova, M.A.; Akopyan, A.A.; Tenditnik, M.V.; Dubrovina, N.I.; Korolenko, T.A. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2019, 177, 1–11. [Google Scholar] [CrossRef]
- Dawoody Nejad, L.; Pioro, E.P. Modeling ALS with Patient-Derived iPSCs: Recent Advances and Future Potentials. Brain Sci. 2025, 15, 134. [Google Scholar] [CrossRef]
- Workman, M.J.; Lim, R.G.; Wu, J.; Frank, A.; Ornelas, L.; Panther, L.; Galvez, E.; Perez, D.; Meepe, I.; Lei, S.; et al. Large-scale differentiation of iPSC-derived motor neurons from ALS and control subjects. Neuron 2023, 111, 1191–1204.e5, Erratum in Neuron 2025, 113, 2028. [Google Scholar] [CrossRef]
- Piao, X.; Li, D.; Liu, H.; Guo, Q.; Yu, Y. Advances in gene and cellular therapeutic approaches for Huntington’s disease. Protein Cell 2024, 16, 307–337, Erratum in Protein Cell 2025, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Brunetti, D.; Kalani, K. Editorial: Mitochondrial therapy in neurodegeneration. Front. Pharmacol. 2023, 14, 1144093. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, R.P.A.; Kliest, T.; McDermott, C.J.; Roes, K.C.B.; Van Damme, P.; Chio, A.; Weber, M.; Ingre, C.; Corcia, P.; Povedano, M.; et al. TRICALS: Creating a highway toward a cure. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 496–501. [Google Scholar] [CrossRef]
- Biogen. A Phase 3 Randomized, Placebo-Controlled Trial with a Longitudinal Natural History Run-In and Open-Label Extension to Evaluate BIIB067 Initiated in Clinically Presymptomatic Adults with a Confirmed Superoxide Dismutase 1 Mutation, clinicaltrials.gov, Clinical Trial Registration NCT04856982, June 2024. Available online: https://clinicaltrials.gov/study/NCT04856982 (accessed on 2 February 2025).
- Willemse, S.W.; Roes, K.C.B.; Van Damme, P.; Hardiman, O.; Ingre, C.; Povedano, M.; Wray, N.R.; Gijzen, M.; de Pagter, M.S.; Demaegd, K.C.; et al. Lithium carbonate in amyotrophic lateral sclerosis patients homozygous for the C-allele at SNP rs12608932 in UNC13A: Protocol for a confirmatory, randomized, group-sequential, event-driven, double-blind, placebo-controlled trial. Trials 2022, 23, 978. [Google Scholar] [CrossRef]
- Stanga, S.; Caretto, A.; Boido, M.; Vercelli, A. Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3719. [Google Scholar] [CrossRef] [PubMed]
- Anwar, I.J.; Berman, D.M.; DeLaura, I.; Gao, Q.; Willman, M.A.; Miller, A.; Gill, A.; Gill, C.; Perrin, S.; Ricordi, C.; et al. The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates. Sci. Transl. Med. 2023, 15, eadf6376. [Google Scholar] [CrossRef]
- Chorev, M.; Haderlein, J.; Chandra, S.; Menon, G.; Burton, B.J.L.; Pearce, I.; McKibbin, M.; Thottarath, S.; Karatsai, E.; Chandak, S.; et al. A Multi-Modal AI-Driven Cohort Selection Tool to Predict Suboptimal Non-Responders to Aflibercept Loading-Phase for Neovascular Age-Related Macular Degeneration: PRECISE Study Report 1. J. Clin. Med. 2023, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, E.; Snow, B.; Bok, A.; Simpson, M.; Smith, A.; Taylor, K.M.; Lockhart, M.; Lam, B.B.J.; Frampton, C.; Finucane, G.; et al. A long-term follow-up of safety and clinical efficacy of NTCELL® [Immunoprotected (Alginate-encapsulated) porcine choroid plexus cells for xenotransplantation] in patients with Parkinson’s disease. Park. Relat. Disord. 2021, 82, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Payra, S.; Singh, S.K. Artificial Intelligence and Machine Learning in Pharmacological Research: Bridging the Gap Between Data and Drug Discovery. Cureus 2023, 15, e44359. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.; Wankhede, N.; Pawar, R.; Ballal, S.; Kumawat, R.; Goswami, M.; Khalid, M.; Taksande, B.; Upaganlawar, A.; Umekar, M.; et al. AI-driven innovations in Alzheimer’s disease: Integrating early diagnosis, personalized treatment, and prognostic modelling. Ageing Res. Rev. 2024, 101, 102497. [Google Scholar] [CrossRef]
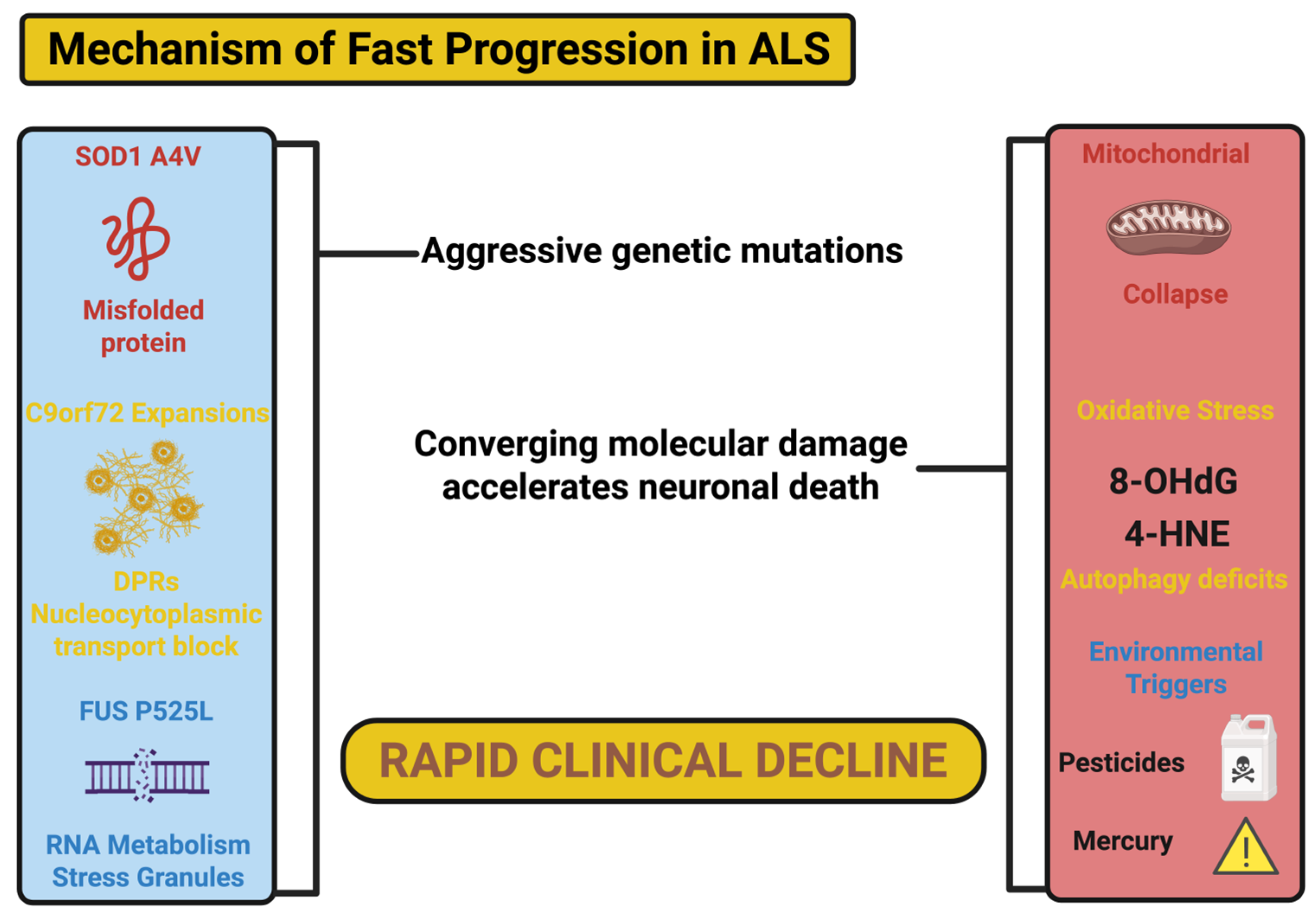

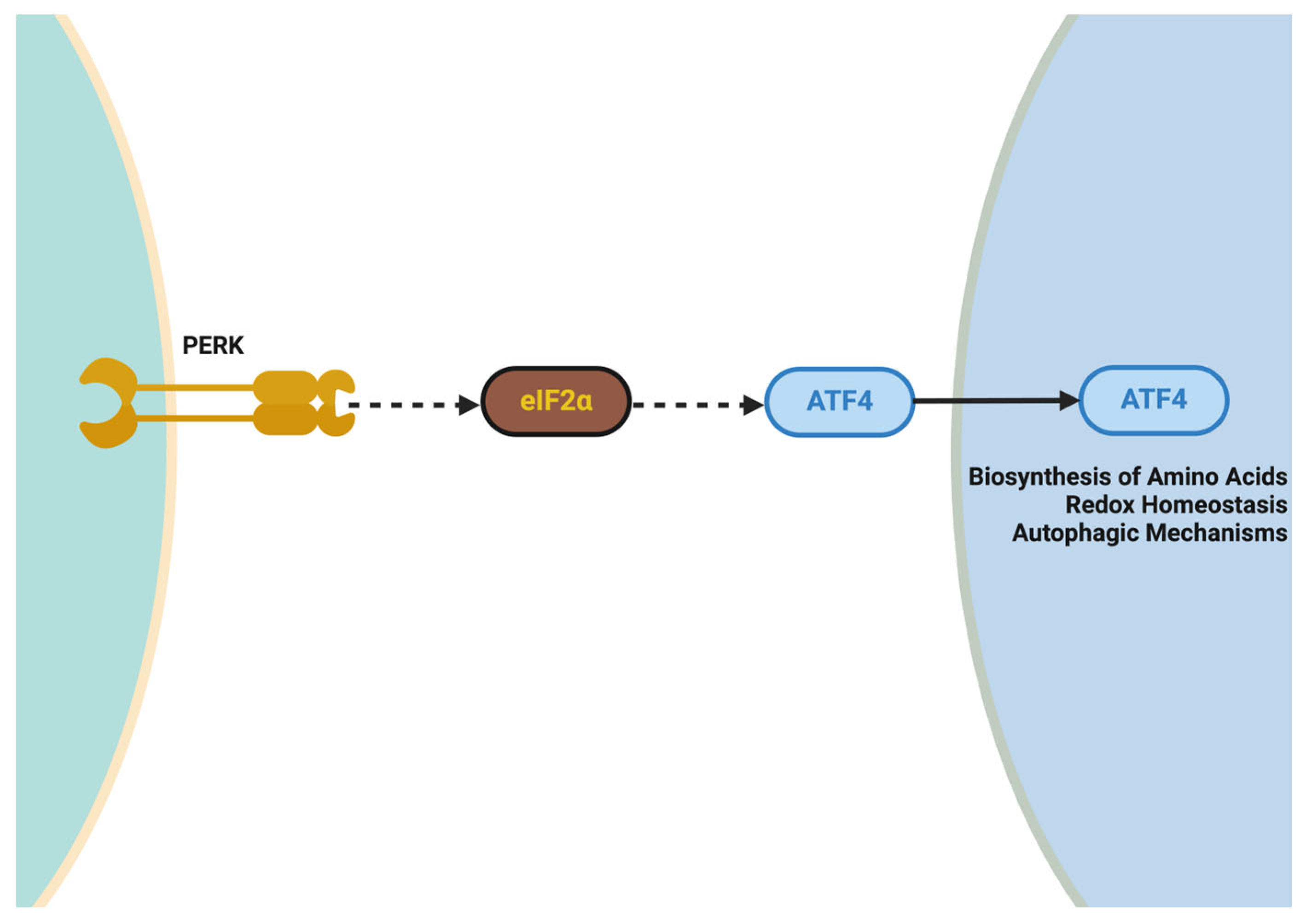
| Key Pathway/Process | Genetic Variants/Targets | Pathophysiological Mechanism | Therapeutic Strategies | References |
|---|---|---|---|---|
| Protein misfolding and aggregation | SOD1 (A4V, G93A variants) | Misfolded SOD1 aggregates induce oxidative stress, impair proteostasis, and disrupt mitochondrial function. | ASOs (tofersen), arimoclomol (HSP induction), SS-31 (mitochondrial stabilizer) | [17,245] |
| RNA dysregulation | C9orf72 expansions | Formation of toxic RNA foci and dipeptide repeat proteins (DPRs) disrupt nucleocytoplasmic transport and protein homeostasis. | Wave ASOs, CRISPR-based genome editing, trehalose (autophagy inducer) | [246,247,248] |
| Neuroinflammation | Immune activation (IL-6, TNF-α) | Chronic microglial activation releases ROS and pro-inflammatory cytokines, contributing to axonal degeneration and cell death. | Tocilizumab (IL-6 blocker), verdiperstat (MPO inhibitor), XPro1595 (TNF-α modulator) | [249,250,251,252] |
| Mitochondrial dysfunction | PGC-1α, SOD1 variants | Impaired oxidative phosphorylation and calcium overload induce ATP depletion and apoptosis. | CNM-Au8 (bioenergetic enhancer), AMX0035 (ER-mitochondrial stabilizer), SS-31 (ROS reduction) | [253,254,255] |
| Defective autophagy | FUS, TARDBP (TDP-43) | Autophagy failure leads to accumulation of protein aggregates, impaired clearance of damaged organelles, and synaptic loss. | Trehalose (autophagy enhancer), rapamycin (mTOR inhibitor), autophagy lysosome enhancers (TFEB activation) | [256,257,258] |
| Nucleocytoplasmic transport | C9orf72, FUS variants | Defects in nuclear pore complexes (NPCs) impair transport of proteins and RNAs, leading to genomic instability and toxicity. | Small molecules targeting NPC stabilization, ASOs for RNA clearance | [259,260] |
| Oxidative stress | SOD1, ATXN2 expansions | Increased ROS production damages lipids, proteins, and DNA, further exacerbating mitochondrial collapse. | Edaravone (antioxidant), AMX0035, SS-31, MitoQ | [261,262] |
| Biomarker Type | Specific Biomarker | Source | Pathophysiological Relevance | Clinical Application | References |
|---|---|---|---|---|---|
| Neurofilament biomarkers | Neurofilament light chain (NfL) | Cerebrospinal fluid (CSF), plasma | Marker of axonal injury; elevated during active neurodegeneration | Prognostic marker for disease progression, therapeutic monitoring in trials (e.g., tofersen, Wave ASOs) | [147,346] |
| Inflammatory biomarkers | IL-6, TNF-α | Plasma, CSF | Elevated in neuroinflammation due to activated microglia and astrocytes | Predicts neuroinflammatory burden; informs use of anti-inflammatory therapies like verdiperstat and XPro1595 | [347,348] |
| Protein aggregation markers | Phosphorylated TDP-43 (pTDP-43) | CSF | Reflects cytoplasmic mislocalization and aggregation, hallmarks of ALS pathology | Diagnostic marker for ALS and disease subtyping; potential for tracking responses to autophagy-based treatments | [349,350] |
| Mitochondrial biomarkers | Lactate, pyruvate ratio | Plasma, CSF | Indicates mitochondrial dysfunction and impaired oxidative phosphorylation, common in ALS fast progressors | Used to assess metabolic interventions targeting mitochondrial function, e.g., CNM-Au8, AMX0035 | [351,352] |
| RNA biomarkers | C9orf72 RNA foci | Neuronal tissues, blood | Toxic RNA aggregates formed due to hexanucleotide expansions, disrupting nucleocytoplasmic transport | Diagnostic and therapeutic monitoring in familial ALS cases with C9orf72 expansions; informs ASO or CRISPR-based therapies | [243,353] |
| Genetic biomarkers | SOD1, FUS, TARDBP variants | Blood, saliva, CSF | Identify genetic subtypes prone to faster progression due to toxic gain-of-function variants | Used for personalized therapeutic selection; informs trial eligibility for gene-based therapies (e.g., tofersen for SOD1) | [146,354] |
| Imaging biomarkers | Brain MRI (corticospinal tract) | Structural MRI | Detects upper motor neuron degeneration and corticospinal tract atrophy | Used for early diagnosis, disease staging, and tracking motor neuron degeneration over time | [355,356] |
| Functional biomarkers | Motor unit number estimation (MUNIX) | Electrophysiological recordings (EMG) | Reflects motor neuron functional integrity and axonal degeneration | Assesses disease progression and response to therapies targeting axonal preservation | [357,358] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS. Int. J. Mol. Sci. 2025, 26, 8072. https://doi.org/10.3390/ijms26168072
Șerban M, Toader C, Covache-Busuioc R-A. Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS. International Journal of Molecular Sciences. 2025; 26(16):8072. https://doi.org/10.3390/ijms26168072
Chicago/Turabian StyleȘerban, Matei, Corneliu Toader, and Răzvan-Adrian Covache-Busuioc. 2025. "Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS" International Journal of Molecular Sciences 26, no. 16: 8072. https://doi.org/10.3390/ijms26168072
APA StyleȘerban, M., Toader, C., & Covache-Busuioc, R.-A. (2025). Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS. International Journal of Molecular Sciences, 26(16), 8072. https://doi.org/10.3390/ijms26168072




