The Relationship Between Inflammation and the Development of Cerebral Ischaemia and Hypoxia in Traumatic Brain Injury—A Narrative Review
Abstract
1. Introduction
2. Inflammation
3. Haemodynamic Changes with Inflammation
4. Inflammatory Responses in the Central Nervous System
5. Glymphatic System
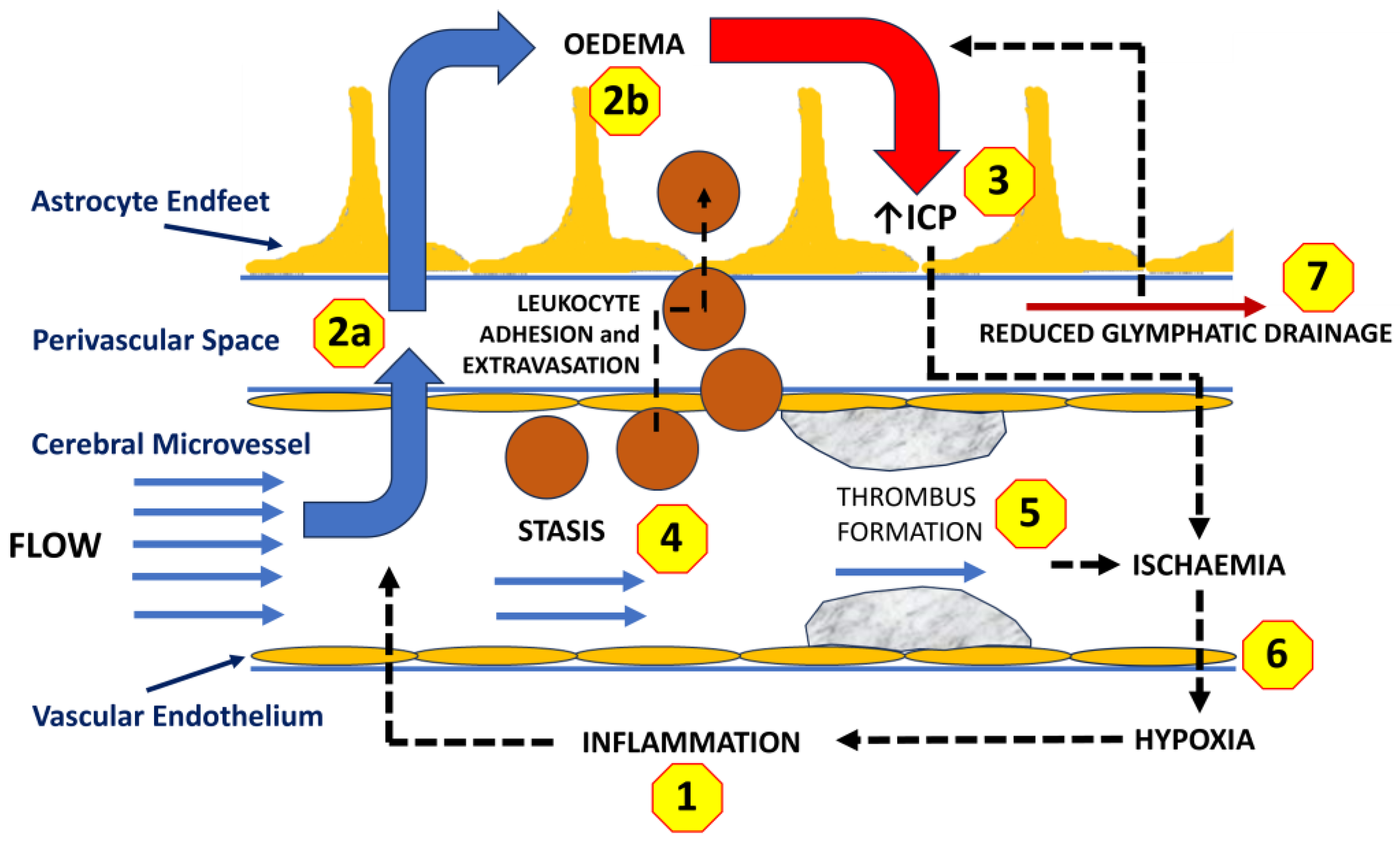
6. Traumatic Brain Injury, Inflammation, and Cerebral Oedema
7. Cerebral Oedema and Raised Intracranial Pressure
8. Intracranial Pressure, Oedema, and Cerebral Hypoxia
9. Potential Strategies to Target Cerebral Oedema and Its Implications
9.1. Inflammation
9.2. Cerebral Oedema
9.3. Microvascular Stasis
9.4. Microthrombus Formation and Bleeding
9.5. Glymphatic Drainage
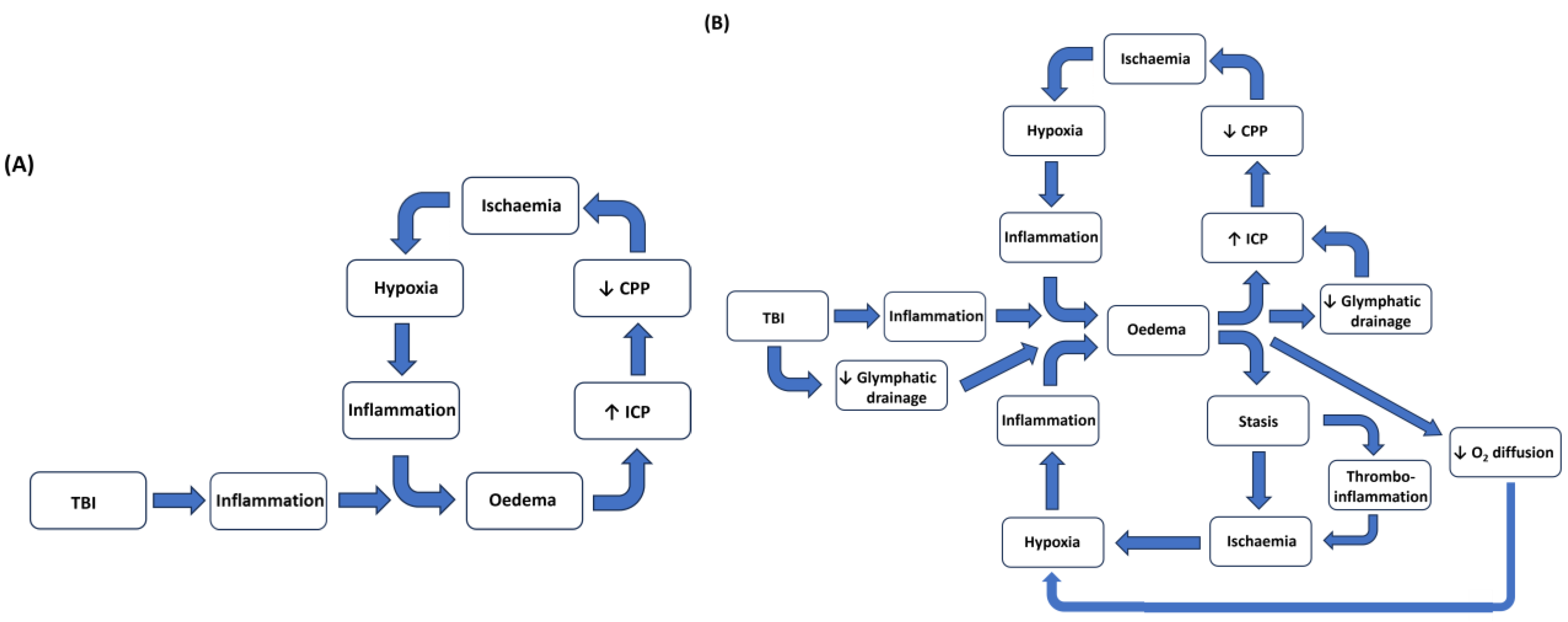
10. Conclusions
11. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| CNS | Central nervous system |
| ICP | Intracranial pressure |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| BBB | Blood–brain barrier |
| VECs | Vascular endothelial cells |
| AQP4 | Aquaporin-4 |
| NVU | Neurovascular unit |
| CSF | Cerebrospinal fluid |
| ISF | Interstitial fluid |
| ICH | Intracerebral hypertension |
| CPP | Cerebral perfusion pressure |
| LDF | Laser doppler flowmetry |
| SP | Substance P |
| NFκB | Nuclear factor κB |
| DRP | Drag-reducing polymers |
| tPAs | Tissue plasminogen activators |
| VEGF-C | Vascular endothelial growth factor C |
References
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef]
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2021, 38, 1411–1440. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arrastia, R.; Kochanek, P.M.; Bergold, P.; Kenney, K.; Marx, C.E.; Grimes, C.J.; Loh, L.T.; Adam, L.T.; Oskvig, D.; Curley, K.C.; et al. Pharmacotherapy of traumatic brain injury: State of the science and the road forward: Report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 2014, 31, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060, Erratum in Lancet Neurol. 2022, 21, E10. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 484040. [Google Scholar] [CrossRef]
- Turner, R.; Nimmo, A. Evidence for the involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System. Receptors 2023, 2, 232–250. [Google Scholar] [CrossRef]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflammation 2016, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Senchenkova, E. Integrated Systems Physiology—From Cell to Function. In Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- van den Tweel, J.G.; Taylor, C.R. A brief history of pathology: Preface to a forthcoming series that highlights milestones in the evolution of pathology as a discipline. Virchows Arch. 2010, 457, 3–10. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Pathologic Basis of Disease, 10th ed.; Elsevier: Philadelphia, PA, USA, 2021; 1379p. [Google Scholar]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Filippi, M.D. Neutrophil transendothelial migration: Updates and new perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Faenza, I.; Blalock, W.L. Innate Immunity: A Balance between Disease and Adaption to Stress. Biomolecules 2022, 12, 737. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Seroogy, C.M.; Gern, J.E. The role of T regulatory cells in asthma. J. Allergy Clin. Immunol. 2005, 116, 996–999. [Google Scholar] [CrossRef]
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24–39. [Google Scholar] [CrossRef]
- Scadding, G.K.; Scadding, G.W. Innate and Adaptive Immunity: ILC2 and Th2 Cells in Upper and Lower Airway Allergic Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 1851–1857. [Google Scholar] [CrossRef]
- Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-D.; Luu, Q.Q.; Park, H.-S. NSAID-Exacerbated Respiratory Disease (NERD): From Pathogenesis to Improved Care. Front. Pharmacol. 2020, 11, 1147. [Google Scholar] [CrossRef]
- Zilberstein, J.; McCurdy, M.T.; Winters, M.E. Anaphylaxis. J. Emerg. Med. 2014, 47, 182–187. [Google Scholar] [CrossRef]
- Kislitsina, O.N.; Rich, J.D.; Wilcox, J.E.; Pham, D.T.; Churyla, A.; Vorovich, E.B.; Ghafourian, K.; Yancy, C.W. Shock—Classification and Pathophysiological Principles of Therapeutics. Curr. Cardiol. Rev. 2019, 15, 102–113. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation Is a Necessary Evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef]
- Ryan, G.B.; Majno, G. Acute inflammation. A review. Am. J. Pathol. 1977, 86, 183–276. [Google Scholar]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Skobe, M.; Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef]
- Sawdon, M.; Kirkman, E. Capillary dynamics and the interstitial fluid–lymphatic system. Anaesth. Intensive Care Med. 2023, 24, 291–297. [Google Scholar] [CrossRef]
- Astiz, M.E.; DeGent, G.E.; Lin, R.Y.; Rackow, E.C. Microvascular function and rheologic changes in hyperdynamic sepsis. Crit. Care Med. 1995, 23, 265–271. [Google Scholar] [CrossRef]
- Spronk, P.E.; Zandstra, D.F.; Ince, C. Bench-to-bedside review: Sepsis is a disease of the microcirculation. Crit. Care 2004, 8, 462. [Google Scholar] [CrossRef][Green Version]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef]
- Benson, B.L.; Li, L.; Myers, J.T.; Dorand, R.D.; Gurkan, U.A.; Huang, A.Y.; Ransohoff, R.M. Biomimetic post-capillary venule expansions for leukocyte adhesion studies. Sci. Rep. 2018, 8, 9328. [Google Scholar] [CrossRef]
- Díaz, N.L.; Finol, H.J.; Torres, S.H.; Zambrano, C.I.; Adjounian, H. Histochemical and ultrastructural study of skeletal muscle in patients with sepsis and multiple organ failure syndrome (MOFS). Histol. Histopathol. 1998, 13, 121–128. [Google Scholar] [PubMed]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. Inflammation: A highly conserved, Janus-like phenomenon-a gastroenterologist’ perspective. J. Mol. Med. 2018, 96, 861–871. [Google Scholar] [CrossRef]
- Soussi, S.; Legrand, M. Hemodynamic coherence in patients with burns. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 437–443. [Google Scholar] [CrossRef]
- Arnemann, P.; Seidel, L.; Ertmer, C. Haemodynamic coherence—The relevance of fluid therapy. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 419–427. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocritical Care 2020, 32, 647–666. [Google Scholar] [CrossRef]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S232–S240. [Google Scholar] [CrossRef]
- Arena, G.; Sharma, K.; Agyeah, G.; Kruger, R.; Grunewald, A.; Fitzgerald, J.C. Neurodegeneration and Neuroinflammation in Parkinson’s Disease: A Self-Sustained Loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef]
- Stuckey, S.M.; Ong, L.K.; Collins-Praino, L.E.; Turner, R.J. Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke? Int. J. Mol. Sci. 2021, 22, 13101. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Berthiaume, A.A.; Grant, R.I.; Harrill, S.A.; Koski, T.; Tieu, T.; McDowell, K.P.; Faino, A.V.; Kelly, A.L.; Shih, A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021, 24, 633–645. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Balança, B.; Desmurs, L.; Grelier, J.; Perret-Liaudet, A.; Lukaszewicz, A.-C. DAMPs and RAGE Pathophysiology at the Acute Phase of Brain Injury: An Overview. Int. J. Mol. Sci. 2021, 22, 2439. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Millan Solano, M.V.; Salinas Lara, C.; Sanchez-Garibay, C.; Soto-Rojas, L.O.; Escobedo-Avila, I.; Tena-Suck, M.L.; Ortiz-Butron, R.; Choreno-Parra, J.A.; Romero-Lopez, J.P.; Melendez Camargo, M.E. Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 11902. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12, 688254. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bacha, R.S.; Baez, E.; Garcia-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Bonomini, F.; Rezzani, R. Aquaporin and blood brain barrier. Curr. Neuropharmacol. 2010, 8, 92–96. [Google Scholar] [CrossRef]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 2009, 190, 159–170. [Google Scholar]
- Uprety, A.; Kang, Y.; Kim, S.Y. Blood-brain barrier dysfunction as a potential therapeutic target for neurodegenerative disorders. Arch. Pharmacal Res. 2021, 44, 487–498. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Pacheco-Herrero, M.; Martinez-Gomez, P.A.; Campa-Cordoba, B.B.; Apatiga-Perez, R.; Villegas-Rojas, M.M.; Harrington, C.R.; de la Cruz, F.; Garces-Ramirez, L.; Luna-Munoz, J. The Neurovascular Unit Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2022. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, P.; Cioni, C.; Santonini, R.; Paccagnini, E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J. Neuroimmunol. 2002, 131, 41–49. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef] [PubMed]
- Hickey, W.F. Leukocyte traffic in the central nervous system: The participants and their roles. Semin. Immunol. 1999, 11, 125–137. [Google Scholar] [CrossRef]
- Jansson, D.; Rustenhoven, J.; Feng, S.; Hurley, D.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.; Dragunow, M. A role for human brain pericytes in neuroinflammation. J. Neuroinflammation 2014, 11, 104, Erratum in J. Neuroinflammation 2015, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnar, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood-Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. Water homeostasis in the brain: Basic concepts. Neuroscience 2004, 129, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Vucevic, D.; Bhatia, K.D.; Kortman, H.G.J.; Krings, T.; Murphy, K.P.; terBrugge, K.G.; Mikulis, D.J. Current Concepts in Intracranial Interstitial Fluid Transport and the Glymphatic System: Part I—Anatomy and Physiology. Radiology 2021, 301, 502–514. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The Interstitial System of the Brain in Health and Disease. Aging Dis. 2020, 11, 200–211. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.-A.; Martens, E.A.; Mori, Y.; et al. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, ra111–ra147. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef]
- Szlufik, S.; Kopeć, K.; Szleszkowski, S.; Koziorowski, D. Glymphatic System Pathology and Neuroinflammation as Two Risk Factors of Neurodegeneration. Cells 2024, 13, 286. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694, Erratum in Nature 2021, 590, E34. [Google Scholar] [CrossRef] [PubMed]
- Boisserand, L.S.B.; Geraldo, L.H.; Bouchart, J.; El Kamouh, M.R.; Lee, S.; Sanganahalli, B.G.; Spajer, M.; Zhang, S.; Lee, S.; Parent, M.; et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J. Exp. Med. 2024, 221, e20221983. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, F.L.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Drieu, A.; Mamuladze, T.; de Lima, K.A.; Dykstra, T.; Wall, M.; Papadopoulos, Z.; Kanamori, M.; Salvador, A.F.; Baker, W.; et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021, 184, 1000–1016.e27. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Tang, X.; Huang, J.; Cai, L.; Zhou, L. The meningeal lymphatic vessels and the glymphatic system: Potential therapeutic targets in neurological disorders. J. Cereb. Blood Flow Metab. 2022, 42, 1364–1382. [Google Scholar] [CrossRef]
- Zou, K.; Deng, Q.; Zhang, H.; Huang, C. Glymphatic system: A gateway for neuroinflammation. Neural Regen. Res. 2024, 19, 2661–2672. [Google Scholar] [CrossRef]
- Palmieri, M.; Frati, A.; Santoro, A.; Frati, P.; Fineschi, V.; Pesce, A. Diffuse Axonal Injury: Clinical Prognostic Factors, Molecular Experimental Models and the Impact of the Trauma Related Oxidative Stress. An Extensive Review Concerning Milestones and Advances. Int. J. Mol. Sci. 2021, 22, 10865. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Rancan, M.; Stahel, P.F.; Kossmann, T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr. Opin. Crit. Care 2002, 8, 101–105. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Zusman, B.E.; Kochanek, P.M.; Jha, R.M. Cerebral Edema in Traumatic Brain Injury: A Historical Framework for Current Therapy. Curr. Treat. Options Neurol. 2020, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.; Aston, J.; Dines, M.; Caraman, E.; Yacyshyn, M.; McCarthy, M.; Olson, J.E. Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury. J. Emerg. Med. 2017, 53, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef]
- Alves, J.L. Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 2014, 92, 141–147. [Google Scholar] [CrossRef]
- Vink, R.; Gabrielian, L.; Thornton, E. The Role of Substance P in Secondary Pathophysiology after Traumatic Brain Injury. Front. Neurol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Thal, S.C.; Neuhaus, W. The blood-brain barrier as a target in traumatic brain injury treatment. Arch. Med. Res. 2014, 45, 698–710. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Almeida, T.; Hernández, M.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Jiménez, A. Serum substance P levels are associated with severity and mortality in patients with severe traumatic brain injury. Crit. Care 2015, 19, 192. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Mestre, H.; Kress, B.T.; Liu, G.; Sweeney, A.M.; Samson, A.J.; Rasmussen, M.K.; Mortensen, K.N.; Bork, P.A.R.; Peng, W.; et al. Cerebrospinal fluid is a significant fluid source for anoxic cerebral oedema. Brain 2022, 145, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Bojarskaite, L.; Nafari, S.; Ravnanger, A.K.; Frey, M.M.; Skauli, N.; Åbjørsbråten, K.S.; Roth, L.C.; Amiry-Moghaddam, M.; Nagelhus, E.A.; Ottersen, O.P.; et al. Role of aquaporin-4 polarization in extracellular solute clearance. Fluids Barriers CNS 2024, 21, 28. [Google Scholar] [CrossRef]
- Stokum, J.A.; Gerzanich, V.; Simard, J.M. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab. 2016, 36, 513–538. [Google Scholar] [CrossRef]
- Cherian, I.; Beltran, M.; Landi, A.; Alafaci, C.; Torregrossa, F.; Grasso, G. Introducing the concept of “CSF-shift edema” in traumatic brain injury. J. Neurosci. Res. 2018, 96, 744–752. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Song, Y.; Xie, G.; Jiao, S.; Shi, L.; Cao, X.; Han, X.; Qu, A. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. 2022, 238, 108186. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.C.J.; Wilson, M.H.; Howlett, W.P. Mountain neurology. Pract. Neurol. 2019, 19, 404–411. [Google Scholar] [CrossRef]
- Levinson, S.; Pulli, B.; Heit, J.J. Neuroinflammation and acute ischemic stroke: Impact on translational research and clinical care. Front. Surg. 2025, 12, 1501359. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef]
- Mutch, C.A.; Talbott, J.F.; Gean, A. Imaging Evaluation of Acute Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 409–439. [Google Scholar] [CrossRef]
- Stein, K.Y.; Froese, L.; Gomez, A.; Sainbhi, A.S.; Vakitbilir, N.; Ibrahim, Y.; Zeiler, F.A. Intracranial Pressure Monitoring and Treatment Thresholds in Acute Neural Injury: A Narrative Review of the Historical Achievements, Current State, and Future Perspectives. Neurotrauma Rep. 2023, 4, 478–494. [Google Scholar] [CrossRef]
- Stocchetti, N.; Maas, A.I. Traumatic intracranial hypertension. N. Engl. J. Med. 2014, 370, 2121–2130. [Google Scholar] [CrossRef]
- Juul, N.; Morris, G.F.; Marshall, S.B.; Marshall, L.F. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J. Neurosurg. 2000, 92, 1–6. [Google Scholar] [CrossRef]
- Ciarrocchi, N.; Quiróz, N.; Traversaro, F.; Roman, E.S.; Risk, M.; Goldemberg, F.; Redelico, F.O. The complexity of intracranial pressure as an indicator of cerebral autoregulation. Commun. Nonlinear Sci. Numer. Simul. 2019, 75, 192–199. [Google Scholar] [CrossRef]
- Tadevosyan, A.; Kornbluth, J. Brain Herniation and Intracranial Hypertension. Neurol. Clin. 2021, 39, 293–318. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Citerio, G.; Hutchinson, P.; Kolias, A.; Meyfroidt, G.; Robba, C.; Stocchetti, N.; Chesnut, R. Intracranial pressure: Current perspectives on physiology and monitoring. Intensive Care Med. 2022, 48, 1471–1481, Erratum in Intensive Care Med. 2023, 49, 384. [Google Scholar] [CrossRef] [PubMed]
- Frisvold, S.; Coppola, S.; Ehrmann, S.; Chiumello, D.; Guérin, C. Respiratory challenges and ventilatory management in different types of acute brain-injured patients. Crit. Care 2023, 27, 247. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. Suppl. 1960, 36, 1–193. [Google Scholar] [CrossRef]
- Miller, J.D.; Becker, D.P.; Ward, J.D.; Sullivan, H.G.; Adams, W.E.; Rosner, M.J. Significance of intracranial hypertension in severe head injury. J. Neurosurg. 1977, 47, 503–516. [Google Scholar] [CrossRef]
- Badri, S.; Chen, J.; Barber, J.; Temkin, N.R.; Dikmen, S.S.; Chesnut, R.M.; Deem, S.; Yanez, N.D.; Treggiari, M.M. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012, 38, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sheng, Y.; Wang, C.; Chen, W.; Shi, X. Global traumatic brain injury intracranial pressure: From monitoring to surgical decision. Front. Neurol. 2024, 15, 1423329. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Crippa, I.A.; Mehta, S.; Menon, D.; Payen, J.F.; Taccone, F.S.; Citerio, G. Optimizing sedation in patients with acute brain injury. Crit. Care 2016, 20, 128. [Google Scholar] [CrossRef]
- Honeybul, S.; Ho, K.M.; Gillett, G.R. Long-term outcome following decompressive craniectomy: An inconvenient truth? Curr. Opin. Crit. Care 2018, 24, 97–104. [Google Scholar] [CrossRef]
- Adams, C.A.; Stein, D.M.; Morrison, J.J.; Scalea, T.M. Does intracranial pressure management hurt more than it helps in traumatic brain injury? Trauma Surg. Acute Care Open 2018, 3, e000142. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.M.; Chiu, Y.L.; Carney, N.; Härtl, R.; Ghajar, J. Marked reduction in mortality in patients with severe traumatic brain injury. J. Neurosurg. 2013, 119, 1583–1590. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Dennis, J.A. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst. Rev. 2019, 12, Cd003983. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef]
- Dunham, C.M.; Ransom, K.J.; Flowers, L.L.; Siegal, J.D.; Kohli, C.M. Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J. Trauma 2004, 56, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; O’Phelan, K.; McArthur, D.; Miller, C.; Eliseo, M.; Hirt, D.; Glenn, T.; Hovda, D.A. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 2007, 35, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Coles, J.P.; Gupta, A.K.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Aigbirhio, F.; Skepper, J.N.; Minhas, P.S.; Hutchinson, P.J.; et al. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004, 32, 1384–1390. [Google Scholar] [CrossRef]
- Stocchetti, N.; Longhi, L. Moving from macro- to microcirculation in head injury. Crit. Care Med. 2004, 32, 1429–1430. [Google Scholar] [CrossRef]
- Vink, R.; Bahtia, K.D.; Reilly, P.L. The relationship between intracranial pressure and brain oxygenation following traumatic brain injury in sheep. Acta Neurochir. Suppl. 2008, 102, 189–192. [Google Scholar]
- Kirkpatrick, P.J.; Smielewski, P.; Czosnyka, M.; Pickard, J.D. Continuous monitoring of cortical perfusion by laser Doppler flowmetry in ventilated patients with head injury. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1382–1388. [Google Scholar] [CrossRef]
- Yri, H.M.; Wegener, M.; Sander, B.; Jensen, R. Idiopathic intracranial hypertension is not benign: A long-term outcome study. J. Neurol. 2012, 259, 886–894. [Google Scholar] [CrossRef]
- Figaji, A.A.; Sandler, S.I.; Fieggen, A.G.; Le Roux, P.D.; Peter, J.C.; Argent, A.C. Continuous monitoring and intervention for cerebral ischemia in tuberculous meningitis. Pediatr. Crit. Care Med. 2008, 9, e25–e30. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Presa, J.L.; Saravia, F.; Bagi, Z.; Filosa, J.A. Vasculo-Neuronal Coupling and Neurovascular Coupling at the Neurovascular Unit: Impact of Hypertension. Front. Physiol. 2020, 11, 584135. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.W.; Lagopoulos, J.; Griffith, J.; Yip, K.; Mudaliar, Y.; Mehdorn, H.M.; Dorsch, N.W. Noninvasive cerebrovascular autoregulation assessment in traumatic brain injury: Validation and utility. J. Neurotrauma 2003, 20, 69–75. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Reinhard, M.; Aries, M.J.; Czosnyka, Z.; Smielewski, P.; Pickard, J.D.; Kirkpatrick, P.J.; Czosnyka, M. Monitoring cerebral autoregulation after head injury. Which Compon. Transcranial Doppler Flow. Veloc. Is. Optim.? Neurocritical Care 2012, 17, 211–218. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Morganti-Kossmann, M.C.; Satgunaseelan, L.; Bye, N.; Kossmann, T. Modulation of immune response by head injury. Injury 2007, 38, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Nattanmai Chandrasekaran, P.; Burton, C.; et al. Acute Traumatic Brain Injury-Induced Neuroinflammatory Response and Neurovascular Disorders in the Brain. Neurotox. Res. 2021, 39, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Salehi, A.; Zhang, J.H.; Obenaus, A. Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 2320–2339. [Google Scholar] [CrossRef]
- Stanimirovic, D.; Satoh, K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 2000, 10, 113–126. [Google Scholar] [CrossRef]
- Crumpler, R.; Roman, R.J.; Fan, F. Capillary Stalling: A Mechanism of Decreased Cerebral Blood Flow in AD/ADRD. J. Exp. Neurol. 2021, 2, 149–153. [Google Scholar] [CrossRef]
- Erdener, Ş.E.; Dalkara, T. Small Vessels Are a Big Problem in Neurodegeneration and Neuroprotection. Front. Neurol. 2019, 10, 889. [Google Scholar] [CrossRef]
- Divecha, Y.A.; Rampes, S.; Tromp, S.; Boyanova, S.T.; Fleckney, A.; Fidanboylu, M.; Thomas, S.A. The microcirculation, the blood-brain barrier, and the neurovascular unit in health and Alzheimer disease: The aberrant pericyte is a central player. Pharmacol. Rev. 2025, 77, 100052. [Google Scholar] [CrossRef]
- Davis, H.; Attwell, D. A tight squeeze: How do we make sense of small changes in microvascular diameter? J. Physiol. 2023, 601, 2263–2272. [Google Scholar] [CrossRef]
- Fruekilde, S.K.; Bailey, C.J.; Lambertsen, K.L.; Clausen, B.H.; Carlsen, J.; Xu, N.L.; Drasbek, K.R.; Gutiérrez-Jiménez, E. Disturbed microcirculation and hyperaemic response in a murine model of systemic inflammation. J. Cereb. Blood Flow Metab. 2022, 42, 2303–2317. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Teramura, Y.; Hamad, O.A.; Asif, S.; Duehrkop, C.; Fromell, K.; Gustafson, E.; Hong, J.; Kozarcanin, H.; Magnusson, P.U.; et al. Dangerous liaisons: Complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 2016, 274, 245–269. [Google Scholar] [CrossRef] [PubMed]
- Albert-Weissenberger, C.; Hopp, S.; Nieswandt, B.; Sirén, A.L.; Kleinschnitz, C.; Stetter, C. How is the formation of microthrombi after traumatic brain injury linked to inflammation? J. Neuroimmunol. 2019, 326, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Aversa, J.; Marsee, M.K.; McCauley, R.; Chitta, S.H.; Vyakaranam, S.; Walsh, M. Changes in Coagulation following Brain Injury. Semin. Thromb. Hemost. 2020, 46, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Gavins, F.N.E. The impact of thrombo-inflammation on the cerebral microcirculation. Microcirculation 2021, 28, e12689. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Leng, S.; Ma, Y.; Jiang, Q.; Wen, Q.; Ju, S.; Hu, J. The relationship between inflammation, impaired glymphatic system, and neurodegenerative disorders: A vicious cycle. Neurobiol. Dis. 2024, 192, 106426. [Google Scholar] [CrossRef]
- Hussain, R.; Tithof, J.; Wang, W.; Cheetham-West, A.; Song, W.; Peng, W.; Sigurdsson, B.; Kim, D.; Sun, Q.; Peng, S.; et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature 2023, 623, 992–1000. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J. Neurosci. 2017, 37, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Harms, A.K.; Tail, M.; Zhang, H.; Nimmo, A.; Skutella, T.; Kiening, K.; Unterberg, A.; Zweckberger, K.; Younsi, A. Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model. Front. Mol. Neurosci. 2023, 16, 1128545. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Ringstad, G. Glymphatic-stagnated edema induced by traumatic brain injury. Trends Pharmacol. Sci. 2024, 45, 388–390. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Oddo, M.; Levine, J.M.; Mackenzie, L.; Frangos, S.; Feihl, F.; Kasner, S.E.; Katsnelson, M.; Pukenas, B.; Macmurtrie, E.; Maloney-Wilensky, E.; et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery 2011, 69, 1037–1045, discussion 1045. [Google Scholar] [CrossRef]
- Bragin, D.E.; Peng, Z.; Bragina, O.A.; Statom, G.L.; Kameneva, M.V.; Nemoto, E.M. Improvement of Impaired Cerebral Microcirculation Using Rheological Modulation by Drag-Reducing Polymers. Adv. Exp. Med. Biol. 2016, 923, 239–244. [Google Scholar]
- Preiksaitis, A.; Krakauskaite, S.; Petkus, V.; Rocka, S.; Chomskis, R.; Dagi, T.F.; Ragauskas, A. Association of Severe Traumatic Brain Injury Patient Outcomes With Duration of Cerebrovascular Autoregulation Impairment Events. Neurosurgery 2016, 79, 75–82. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Aries, M.; Czosnyka, M.; Smielewski, P. Cerebral Autoregulation Monitoring in Traumatic Brain Injury: An Overview of Recent Advances in Personalized Medicine. J. Neurotrauma 2022, 39, 1477–1494. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A.; Datta, D. An overview of preclinical models of traumatic brain injury (TBI): Relevance to pathophysiological mechanisms. Front. Cell Neurosci. 2024, 18, 1371213. [Google Scholar] [CrossRef]
- Beauchamp, K.; Mutlak, H.; Smith, W.R.; Shohami, E.; Stahel, P.F. Pharmacology of traumatic brain injury: Where is the “golden bullet”? Mol. Med. 2008, 14, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Yates, D.; Sandercock, P.; Farrell, B.; Wasserberg, J.; Lomas, G.; Cottingham, R.; Svoboda, P.; Brayley, N.; Mazairac, G.; et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet 2004, 364, 1321–1328. [Google Scholar] [PubMed]
- Ali, M.A. NSAIDS related hyponatremia in brain injury patients: A silent factor for their deterioration. Ann. Phys. Rehabil. Med. 2018, 61, e271–e272. [Google Scholar] [CrossRef]
- Hatton, G.E.; Bell, C.; Wei, S.; Wade, C.E.; Kao, L.S.; Harvin, J.A. Do early non-steroidal anti-inflammatory drugs for analgesia worsen acute kidney injury in critically ill trauma patients? An inverse probability of treatment weighted analysis. J. Trauma Acute Care Surg. 2020, 89, 673–678. [Google Scholar] [CrossRef]
- Bergold, P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016, 275, 367–380. [Google Scholar] [CrossRef]
- Vink, R.; Nimmo, A.J. Multifunctional drugs for head injury. Neurotherapeutics 2009, 6, 28–42. [Google Scholar] [CrossRef]
- Stein, D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015, 29, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.; Leon, M.; van der Horn, H.J.; van der Naalt, J.; Sommer, I. Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: A meta-analysis. Sci. Rep. 2020, 10, 16179. [Google Scholar] [CrossRef]
- Kalra, S.; Malik, R.; Singh, G.; Bhatia, S.; Al-Harrasi, A.; Mohan, S.; Albratty, M.; Albarrati, A.; Tambuwala, M.M. Pathogenesis and management of traumatic brain injury (TBI): Role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology 2022, 30, 1153–1166. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef] [PubMed]
- Donkin, J.J.; Vink, R. Mechanisms of cerebral edema in traumatic brain injury: Therapeutic developments. Curr. Opin. Neurol. 2010, 23, 293–299. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Suarez, J.I. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit. Care Med. 2000, 28, 3301–3313. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.; Sagare, A.P.; Mingzi, Z.; Zlokovic, B.V.; Kisler, K. The blood–brain barrier as a treatment target for neurodegenerative disorders. Expert. Opin. Drug Deliv. 2025, 22, 673–692. [Google Scholar] [CrossRef]
- Jha, R.M.; Puccio, A.M.; Chou, S.H.; Chang, C.H.; Wallisch, J.S.; Molyneaux, B.J.; Zusman, B.E.; Shutter, L.A.; Poloyac, S.M.; Janesko-Feldman, K.L. Sulfonylurea Receptor-1: A Novel Biomarker for Cerebral Edema in Severe Traumatic Brain Injury. Crit. Care Med. 2017, 45, e255–e264. [Google Scholar] [CrossRef]
- Khalili, H.; Derakhshan, N.; Niakan, A.; Ghaffarpasand, F.; Salehi, M.; Eshraghian, H.; Shakibafard, A.; Zahabi, B. Effects of Oral Glibenclamide on Brain Contusion Volume and Functional Outcome of Patients with Moderate and Severe Traumatic Brain Injuries: A Randomized Double-Blind Placebo-Controlled Clinical Trial. World Neurosurg. 2017, 101, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Frisina, R.D.; Zhu, X.; Sakai, Y.; Sokolowski, B.; Walton, J.P. Direct control of Na(+)-K(+)-2Cl(-)-cotransport protein (NKCC1) expression with aldosterone. Am. J. Physiol. Cell Physiol. 2014, 306, C66–C75. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Wang, X.; Li, W.; Chen, J.; Sun, H. Pretreatment with AQP4 and NKCC1 Inhibitors Concurrently Attenuated Spinal Cord Edema and Tissue Damage after Spinal Cord Injury in Rats. Front. Physiol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Wilkinson, C.M.; Fedor, B.A.; Aziz, J.R.; Nadeau, C.A.; Brar, P.S.; Clark, J.J.A.; Colbourne, F. Failure of bumetanide to improve outcome after intracerebral hemorrhage in rat. PLoS ONE 2019, 14, e0210660. [Google Scholar] [CrossRef]
- Marmarou, C.R.; Liang, X.; Abidi, N.H.; Parveen, S.; Taya, K.; Henderson, S.C.; Young, H.F.; Filippidis, A.S.; Baumgarten, C.M. Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain Res. 2014, 1581, 89–102. [Google Scholar] [CrossRef]
- Morrison, T.R.; Kulkarni, P.; Cai, X.; Iriah, S.; Aggarwal, D.; Lu, S.-F.; Simon, N.G.; Madularu, D.; Ferris, C.F. Treating head injury using a novel vasopressin 1a receptor antagonist. Neurosci. Lett. 2020, 714, 134565. [Google Scholar] [CrossRef]
- Galton, C.; Deem, S.; Yanez, N.D.; Souter, M.; Chesnut, R.; Dagal, A.; Treggiari, M. Open-label randomized trial of the safety and efficacy of a single dose conivaptan to raise serum sodium in patients with traumatic brain injury. Neurocritical Care 2011, 14, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell Neurosci. 2016, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.; Paiva Dos Santos, B.; Leng, J.; Oliveira, H.; Amedee, J. Sensory neurons from dorsal root ganglia regulate endothelial cell function in extracellular matrix remodelling. Cell Commun. Signal 2020, 18, 162. [Google Scholar] [CrossRef]
- Chowdari Gurram, P.; Satarker, S.; Nampoothiri, M. Recent advances in the molecular signaling pathways of Substance P in Alzheimer’s disease: Link to neuroinflammation associated with toll-like receptors. Biochem. Biophys. Res. Commun. 2024, 733, 150597. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 1388–1398. [Google Scholar] [CrossRef]
- Donkin, J.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. A substance P antagonist reduces axonal injury and improves neurologic outcome when administered up to 12 hours after traumatic brain injury. J. Neurotrauma 2011, 28, 217–224. [Google Scholar] [CrossRef]
- Gabrielian, L.; Helps, S.C.; Thornton, E.; Turner, R.J.; Leonard, A.V.; Vink, R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir. Suppl. 2013, 118, 201–204. [Google Scholar]
- Sorby-Adams, A.J.; Leonard, A.V.; Hoving, J.W.; Yassi, N.; Vink, R.; Wells, A.J.; Turner, R.J. NK1-r Antagonist Treatment Comparable to Decompressive Craniectomy in Reducing Intracranial Pressure Following Stroke. Front. Neurosci. 2019, 13, 681. [Google Scholar] [CrossRef]
- Vink, R.; Nimmo, A. Identification of an Intravenous Injectable NK1 Receptor Antagonist for Use in Traumatic Brain Injury. Int. J. Mol. Sci. 2024, 25, 3535. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-K.; Hoon Roh, T.; Kim, H.; Jin Ha, E.; Yoon, D.; Min Park, C.; Kim, S.-H.; You, N.; Kim, D.-J. Hyperosmolar therapy response in traumatic brain injury: Explainable artificial intelligence based long-term time series forecasting approach. Expert. Syst. Appl. 2024, 255, 124795. [Google Scholar] [CrossRef]
- Schwarzmaier, S.M.; de Chaumont, C.; Balbi, M.; Terpolilli, N.A.; Kleinschnitz, C.; Gruber, A.; Plesnila, N. The Formation of Microthrombi in Parenchymal Microvessels after Traumatic Brain Injury Is Independent of Coagulation Factor XI. J. Neurotrauma 2016, 33, 1634–1644. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Dong, J.F.; Cruz, M.A.; Rumbaut, R.E. Links between thrombosis and inflammation in traumatic brain injury. Thromb. Res. 2021, 198, 62–71. [Google Scholar] [CrossRef]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef]
- Nagata, K.; Browne, K.D.; Suto, Y.; Kumasaka, K.; Cognetti, J.; Johnson, V.E.; Marks, J.; Smith, D.H.; Pascual, J.L. Early heparin administration after traumatic brain injury: Prolonged cognitive recovery associated with reduced cerebral edema and neutrophil sequestration. J. Trauma Acute Care Surg. 2017, 83, 406–412. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Jacovides, C.L.; Suto, Y.; Leone, R.M.; Roche, A.; Weber, M.T.; Johnson, V.E.; Kaplan, L.J.; Smith, D.H.; Pascual, J.L. Low-Anticoagulant Heparin Improves Outcomes after Traumatic Brain Injury: Balancing Inflammation Mitigation and Bleeding Potentiation. J. Am. Coll. Surg. 2018, 227, S267. [Google Scholar] [CrossRef]
- Sonderegger, P.; Matsumoto-Miyai, K. Activity-controlled proteolytic cleavage at the synapse. Trends Neurosci. 2014, 37, 413–423. [Google Scholar] [CrossRef]
- Xia, Y.; Pu, H.; Leak, R.K.; Shi, Y.; Mu, H.; Hu, X.; Lu, Z.; Foley, L.M.; Hitchens, T.K.; Dixon, C.E.; et al. Tissue plasminogen activator promotes white matter integrity and functional recovery in a murine model of traumatic brain injury. Proc. Natl. Acad. Sci. USA 2018, 115, E9230–E9238. [Google Scholar] [CrossRef] [PubMed]
- CRASH, T. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Fitzgerald, M.; Gao, G.; Gupta, D.; Hutchinson, P.; Manley, G.T.; Menon, D.K. Traumatic brain injury over the past 20 years: Research and clinical progress. Lancet Neurol. 2022, 21, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Pergakis, M.; Badjatia, N.; Chaturvedi, S.; Cronin, C.A.; Kimberly, W.T.; Sheth, K.N.; Simard, J.M. BIIB093 (IV glibenclamide): An investigational compound for the prevention and treatment of severe cerebral edema. Expert. Opin. Investig. Drugs 2019, 28, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
| Main Categories of Cerebral Oedema | |||
|---|---|---|---|
| Category | Mechanism | Contribution to ICP | References |
| Vasogenic | Increased interstitial fluid volume associated with increased BBB permeability and the extravasation of plasma proteins and water into the interstitial space | Potential to cause a significant increase in ICP due to increased interstitial fluid volume | [24,46,102,104] |
| Cytotoxic (cellular) | Increased intracellular fluid volume—primarily associated with changed osmotic gradients associated with cellular energy failure and Na+/K+-ATPase dysfunction | Little direct impact on ICP, since it is associated with a fluid shift from the extracellular to intracellular compartment | [46,98] |
| Ionic (osmotic) | The osmotic forces that drive cytotoxic oedema, in turn, drive the movement of extracellular water and ions into the interstitial space—this may come from the vasculature or CSF | Potential to cause a significant increase in ICP due to increased interstitial fluid volume | [46,108] |
| Interstitial (CSF-shift) | Associated with transependymal CSF flow and CSF-shift oedema linked to impaired glymphatic clearance | Increased ICP associated with increased parenchymal volume, particularly in periventricular and subcortical regions | [106,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimmo, A.; Younsi, A. The Relationship Between Inflammation and the Development of Cerebral Ischaemia and Hypoxia in Traumatic Brain Injury—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 8066. https://doi.org/10.3390/ijms26168066
Nimmo A, Younsi A. The Relationship Between Inflammation and the Development of Cerebral Ischaemia and Hypoxia in Traumatic Brain Injury—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(16):8066. https://doi.org/10.3390/ijms26168066
Chicago/Turabian StyleNimmo, Alan, and Alexander Younsi. 2025. "The Relationship Between Inflammation and the Development of Cerebral Ischaemia and Hypoxia in Traumatic Brain Injury—A Narrative Review" International Journal of Molecular Sciences 26, no. 16: 8066. https://doi.org/10.3390/ijms26168066
APA StyleNimmo, A., & Younsi, A. (2025). The Relationship Between Inflammation and the Development of Cerebral Ischaemia and Hypoxia in Traumatic Brain Injury—A Narrative Review. International Journal of Molecular Sciences, 26(16), 8066. https://doi.org/10.3390/ijms26168066






