Optimization of Photodynamic Therapy in Dermatology: The Role of Light Fractionation
Abstract
1. Introduction
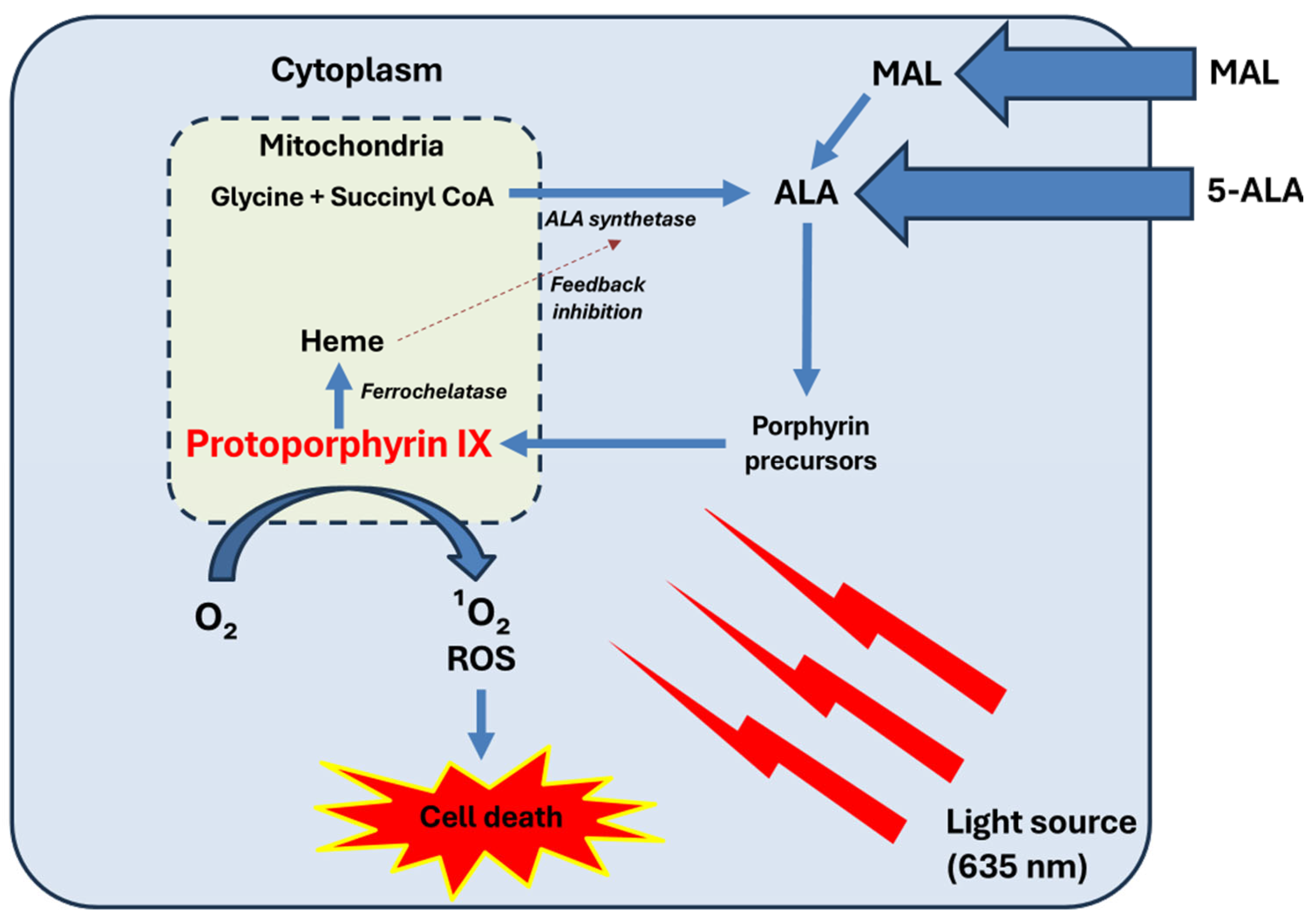
2. Fundamentals of PDT
- −
- PS factors: type, concentration, incubation time, and temperature.
- −
- Light parameters: wavelength, fluence, irradiance, and exposure duration.
- −
- Tissue characteristics: oxygen availability, pigmentation, and vascularization.
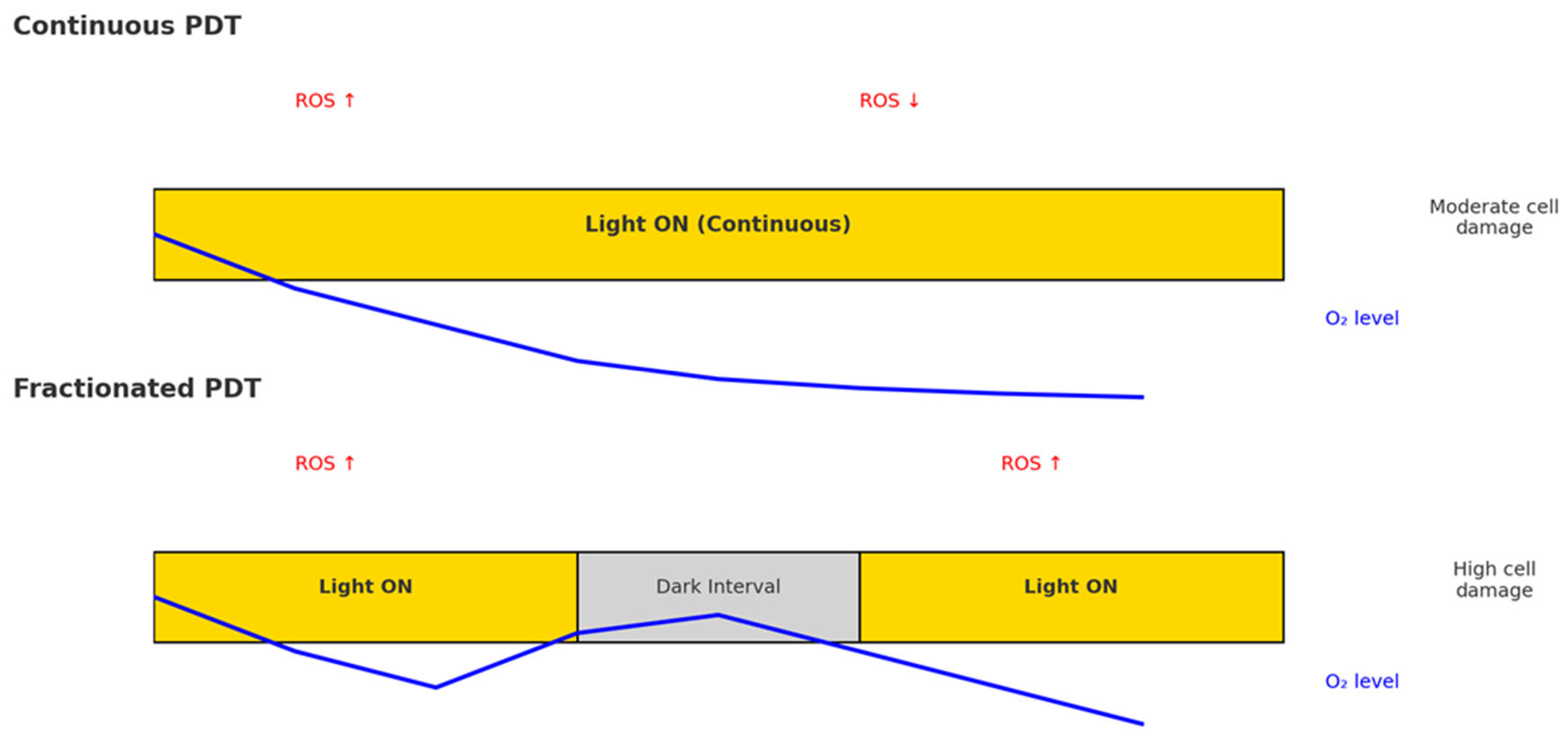
3. Light Fractionation Concept
4. Mechanistic Insights from Preclinical Models of Light Fractionation in PDT
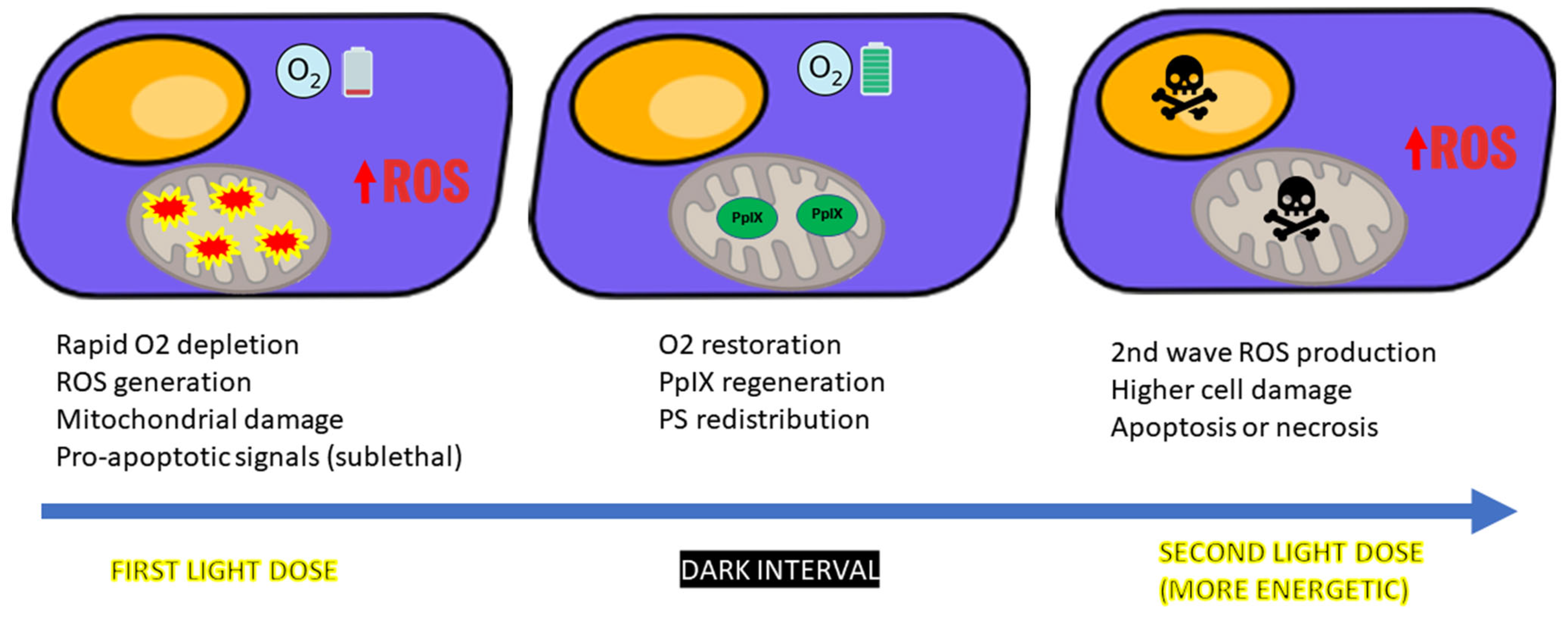
4.1. ROS Dynamics and Tissue Reoxygenation
4.2. Sublethal Damage and PpIX Dynamics
4.3. Vascular Disruption
5. Clinical Evidence for Light Fractionation in PDT
6. Influencing Factors, Limitations, Clinical Implications, and Future Directions of Light Fractionation in PDT
6.1. Influencing Factors
- −
- PS type and pharmacokinetics: ALA-based protocols tend to benefit more from light fractionation than MAL-based ones, largely due to differences in PpIX accumulation, subcellular localization, and vascular targeting. ALA leads to broader and deeper PpIX distribution, especially in endothelial and perivascular zones, which enhances the cytotoxic effect of the second illumination.
- −
- Tissue oxygenation and perfusion: The therapeutic window between light fractions must be adequate to allow for tissue reoxygenation. This is influenced by lesion vascularity, anatomical site, and underlying patient conditions. Poorly perfused or hypoxic tissues may fail to benefit fully from fractionation unless additional oxygenation strategies are employed.
- −
- Tumor or lesion characteristics: Superficial lesions such as grade I/II AK or sBCC respond more favorably to fractionated PDT. In contrast, thicker or nodular tumors, while potentially responsive, may require debulking or enhanced delivery strategies to achieve comparable efficacy.
- −
- Protocol design: Fractionation schedules typically involve a low initial fluence, a 2 h dark interval, and a higher second fluence. However, variations exist, including multifractionated schemes or personalized intervals based on real-time oxygen monitoring, which remain under investigation.
6.2. Limitations
6.3. Clinical Implications and Future Directions
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1O2 | singlet oxygen |
| ALA | 5-aminolevulinic acid |
| AK | actinic keratosis |
| BCC | basal cell carcinoma |
| BD | Bowen’s disease |
| CR | complete response |
| DFO | desferrioxamine |
| MAL | methyl aminolevulinate |
| nBCC | nodular basal cell carcinoma |
| PDT | photodynamic therapy |
| PpIX | protoporphyrin IX |
| PS | photosensitizer(s) |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| sBCC | superficial basal cell carcinoma |
References
- Fernández-Guarino, M.; García-Morales, I.; Harto, A.; Montull, C.; Pérez-García, B.; Jaén, P. Photodynamic Therapy: New Indications. Actas Dermo-Sifiliográficas (Engl. Ed.) 2007, 98, 377–395. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications—Actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2225–2238. [Google Scholar] [CrossRef]
- Akhtar, F.; Misba, L.; Khan, A.U. The dual role of photodynamic therapy to treat cancer and microbial infection. Drug Discov. Today 2024, 29, 104099. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ohgari, Y.; Nakayasu, Y.; Kitajima, S.; Sawamoto, M.; Mori, H.; Shimokawa, O.; Matsui, H.; Taketani, S. Mechanisms involved in δ-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: Relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem. Pharmacol. 2005, 71, 42–49. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, H.S.; Brooks, S.; Van Der Ploeg-Van Den Heuvel, A.; Ten Hagen, T.L.M.; De Haas, E.R.M.; Robinson, D.J. Light fractionation significantly increases the efficacy of photodynamic therapy using BF-200 ALA in normal mouse skin. PLoS ONE 2016, 11, e0148850. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Aguilar, M.; Almagro, M.; Correia, O.; Guillén, C.; Harto, A.; Pérez-García, B.; Pérez-Pérez, L.; Redondo, P.; Sánchez-Carpintero, I.; et al. Spanish-Portuguese consensus statement on use of daylight-mediated photodynamic therapy with methyl aminolevulinate in the treatment of actinic keratosis. Actas Dermo-Sifiliográficas 2015, 106, 623–631. [Google Scholar] [CrossRef]
- Garcia-Mouronte, E.; Naharro-Rodriguez, J.; Alonso-Mtz de Salinas, L.; Pérez-González, L.A.; Fernández-Guarino, M. Self-Applied Daylight Photodynamic Therapy: A Paradigm Shift? Int. J. Mol. Sci. 2025, 26, 628. [Google Scholar] [CrossRef]
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Noodt, B.B.; Berg, K.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.L.; Levin, M.K.; Marmur, E.S. The Two Faces of Fractionated Photodynamic Therapy: Increasing Efficacy With Light Fractionation or Adjuvant Use of Fractional Laser Technology. J. Drugs Dermatol. 2016, 15, 1324–1328. [Google Scholar] [PubMed]
- De Haas, E.R.M.; Kruijt, B.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J. Fractionated illumination significantly improves the response of superficial basal cell carcinoma to aminolevulinic acid photodynamic therapy. J. Investig. Dermatol. 2006, 126, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, J.; Luo, Y.; Guo, T.; Zhang, C.; Ou, S.; Long, Y.; Hu, Z. Recent Advances in Strategies for Addressing Hypoxia in Tumor Photodynamic Therapy. Biomolecules 2022, 12, 81. [Google Scholar] [CrossRef]
- de Haas, E.R.M.; de Vijlder, H.C.; Sterenborg, H.J.C.M.; Neumann, H.A.M.; Robinson, D.J. Fractionated aminolevulinic acid-photodynamic therapy provides additional evidence for the use of PDT for non-melanoma skin cancer. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 426–430. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Chovarda, E.; Goussi, C.; Trigoni, A.; Ioannides, D. Single vs. fractionated photodynamic therapy for face and scalp actinic keratoses: A randomized, intraindividual comparison trial with 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 36–40. [Google Scholar] [CrossRef]
- Puizina-Ivić, N.; Zorc, H.; Vanjaka-Rogošić, L.; Mirić, L.; Peršin, A. Fractionated Illumination Improves the Outcome in the Treatment of Precancerous Lesions with Photodynamic Therapy. Coll. Antropol. 2008, 32, 67–73. [Google Scholar]
- Kessels, J.P.H.M.; Kreukels, H.; Nelemans, P.J.; Roozeboom, M.H.; van Pelt, H.; Mosterd, K.; de Haas, E.R.M.; Kelleners-Smeets, N.W.J. Treatment of superficial basal cell carcinoma by topical photodynamic therapy with fractionated 5-aminolaevulinic acid 20% vs. two-stage topical methyl aminolaevulinate: Results of a randomized controlled trial. Br. J. Dermatol. 2018, 178, 1056–1063. [Google Scholar] [CrossRef]
- Naghavi, N.; Miranbaygi, M.H.; Sazgarnia, A. Simulation of fractionated and continuous irradiation in photodynamic therapy: Study the differences between photobleaching and singlet oxygen dose deposition. Australas. Phys. Eng. Sci. Med. 2011, 34, 203–211. [Google Scholar] [CrossRef]
- De Souza, A.L.R.; Marra, K.; Gunn, J.; Samkoe, K.S.; Kanick, S.C.; Davis, S.C.; Chapman, M.S.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer. Br. J. Cancer 2016, 115, 805–813. [Google Scholar] [CrossRef]
- Sun, H.; Ong, Y.H.; Zhu, T.C. Reactive oxygen species explicit dosimetry (ROSED) for fractionated photofrin-mediated photodynamic therapy (PDT). Proc. SPIE Int. Soc. Opt. Eng. 2022, 11940, 35–40. [Google Scholar] [CrossRef]
- Sun, H.; Rastogi, V.; Zhu, T.C. Evaluation of fractionated PHOTOFRIN-mediated photodynamic therapy using different light fluences with Reactive Oxygen Species Explicit Dosimetry (ROSED). Proc. SPIE Int. Soc. Opt. Eng. 2023, 12359, 33–38. [Google Scholar] [CrossRef]
- Sun, H.; Yang, W.; Ong, Y.; Busch, T.M.; Zhu, T.C. Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival. Cancers 2023, 15, 5682. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, H.S.; De Haas, E.R.M.; Hebeda, K.M.; Van Der Ploeg-Van Den Heuvel, A.; Sterenborg, H.J.C.M.; Neumann, H.A.M.; Robinson, D.J. Light fractionation does not enhance the efficacy of methyl 5-aminolevulinate mediated photodynamic therapy in normal mouse skin. Photochem. Photobiol. Sci. 2007, 6, 1325–1331. [Google Scholar] [CrossRef]
- Grebeňová, D.; Kuželová, K.; Smetana, K.; Pluskalová, M.; Cajthamlová, H.; Marinov, I.; Fuchs, O.; Souček, J.; Jarolím, P.; Hrkal, Z. Mitochondrial and endoplasmic reticulum stress-induced apoptotic pathways are activated by 5-aminolevulinic acid-based photodynamic therapy in HL60 leukemia cells. J. Photochem. Photobiol. B Biol. 2003, 69, 71–85. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, H.S.; Casas, A.G.; Di Venosa, G.; Gandara, L.; Sterenborg, H.J.C.M.; Batlle, A.; Robinson, D.J. Light fractionated ALA-PDT enhances therapeutic efficacy in vitro; The influence of PpIX concentration and illumination parameters. Photochem. Photobiol. Sci. 2013, 12, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; de Bruijn, H.S.; Star, W.M.; Sterenborg, H.J.C.M. Dose and Timing of the First Light Fraction in Two-fold Illumination Schemes for Topical ALA-mediated Photodynamic Therapy of Hairless Mouse Skin. Photochem. Photobiol. 2003, 77, 319. [Google Scholar] [CrossRef]
- Star, W.M.; Van’t Veen, A.J.; Robinson, D.J.; Munte, K.; De Haas, E.R.M.; Sterenborg, H.J.C.M. Topical 5-aminolaevulinic acid mediated photodynamic therapy of superficial basal cell carcinoma using two light fractions with a two-hour interval: Long-term follow-up. Acta. Derm. Venereol. 2006, 86, 412–417. [Google Scholar] [CrossRef]
- Aguilar Cosme, J.R.; Gagui, D.C.; Green, N.H.; Bryant, H.E.; Claeyssens, F. In Vitro Low-Fluence Photodynamic Therapy Parameter Screening Using 3D Tumor Spheroids Shows that Fractionated Light Treatments Enhance Phototoxicity. ACS Biomater. Sci. Eng. 2021, 7, 5078–5089. [Google Scholar] [CrossRef]
- Middelburg, T.A.; De Vijlder, H.C.; De Bruijn, H.S.; Van Der Ploeg-Van Den Heuvel, A.; Neumann, H.A.M.; De Haas, E.R.M.; Robinson, D.J. Topical photodynamic therapy using different porphyrin precursors leads to differences in vascular photosensitization and vascular damage in normal mouse skin. Photochem. Photobiol. 2014, 90, 896–902. [Google Scholar] [CrossRef]
- van der Veen, N.; van Leengoed, H.L.L.M.; Star, W.M. In vivo fluorescence kinetics and photodynamic therapy using 5-aminolaevulinic acid-induced porphyrin: Increased damage after multiple irradiations. Br. J. Cancer 1994, 70, 867–872. [Google Scholar] [CrossRef]
- De Vijlder, H.C.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J.; De Haas, E.R.M. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: Five-year follow-up of a randomized, prospective trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]
- Van Delft, L.C.J.; Nelemans, P.J.; Kessels, J.P.H.M.; Kreukels, H.; Roozeboom, M.H.; De Rooij, M.J.M.; Mosterd, K.; De Haas, E.R.M.; Kelleners-Smeets, N.W.J. Long-Term Efficacy of Photodynamic Therapy with Fractionated 5-Aminolevulinic Acid 20% versus Conventional Two-Stage Topical Methyl Aminolevulinate for Superficial Basal-Cell Carcinoma. Dermatology 2022, 238, 1044–1049. [Google Scholar] [CrossRef]
- Roozeboom, M.H.; Aardoom, M.A.; Nelemans, P.J.; Thissen, M.R.T.M.; Kelleners-Smeets, N.W.J.; Kuijpers, D.I.M.; Mosterd, K. Fractionated 5-aminolevulinic acid photodynamic therapy after partial debulking versus surgical excision for nodular basal cell carcinoma: A randomized controlled trial with at least 5-year follow-up. J. Am. Acad. Dermatol. 2013, 69, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, K.; Thissen, M.R.T.M.; Nelemans, P.; Kelleners-Smeets, N.W.J.; Janssen, R.L.L.T.; Broekhof, K.G.M.E.; Neumann, H.A.M.; Steijlen, P.M.; Kuijpers, D.I.M. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: Results of a randomized controlled trial. Br. J. Dermatol. 2008, 159, 864–870. [Google Scholar] [CrossRef] [PubMed]
- De Haas, E.R.M.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J. Response of Bowen disease to ALA-PDT using a single and a 2-fold illumination scheme. Arch. Dermatol. 2007, 143, 264–265. [Google Scholar] [CrossRef]
- SuÁrez-Pérez, J.A.; LÓpez-Navarro, N.; Herrera-Acosta, E.; Aguilera, J.; Gallego, E.; Bosch, R.; Herrera, E. Treatment of actinic cheilitis with methyl aminolevulinate photodynamic therapy and light fractionation: A prospective study of 10 patients. Eur. J. Dermatol. 2015, 25, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Smits, T.; Kleinpenning, M.M.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M.; Gerritsen, M.J.P. A placebo-controlled randomized study on the clinical effectiveness, immunohistochemical changes and protoporphyrin IX accumulation in fractionated 5-aminolaevulinic acid-photodynamic therapy in patients with psoriasis. Br. J. Dermatol. 2006, 155, 429–436. [Google Scholar] [CrossRef]
- Khan, K.; Khan, A.U.; Ghufran; Khan, A.; Khan, M.; Ahmad, I. Fractionated illumination improves the treatment outcomes of photodynamic therapy for high grade cutaneous leishmaniasis. Photodiagn. Photodyn. Ther. 2020, 29, 101622. [Google Scholar] [CrossRef]
- Vicentini, C.; Vignion-Dewalle, A.S.; Thecua, E.; Lecomte, F.; Maire, C.; Deleporte, P.; Béhal, H.; Kerob, D.; Duhamel, A.; Mordon, S.; et al. Photodynamic therapy for actinic keratosis of the forehead and scalp: A randomized, controlled, phase II clinical study evaluating the noninferiority of a new protocol involving irradiation with a light-emitting, fabric-based device (the Flexitheralight protocol) compared with the conventional protocol involving irradiation with the Aktilite CL 128 lamp. Br. J. Dermatol. 2019, 180, 765–773. [Google Scholar] [CrossRef]
- Ramirez, D.P.; Moriyama, L.T.; de Oliveira, E.R.; Inada, N.M.; Bagnato, V.S.; Kurachi, C.; Salvio, A.G. Single visit PDT for basal cell carcinoma—A new therapeutic protocol. Photodiagn. Photodyn. Ther. 2019, 26, 375–382. [Google Scholar] [CrossRef]
- Salvio, A.G.; Veneziano, D.B.; Moriyama, L.T.; Inada, N.M.; Grecco, C.; Kurachi, C.; Bagnato, V.S. A new photodynamic therapy protocol for nodular basal cell carcinoma treatment: Effectiveness and long-term follow-up. Photodiagn. Photodyn. Ther. 2022, 37, 102668. [Google Scholar] [CrossRef] [PubMed]
- Oberdanner, C.B.; Plaetzer, K.; Kiesslich, T.; Krammer, B. Photodynamic Treatment with Fractionated Light Decreases Production of Reactive Oxygen Species and Cytotoxicity in vitro via Regeneration of Glutathione. Photochem. Photobiol. 2005, 81, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Turan, I.S.; Yildiz, D.; Turksoy, A.; Gunaydin, G.; Akkaya, E.U. A Bifunctional Photosensitizer for Enhanced Fractional Photodynamic Therapy: Singlet Oxygen Generation in the Presence and Absence of Light. Angew. Chem.—Int. Ed. 2016, 55, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, L.; Zhu, J.; Li, X.; Yang, Z.; Huang, W.; Chen, X. Singlet Oxygen “Afterglow” Therapy with NIR-II Fluorescent Molecules. Adv. Mater. 2021, 33, 2103627. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Mao, R.; Wang, Y.; Yan, X.; Liu, J. Persistent luminescent nanoparticles as energy mediators for enhanced photodynamic therapy with fractionated irradiation. J. Mater. Chem. B 2017, 5, 5793–5805. [Google Scholar] [CrossRef]
- Li, A.; Liang, C.; Xu, L.; Wang, Y.; Liu, W.; Zhang, K.; Liu, J.; Shi, J. Boosting 5-ALA-based photodynamic therapy by a liposomal nanomedicine through intracellular iron ion regulation. Acta Pharm. Sin. B 2021, 11, 1329–1340. [Google Scholar] [CrossRef]
- Kan, D.; Ding, R.; Yang, H.; Jia, Y.; Lei, K.; Wang, Z.; Zhang, W.; Yang, C.; Liu, Z.; Xie, F.; et al. Synergistic strategies in photodynamic combination therapy for cancer: Mechanisms, nanotechnology, and clinical translation. Front. Oncol. 2025, 15, 1607259. [Google Scholar] [CrossRef]
- Lindholm, V.; Salmivuori, M.; Hahtola, S.; Mäkelä, K.; Pitkänen, S.; Isoherranen, K. Ablative Fractional Laser Enhances Artificial or Natural Daylight Photodynamic Therapy of Actinic Field Cancerization: A Randomized and Investigator-initiated Half-side Comparative Study. Acta Derm.-Venereol. 2023, 103, adv6579. [Google Scholar] [CrossRef]
- Yi, J.; Liu, L.; Gao, W.; Zeng, J.; Chen, Y.; Pang, E.; Lan, M.; Yu, C. Advances and perspectives in phototherapy-based combination therapy for cancer treatment. J. Mater. Chem. B 2024, 12, 6285–6304. [Google Scholar] [CrossRef]
- Collier, N.J.; Haylett, A.K.; Wong, T.H.; Morton, C.A.; Ibbotson, S.H.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Seukeran, D.; Ward, K.A.; et al. Conventional and combination topical photodynamic therapy for basal cell carcinoma: Systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 1277–1296. [Google Scholar] [CrossRef]
- Qiao, S.; Tang, H.; Xia, J.; Ding, M.; Qiao, S.; Niu, Y.; Jiang, G. Efficacy and safety of microneedling, fractional CO2 laser, and cryotherapy combined with 5-aminolevulinic acid photodynamic therapy in the treatment of actinic keratosis: A multicenter prospective randomized controlled study. Photodiagn. Photodyn. Ther. 2023, 43, 103700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Qu, Y.; Jin, Y.; Cao, J.; Zhan, J.; Li, Z.; Chai, C.; Huang, C.; Li, M. Ferroptosis: A novel cell death modality as a synergistic therapeutic strategy with photodynamic therapy. Photodiagn. Photodyn. Ther. 2025, 51, 104463. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dou, W.T.; Yang, C.; Zhou, L.; Wang, F.; He, L.; Qian, X.; Xu, L. Small molecule photosensitizers: Paving the way for improved photodynamic therapy in dermatology. Coord. Chem. Rev. 2025, 541, 216839. [Google Scholar] [CrossRef]
- Mohanty, S.; Desai, V.M.; Jain, R.; Agrawal, M.; Dubey, S.K.; Singhvi, G. Unveiling the potential of photodynamic therapy with nanocarriers as a compelling therapeutic approach for skin cancer treatment: Current explorations and insights. RSC Adv. 2024, 14, 21915–21937. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Meng, C.; Feng, F. Recent advances for enhanced photodynamic therapy: From new mechanisms to innovative strategies. Chem. Sci. 2024, 15, 12234–12257. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, 2308374121. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Jetten, A.M.; Slominski, A.T. Neuro-immuno-endocrinology of the skin: How environment regulates body homeostasis. Nat. Rev. Endocrinol. 2025, 21, 495–509. [Google Scholar] [CrossRef]
| Clinical Indication | Number of Lesions | Photo- Sensitizer | Fractionation Protocol | Comparator | Results | |
|---|---|---|---|---|---|---|
| Sotiriou et al. [16] | AK | 266 | ALA | 20 + 80 J/cm2, 2 h dark interval | 75 J/cm2 × 2 (7 days apart) | Higher CR at 3 and 12 months (93.8% vs. 85.4%) |
| de Haas et al. [15] | AK, BD, sBCC, nBCC | 552 (70 AK, 32 BD, 430 sBCC, 20 nBCC) | ALA | 20 + 80 J/cm2, 2 h interval | None | CR: AK 98%, BD 84%, sBCC 97%, nBCC 80% at 2 years |
| Puizina-Ivić et al. [17] | AK, BD | 51 (36 AK, 15 BD) | ALA | 50 + 50 J/cm2, 2 h dark interval | 100 J/cm2 single illumination | Significantly less residual tumor in fractionated group at 24 weeks (4% vs. 73%) |
| de Haas et al. [13]; C. de Vijlder et al. [32] | sBCC | 505 | ALA | 20 + 80 J/cm2, 2 h dark interval | 75 J/cm2 single illumination | CR: 97% vs. 89% at 12 months; 88% vs. 75% at 5 years |
| Kessels et al. [18]; van Delft et al. [33] | sBCC | 162 | ALA vs. MAL | ALA: 20 + 80 J/cm2 | MAL: 37 J/cm2 × 2 (7 days apart) | Higher CR at 12 months (92.3% vs. 82.4%; not statistically significant), but lower long-term tumor-free survival after 5 years (70.7% vs. 76.5%) |
| Roozeboom et al. [34]; Mosterd et al. [35] | nBCC | 173 | ALA | 75 + 75 J/cm2, 1 h dark interval; 3 weeks after debulking | Surgical excision | Higher recurrence at 5 years (30.7% vs. 2.3%). Better recurrence-free survival with PDT in tumors ≤ 0.7 mm (94.4% vs. 65%) |
| de Haas et al. [36] | BD | 50 | ALA | 20 + 80 J/cm2, 2 h dark interval | 75 J/cm2 single illumination | Higher CR at 12 months (88% vs. 80%; not statistically significant) |
| Suárez-Pérez et al. [37] | Actinic cheilitis | 10 | MAL | 20 + 80 J/cm2, 2 h dark interval | None | CR: 80% at 3 months; 60% at 18 months. |
| Smits et al. [38] | Psoriasis (stable plaques) | 8 | ALA | 2 + 8 J/cm2 weekly × 4 | Placebo | Clinical + histological improvement; Koebnerization (25%) |
| Khan et al. [39] | Cutaneous leishmaniasis | 104 | MAL | 30 + 30+ 30 J/cm2 (0 h, 2 h, 16 h) | 90 J/cm2 single illumination | Higher CR at 9 months (91.4% vs. 76%); less pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Mtz de Salinas, L.; Garcia-Mouronte, E.; Naharro-Rodriguez, J.; Perez-Gonzalez, L.A.; Fernández-Guarino, M. Optimization of Photodynamic Therapy in Dermatology: The Role of Light Fractionation. Int. J. Mol. Sci. 2025, 26, 8054. https://doi.org/10.3390/ijms26168054
Alonso-Mtz de Salinas L, Garcia-Mouronte E, Naharro-Rodriguez J, Perez-Gonzalez LA, Fernández-Guarino M. Optimization of Photodynamic Therapy in Dermatology: The Role of Light Fractionation. International Journal of Molecular Sciences. 2025; 26(16):8054. https://doi.org/10.3390/ijms26168054
Chicago/Turabian StyleAlonso-Mtz de Salinas, Luis, Emilio Garcia-Mouronte, Jorge Naharro-Rodriguez, Luis Alfonso Perez-Gonzalez, and Montserrat Fernández-Guarino. 2025. "Optimization of Photodynamic Therapy in Dermatology: The Role of Light Fractionation" International Journal of Molecular Sciences 26, no. 16: 8054. https://doi.org/10.3390/ijms26168054
APA StyleAlonso-Mtz de Salinas, L., Garcia-Mouronte, E., Naharro-Rodriguez, J., Perez-Gonzalez, L. A., & Fernández-Guarino, M. (2025). Optimization of Photodynamic Therapy in Dermatology: The Role of Light Fractionation. International Journal of Molecular Sciences, 26(16), 8054. https://doi.org/10.3390/ijms26168054







