Cytidine and dCMP Deaminases—Current Methods of Activity Analysis
Abstract
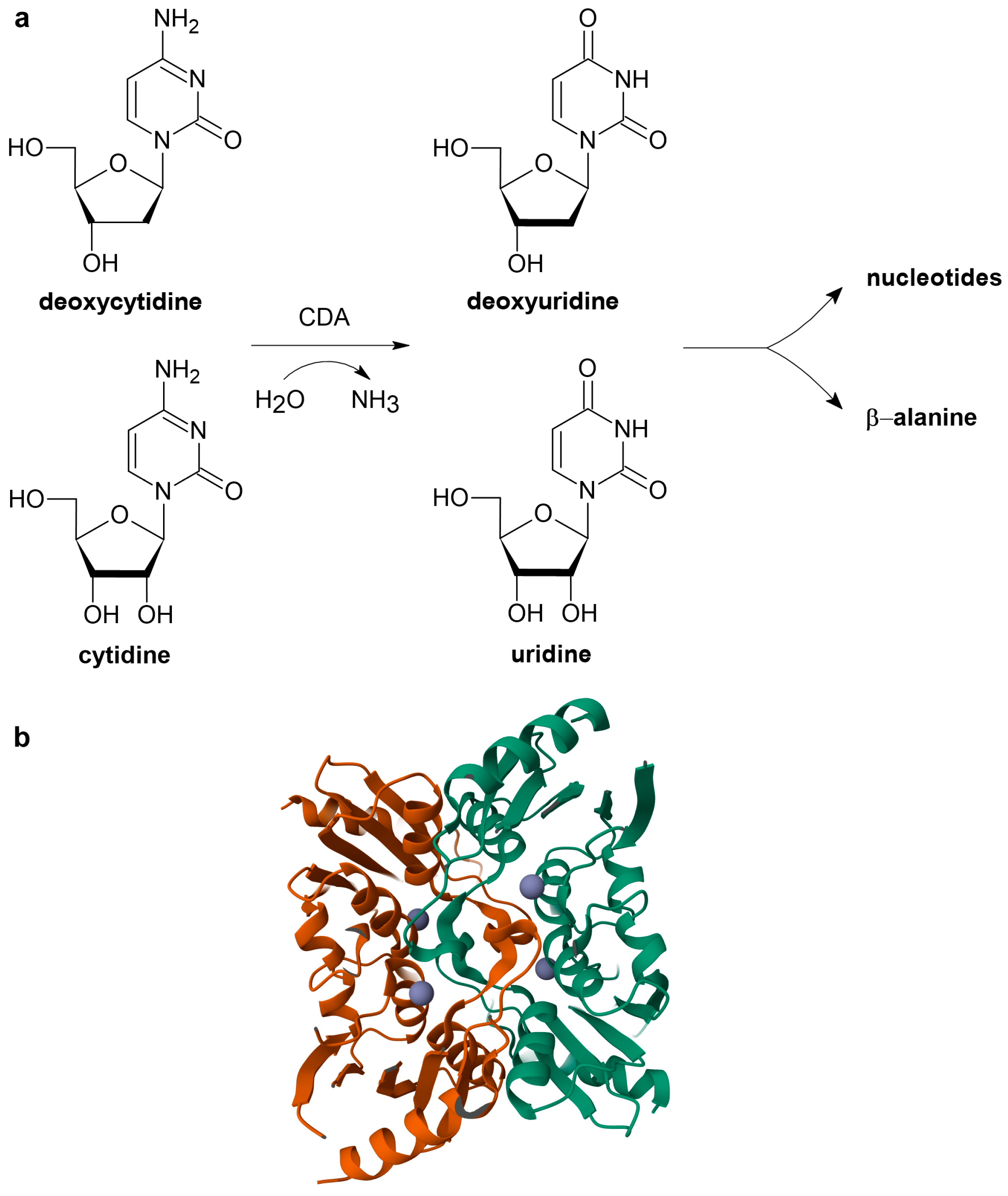
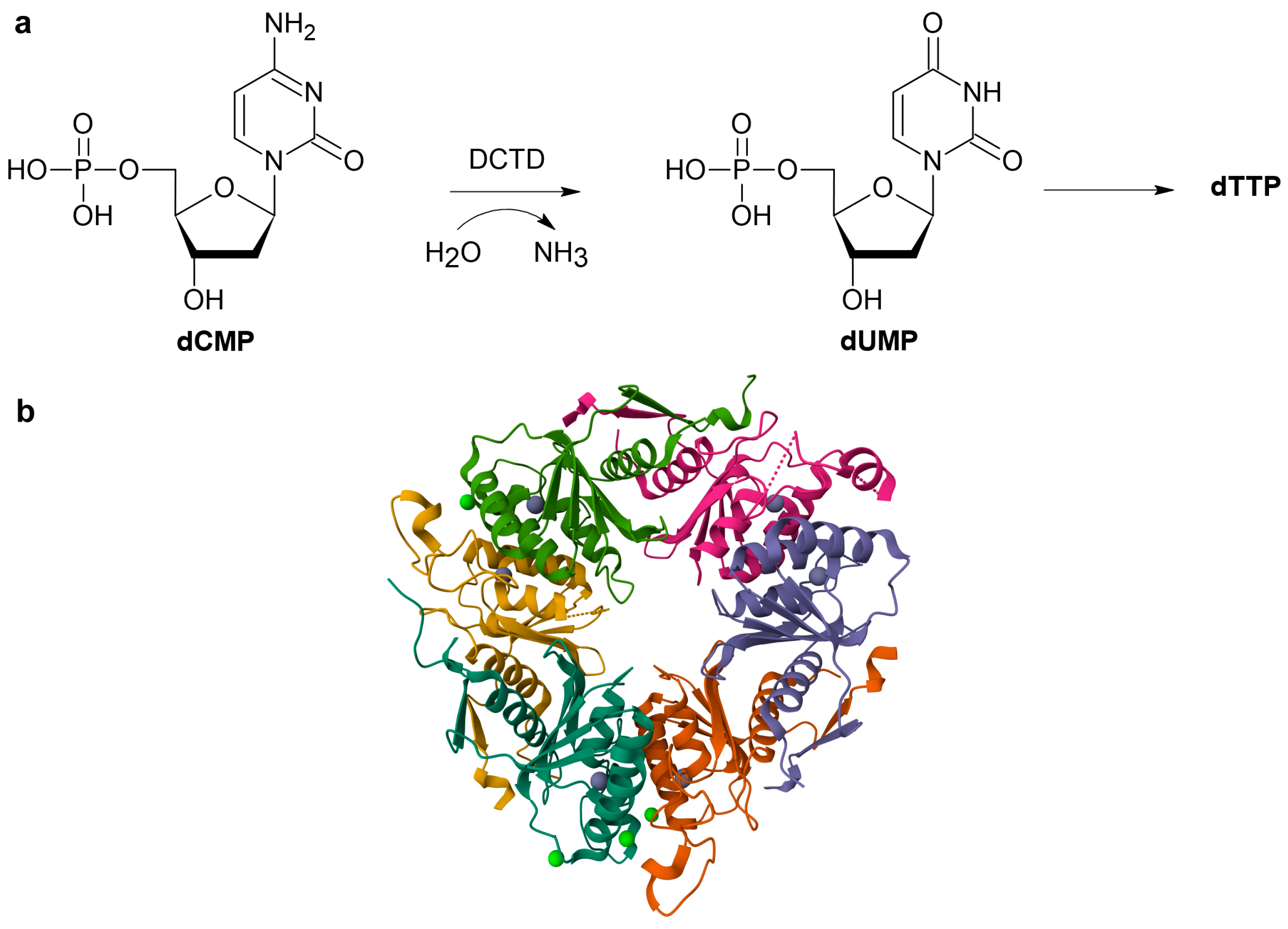
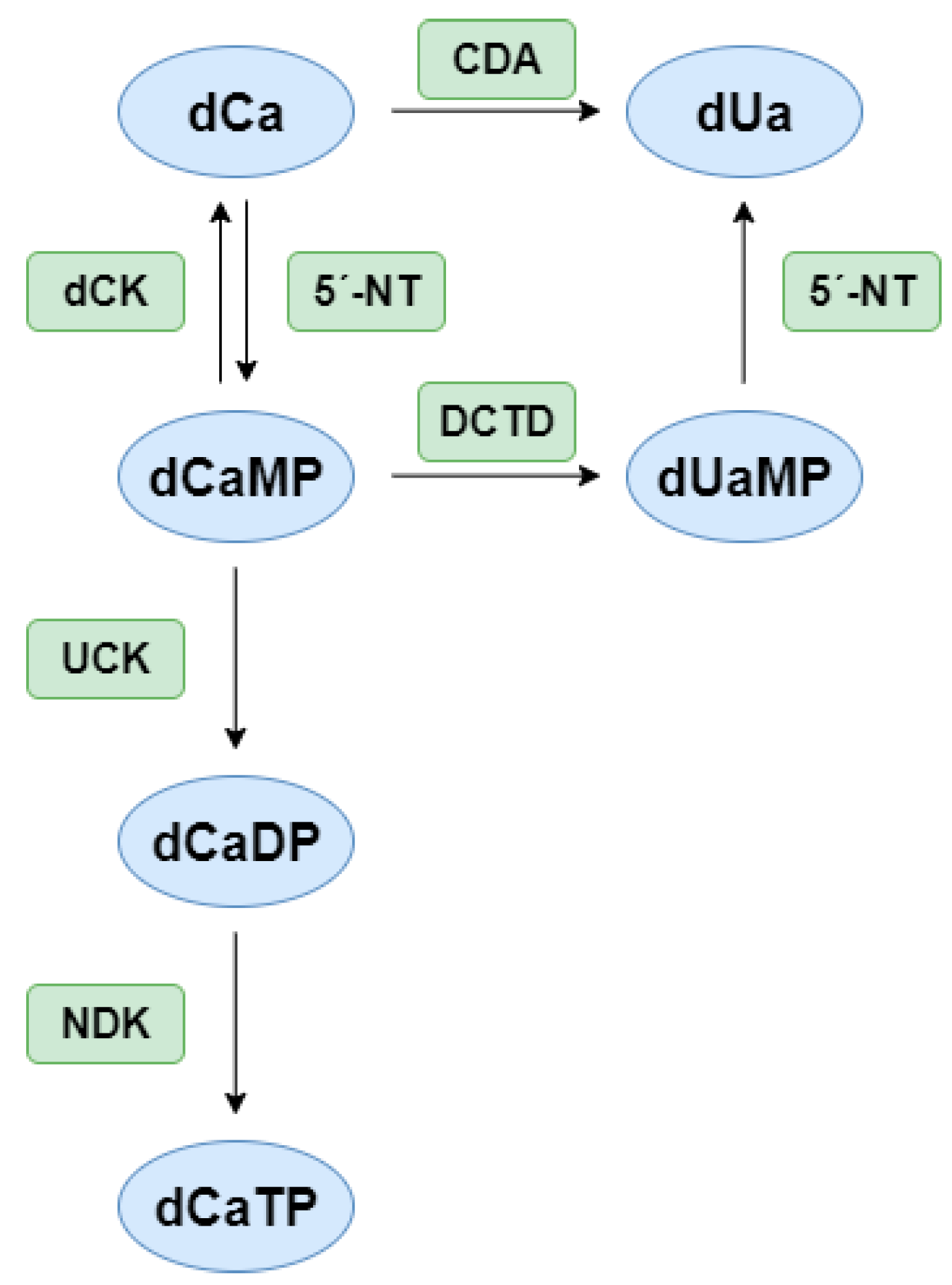
1. Introduction
2. Spectrophotometric and Fluorimetric Assays
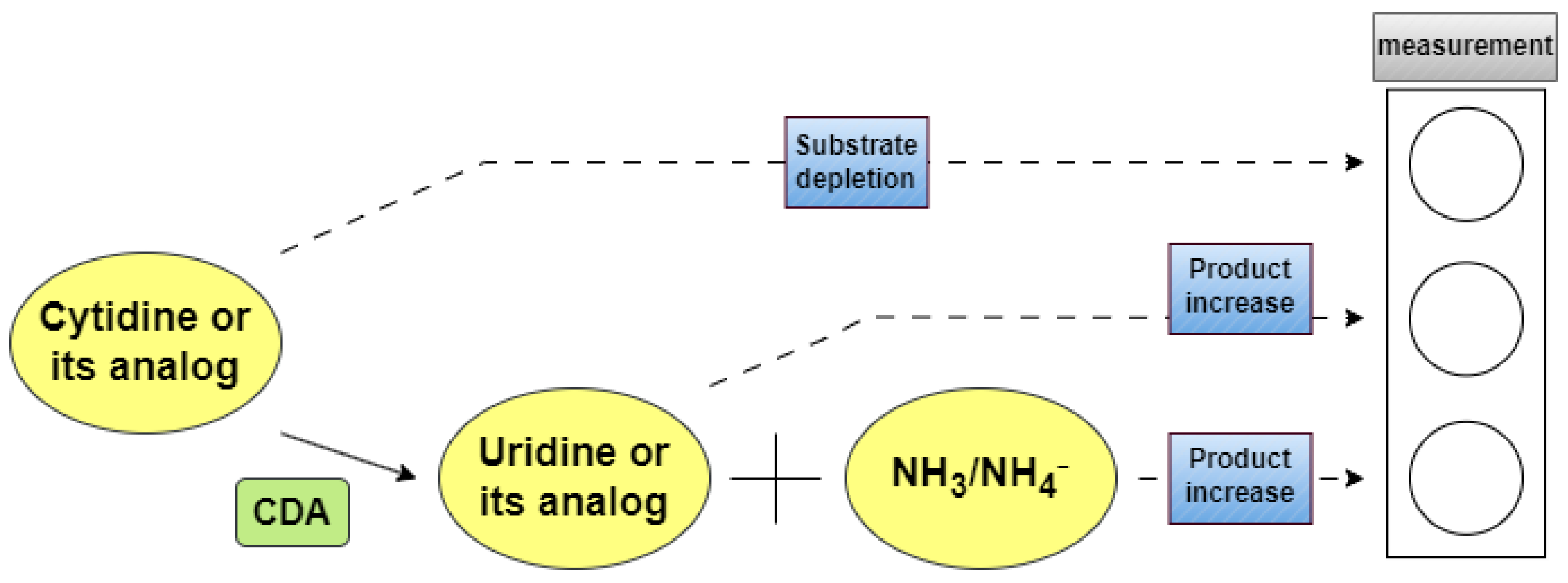
2.1. Methods Based on Absorption Spectrophotometry
2.1.1. Direct UV-Vis Spectrophotometric Assays
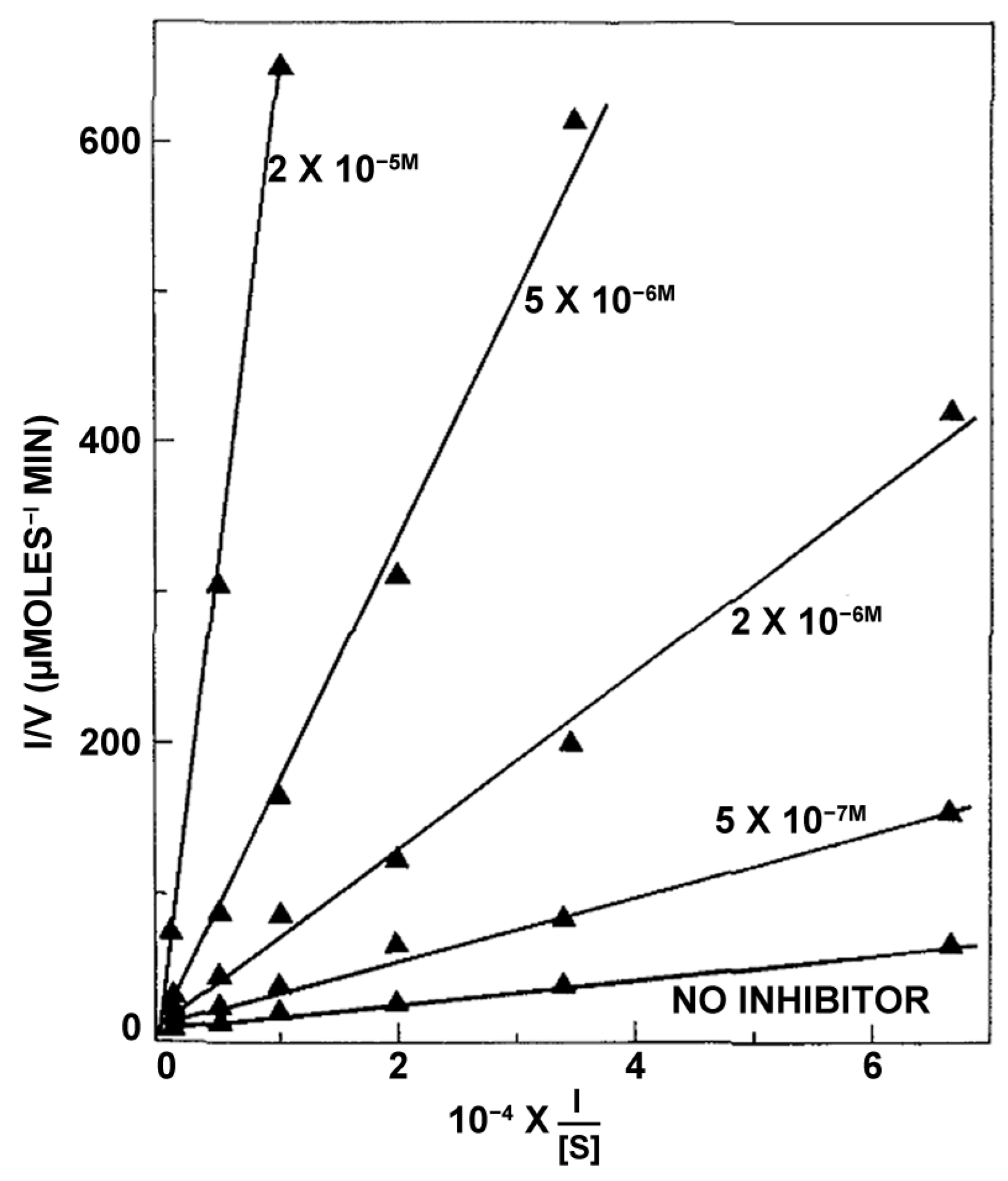
2.1.2. Indirect Spectrophotometric Assays
2.2. Fluorescence-Based Methods
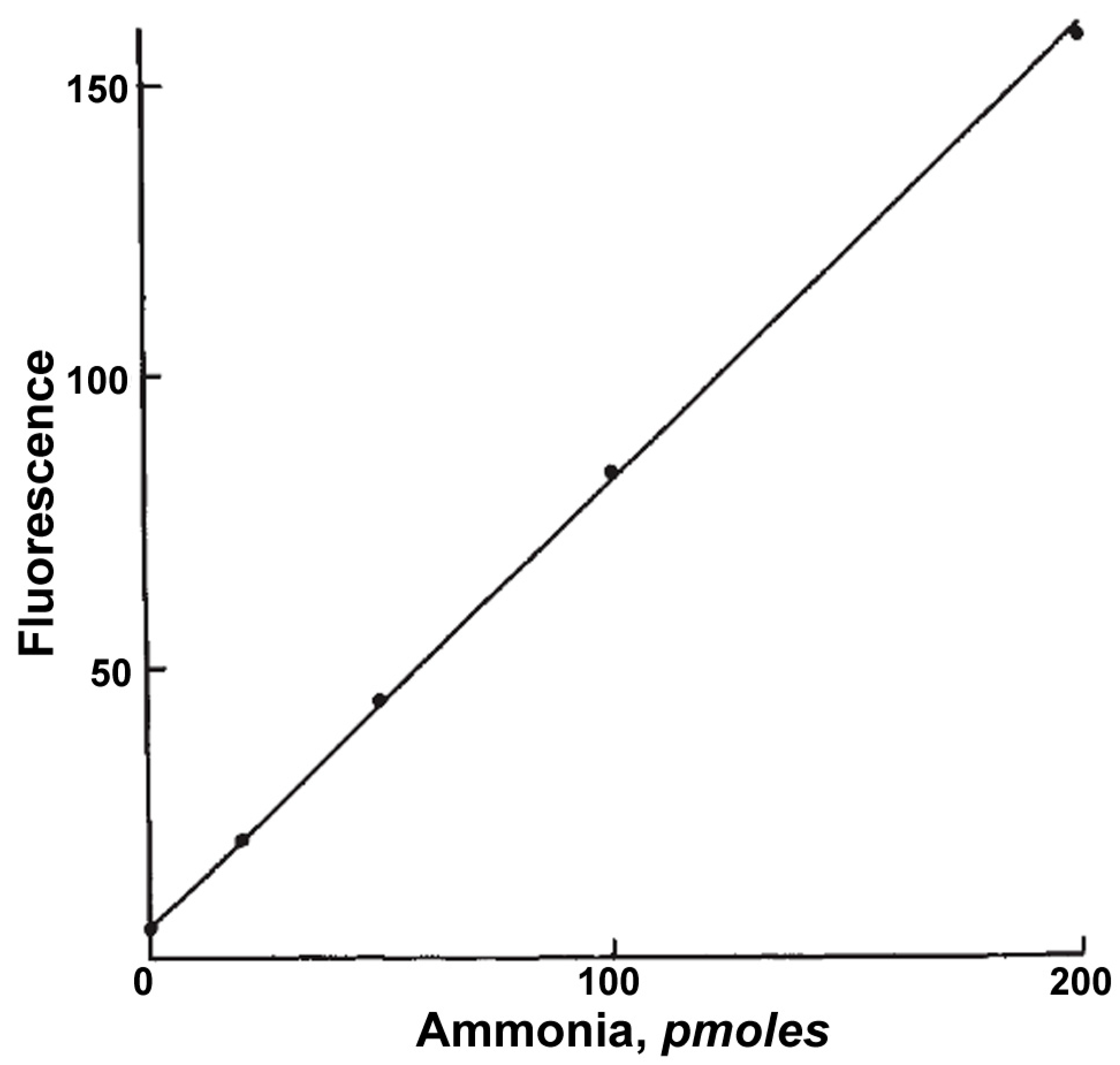
2.2.1. Indirect Fluorimetric Assays
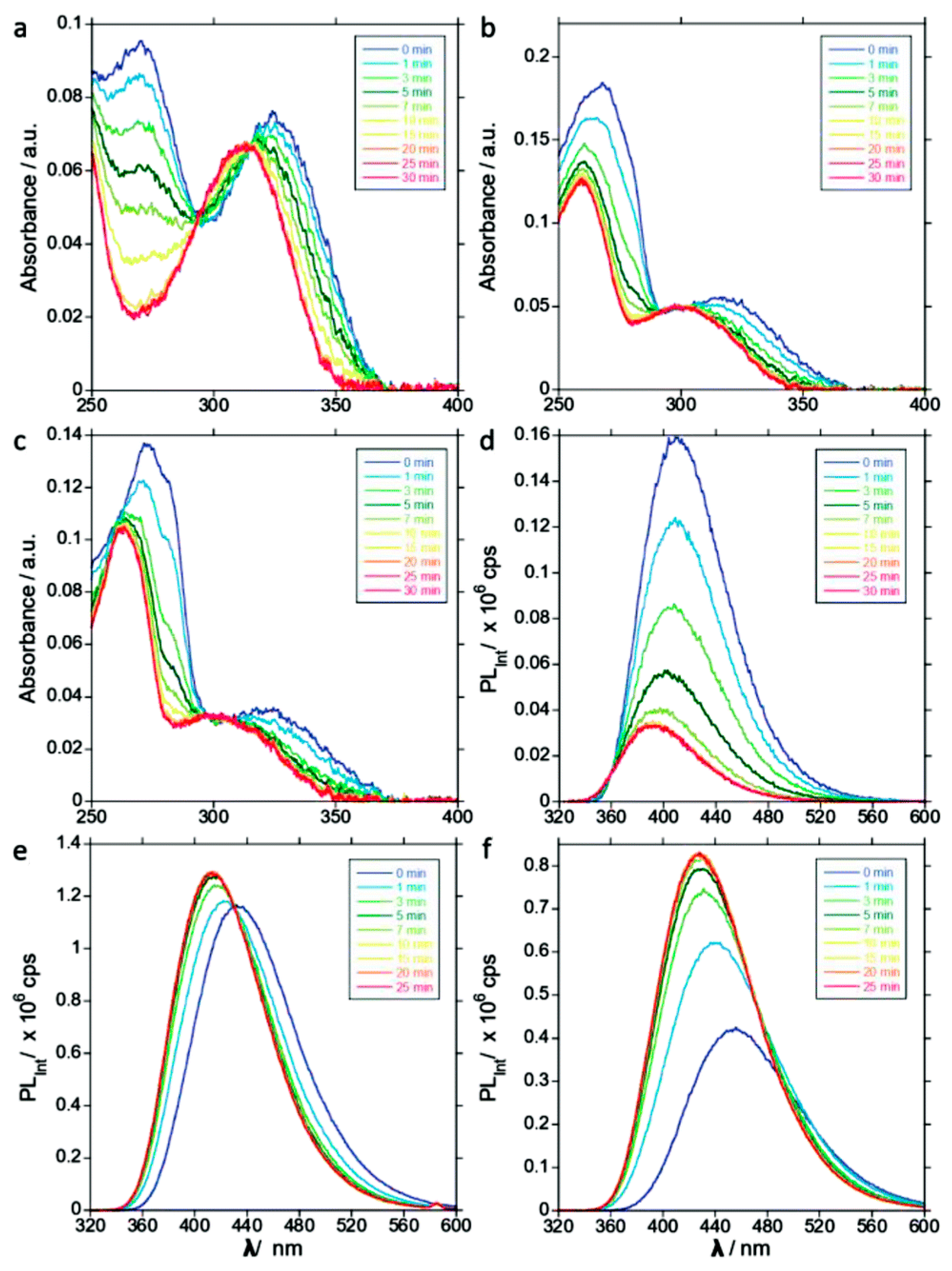
2.2.2. Direct Fluorimetric Assays
2.3. Summary and Comparison of Spectrophotometric and Fluorimetric Approaches
3. Liquid Chromatography and LC-Coupled Detection Methods
3.1. Separation Modes for CDA/DCTD Substrates and Products
Column Selection
3.2. LC-UV/Vis and LC-Fluorescence Based Assays
3.2.1. LC-UV/Vis
3.2.2. LC-FLD
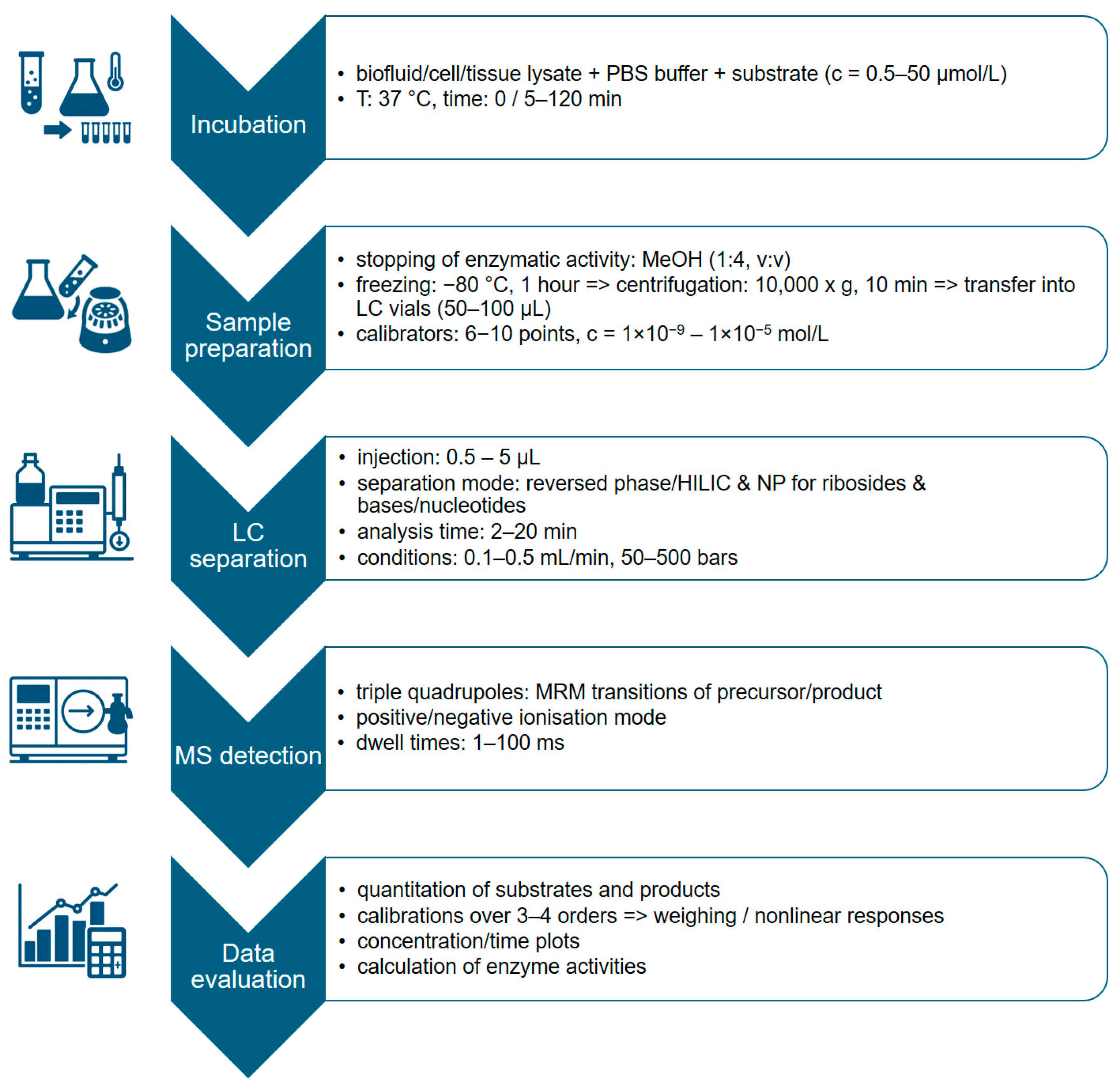
3.3. LC-MS Based Assays
Measurement of Enzyme Activity Using LC-MS
3.4. Summary and Comparison of Liquid Chromatography-Based Methods for CDA and DCTD Activity Analysis
4. Radiometric Assays
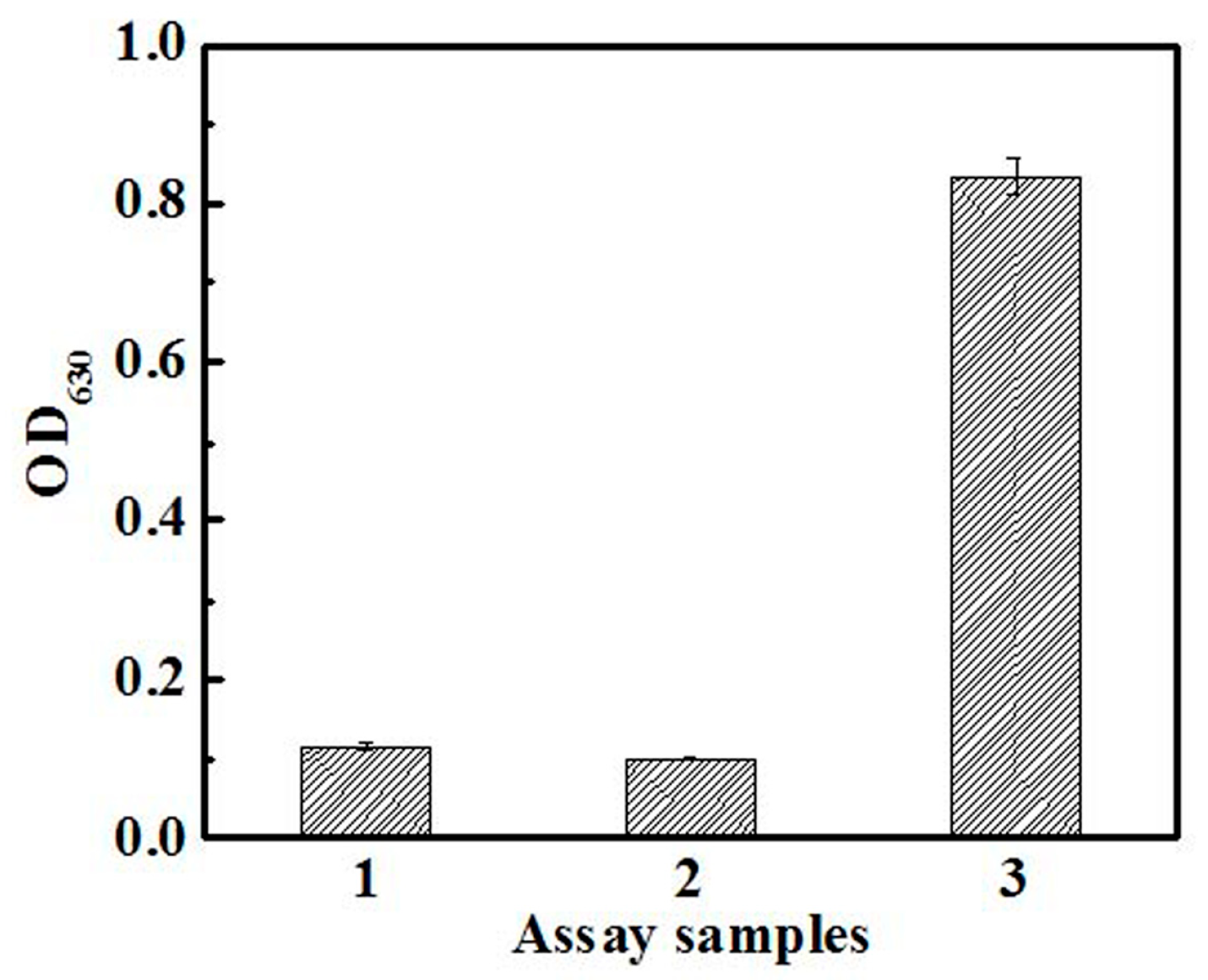
5. Cell-Based Assays
6. Considerations for Method Selection and Application
7. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDA | Cytidine deaminase |
| DCTD | Deoxycytidine monophosphate deaminase; dCMP deaminase |
| dCTP | 2′-Deoxycytidine 5′-triphosphate |
| ATP | Adenosine 5′-triphosphate |
| dCMP | 2′-Deoxycytidine 5′-monophosphate |
| dUMP | 2′-Deoxyuridine 5′-monophosphate |
| dTTP | Thymidine 5′-triphosphate |
| hmdCMP | 5-Hydroxymethyl-2′-deoxycytidine 5′-monophosphate |
| hmdUMP | 5-Hydroxymethyl-2′-deoxyuridine 5′-monophosphate |
| dNTP | Deoxynucleotide triphosphate |
| MDS | Myelodysplastic syndrome |
| AML | Acute myeloid leukemia |
| dCa | 2′-Deoxycytidine analog |
| dUa | 2′-Deoxyuridine analog |
| dCaMP | Monophosphate form of dCa |
| dCaDP | Diphosphate form of dCa |
| dCaTP | Triphosphate form of dCa |
| dCK | Deoxycytidine kinase |
| UCK | UMP-CMP kinase |
| NDK | Nucleoside diphosphate kinase; |
| 5′-NT | 5′-Nucleotidases |
| dUaMP | Monophosphate form of dUa |
| FdUMP | 5-Fluoro-2′-deoxyuridine monophosphate |
| 5hmdC | 5-Hydroxymethyl-2ʹ-deoxycytidine |
| 5fdC | 5-Formyl-2ʹ-deoxycytidine |
| 5hmdU | 5-Hydroxymethyl-2ʹ-deoxyuridine |
| 5fdU | 5-Formyl-2ʹ-deoxyuridine |
| SNP | Single nucleotide polymorphism |
| UV | Ultraviolet |
| Vis | Visible |
| GLDH | Glutamate dehydrogenase |
| NADH | Nicotinamide adenine dinucleotide |
| OPA | o-Phthaldialdehyde |
| EdC | 5-Ethynyl-2ʹ-deoxycytidine |
| FC | 5-Fluorocytidine |
| EdU | 5-Ethynyl-2ʹ-deoxyuridine |
| FU | 5-Fluorouridine |
| EdUTP | 5-Ethynyl-2ʹ-deoxyuridine triphosphate |
| EdCMP | 5-Ethynyl-2ʹ-deoxycytidine monophosphate |
| EdUMP | 5-Ethynyl-2ʹ-deoxyuridine monophosphate |
| BrdU | 5-Bromo-2ʹ-deoxyuridine |
| THU | Tetrahydrouridine |
| HPLC | High-performance liquid chromatography |
| RP-LC | Reversed-phase liquid chromatography |
| NP-LC | Normal-phase liquid chromatography |
| HILIC | Hydrophilic interaction chromatography |
| OMB-COCl | 2-(5-Chlorocarbonyl-2-oxazolyl)-5,6-methylenedioxybenzofuran |
| LC-MS/MS | Liquid chromatography with tandem mass spectrometry |
| rNTP | Ribonucleotide triphosphate |
| ADP | Adenosine 5′-diphosphate |
| PGC | Porous graphitic carbon column |
| CMP | Cytidine 5′-monophosphate |
| CTP | Cytidine 5′-triphosphate |
| dFdC | 2′,2′-Difluoro-2′-deoxycytidine |
| dFdCTP | Gemcitabine triphosphate (2′,2′-Difluorodeoxycytidine 5′-triphosphate) |
| dFdU | 2′,2′-Difluoro-2′-deoxyuridine |
| dFdCMP | Gemcitabine monophosphate (2′,2′-Difluorodeoxycytidine 5′-monophosphate) |
| ara-CMP | Cytarabine monophosphate |
| ara-CDP | Cytarabine diphosphate |
| ara-CTP | Cytarabine triphosphate |
| MALDI | Matrix-assisted laser desorption/ionization |
| ESI | Electrospray ionization |
| LB medium | The Lysogeny Broth medium |
| M9 medium | M9 minimal salts medium |
| tzC | Isothiazolo[4,3-d]pyrimidine analog of cytidine |
| thC | Thieno[3,4-d]pyrimidine analog of cytidine |
| mthC | Methylthieno[3,4-d]pyrimidine analog of cytidine |
| UV-Vis | Ultraviolet-Visible |
| TS | Thymidylate synthase |
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| FLD | Fluorescence detection |
| MS/MS | Tandem mass spectrometry |
| dFdUMP | 2′,2′-Difluoro-2′-deoxyuridine monophosphate |
| Cytarabine | Ara-C; 1-β-D-arabinofuranosylcytosine |
| MRM | Multiple reaction monitoring |
| LOD | Limit of detection |
| ALL | Acute lymphoblastic leukemia |
References
- Ligasova, A.; Kocianova, M.; Koberna, K. A Rapid Approach for Identifying Cell Lines Lacking Functional Cytidine Deaminase. Int. J. Mol. Sci. 2025, 26, 3344. [Google Scholar] [CrossRef]
- Frances, A.; Cordelier, P. The Emerging Role of Cytidine Deaminase in Human Diseases: A New Opportunity for Therapy? Mol. Ther. 2020, 28, 357–366. [Google Scholar] [CrossRef]
- Nygaard, P. On the Role of Cytidine Deaminase in Cellular Metabolism. In Purine and Pyrimidine Metabolism in Man V; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 1986; Volume 195B, pp. 415–420. [Google Scholar] [CrossRef]
- Ruan, H.M.; Qiu, S.B.; Beard, B.C.; Black, M.E. Creation of Zebularine-Resistant Human Cytidine Deaminase Mutants to Enhance the Chemoprotection of Hematopoietic Stem Cells. Protein Eng. Des. Sel. 2016, 29, 573–582. [Google Scholar] [CrossRef]
- Costanzi, S.; Vincenzetti, S.; Vita, A.; Lambertucci, C.; Taffi, S.; Volpini, R.; Vittori, S.; Cristalli, G. Human Cytidine Deaminase: Understanding the Catalytic Mechanism. Nucleosides Nucleotides Nucleic Acids 2003, 22, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Micozzi, D.; Pucciarelli, S.; Carpi, F.M.; Costanzi, S.; De Sanctis, G.; Polzonetti, V.; Natalini, P.; Santarelli, I.F.; Vita, A.; Vincenzetti, S. Role of Tyrosine 33 Residue for the Stabilization of the Tetrameric Structure of Human Cytidine Deaminase. Int. J. Biol. Macromol. 2010, 47, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, N.; Sarwar, R. An Overview of Cytidine Deaminases. Int. J. Hematol. 2006, 83, 195–200. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Quadrini, B.; Mariani, P.; De Sanctis, G.; Cammertoni, N.; Polzonetti, V.; Pucciarelli, S.; Natalini, P.; Vita, A. Modulation of Human Cytidine Deaminase by Specific Aminoacids Involved in the Intersubunit Interactions. Proteins 2008, 70, 144–156. [Google Scholar] [CrossRef]
- Vincenzetti, S.; De Sanctis, G.; Costanzi, S.; Cristalli, G.; Mariani, P.; Mei, G.; Neuhard, J.; Natalini, P.; Polzonetti, V.; Vita, A. Functional Properties of Subunit Interactions in Human Cytidine Deaminase. Protein Eng. 2003, 16, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Betts, L.; Xiang, S.; Short, S.A.; Wolfenden, R.; Carter, C.W., Jr. Cytidine Deaminase. The 2·3 Å Crystal Structure of an Enzyme: Transition-State Analog Complex. J. Mol. Biol. 1994, 235, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Gemble, S.; Ahuja, A.; Buhagiar-Labarchede, G.; Onclercq-Delic, R.; Dairou, J.; Biard, D.S.; Lambert, S.; Lopes, M.; Amor-Gueret, M. Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits Parp-1 Activity, Leading to the under Replication of DNA. PLoS Genet. 2015, 11, e1005384. [Google Scholar] [CrossRef]
- Frances, A.; Lumeau, A.; Bery, N.; Gayral, M.; Stuani, L.; Sorbara, M.; Saland, E.; Pagan, D.; Hanoun, N.; Torrisani, J.; et al. Cytidine Deaminase-Dependent Mitochondrial Biogenesis as a Potential Vulnerability in Pancreatic Cancer Cells. Commun. Biol. 2024, 7, 1065. [Google Scholar] [CrossRef]
- Weiner, K.X.; Weiner, R.S.; Maley, F.; Maley, G.F. Primary Structure of Human Deoxycytidylate Deaminase and Overexpression of Its Functional Protein in Escherichia coli. J. Biol. Chem. 1993, 268, 12983–12989. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Sharma, S.; Rozenzhak, S.; Roguev, A.; Krogan, N.J.; Chabes, A.; Russell, P. Replication Fork Collapse and Genome Instability in a Deoxycytidylate Deaminase Mutant. Mol. Cell. Biol. 2012, 32, 4445–4454. [Google Scholar] [CrossRef]
- Yague-Capilla, M.; Rudd, S.G. Understanding the Interplay between Dntp Metabolism and Genome Stability in Cancer. Dis. Model. Mech. 2024, 17, dmm050775. [Google Scholar] [CrossRef]
- Maley, G.F.; Lobo, A.P.; Maley, F. Properties of an Affinity-Column-Purified Human Deoxycytidylate Deaminase. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1993, 1162, 161–170. [Google Scholar] [CrossRef]
- Slyvka, A.; Rathore, I.; Yang, R.; Gewartowska, O.; Kanai, T.; Lountos, G.T.; Skowronek, K.; Czarnocki-Cieciura, M.; Wlodawer, A.; Bochtler, M. Activity and Structure of Human (D)Ctp Deaminase Cdadc1. Proc. Natl. Acad. Sci. USA 2025, 122, e2424245122. [Google Scholar] [CrossRef]
- Niu, M.; Wang, Y.H.; Wang, C.M.; Lyu, J.; Wang, Y.L.; Dong, H.; Long, W.H.; Wang, D.; Kong, W.Y.; Wang, L.W.; et al. ALR Encoding Dcmp Deaminase Is Critical for DNA Damage Repair, Cell Cycle Progression and Plant Development in Rice. J. Exp. Bot. 2017, 68, 5773–5786. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Tang, C.J.; Luo, M.Z.; Jia, G.Q.; Zhi, H.; Diao, X.M. SisSTL2 Is Required for Cell Cycle, Leaf Organ Development, Chloroplast Biogenesis, and Has Effects on C4 Photosynthesis in Setaria italica (L.) P. Beauv. Front. Plant Sci. 2018, 9, 1103. [Google Scholar] [CrossRef]
- Lachmann, N.; Brennig, S.; Phaltane, R.; Flasshove, M.; Dilloo, D.; Moritz, T. Myeloprotection by Cytidine Deaminase Gene Transfer in Antileukemic Therapy. Neoplasia 2013, 15, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ligasova, A.; Pisklakova, B.; Friedecky, D.; Koberna, K. A New Technique for the Analysis of Metabolic Pathways of Cytidine Analogues and Cytidine Deaminase Activities in Cells. Sci. Rep. 2023, 13, 20530. [Google Scholar] [CrossRef] [PubMed]
- de Vos, D.; van Overveld, W. Decitabine: A Historical Review of the Development of an Epigenetic Drug. Ann. Hematol. 2005, 84 (Suppl. 1), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Salavaggione, O.E.; Ji, Y.; Pelleymounter, L.L.; Eckloff, B.W.; Wieben, E.D.; Ames, M.M.; Weinshilboum, R.M. Gemcitabine Pharmacogenomics: Cytidine Deaminase and Deoxycytidylate Deaminase Gene Resequencing and Functional Genomics. Clin. Cancer Res. 2006, 12, 1794–1803. [Google Scholar] [CrossRef]
- Hamada, A.; Kawaguchi, T.; Nakano, M. Clinical Pharmacokinetics of Cytarabine Formulations. Clin. Pharmacokinet. 2002, 41, 705–718. [Google Scholar] [CrossRef]
- Heinemann, V.; Xu, Y.Z.; Chubb, S.; Sen, A.; Hertel, L.W.; Grindey, G.B.; Plunkett, W. Cellular Elimination of 2′,2′-Difluorodeoxycytidine 5′-Triphosphate: A Mechanism of Self-Potentiation. Cancer Res. 1992, 52, 533–539. [Google Scholar] [PubMed]
- Lamba, J.K. Pharmacogenomics of Cytarabine in Childhood Leukemia. Pharmacogenomics 2011, 12, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Plunkett, W. Modulation of Deoxycytidylate Deaminase in Intact Human Leukemia Cells: Action of 2′,2′-Difluorodeoxycytidine. Biochem. Pharmacol. 1992, 44, 1819–1827. [Google Scholar] [CrossRef]
- Estey, E.H. Acute Myeloid Leukemia: 2014 Update on Risk-Stratification and Management. Am. J. Hematol. 2014, 89, 1063–1081. [Google Scholar] [CrossRef]
- Ferrara, F.; Vitagliano, O. Induction Therapy in Acute Myeloid Leukemia: Is It Time to Put Aside Standard 3 + 7? Hematol. Oncol. 2019, 37, 558–563. [Google Scholar] [CrossRef]
- Budman, D.R.; Meropol, N.J.; Reigner, B.; Creaven, P.J.; Lichtman, S.M.; Berghorn, E.; Behr, J.; Gordon, R.J.; Osterwalder, B.; Griffin, T. Preliminary Studies of a Novel Oral Fluoropyrimidine Carbamate: Capecitabine. J. Clin. Oncol. 1998, 16, 1795–1802. [Google Scholar] [CrossRef]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a Novel Oral Fluoropyrimidine Carbamate, Capecitabine, Which Generates 5-Fluorouracil Selectively in Tumours by Enzymes Concentrated in Human Liver and Cancer Tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Grem, J.L.; Keith, B. Mechanisms of Action of Cancer Chemotherapeutic Agents: Antimetabolites. In The Cancer Handbook; Alison, M.R., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ishikawa, T.; Sekiguchi, F.; Fukase, Y.; Sawada, N.; Ishitsuka, H. Positive Correlation between the Efficacy of Capecitabine and Doxifluridine and the Ratio of Thymidine Phosphorylase to Dihydropyrimidine Dehydrogenase Activities in Tumors in Human Cancer Xenografts. Cancer Res. 1998, 58, 685–690. [Google Scholar]
- Terranova-Barberio, M.; Roca, M.S.; Zotti, A.I.; Leone, A.; Bruzzese, F.; Vitagliano, C.; Scogliamiglio, G.; Russo, D.; D’Angelo, G.; Franco, R.; et al. Valproic Acid Potentiates the Anticancer Activity of Capecitabine In Vitro and In Vivo in Breast Cancer Models Via Induction of Thymidine Phosphorylase Expression. Oncotarget 2016, 7, 7715–7731. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J. Rational Design of New Tumoractivated Cytotoxic Agents. Oncology 1999, 57 (Suppl. 1), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zauri, M.; Berridge, G.; Thézénas, M.L.; Pugh, K.M.; Goldin, R.; Kessler, B.M.; Kriaucionis, S. Cda Directs Metabolism of Epigenetic Nucleosides Revealing a Therapeutic Window in Cancer. Nature 2015, 524, 114–118. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Jiang, W.; Gao, H.; Pang, G.Z.; Wu, Y.S.; Wang, Y.X.; Sheng, M.Y.; Xie, J.Y.; Wu, W.L.; Ji, Z.J.; et al. Dck Confers Sensitivity of Dctd-Positive Cancer Cells to Oxidized Methylcytidines. Protein Cell 2023, 14, 532–537. [Google Scholar] [CrossRef]
- Lamba, J.K. Genetic Factors Influencing Cytarabine Therapy. Pharmacogenomics 2009, 10, 1657–1674. [Google Scholar] [CrossRef]
- Mahfouz, R.Z.; Jankowska, A.; Ebrahem, Q.; Gu, X.; Visconte, V.; Tabarroki, A.; Terse, P.; Covey, J.; Chan, K.; Ling, Y.; et al. Increased Cda Expression/Activity in Males Contributes to Decreased Cytidine Analog Half-Life and Likely Contributes to Worse Outcomes with 5-Azacytidine or Decitabine Therapy. Clin. Cancer Res. 2013, 19, 938–948. [Google Scholar] [CrossRef]
- Sugiyama, E.; Kaniwa, N.; Kim, S.R.; Hasegawa, R.; Saito, Y.; Ueno, H.; Okusaka, T.; Ikeda, M.; Morizane, C.; Kondo, S.; et al. Population Pharmacokinetics of Gemcitabine and Its Metabolite in Japanese Cancer Patients: Impact of Genetic Polymorphisms. Clin. Pharmacokinet. 2010, 49, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, T.A.; Schultz, E.; Borland, M.G.; Pugh, M.E. Applied Spectrophotometry: Analysis of a Biochemical Mixture. Biochem. Mol. Biol. Educ. 2013, 41, 242–250. [Google Scholar] [CrossRef]
- Zacharioudaki, D.E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Cohen, R.M.; Wolfenden, R. Cytidine Deaminase from Escherichia coli. Purification, Properties and Inhibition by the Potential Transition State Analog 3,4,5,6-Tetrahydrouridine. J. Biol. Chem. 1971, 246, 7561–7565. [Google Scholar] [CrossRef]
- Vita, A.; Amici, A.; Cacciamani, T.; Lanciotti, M.; Magni, G. Cytidine Deaminase from Escherichia coli B. Purification and Enzymatic and Molecular Properties. Biochemistry 1985, 24, 6020–6024. [Google Scholar] [CrossRef] [PubMed]
- Tom, J. UV-Vis Spectroscopy: Principle, Strengths and Limitations and Applications. Available online: https://www.technologynetworks.com/analysis/articles/uv-vis-spectroscopy-principle-strengths-and-limitations-and-applications-349865 (accessed on 26 May 2025).
- Ressler, N. A Simple and Sensitive Method for the Measurement of Deoxycytidylate Deaminase Activity. Clin. Chem. 1969, 15, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.F.; Jones, D.D. Deoxycytidylate Deaminase in Pregnancy. Br. Med. J. 1975, 2, 10–12, Erratum in Br. Med. J. 1975, 2, 10–12. [Google Scholar] [CrossRef]
- Targett-Adams, L.; Jones, D.D.; Williams, G.F. A Rapid Method for the Determination of Deoxycytidylate Deaminase Activity in Pregnancy Serum. Clin. Chim. Acta 1975, 63, 377–382. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Zu, X.; Li, N.; Li, F.; Zhang, D. An Enzymatic Assay for High-Throughput Screening of Cytidine-Producing Microbial Strains. PLoS ONE 2015, 10, e0121612. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Roman, R.J.; Lechene, C. Fluorescence Assay for Picomole Quantities of Ammonia. Kidney Int. 1982, 21, 524–527. [Google Scholar] [CrossRef]
- Taylor, S.; Ninjoor, V.; Dowd, D.M.; Tappel, A.L. Cathepsin B2 Measurement by Sensitive Fluorometric Ammonia Analysis. Anal. Biochem. 1974, 60, 153–162. [Google Scholar] [CrossRef]
- Shin, D.; Sinkeldam, R.W.; Tor, Y. Emissive Rna Alphabet. J. Am. Chem. Soc. 2011, 133, 14912–14915. [Google Scholar] [CrossRef]
- Ludford, P.T.; Li, Y.; Yang, S.H.; Tor, Y. Cytidine Deaminase Can Deaminate Fused Pyrimidine Ribonucleosides. Org. Biomol. Chem. 2021, 19, 6237–6243. [Google Scholar] [CrossRef] [PubMed]
- Tor, Y. Isomorphic Fluorescent Nucleosides. Acc. Chem. Res. 2024, 57, 1325–1335. [Google Scholar] [CrossRef]
- Kowalska, S.; Krupczynska, K.; Buszewski, B. Some Remarks on Characterization and Application of Stationary Phases for Rp-Hplc Determination of Biologically Important Compounds. Biomed. Chromatogr. 2006, 20, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Singh, P.K.; Upadhyay, S. A Comprehensive Review on High-Performance Liquid Chromatography (HPLC). Ijppr. Hum. 2023, 27, 312–324. [Google Scholar]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. Lc-Ms-Based Metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarian, F.; Sharma, S.; Falappa, G.; Taruschio, W.; Chabes, A.; Hofer, A. Isocratic Hplc Analysis for the Simultaneous Determination of Dntps, Rntps and Adp in Biological Samples. Nucleic Acids Res. 2022, 50, e18. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, J.L.; Zhang, L.; Siepmann, J.I.; Schure, M.R. Retention Mechanism in Reversed-Phase Liquid Chromatography: A Molecular Perspective. Anal. Chem. 2007, 79, 6551–6558. [Google Scholar] [CrossRef]
- Zuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba Czek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B.; Heberger, K. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)—A Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Jandera, P.; Janas, P. Recent Advances in Stationary Phases and Understanding of Retention in Hydrophilic Interaction Chromatography. A Review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Cubbon, S.; Bradbury, T.; Wilson, J.; Thomas-Oates, J. Hydrophilic Interaction Chromatography for Mass Spectrometric Metabonomic Studies of Urine. Anal. Chem. 2007, 79, 8911–8918. [Google Scholar] [CrossRef]
- Di Pierro, D.; Tavazzi, B.; Perno, C.F.; Bartolini, M.; Balestra, E.; Calio, R.; Giardina, B.; Lazzarino, G. An Ion-Pairing High-Performance Liquid Chromatographic Method for the Direct Simultaneous Determination of Nucleotides, Deoxynucleotides, Nicotinic Coenzymes, Oxypurines, Nucleosides, and Bases in Perchloric Acid Cell Extracts. Anal. Biochem. 1995, 231, 407–412. [Google Scholar] [CrossRef]
- Cohen, S.; Jordheim, L.P.; Megherbi, M.; Dumontet, C.; Guitton, J. Liquid Chromatographic Methods for the Determination of Endogenous Nucleotides and Nucleotide Analogs Used in Cancer Therapy: A Review. J. Chromatogr. B 2010, 878, 1912–1928. [Google Scholar] [CrossRef]
- Gouy, M.H.; Fabre, H.; Blanchin, M.D.; Peyrottes, S.; Périgaud, C.; Lefebvre, I. Quantification of 5′-Monophosphate Cytosine Arabinoside (Ara-Cmp) in Cell Extracts Using Liquid Chromatography-Electrospray Mass Spectrometry. Anal. Chim. Acta 2006, 566, 178–184. [Google Scholar] [CrossRef]
- Huang, S.; Liu, L.; Liu, X.; Song, L.; Huang, C.; Miao, L. Development and Application of a Rapid and Sensitive Liquid Chromatography-Mass Spectrometry Method for Simultaneous Analysis of Cytarabine, Cytarabine Monophosphate, Cytarabine Diphosphate and Cytarabine Triphosphate in the Cytosol and Nucleus. J. Pharm. Biomed. Anal. 2022, 211, 114582. [Google Scholar] [CrossRef]
- Zbornikova, E.; Knejzlik, Z.; Hauryliuk, V.; Krasny, L.; Rejman, D. Analysis of Nucleotide Pools in Bacteria Using HPLC-MS in HILIC Mode. Talanta 2019, 205, 120161. [Google Scholar] [CrossRef]
- Crauste, C.; Lefebvre, I.; Hovaneissian, M.; Puy, J.Y.; Roy, B.; Peyrottes, S.; Cohen, S.; Guitton, J.; Dumontet, C.; Perigaud, C. Development of a Sensitive and Selective LC/MS/MS Method for the Simultaneous Determination of Intracellular 1-Beta-D-Arabinofuranosylcytosine Triphosphate (araCTP), Cytidine Triphosphate (CTP) and Deoxycytidine Triphosphate (dCTP) in a Human Follicular Lymphoma Cell Line. J. Chromatogr. B 2009, 877, 1417–1425. [Google Scholar] [CrossRef]
- Crotti, S.; Posocco, B.; Marangon, E.; Nitti, D.; Toffoli, G.; Agostini, M. Mass Spectrometry in the Pharmacokinetic Studies of Anticancer Natural Products. Mass Spectrom. Rev. 2017, 36, 213–251. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Nohta, H.; Saito, M.; Ohkura, Y. High-Performance Liquid Chromatographic Determination of Ribonucleosides and 2′-Deoxyribonucleosides Based on Precoiumn Fluorescence Derivatization of the Sugar Moieties. Anal. Sci. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Banoub, J.H.; Newton, R.P.; Esmans, E.; Ewing, D.F.; Mackenzie, G. Recent Developments in Mass Spectrometry for the Characterization of Nucleosides, Nucleotides, Oligonucleotides, and Nucleic Acids. Chem. Rev. 2005, 105, 1869–1916. [Google Scholar] [CrossRef]
- Wang, E.H.; Combe, P.C.; Schug, K.A. Multiple Reaction Monitoring for Direct Quantitation of Intact Proteins Using a Triple Quadrupole Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2016, 27, 886–896. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, Q.; Wang, W.; Zhang, X.; Zhou, F.; Sun, J.; Wang, G.; Peng, Y. Sensitive Analysis and Pharmacokinetic Study of a Novel Gemcitabine Carbamate Prodrug and Its Active Metabolite Gemcitabine in Rats Using LC-ESI-MS/MS. J. Chromatogr. B 2018, 1083, 249–257. [Google Scholar] [CrossRef]
- Donnette, M.; Osanno, L.; Giocanti, M.; Venton, G.; Farnault, L.; Berda-Haddad, Y.; Costello, R.; Caroline, S.; Ouafik, L.; Ciccolini, J.; et al. Determination of 5-Azacitidine in Human Plasma by LC-MS/MS: Application to Pharmacokinetics Pilot Study in MDS/AML Patients. Cancer Chemother. Pharmacol. 2023, 91, 231–238. [Google Scholar] [CrossRef]
- Parise, R.A.; Egorin, M.J.; Eiseman, J.L.; Joseph, E.; Covey, J.M.; Beumer, J.H. Quantitative Determination of the Cytidine Deaminase Inhibitor Tetrahydrouridine (THU) in Mouse Plasma by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1991–1997. [Google Scholar] [CrossRef]
- Peters, G.J.; Giovannetti, E.; Honeywell, R.J.; Ciccolini, J. Can Cytidine Deaminase Be Used as Predictive Biomarker for Gemcitabine Toxicity and Response? Br. J. Clin. Pharmacol. 2019, 85, 1213–1214. [Google Scholar] [CrossRef]
- Liesener, A.; Karst, U. Monitoring Enzymatic Conversions by Mass Spectrometry: A Critical Review. Anal. Bioanal. Chem. 2005, 382, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Saunders, K.C. A Review of LC-MS Techniques and High-Throughput Approaches Used to Investigate Drug Metabolism by Cytochrome P450s. J. Chromatogr. B 2010, 878, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Krijt, J.; Duta, A.; Kozich, V. Determination of S-Adenosylmethionine and S-Adenosylhomocysteine by LC-MS/MS and Evaluation of Their Stability in Mice Tissues. J. Chromatogr. B 2009, 877, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.T.; Korany, M.A.; Maher, H.M. High Performance Liquid Chromatographic Determination of Some Co-Administered Anticancer Drugs in Pharmaceutical Preparations and in Spiked Human Plasma. J. Pharm. Biomed. Anal. 2004, 34, 1099–1107. [Google Scholar] [CrossRef]
- de Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and Molecular Mechanisms of Action, Sensitivity and Chemoresistance in Pancreatic Cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef]
- Saiki, Y.; Hirota, S.; Horii, A. Attempts to Remodel the Pathways of Gemcitabine Metabolism: Recent Approaches to Overcoming Tumours with Acquired Chemoresistance. Cancer Drug Resist. 2020, 3, 819–831. [Google Scholar] [CrossRef]
- Gu, X.R.; Tohme, R.; Tomlinson, B.; Sakre, N.; Hasipek, M.; Durkin, L.; Schuerger, C.; Grabowski, D.; Zidan, A.M.; Radivoyevitch, T.; et al. Decitabine- and 5-Azacytidine Resistance Emerges from Adaptive Responses of the Pyrimidine Metabolism Network. Leukemia 2021, 35, 1023–1036. [Google Scholar] [CrossRef]
- Abbara, C.; Drevin, G.; Ferec, S.; Ghamrawi, S.; Souchet, S.; Robin, J.B.; Schmidt, A.; Hunault-Berger, M.; Guardiola, P.; Briet, M. Slower Degradation Rate of Cytarabine in Blood Samples from Acute Myeloid Leukemia by Comparison with Control Samples. Cancer Chemother. Pharmacol. 2020, 86, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, L.P.; Dumontet, C. Review of Recent Studies on Resistance to Cytotoxic Deoxynucleoside Analogues. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1776, 138–159. [Google Scholar] [CrossRef]
- Pavelka, S. Radiometric Enzyme Assays: Development of Methods for Extremely Sensitive Determination of Types 1, 2 and 3 Iodothyronine Deiodinase Enzyme Activities. J. Radioanal. Nucl. Chem. 2010, 286, 861–865. [Google Scholar] [CrossRef]
- Maguire, M.H.; Aronson, D.M. Measurement of Human Placental 5′-Amp Deaminase Activity by Radiometric Assay. Anal. Biochem. 1981, 116, 174–180. [Google Scholar] [CrossRef]
- Meier, W.; Conscience, J.F. A Fast and Simple Radiometric Assay for Adenosine Deaminase Using Reversed-Phase Thin-Layer Chromatography. Anal. Biochem. 1980, 105, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Perignon, J.L.; Chaleon, J.; Leverger, G.; Houllier, A.M.; Thuillier, L.; Cartier, P.H. Cytidine Deaminase Activity of Human Normal and Malignant Lymphoid Cells. Clin. Chim. Acta 1985, 147, 67–74. [Google Scholar] [CrossRef]
- Chabner, B.A.; Johns, D.G.; Coleman, C.N.; Drake, J.C.; Evans, W.H. Purification and Properties of Cytidine Deaminase from Normal and Leukemic Granulocytes. J. Clin. Investig. 1974, 53, 922–931. [Google Scholar] [CrossRef]
- Chou, T.C.; Arlin, Z.; Clarkson, B.D.; Philips, F.S. Metabolism of 1-Beta-D-Arabinofuranosylcytosine in Human Leukemic-Cells. Cancer Res. 1977, 37, 3561–3570. [Google Scholar]
- Giusti, G.; Mangoni, C.; de Petrocellis, B.; Scarano, E. Deoxycytidylate Deaminase and Deoxycytidine Deaminase in Normal and Neoplastic Human Tissues. Enzymol. Biol. Clin. 1970, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Maley, G.F.; Maley, F. The Purification and Properties of Deoxycytidylate Deaminase from Chick Embryo Extracts. J. Biol. Chem. 1964, 239, 1168–1176. [Google Scholar] [CrossRef]
- Scocca, J.J.; Panny, S.R.; Bessman, M.J. Studies of Deoxycytidylate Deaminase from T4-Infected Escherichia coli. J. Biol. Chem. 1969, 244, 3698–3706. [Google Scholar] [CrossRef]
- Ellims, P.H.; Medley, G. Deoxycytidylate Deaminase Activity in Lymphoproliferative Disorders. Leuk. Res. 1984, 8, 123–128. [Google Scholar] [CrossRef]
- Salic, A.; Mitchison, T.J. A Chemical Method for Fast and Sensitive Detection of DNA Synthesis in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef]
- Qu, D.; Wang, G.; Wang, Z.; Zhou, L.; Chi, W.; Cong, S.; Ren, X.; Liang, P.; Zhang, B. 5-Ethynyl-2′-Deoxycytidine as a New Agent for DNA Labeling: Detection of Proliferating Cells. Anal. Biochem. 2011, 417, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ligasova, A.; Liboska, R.; Friedecky, D.; Micova, K.; Adam, T.; Ozdian, T.; Rosenberg, I.; Koberna, K. Dr Jekyll and Mr Hyde: A Strange Case of 5-Ethynyl-2′-Deoxyuridine and 5-Ethynyl-2′-Deoxycytidine. Open Biol. 2016, 6, 150172. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.T. Radiometric Assays. In Enzyme Assays: A Practical Approach, 2nd ed.; Eisenthal, R., Danson, M.J., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 79–103. [Google Scholar]
| Method Type | Advantages | Disadvantages | Ref. | Sensitivity (LOD) | Feasibility/Cost | Assay Type |
|---|---|---|---|---|---|---|
| Spectrophotometric | ||||||
| Direct | Simple, rapid, cost-effective, real-time, non-destructive. | Sensitivity to other UV-absorbers, requires pure samples, light scattering issues, limited sensitivity. | [43,44] | LOD: ~1–10 µM (e.g., for cytidine/uridine) | Low | Kinetic |
| Indirect | High sensitivity, complex sample applicability, high-throughput adaptable. | Endogenous ammonia/metabolic interference, high background, long protocols, enzyme coupling optimization needed. | [46,47,48,49] | LOD: ~50 µM (for ammonia) | Low | Endpoint |
| Fluorimetric | ||||||
| Direct | High sensitivity, real-time monitoring, suited for kinetics/inhibitor screening, red-shifted spectra. | Complex/costly substrate synthesis, kinetic parameters may differ from native substrates. | [53,54] | LOD: ~0.1–1 µM (for fluorescent analog) | Medium-High | Kinetic |
| Indirect | Very high sensitivity (picomolar to subpicomolar detection limits for ammonia). | Endogenous ammonia interference, limited dynamic range. | [50,51] | LOD: ~10–100 nM (for ammonia) | Low-Medium | Endpoint |
| Liquid Chromatography-Based Assays | ||||||
| High resolution and selectivity, suitable for complex samples, adaptable to various detection modes. | Time-consuming method optimization, high reagent/equipment cost. | |||||
| LC-UV/Vis | No derivatization, simpler sample preparation. | Sample clarity required, limited sensitivity. | [58] | LOD: ~0.1–10 µM (e.g., for cytidine/uridine, deoxycytidine/deoxyuridine) | Medium | Endpoint |
| LC-FLD | High sensitivity (picomole level), high selectivity, low background noise. | Requires derivatization, sensitive to fluorescence quenching/autofluorescence. | [71] | LOD: ~0.1–1 µM (e.g., for cytidine/uridine derivatized by fluorescent reagents) | Medium-High | Endpoint |
| LC-MS | Highest sensitivity and specificity, no derivatization, complex matrix applicability, simultaneous inhibitor detection. | High cost of equipment, time-consuming method development/sample preparation, susceptible to matrix effect (interference with ionization efficiency). | [70,72] | LOD: ~1–100 nM (e.g., for cytidine/uridine, deoxycytidine/deoxyuridine) | High | Endpoint |
| Radiometric Assays | ||||||
| Radiometric Assays | High sensitivity for low concentrations of enzymes. | Radiation safety concerns, specialized facilities/protocols, hazardous waste generation, declining use. | [91,100] | LOD: <1 nM; enables detection of low analyte levels in complex mixtures | Medium-High | Endpoint |
| Cell-Based Assays | ||||||
| Physiological context study, compatible with fluorescence microscopy/microplate readers, high sensitivity. | Product amount reflects multiple cellular processes (transport, metabolism), limiting precise quantification. | High (biological context); ability to detect activity at the single-cell level | Medium | Endpoint | ||
| EdC-to-EdU conversion assay | Rapid detection (“click” chemistry), no DNA denaturation, simultaneous cytotoxicity/replication testing. | Replication-dependent (limits analysis to proliferating cells). | [21] | |||
| FC-to-FU conversion assay | Transcription-dependent (broader applicability across cell cycle phases/quiescent cells). | Antibody use. | [1] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligasová, A.; Horejšová, M.; Brumarová, R.; Friedecký, D.; Koberna, K. Cytidine and dCMP Deaminases—Current Methods of Activity Analysis. Int. J. Mol. Sci. 2025, 26, 8045. https://doi.org/10.3390/ijms26168045
Ligasová A, Horejšová M, Brumarová R, Friedecký D, Koberna K. Cytidine and dCMP Deaminases—Current Methods of Activity Analysis. International Journal of Molecular Sciences. 2025; 26(16):8045. https://doi.org/10.3390/ijms26168045
Chicago/Turabian StyleLigasová, Anna, Martina Horejšová, Radana Brumarová, David Friedecký, and Karel Koberna. 2025. "Cytidine and dCMP Deaminases—Current Methods of Activity Analysis" International Journal of Molecular Sciences 26, no. 16: 8045. https://doi.org/10.3390/ijms26168045
APA StyleLigasová, A., Horejšová, M., Brumarová, R., Friedecký, D., & Koberna, K. (2025). Cytidine and dCMP Deaminases—Current Methods of Activity Analysis. International Journal of Molecular Sciences, 26(16), 8045. https://doi.org/10.3390/ijms26168045





