Therapeutic Potential of Propolis in Preclinical Models of Cancer and Infectious Diseases: A Review
Abstract
1. Introduction
2. Effect of Propolis Monotherapy on Tumor Xenograft Models
2.1. Breast Cancer
2.2. Cervical Cancer
2.3. Colorectal and Gastrointestinal Cancer
2.4. Epidermoid Carcinoma
2.5. Fibrosarcoma
2.6. Hodgkin Lymphoma
3. Preclinical Evaluation of Propolis-Based Combination Therapies in Cancer Treatment
3.1. Methotrexate
3.2. Fluorouracil
- In a BALB/c mouse model of chemically induced colorectal cancer, co-administration of Iranian propolis (90 mg/kg, i.p., five times per week) and 5-FU (50 mg/kg, i.p., once weekly) for eight weeks significantly reduced tumor burden. Histopathological evaluation showed reduced β-catenin, COX-2, and iNOS expression in the combination therapy group compared to monotherapy groups, suggesting improved chemotherapeutic efficacy and inflammation control [52].
- In a LoVo colorectal cancer xenograft model, the combination of Anatolian propolis extract (400 mg/kg, i.p.) with 5-FU (10 mg/kg, i.p.), administered daily for three weeks, demonstrated superior tumor inhibition relative to either agent alone. Intraperitoneal administration of propolis produced better outcomes than oral administration. Notably, serum levels of pro-inflammatory cytokines (TNF-α, IL-1, IL-6) were reduced in the combination and propolis-alone groups. Additionally, the activity of liver enzymes: ALT (alanine aminotransferase) and AST (aspartate aminotransferase) was significantly lower, indicating reduced hepatotoxicity [53].
- A separate study explored sex-dependent effects of propolis combined with 5-FU. Mice pretreated with crude Croatian propolis powder (300 mg/kg in the diet) for 14 days prior to 4T1 mammary carcinoma inoculation received 5-FU (200 mg/kg, i.p.) one day post-injection. In male mice, the combination significantly reduced tumor growth and metastatic spread, a benefit not observed in female mice. This sex-specific difference may be attributed to lower dihydropyrimidine dehydrogenase (DPD) activity in males, resulting in higher 5-FU sensitivity [54].
3.3. Irinotecan
3.4. Dual Model: Cancer and Candida Albicans Infection
3.5. Photodynamic Therapy
4. Adjuvant Use of Propolis in Oncology: Mitigating Treatment-Related Side Effects
4.1. 5-Fluorouracil-Induced Cardiotoxicity
4.2. Mitomycin C-Induced Toxicity
4.3. Protection Against Common Chemotherapeutic Agents
4.3.1. Cisplatin
4.3.2. Irinotecan
4.3.3. Doxorubicin
4.3.4. Cyclophosphamide
4.4. Radiotherapy-Induced Toxicity
- In a 40-week rat model of bladder cancer, propolis (300 mg/kg/day, i.p.) significantly inhibited microvascular density, indicating suppression of tumor-induced angiogenesis [74].
- Brazilian green propolis (300 mg/kg, s.c., for 5 days) reduced retinal neovascularization in a murine retinopathy model without impairing physiological revascularization [75].
- Brazilian red propolis (200 mg/kg, oral gavage for 11 days) also inhibited angiogenesis in a hamster cheek pouch tumor model [76].
5. Antiparasitic Effect of Propolis
5.1. Toxoplasma Gondii
5.2. Leishmania spp.
5.3. Schistosoma mansonii
5.4. Trypanosoma spp.
5.5. Plasmodium spp.
5.6. Other Parasites
6. Antibacterial Effect of Propolis
6.1. Gram-Positive Bacteria
6.2. Gram-Negative Bacteria
6.3. Polymicrobial Infections
7. Antifungal Effect of Propolis
7.1. Candida Albicans
7.2. Other Fungal Infections
8. Antiviral Effect of Propolis
9. Methods
10. Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanahan, M.; Spivak, M.; Satta, A.; Theodorou, P. Resin Use by Stingless Bees: A Review. Insects 2021, 12, 719. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an Old Remedy Used in Modern Medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid. Based Complement. Alternat. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid.-Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.S.; Benchaabane, S.; Daas, T.; Smagghe, G.; Loucif-Ayad, W. Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life 2025, 15, 764. [Google Scholar] [CrossRef]
- de Santana Neto, D.C.; Silva Paiva, T.; de Souza Tasso, I.; Matos Nascimento, K.; de Magalhães Cordeiro, Â.M.T.; de Albuquerque Meireles, B.R.L.; da Silva, F.A.P.; de Matos Jorge, L.M.; Maria Matos Jorge, R. A Comparison of the Antioxidant Properties of Two Different Brazilian Propolis. Microchem. J. 2024, 200, 110352. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Bollin, P.; Kuś, P.M.; Okińczyc, P.; Van Dijck, P.; Szweda, P. Identification of Potential Markers of Elevated Anticandidal Activity of Propolis Extracts. J. Ethnopharmacol. 2025, 347, 119799. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-Type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef]
- Cheung, K.W.; Sze, D.M.Y.; Chan, W.K.; Deng, R.X.; Tu, W.; Chan, G.C.F. Brazilian Green Propolis and Its Constituent, Artepillin C Inhibits Allogeneic Activated Human CD4 T Cells Expansion and Activation. J. Ethnopharmacol. 2011, 138, 463–471. [Google Scholar] [CrossRef]
- de Morais, D.V.; Rosalen, P.L.; Ikegaki, M.; Silva, A.P.d.S.; Massarioli, A.P.; de Alencar, S.M. Active Antioxidant Phenolics from Brazilian Red Propolis: An Optimization Study for Their Recovery and Identification by LC-ESI-QTOF-MS/MS. Antioxidants 2021, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Queiroga, M.C.; Laranjo, M.; Andrade, N.; Marques, M.; Costa, A.R.; Antunes, C.M. Antimicrobial, Antibiofilm and Toxicological Assessment of Propolis. Antibiotics 2023, 12, 347. [Google Scholar] [CrossRef]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Ptak, J.; Miśkiewicz, M.; Noga, R.; Marcinkowska, J.; Herc, A.; Koczkodon, K.; Teska, V.; Perłowski, J.; Sawczuk, M.; Krompiewski, M. Propolis in Human Health: Unraveling Chemistry, Applications, and Efficacy. Qual. Sport 2024, 21, 54248. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis-Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Kabala-Dzik, A.; Mozdzierz, A.; Kubina, R.; Wojtyczka, R.D.; Stojko, R.; Dziedzic, A.; Jastrzebska-Stojko, Z.; Jurzak, M.; Buszman, E.; et al. Caffeic Acid Phenethyl Ester and Ethanol Extract of Propolis Induce the Complementary Cytotoxic Effect on Triple-Negative Breast Cancer Cell Lines. Molecules 2015, 20, 9242. [Google Scholar] [CrossRef]
- Motomura, M.; Kwon, K.M.; Suh, S.J.; Lee, Y.C.; Kim, Y.K.; Lee, I.S.; Kim, M.S.; Kwon, D.Y.; Suzuki, I.; Kim, C.H. Propolis Induces Cell Cycle Arrest and Apoptosis in Human Leukemic U937 Cells through Bcl-2/Bax Regulation. Environ. Toxicol. Pharmacol. 2008, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Khabeer, A.; Faridi, M.A.; Makhdoom, G. Effectiveness of Propolis in Maintaining Oral Health: A Scoping Review. Can. J. Dent. Hyg. 2021, 55, 167–176. [Google Scholar] [PubMed]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.A.S. Applications of Propolis-Based Materials in Wound Healing. Arch. Dermatol. Res. 2023, 316, 61. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Contieri, L.S.; de Souza Mesquita, L.M.; Sanches, V.L.; Viganó, J.; Martinez, J.; da Cunha, D.T.; Rostagno, M.A. Standardization Proposal to Quality Control of Propolis Extracts Commercialized in Brazil: A Fingerprinting Methodology Using a UHPLC-PDA-MS/MS Approach. Food Res. Int. 2022, 161, 111846. [Google Scholar] [CrossRef]
- Mesbah, L.; Samia, A. Bioavailability and Pharmacokinetic of the Algerian Propolis Constituent Naringenin in Rats after Oral Administration. Planta Med. 2011, 77, PA11. [Google Scholar] [CrossRef]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma Mice Model for Studying Cancer-Induced Cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef]
- Badr, M.O.T.; Edrees, N.M.M.; Abdallah, A.A.M.; El-Deen, N.A.M.N.; Neamat-Allah, A.N.F.; Ismail, H.T.H. Anti-tumour Effects of Egyptian Propolis on Ehrlich Ascites Carcinoma. Vet. Ital. 2011, 47, 341–350. [Google Scholar]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Shaker, S.A.; Alshufta, S.M.; Gowayed, M.A.; El-Salamouni, N.S.; Bassam, S.M.; Megahed, M.A.; El-Tahan, R.A. Propolis-Loaded Nanostructured Lipid Carriers Halt Breast Cancer Progression through MiRNA-223 Related Pathways: An in-Vitro/in-Vivo Experiment. Sci. Rep. 2023, 13, 15752. [Google Scholar] [CrossRef] [PubMed]
- Swase, T.D.; Fasogbon, I.V.; Eseoghene, I.J.; Etukudo, E.M.; Mbina, S.A.; Joan, C.; Dangana, R.S.; Anyanwu, C.; Vandu, C.D.; Agbaje, A.B.; et al. The Impact of HPV/HIV Co-Infection on Immunosuppression, HPV Genotype, and Cervical Cancer Biomarkers. BMC Cancer 2025, 25, 202. [Google Scholar] [CrossRef] [PubMed]
- Khacha-ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Propolis Extracts from the Northern Region of Thailand Suppress Cancer Cell Growth through Induction of Apoptosis Pathways. Investig. New Drugs 2016, 34, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Bronte, F.; Giunta, E.F. Editorial: Reviews in Gastrointestinal Cancers. Front. Oncol. 2023, 13, 1252665. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Ad’hiah, A.H.; Al-Sammarrae, K.W.; Bagnati, R.; Frapolli, R.; Bello, E.; Uboldi, S.; Romano, M.; Panini, N.; Scanziani, E.; et al. Assessing the Anti-Tumour Properties of Iraqi Propolis In Vitro and In Vivo. Food Chem. Toxicol. 2012, 50, 1632–1641. [Google Scholar] [CrossRef]
- Desamero, M.J.; Kakuta, S.; Tang, Y.; Chambers, J.K.; Uchida, K.; Estacio, M.A.; Cervancia, C.; Kominami, Y.; Ushio, H.; Nakayama, J.; et al. Tumor-Suppressing Potential of Stingless Bee Propolis in In Vitro and In Vivo Models of Differentiated-Type Gastric Adenocarcinoma. Sci. Rep. 2019, 9, 19635. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Jia, L.; Qiao, J.; El-Seedi, H.R.; Zhang, Y.; Zhang, H. Supercritical CO2 Extracts of Propolis Inhibits Tumor Proliferation and Enhances the Immunomodulatory Activity via Activating the TLR4-MAPK/NF-ΚB Signaling Pathway. Food Res. Int. 2024, 196, 115137. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. Epidermal Growth Factor. J. Biol. Chem. 1990, 265, 7709–7712. [Google Scholar] [CrossRef]
- Kaniowski, D.; Suwara, J.; Ebenryter-Olbińska, K.; Jakóbik-Kolon, A.; Nawrot, B. EGFR-Targeted Cellular Delivery of Therapeutic Nucleic Acids Mediated by Boron Clusters. Int. J. Mol. Sci. 2022, 23, 14793. [Google Scholar] [CrossRef]
- Kaniowski, D.; Nska, K.E.O.; Sobczak, M.; Wojtczak, B.; Janczak, S.; Lésnikowski, Z.J.; Nawrot, B. High Boron-Loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents. Molecules 2017, 22, 1393. [Google Scholar] [CrossRef]
- Kaniowski, D.; Ebenryter-Olbińska, K.; Suwara, J.; Kulik, K.; Hall, J.; Wang, D.; Kang, E.Y.; Dolot, R.; Somenzi, A.; Jakóbik-Kolon, A.; et al. EGFR-Targeted Antisense Oligonucleotides Modified with Boron Clusters Offer an Innovative Approach to Cancer Chemo-Radiotherapy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Z.; Lv, W.; Pan, H. The Role of Anti-EGFR Monoclonal Antibody in MCRC Maintenance Therapy. Front. Mol. Biosci. 2022, 9, 870395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Cui, H.; Wang, Y.; Yang, J.; Lin, C.; Shi, X.; Zou, Y.; Chen, J.; Jia, X.; Su, L. The Advance of the Third-generation EGFR-TKI in the Treatment of Non-small Cell Lung Cancer. Oncol. Rep. 2024, 51, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, Y.; Yang, A.; Tan, W.; Liu, X.; Yang, W. Antitumor Effect of Poplar Propolis on Human Cutaneous Squamous Cell Carcinoma A431 Cells. Int. J. Mol. Sci. 2023, 24, 16753. [Google Scholar] [CrossRef]
- Davis, D.D.; Taqi, M.; Kane, S.M. Fibrosarcoma. In Bone Tumors Diagnosis and Therapy Today; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 127–128. [Google Scholar] [CrossRef]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer Activity of the Supercritical Extract of Brazilian Green Propolis and Its Active Component, Artepillin C: Bioinformatics and Experimental Analyses of Its Mechanisms of Action. Int. J. Oncol. 2018, 52, 925–932. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.H.; Bartlett, N.L. Hodgkin Lymphoma. Nat. Rev. Dis. Prim. 2020, 6, 61. [Google Scholar] [CrossRef]
- Kapare, H.; Lohidasan, S.; Sinnathambi, A.; Mahadik, K. Standardization, Anti-Carcinogenic Potential and Biosafety of Indian Propolis. J. Ayurveda Integr. Med. 2019, 10, 81–87. [Google Scholar] [CrossRef]
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.M.; Mohamed, T.M. Propolis Potentiates Methotrexate Anticancer Mechanism and Reduces Its Toxic Effects. Nutr. Cancer 2020, 72, 460–480. [Google Scholar] [CrossRef]
- Rudy, B.C.; Senkowski, B.Z. Fluorouracil. Anal. Profiles Drug Subst. Excip. 2024, 2, 221–244. [Google Scholar] [CrossRef]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-Administration of 5FU and Propolis on AOM/DSS Induced Colorectal Cancer in BALB-c Mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Durmus, E.; Yenigun, V.B.; Kanimdan, E.; Ozman, Z.; Yasar, O.; Goren, A.C.; Hekimoglu, E.R.; Oruc, H.H.; et al. Propolis Enhances 5-Fluorouracil Mediated Antitumor Efficacy and Reduces Side Effects in Colorectal Cancer: An In Vitro and In Vivo Study. Chem. Biodivers. 2023, 20, e202300591. [Google Scholar] [CrossRef]
- Sobočanec, S.; Balog, T.; Šari, A.; MačAk-Šafranko, Ž.; Štroser, M.; Žarković, K.; Žarković, N.; Stojković, R.; Ivanković, S.; Marotti, T. Antitumor Effect of Croatian Propolis as a Consequence of Diverse Sex-Related Dihydropyrimidine Dehydrogenase (DPD) Protein Expression. Phytomedicine 2011, 18, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.E.; Tayea, N.A.; Elwakil, B.H.; Elshewemi, S.S.; Gad, A.A.E.M.; Abdulmalek, S.A.; Ghareeb, D.A.; Olama, Z.A. In Vitro and in Vivo Anti-Colorectal Cancer Effect of the Newly Synthesized Sericin/Propolis/Fluorouracil Nanoplatform through Modulation of PI3K/AKT/MTOR Pathway. Sci. Rep. 2024, 14, 2433. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Lisičić, D.; Benković, V.; Đikić, D.; Blažević, A.S.; Mihaljević, J.; Oršolić, N.; Knežević, A.H. Addition of Propolis to Irinotecan Therapy Prolongs Survival in Ehrlich Ascites Tumor-Bearing Mice. Cancer Biother. Radiopharm. 2014, 29, 62–69. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Darvishi, S.; Taghavi, M. Immunomodulatory Efficacy of Ethanol Extract of Propolis on Tumor-Bearing Mice with Disseminated Candidiasis. J. Med. Mycol. 2014, 24, e143–e148. [Google Scholar] [CrossRef]
- Onur, E.; Gökmen, G.G.; Nalbantsoy, A.; Kışla, D. Investigation of the Supportive Therapy Potential of Propolis Extract and Lactobacillus Acidophilus LA-5 Milk Combination against Breast Cancer in Mice. Cytokine 2022, 149, 155743. [Google Scholar] [CrossRef]
- Al-Jamal, A.N.; Al-Hussainy, A.F.; Mohammed, B.A.; Abbas, H.H.; Kadhim, I.M.; Ward, Z.H.; Mahapatra, D.K.; Joseph, T.M.; Kianfar, E.; Thomas, S. Photodynamic Therapy (PDT) in Drug Delivery: Nano-Innovations Enhancing Treatment Outcomes. Heal. Sci. Rev. 2025, 14, 100218. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, Y.X.; Yu, N.Q.; Hu, D.; Wang, X.Y.; Chen, X.G.; Liao, Y.W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-Mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-ΚB and COX-2. Molecules 2017, 22, 732. [Google Scholar] [CrossRef]
- Barary, M.; Hosseinzadeh, R.; Kazemi, S.; Liang, J.J.; Mansouri, R.; Sio, T.T.; Hosseini, M.; Moghadamnia, A.A. The Effect of Propolis on 5-Fluorouracil-Induced Cardiac Toxicity in Rats. Sci. Rep. 2022, 12, 8661, Correction in Sci. Rep. 2022, 12, 12531. [Google Scholar] [CrossRef]
- Kumari, S.; Naik, P.; Vishma, B.L.; Salian, S.R.; Devkar, R.A.; Khan, S.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Mitigating Effect of Indian Propolis against Mitomycin C Induced Bone Marrow Toxicity. Cytotechnology 2016, 68, 1789–1800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumari, S.; Nayak, G.; Lukose, S.T.; Kalthur, S.G.; Bhat, N.; Hegde, A.R.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Indian Propolis Ameliorates the Mitomycin C-Induced Testicular Toxicity by Reducing DNA Damage and Elevating the Antioxidant Activity. Biomed. Pharmacother. 2017, 95, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Okuma, Y.; Hakozaki, T.; Mirokuji, K.; Yomota, M.; Hishima, T.; Hosomi, Y. Cisplatin and Irinotecan as First-Line Chemotherapy for Previously Untreated Metastatic Thymic Carcinoma: Updated Analysis. Front. Oncol. 2022, 11, 779700. [Google Scholar] [CrossRef] [PubMed]
- Gadisa, D.A.; Assefa, M.; Wang, S.H.; Yimer, G. Toxicity Profile of Doxorubicin-Cyclophosphamide and Doxorubicin-Cyclophosphamide Followed by Paclitaxel Regimen and Its Associated Factors among Women with Breast Cancer in Ethiopia: A Prospective Cohort Study. J. Oncol. Pharm. Pract. 2020, 26, 1912–1920. [Google Scholar] [CrossRef]
- Oršolic, N.; Car, N.; Lisičić, D.; Benković, V.; Knežević, A.H.; Domagoj, D.; Petrik, J. Synergism between Propolis and Hyperthermal Intraperitoneal Chemotherapy with Cisplatin on Ehrlich Ascites Tumor in Mice. J. Pharm. Sci. 2013, 102, 4395–4405. [Google Scholar] [CrossRef]
- Yuluğ, E.; Türedi, S.; Yıldırım, E.; Yenilmez, E.; Aliyazıcıoğlu, Y.; Demir, S.; Özer-Yaman, S.; Menteşe, A. Biochemical and Morphological Evaluation of the Effects of Propolis on Cisplatin Induced Kidney Damage in Rats. Biotech. Histochem. 2019, 94, 204–213. [Google Scholar] [CrossRef]
- Oršolić, N.; Benković, V.; Lisičić, D.; Dikić, D.; Erhardt, J.; Knežević, A.H. Protective Effects of Propolis and Related Polyphenolic/Flavonoid Compounds against Toxicity Induced by Irinotecan. Med. Oncol. 2010, 27, 1346–1358. [Google Scholar] [CrossRef]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A.M. Propolis Attenuates Doxorubicin-Induced Testicular Toxicity in Rats. Food Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef]
- Singla, S.; Kumar, N.; Kaur, J. In Vivo Studies on the Protective Effect of Propolis on Doxorubicin-Induced Toxicity in Liver of Male Rats. Toxicol. Int. 2014, 21, 191–195. [Google Scholar] [CrossRef]
- El-Naggar, S.A.; Alm-Eldeen, A.A.; Germoush, M.O.; El-Boray, K.F.; Elgebaly, H.A. Ameliorative Effect of Propolis against Cyclophosphamide-Induced Toxicity in Mice. Pharm. Biol. 2015, 53, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, S.S.; Brooks, J.; Zuro, D.; Su, Y.-L.; Kaniowski, D.; Ghimire, H.; Lim, J.E.; Mohamed, A.; Abdelhamid, H.; Kuo, Y.-H.; et al. Targeted Irradiation and STAT3 Inhibition Reprogram the AML Microenvironment and Extend Survival: Toward Translational Immunoradiotherapy. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, C.A.; Fechine-Jamacaru, F.V.; Albuquerque, I.L.; Magalhães, H.I.F.; Dias, T.A.; Faria, M.H.G.; Alves, M.K.S.; Rabenhorst, S.H.B.; Almeida, P.R.C.d.; de Lemos, T.L.G.; et al. Angiogenesis Inhibition by Green Propolis and the Angiogenic Effect of L-Lysine on Bladder Cancer in Rats. Acta Cir. Bras. 2012, 27, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Chikaraishi, Y.; Izuta, H.; Shimazawa, M.; Mishima, S.; Hara, H. Angiostatic Effects of Brazilian Green Propolis and Its Chemical Constituents. Mol. Nutr. Food Res. 2010, 54, 566–575. [Google Scholar] [CrossRef]
- Juanes, C.d.C.; Souza, S.M.d.; Braga, V.N.L.; Barreto, F.S.; Aguiar, G.R.; Pimentel, K.D.G.; Fechine, F.V.; Dornelas, C.A. Red Propolis and L-Lysine on Angiogenesis and Tumor Growth in a New Model of Hamster Cheek Pouch Inoculated with Walker 256 Tumor Cells. Einstein 2019, 17, eAO4576. [Google Scholar] [CrossRef]
- Çolak, S.; Erdil, A.; Gevrek, F. Effects of Systemic Anatolian Propolis Administration on a Rat-Irradiated Osteoradionecrosis Model. J. Appl. Oral Sci. 2023, 31, e20230231. [Google Scholar] [CrossRef]
- Guler Avci, G.; Erdim, I.; Ozmen, Z.C.; Gevrek, F.; Colak, S.; Demirsoy, M.S.; Bozkurt, H. The Effect of Systemic Application of Propolis on Tongue Damage and Oral Mucositis in Rats Exposed to Radiation. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1043–1052. [Google Scholar] [CrossRef]
- Motallebnejad, M.; Abedi, S.M.; Seyedmajidi, M.; Moghadamnia, A.A.; Ashrafpour, M.; Salehi, M.; Moslemi, D.; Ghorbani, A. Evaluation of Protective Effect of Propolis on Parotid Salivary Glands in Gamma-Irradiated Rats. J. Contemp. Dent. Pract. 2014, 15, 8–11. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma Gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human Schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug Repurposing and Human Parasitic Protozoan Diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Hagras, N.A.E.; Mogahed, N.M.F.H.; Sheta, E.; Darwish, A.A.E.; El-Hawary, M.A.; Hamed, M.T.; Elwakil, B.H. The Powerful Synergistic Effect of Spiramycin/Propolis Loaded Chitosan/Alginate Nanoparticles on Acute Murine Toxoplasmosis. PLoS Negl. Trop. Dis. 2022, 16, e0010268. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.M.; Fadaly, H.A.M.E.; Gareh, A.; Abd El-Razik, K.A.; Ali, F.A.Z.; Saleh, A.A.; Sadek, S.A.S.; Dahran, N.; El-Gendy, A.E.N.G.; El-Khadragy, M.F.; et al. Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis. Animals 2022, 12, 3069. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; El Fadaly, H.A.M.; Soror, A.H.; Ali, F.A.Z.; Abd El-Razik, K.A.; Soliman, Y.A.; Alkhaldi, A.A.M.; Albezrah, N.K.A.; Barakat, A.M. Novel Insights on the Potential Activity of Propolis and Wheat Germ Oil against Chronic Toxoplasmosis in Experimentally Infected Mice. Biomed. Pharmacother. 2022, 156, 113811. [Google Scholar] [CrossRef]
- Barakat, A.M.; El-Razik, K.A.A.; El Fadaly, H.A.M.; Saleh, W.M.; Ali, F.A.Z.; Gouda, A.A.; Sadek, S.A.S.; Dahran, N.; El-khadragy, M.F.; Elmahallawy, E.K. Parasitological, Molecular, and Histopathological Investigation of the Potential Activity of Propolis and Wheat Germ Oil against Acute Toxoplasmosis in Mice. Pharmaceutics 2023, 15, 478. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Ali, F.A.Z.; Raya-Álvarez, E.; Fehaid, A.; Abd El-Razik, K.A.; El Fadaly, H.A.M.; El-Khadragy, M.F.; Sayed, A.S.M.; Soror, A.H.; Alhegaili, A.S.; et al. Ameliorative Effects of Propolis and Wheat Germ Oil on Acute Toxoplasmosis in Experimentally Infected Mice Are Associated with Reduction in Parasite Burden and Restoration of Histopathological Changes in the Brain, Uterus, and Kidney. Front. Vet. Sci. 2024, 11, 1357947. [Google Scholar] [CrossRef]
- Ayres, D.C.; Fedele, T.A.; Marcucci, M.C.; Giorgio, S. Potential Utility of Hyperbaric Oxygen Therapy and Propolis in Enhancing the Leishmanicidal Activity of Glucantime. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 329–334. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Amorim, N.A.; Jesus-Santos, F.H.; de Lima, J.A.; Lima, J.B.; Berretta, A.A.; Borges, V.M. Leishmanicidal and Immunomodulatory Properties of Brazilian Green Propolis Extract (EPP-AF®) and a Gel Formulation in a Pre-Clinical Model. Front. Pharmacol. 2023, 14, 1013376. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Castro, R.A.O.; Batista, M.A.; Rossi, F.M.O.; Silveira-Lemos, D.; Frézard, F.; Moura, S.A.L.; Rezende, S.A. Association of Water Extract of Green Propolis and Liposomal Meglumine Antimoniate in the Treatment of Experimental Visceral Leishmaniasis. Parasitol. Res. 2014, 113, 533–543. [Google Scholar] [CrossRef]
- Saberi, R.; Zadeh, A.G.; Afshar, M.J.A.; Fakhar, M.; Keighobadi, M.; Mohtasebi, S.; Rahimi-Esboei, B. In Vivo Anti-Leishmanial Activity of Concocted Herbal Topical Preparation against Leishmania Major (MRHO/IR/75/ER). Ann. Parasitol. 2021, 67, 483–488. [Google Scholar] [CrossRef]
- Mahmoud, T.Y.; Rizk, S.M.; Maghraby, A.S.; Shaheen, A.A. Propolis Enhances the Effectiveness of Praziquantel in Experimental Schistosomiasis: Biochemical and Histopathological Study. Parasitol. Res. 2014, 113, 4513–4523. [Google Scholar] [CrossRef]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; de Moraes, J. Brazilian Red Propolis Exhibits Antiparasitic Properties In Vitro and Reduces Worm Burden and Egg Production in an Mouse Model Harboring Either Early or Chronic Schistosoma Mansoni Infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef]
- De Castro, S.L.; Salomão, K.; De Souza, E.M.; Henriques-Pons, A.; Barbosa, H.S. Brazilian Green Propolis: Effects In Vitro and In Vivo on Trypanosoma Cruzi. Evid.-Based Complement. Altern. Med. 2011, 2011, 185918. [Google Scholar] [CrossRef]
- Gressler, L.T.; Da Silva, A.S.; Machado, G.; Rosa, L.D.; Dorneles, F.; Gressler, L.T.; Oliveira, M.S.; Zanette, R.A.; de Vargas, A.C.P.; Monteiro, S.G. Susceptibility of Trypanosoma Evansi to Propolis Extract In Vitro and in Experimentally Infected Rats. Res. Vet. Sci. 2012, 93, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- AlGabbani, Q.; Mansour, L.; Elnakady, Y.A.; Al-Quraishy, S.; Alomar, S.; Al-Shaebi, E.M.; Abdel-Baki, A.A.S. In Vivo Assessment of the Antimalarial and Spleen-Protective Activities of the Saudi Propolis Methanolic Extract. Parasitol. Res. 2017, 116, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Afrouzan, H.; Zakeri, S.; Mehrizi, A.A.; Molasalehi, S.; Tahghighi, A.; Shokrgozar, M.A.; Es-Haghi, A.; Djadid, N.D. Anti-Plasmodial Assessment of Four Different Iranian Propolis Extracts. Arch. Iran. Med. 2017, 20, 270–281. [Google Scholar] [PubMed]
- El-aziz, T.H.A.; El-beih, N.M.; Soufy, H.; Nasr, S.M.; Khalil, F.A.M.; Sharaf, M.; Street, E.; Box, P.O.; Street, E.; Box, P.O. Effect of Egyptian Propolis on Lipid Profile and Oxidative Status in Comparison with Nitazoxanide in Immunosuppressed Rats Infected with Cryptosporidium spp. Glob. Vet. 2014, 13, 17–27. [Google Scholar]
- Soufy, H.; El-Beih, N.M.; Nasr, S.M.; Abd El-Aziz, T.H.; Khalil, F.A.M.; Ahmed, Y.F.; Abou Zeina, H.A.A. Effect of Egyptian Propolis on Cryptosporidiosis in Immunosuppressed Rats with Special Emphasis on Oocysts Shedding, Leukogram, Protein Profile and Ileum Histopathology. Asian Pac. J. Trop. Med. 2017, 10, 253–262. [Google Scholar] [CrossRef]
- Sarhan, M.H.; Farghaly, A.; Abd El-Aal, N.F.; Mohammed Farag, S.; Ahmed Ali, A.; Farag, T.I. Egyptian Propolis and Selenium Nanoparticles against Murine Trichinosis: A Novel Therapeutic Insight. J. Helminthol. 2022, 96, e50. [Google Scholar] [CrossRef]
- Al-Ghandour, A.M.F.; Ahmed, H.K.; Salem, A.; Tealeb, A.S.M.; Mohamed, R.M.S.; Yousef, A.M. Efficacy of Olibanum and Propolis Medicinal Extracts versus Metronidazole in Giardia Lamblia Experimentally Infected Mice. Microbes Infect. Dis. 2020, 1, 209–220. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter Pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Van Der Poll, T.; Van De Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental Plaque: Biological Significance of a Biofilm and Community Life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; El-Ezz, R.F.A.; Ramadan, E.; El-Kased, R.F. In Vitro and in Vivo Burn Healing Study of Standardized Propolis: Unveiling Its Antibacterial, Antioxidant and Antiinflammatory Actions in Relation to Its Phytochemical Profiling. PLoS ONE 2024, 19, e0302795. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Muniz, I.P.R.; Galantini, M.P.L.; Gonçalves, C.V.; Lima, P.H.B.; Silva, N.R.; de Oliveira, S.L.; Nunes, M.S.; Novaes, A.K.S.; de Oliveira, M.E.S.; et al. Antimicrobial Photodynamic Therapy with Brazilian Green Propolis Controls Intradermal Infection Induced by Methicillin-Resistant Staphylococcus Aureus and Modulates the Inflammatory Response in a Murine Model. Photochem. Photobiol. Sci. 2024, 23, 561–573. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Han, Y.M.; Kim, E.H. Anti-Inflammatory Effect of Korean Propolis on Helicobacter Pylori-Infected Gastric Mucosal Injury Mice Model. Nutrients 2022, 14, 4644. [Google Scholar] [CrossRef]
- Santiago, M.B.; Leandro, L.F.; Rosa, R.B.; Silva, M.V.; Teixeira, S.C.; Servato, J.P.S.; Ambrósio, S.R.; Veneziani, R.C.S.; Aldana-Mejía, J.A.; Bastos, J.K.; et al. Brazilian Red Propolis Presents Promising Anti-H. Pylori Activity in In Vitro and In Vivo Assays with the Ability to Modulate the Immune Response. Molecules 2022, 27, 7310. [Google Scholar] [CrossRef]
- Kiani, R.; Mojgani, N.; Kobarfard, F.; Saffarian, P.; Ayatollahi, S.A.; Khoramjouy, M. Evaluating the Inhibitory Effects of Probiotic Bacteria and Propolis Extracts on the Growth and Histopathological Changes in Gastric Tissues of Helicobacter Pylori Challenged Wistar Rats. Iran. J. Pharm. Res. 2024, 23, e148158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, G.; Yushanjiang, S.; Zhao, M.; Yang, H.; Lu, R.; Qu, R.; Dai, Y.; Yang, L. In Vitro and In Vivo Study of Antibacterial and Anti-Encrustation Coating on Ureteric Stents. BJU Int. 2024, 134, 72–80. [Google Scholar] [CrossRef]
- Kalia, P.; Kumar, N.R.; Harjai, K. Effect of Propolis Extract on Hematotoxicity and Histological Changes Induced by Salmonella Enterica Serovar Typhimurium in BALB/c Mice. Arch. Biol. Sci. 2016, 68, 385–391. [Google Scholar] [CrossRef]
- Kalia, P.; Kumar, N.R.; Harjai, K. Studies on the Therapeutic Effect of Propolis along with Standard Antibacterial Drug in Salmonella Enterica Serovar Typhimurium Infected BALB/c Mice. BMC Complement. Altern. Med. 2016, 16, 485, Erratum in BMC Complement. Altern. Med. 2017, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Garbis, D.V.O.; Fortes, T.S.; Brito, J.M.; Silva, L.D.M.; Trovão, L.d.O.; Oliveira, A.S.; Alves, P.C.S.; Vale, A.A.M.; Reis, A.S.; Azevedo-Santos, A.P.S.; et al. Prophylactic Use of Standardized Extract of Propolis of Apis Mellifera (EPP-AF®) Reduces Lung Inflammation and Improves Survival in Experimental Lethal Sepsis. J. Ethnopharmacol. 2024, 331, 118294. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Capcha, J.M.C.; Sanches, T.R.; de Sousa Moreira, R.; Garnica, M.S.; Shimizu, M.H.; Berretta, A.; Teles, F.; Noronha, I.L.; Andrade, L. Green Propolis Extract Attenuates Acute Kidney Injury and Lung Injury in a Rat Model of Sepsis. Sci. Rep. 2021, 11, 5925. [Google Scholar] [CrossRef]
- Nazeri, R.; Ghaiour, M. Evaluation of Antibacterial Effect of Propolis and Its Application in Mouthwash Production. Front. Dent. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Imani, Z.; Sodagar, A.; Pourhajibagher, M.; Nader, A.H.; Bahador, A. Evaluation of Antibacterial Effect of the Orthodontic Composite Containing Propolis Nanoparticles in Rat as an Animal Model. Folia Med. 2023, 65, 131–139. [Google Scholar] [CrossRef]
- El-Tayeb, M.M.; Abu-Seida, A.M.; El Ashry, S.H.; El-Hady, S.A. Evaluation of Antibacterial Activity of Propolis on Regenerative Potential of Necrotic Immature Permanent Teeth in Dogs. BMC Oral Health 2019, 19, 174. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Baer, S.L.; Pappas, P.G. Hematogenously Disseminated Fungal Infections. In Clinical Mycology with CD-ROM; Elsevier: Amsterdam, The Netherlands, 2009; pp. 609–622. [Google Scholar] [CrossRef]
- Fatahinia, M.; Khosravi, A.R.; Shokri, H. Propolis Efficacy on TNF-α, IFN-γ and IL2 Cytokines Production in Old Mice with and without Systemic Candidiasis. J. Mycol. Med. 2012, 22, 237–242. [Google Scholar] [CrossRef]
- Berretta, A.A.; De Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-Oliveira, F.; Pedrazzi, V.; Ramalho, L.N.Z.; Goldman, G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvovaginal Infection. Evid.-Based Complement. Altern. Med. 2013, 2013, 641480. [Google Scholar] [CrossRef]
- De Castro, P.A.; Bom, V.L.P.; Brown, N.A.; de Almeida, R.S.C.; Ramalho, L.N.Z.; Savoldi, M.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Identification of the Cell Targets Important for Propolis-Induced Cell Death in Candida Albicans. Fungal Genet. Biol. 2013, 60, 74–86. [Google Scholar] [CrossRef]
- Bonfim, A.P.; Sakita, K.M.; Faria, D.R.; Arita, G.S.; Vendramini, F.A.V.R.; Capoci, I.R.G.; Braga, A.G.; dos Santos, R.S.; Bruschi, M.L.; Becker, T.C.A.; et al. Preclinical Approaches in Vulvovaginal Candidiasis Treatment with Mucoadhesive Thermoresponsive Systems Containing Propolis. PLoS ONE 2020, 15, e0243197. [Google Scholar] [CrossRef]
- Dos Reis Teixeira, A.; De Vasconcelos Quaresma, A.; Branquinho, R.T.; De Oliveira, P.C.; García Suárez, J.A.; Brandão, G.C.; Barboza, A.P.M.; De Freitas Araújo, M.G.; De Magalhães, J.T.; De Moura, S.A.L.; et al. Brazilian Green Propolis Extract-Loaded Poly(Ε-Caprolactone) Nanoparticles Coated with Hyaluronic Acid: Antifungal Activity in a Murine Model of Vulvovaginal Candidiasis. AAPS PharmSciTech 2025, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Grisolia, J.C.; Nascimento Gomes, B.J.; Blosfeld-Lopes, L.; Ikegaki, M.; de Alencar, S.M.; Burger, E. Brazilian Red Propolis Shows Antifungal and Immunomodulatory Activities against Paracoccidioides Brasiliensis. J. Ethnopharmacol. 2021, 277, 114181. [Google Scholar] [CrossRef] [PubMed]
- Thammasit, P.; Tharinjaroen, C.S.; Tragoolpua, Y.; Rickerts, V.; Georgieva, R.; Bäumler, H.; Tragoolpua, K. Targeted Propolis-Loaded Poly (Butyl) Cyanoacrylate Nanoparticles: An Alternative Drug Delivery Tool for the Treatment of Cryptococcal Meningitis. Front. Pharmacol. 2021, 12, 723727. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.E.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2010, 55, 459. [Google Scholar] [CrossRef]
- Kurokawa, M.; Shimizu, T.; Takeshita, Y.; Takamori, Y.; Kai, H.; Sawamura, R.; Yoshida, H.; Watanabe, W.; Tsutsumi, A.; Park, Y.K.; et al. Efficacy of Brazilian Propolis against Herpes Simplex Virus Type 1 Infection in Mice and Their Modes of Antiherpetic Efficacies. Evid.-Based Complement. Altern. Med. 2011, 2011, 976196. [Google Scholar] [CrossRef]
- Mazia, R.S.; De Araújo Pereira, R.R.; De Francisco, L.M.B.; Natali, M.R.M.; Dias Filho, B.P.; Nakamura, C.V.; Bruschi, M.L.; Ueda-Nakamura, T. Formulation and Evaluation of a Mucoadhesive Thermoresponsive System Containing Brazilian Green Propolis for the Treatment of Lesions Caused by Herpes Simplex Type I. J. Pharm. Sci. 2016, 105, 113–121. [Google Scholar] [CrossRef]
- Sartori, G.; Pesarico, A.P.; Pinton, S.; Dobrachinski, F.; Roman, S.S.; Pauletto, F.; Rodrigues, L.C.; Prigol, M. Protective Effect of Brown Brazilian Propolis against Acute Vaginal Lesions Caused by Herpes Simplex Virus Type 2 in Mice: Involvement of Antioxidant and Anti-Inflammatory Mechanisms. Cell Biochem. Funct. 2012, 30, 1–10. [Google Scholar] [CrossRef]
- Kuwata, K.; Takemura, T.; Urushisaki, T.; Fukuoka, M.; Hosokawa-Muto, J.; Hata, T.; Okuda, Y.; Hori, S.; Tazawa, S.; Araki, Y. 3,4-Dicaffeoylquinic Acid, a Major Constituent of Brazilian Propolis, Increases TRAIL Expression and Extends the Lifetimes of Mice Infected with the Influenza a Virus. Evid.-Based Complement. Altern. Med. 2012, 2012, 946867. [Google Scholar] [CrossRef]
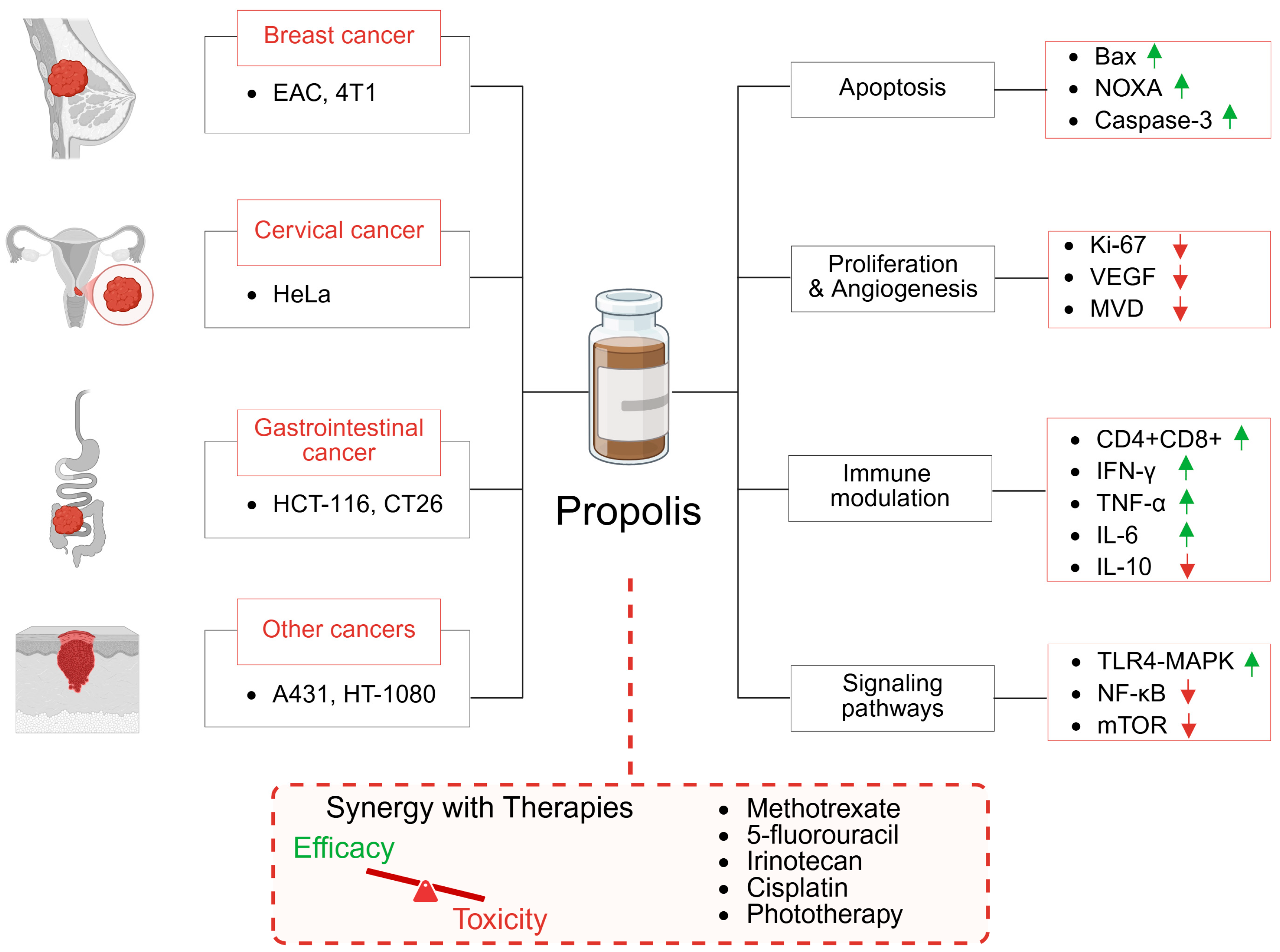
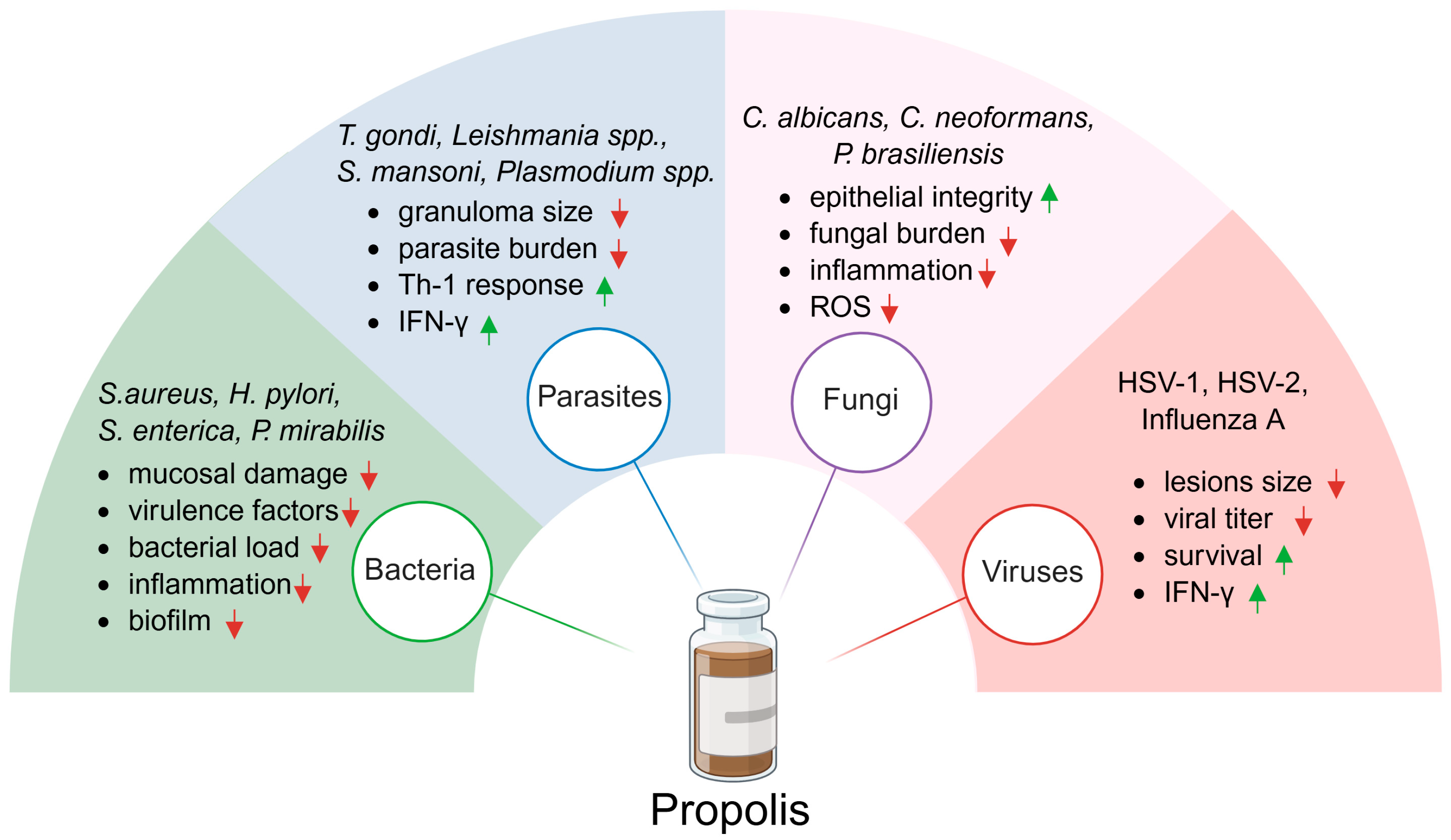
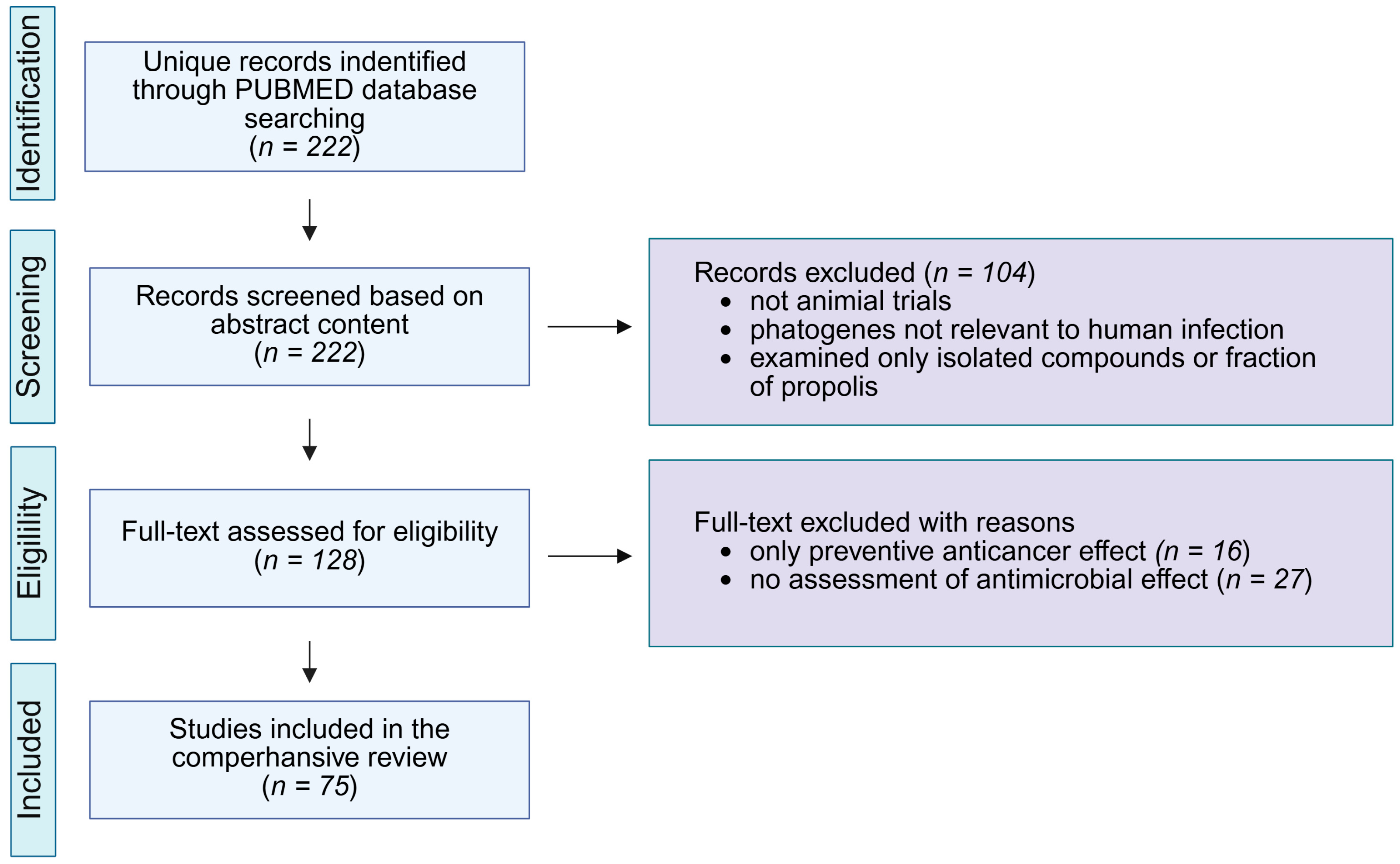
| Cancer | Cell Line | Type of Propolis | Administration | Dose [mg/kg] | Treatment Duration | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Breast cancer | EAC | Egyptian—water-soluble | oral | 50 | 11 days | ⬆ survival ⬇ tumor burden | [30] |
| Breast cancer | EAC | Egyptian—ethanolic extract | oral | 500 | 2 weeks | ⬆ survival ⬇ tumor volume ⬇ methotrexate toxicity | [50] |
| Cervical cancer | HeLa | Thai—ethanolic extract | oral | 24 | ~1000 mm3 | ⬆ survival ⬆ apoptosis | [34] |
| Colorectal cancer | HCT-116 | Iraq—unknown extract | oral | 500, 1000 | 3 weeks | ⬇ tumor volume ⬇ mitosis | [36] |
| Gastrointestinal cancer | A4gnt KO | Philippine stingless bee—ethanolic extract | oral | 100 | 30 days | ⬇ tumor growth ⬇ T-lymphocyte infiltration | [37] |
| Colorectal cancer | CT26 | supercritical CO2 extract of propolis | oral | 100–300 | 1 week | ⬇ tumor volume ⬆ immune stimulation | [38] |
| Epidermoid carcinoma | A431 | Poplar—ethanolic extract | oral | 50, 100 | 12 days | ⬇ tumor volume ⬆ necrosis | [45] |
| Fibrosarcoma | HT1080 | Brazilian green—supercritical extract | oral | 100 | 3 weeks | ⬇ tumor volume ⬇ cell migration | [47] |
| Hodgkin lymphoma | DLA | Indian—standardized ethanolic extract | i.p. | 100–400 | 14 days | ⬆ life span ⬇ tumor growth | [49] |
| Colorectal cancer | Induced | Iranian—ethanolic extract | i.p. | 10–90 | 8 weeks | ⬇ progression ⬆ efficiency of 5-FU | [52] |
| Colorectal cancer | LoVo-luc | Anatolian—ethanolic extract | i.p./oral | 400, 800 | 3 weeks | ⬇ tumor size ⬆ efficiency of 5-FU | [53] |
| Mammary carcinoma | 4T1 | Croatian—powder | in food | 300 | 38 days | ⬇ tumor size with 5-FU ⬇ metastasis with 5-FU | [54] |
| Colorectal cancer | Induced | Sericin/propolis nanoparticles | oral | 75 | 8 weeks | ⬆ autophagy ⬆ apoptosis ⬇ oxidative stress | [55] |
| Breast cancer | EAC | Poplar-type—water and ethanolic extracts | i.p. | 100 | 3 days | ⬆ survival with Irinotecan ⬆ hematopoietic activity | [57] |
| Mammary tumor | SMMT | Iranian—ethanolic extract | oral | 100 | 10 days | ⬇ tumor size ⬇ Th2 responses ⬆ pro-inflammatory cytokines | [58] |
| Breast cancer | 4T1 | Neptune™—water extract | oral | 66 | 14 days | ⬇ tumor volume ⬆ immune stimulation | [59] |
| Epidermoid carcinoma | A431 | Brazilian green—ethanolic extract | cells pre-treatment | 75 µg/mL | pre-treatment | ⬇ tumour volume with PpIX-PDT ⬆ apoptosis with PpIX-PDT | [61] |
| Drug | Dose of Drug [mg/kg] | Type of Propolis | Administration | Dose of Propolis [mg/kg] | Treatment Duration | Main Effect | Reference |
|---|---|---|---|---|---|---|---|
| 5-fluorouracil | 125 | Irania—ethanolic extract | oral | 250 | 14 days | ameliorates the cardiotoxic effects | [62] |
| Mitomycin C | 8 | Indian—hydroethanolic extract | i.p. | 100–800 | 1 h before | mitigates the genotoxic and cytotoxic effects | [63] |
| Mitomycin C | 2–8 | Indian—hydroethanolic extract | i.p. | 400 | 1 h before | mitigates testicular damage | [64] |
| Cisplatin | 7 | Turkish—water extract | oral | 50, 100 | 14 days | protects against nephrotoxic effects | [68] |
| Irinotecan | 50 | Croatian—water and ethanolic extracts | i.p. | 100 | 3 days before | ameliorates haematological, liver, and kidney toxicity | [69] |
| Doxorubicin | 3 | Egyptia—ethanolic extract | oral | 200 | 3 weeks | alleviates toxicity to the testis | [70] |
| Doxorubicin | 25 | Indian—ethanolic extract | oral | 250 | 14 days | protects against liver toxicity and oxidative stress | [71] |
| Cyclophosphamide | 200 | Saudi Arabia—ethanolic extract | i.v. | 100 | 7 days | ameliorates haematological, liver, and kidney toxicity | [72] |
| Radio-therapy | 35 Gy | Anatolian—water-soluble droplets | oral | 100, 200 | 3–7 weeks | reduces the severity of osteoradionecrosis | [77] |
| Radio-therapy | 15 Gy | Turkish—water soluble extract | i.v. | 100, 200 | 2 weeks | reduces acute mucositis | [78] |
| Radio-therapy | 15 Gy | Unknown | i.p. | 400 | 3 days | protects salivary gland function and reduces mucositis | [79] |
| Infectious Agent | Route of Infection | Type of Propolis | Administration | Dose [mg/kg] | Treatment Duration | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Toxoplasma gondii | i.p. | Egyptian—ethanolic extract | oral | 150 | 7 days | ⬆ survival ⬇ parasite count | [84] |
| Toxoplasma gondii | i.p. | Egyptian—unknown extract | oral | 0.1 mL/day | 10 days | ⬇ parasite load ⬇ changes in liver and lung | [85] |
| Toxoplasma gondii | i.p. | Egyptian—unknown extract | oral | 0.1 mL/day | 10 days | ⬇ parasite load ⬇ lesions in brain, uterus, kidneys | [86] |
| Toxoplasma gondii | unknown | Unknown | oral | 0.1 mL/day | 10 days | ⬇ parasite load ⬆ histopathology of liver, spleen, lungs | [87] |
| Toxoplasma gondii | i.p. | Egyptian—commercial extract | oral | 0.1 mL/day | 10 days | ⬇ parasite load ⬇ changes in brain, uterus, kidneys | [88] |
| Leishmania amazonensis | s.c. | Brazilian Red—ethanolic extract | topical | 2.5% gel | 20 days | ⬇ lesions (combined with Glucantime) | [89] |
| Leishmania amazonensis | i.d. | Brazilian Green—glycolic extract | topical | 3.6% gel | 3 weeks | ⬇ lesion size ⬇ inflammation | [90] |
| Leishmania infantum | i.v. | Brazilian Green—water extract | oral | 500 | 14 days | ⬇ parasite load in liver ⬇ lesions in liver and spleen | [91] |
| Leishmania major | s.c. | Iranian in mixture with herbs | topical | twice daily | unknown | ⬇ parasite burden ⬇ lesion size | [92] |
| Schistosoma mansoni | s.c. | Egyptian—ethanolic extract | oral | 300 | 4 weeks | ⬇ hepatic granuloma number ⬇ lymphocytic infiltration and aggregation ⬆ Praziquantel activity | [93] |
| Schistosoma mansoni | s.c. | Brazilian Red—crude extract | oral | 400 | single dose | ⬇ total worm burden ⬇ eggs number in the intestine and feces | [94] |
| Trypanosoma cruzi | i.p. | Brazilian Green—ethanolic extract | oral | 25–300 | 10 days | ⬆ survival ⬇ metacyclogenesis | [95] |
| Trypanosoma evansi | i.p. | Brazilian—ethanolic extract | oral | 100–400 | 10 days | ⬆ longevity ⬇ parasitaemia levels | [96] |
| Plasmodium chabaudi | i.p. | Saudi—methanolic extract | oral | 25–100 | 7 days | ⬇ parasitaemia levels ⬇ oxidative damage ⬆ splenic architecture | [97] |
| Plasmodium berghei | i.p. | Iranian—dichloromethane and ethanolic extracts | i.p. | 50–200 | 5 days | ⬆ survival ⬇ parasite count | [98] |
| Cryptosporidium spp. | oral | Egyptian—ethanolic and water extracts | oral | 50 | 7 days | ⬇ mortality rate ⬇ oxidative stress | [99] |
| Cryptosporidium spp. | oral | Egyptian—ethanolic and water extracts | oral | 50 | 7 days | ⬇ oocysts count ⬆ total leukocytic count | [100] |
| Trichinella spiralis | oral | Egyptian—ethanolic extract | oral | 250 | 34 days | ⬇ worm and larval count ⬇ inflammation and angiogenesis | [101] |
| Giardia lamblia | oral | ‘Biopropolis‘ tablets—aqueous suspension | oral | 1.04 mg/ 0.2 mL/mouse | 7 days | ⬇ trophozoite count ⬇ changes in jejunal mucosa | [102] |
| Staphylococcus aureus | topical | South Asian—hydroalcoholic extract | topical | 50, 100% | 13 days | ⬇ wound diameter ⬇ bacterial load | [108] |
| Staphylococcus aureus | i.d. | Brazilian Green—glycolic extract | topical | 10 µg | single dose | ⬇ bacterial load (combined with aPDT) ⬇ weight loss | [109] |
| Helicobacter pylori | oral | Korean—ethanolic extract | oral | 200 | 4 weeks | ⬇ bacterial growth and virulence factors ⬇ lesions and inflammation | [110] |
| Helicobacter pylori | oral | Brazilian Red—hydroalcoholic extract | oral | 18–300 | 7 days | ⬇ bacterial load ⬇ chronic inflammation | [111] |
| Helicobacter pylori | oral | Iranian—ethanolic extract | oral | 75–300 | 21 days | ⬇ bacterial load ⬇ changes in gastric tissue | [112] |
| Proteus mirabilis | intravesical | Chinese—ethanolic extract | coating on ureteric stents | 128 mg/mL | prevention | ⬇ bacteria, stones, salt deposits ⬇ inflammation | [113] |
| Salmonella enterica serovar Typhimurium | i.p. | Indian—ethanolic extract | oral | 100, 300 | 30 days | ⬇ bacterial load ⬇ biochemical and histological changes | [114] |
| Salmonella enterica serovar Typhimurium | i.p. | Indian—ethanolic extract | oral | 300 | 30 days | ⬇ bacterial count ⬆ biochemical, haematological parameters | [115] |
| Polymicrobial-sepsis | CLP | Brazilian—standardized extract | s.c. | 10, 100 | single dose | ⬆ survival ⬇ lung inflammation | [116] |
| Polymicrobial-sepsis | CLP | Brazilian Green—ethanolic extract | i.p. | 500 | single dose | ⬆ survival ⬇ acute kidney and lung injury | [117] |
| Cariogenic bacteria | – | Iranian—ethanolic extract | oral | 3 mg | 2 weeks | ⬇ bacterial count | [118] |
| Cariogenic bacteria | oral | Iranian—nanoparticles | tooth surface | 1–10% | single dose | ⬇ bacterial count | [119] |
| Polymicrobialteeth | – | Egyptian—unknown extract | tooth canal | 150 | single dose | ⬇ bacterial count ⬆ teeth regeneration | [120] |
| Candida albicans | i.v. | Iranian—ethanolic extract | oral | 100 | 18 days | ⬇ fungal burden ⬇ proinflammatory cytokines | [123] |
| Candida albicans | i.vag. | Brazilian—ethanolic and water extracts | i.vag. | 1% 60 µL | 10 days | ⬇ fungal burden | [124] |
| Candida albicans | i.vag. | Brazilian—standardized extract | i.vag. | 2–3% | 10 days | ⬇ fungal burden ⬇ changes in vaginal tissue | [125] |
| Candida albicans | i.vag. | Brazilian—ethanolic extract | i.vag. | 14–16% 60 µL | 14 days | ⬇ fungal burden | [126] |
| Candida albicans | i.vag. | Brazilian Green—nanoparticles | i.vag. | 4.26 mg | 24 h | ⬇ fungal burden (full composition) | [127] |
| Paracoccidioides brasiliensis | s.c. | Brazilian Red—ethanolic extract | s.c. | 50–500 | single dose | ⬇ fungal burden ⬆ neutrophils activity | [128] |
| Cryptococcus neoformans | i.v. | Thai—nanoparticles | i.v. | 30.75 mg | 8 days | ⬇ fungal burden in brain ⬇ lesions number in brain | [129] |
| Herpes Simplex—Type 1 | s.c. | Brazilian—ethanolic extracts | oral | 10 | 6 days | ⬇ virus titers ⬇ skin lesions | [132] |
| Herpes Simplex—Type 1 | topical | Brazilian Green—unknown extract | topical | 8% | 10 days | ⬇ lesion score | [133] |
| Herpes Simplex—Type 2 | i.vag. | Brazilian Brown—hydroalcoholic extract | oral | 50 | 5 days | ⬇ lesion score ⬆ longevity ⬇ inflammation | [134] |
| Influenza A virus | intranasal | Brazilian Green—ethanolic and water extracts | oral | 100 | 6 days | ⬆ survival ⬆ viral clearance | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierański, M.K.; Kaniowski, D.; Szweda, P. Therapeutic Potential of Propolis in Preclinical Models of Cancer and Infectious Diseases: A Review. Int. J. Mol. Sci. 2025, 26, 8041. https://doi.org/10.3390/ijms26168041
Pierański MK, Kaniowski D, Szweda P. Therapeutic Potential of Propolis in Preclinical Models of Cancer and Infectious Diseases: A Review. International Journal of Molecular Sciences. 2025; 26(16):8041. https://doi.org/10.3390/ijms26168041
Chicago/Turabian StylePierański, Michał K., Damian Kaniowski, and Piotr Szweda. 2025. "Therapeutic Potential of Propolis in Preclinical Models of Cancer and Infectious Diseases: A Review" International Journal of Molecular Sciences 26, no. 16: 8041. https://doi.org/10.3390/ijms26168041
APA StylePierański, M. K., Kaniowski, D., & Szweda, P. (2025). Therapeutic Potential of Propolis in Preclinical Models of Cancer and Infectious Diseases: A Review. International Journal of Molecular Sciences, 26(16), 8041. https://doi.org/10.3390/ijms26168041






