A High-Fat Diet Increases Kidney Fibrosis Through Regulating TGF-β and PDGF-β Signaling Pathways in Normotensive and Hypertensive Rat Models
Abstract
1. Introduction
2. Results
2.1. The Effects of a High-Fat Diet on Kidney Histology and Fibrosis in Normotensive and Hypertensive Rat Models
2.2. The Effects of a High-Fat Diet on Kidney Injury in Normotensive and Hypertensive Rat Models
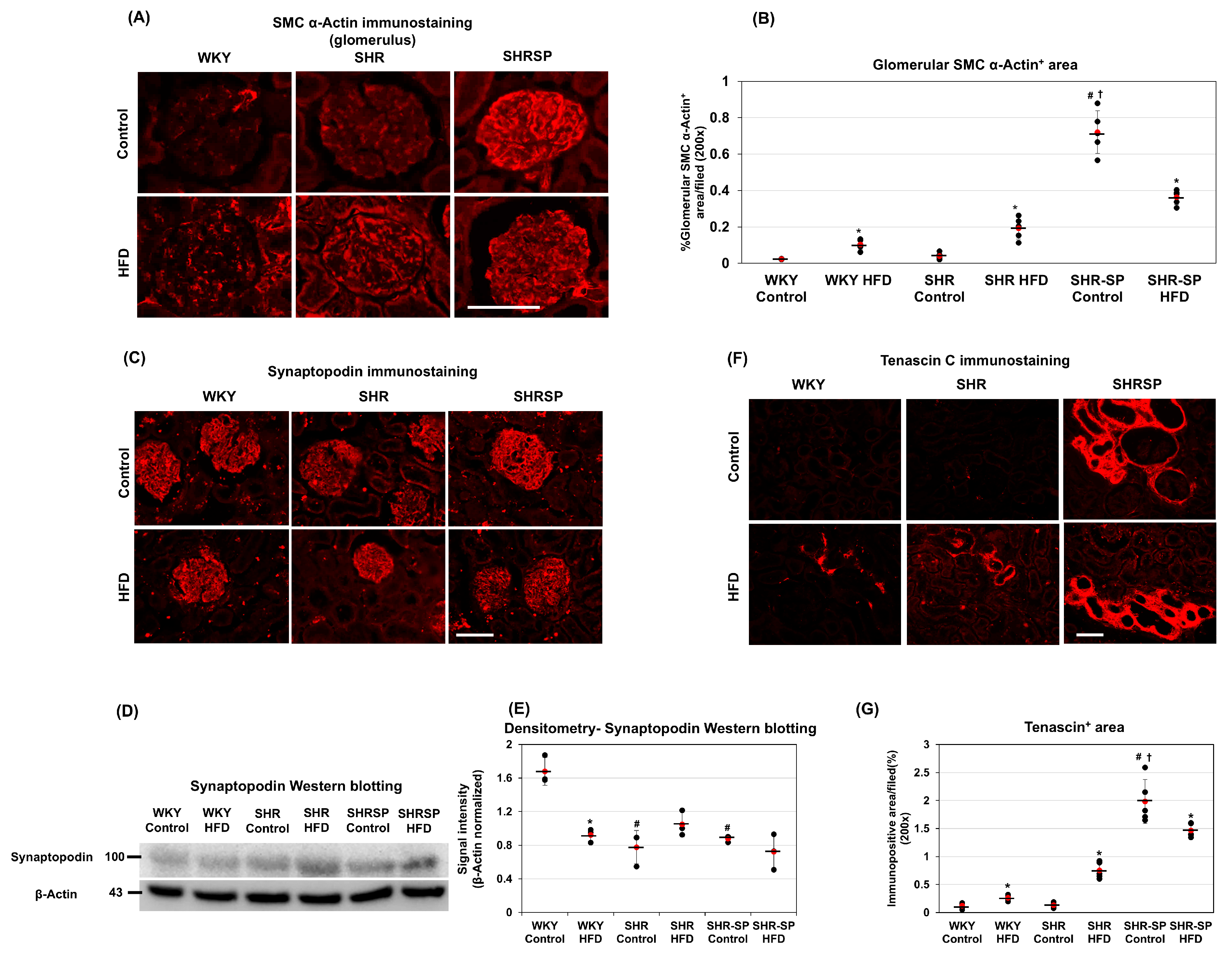
2.3. The Effects of a High-Fat Diet on Kidney Fibrosis Types in Normotensive and Hypertensive Rat Models
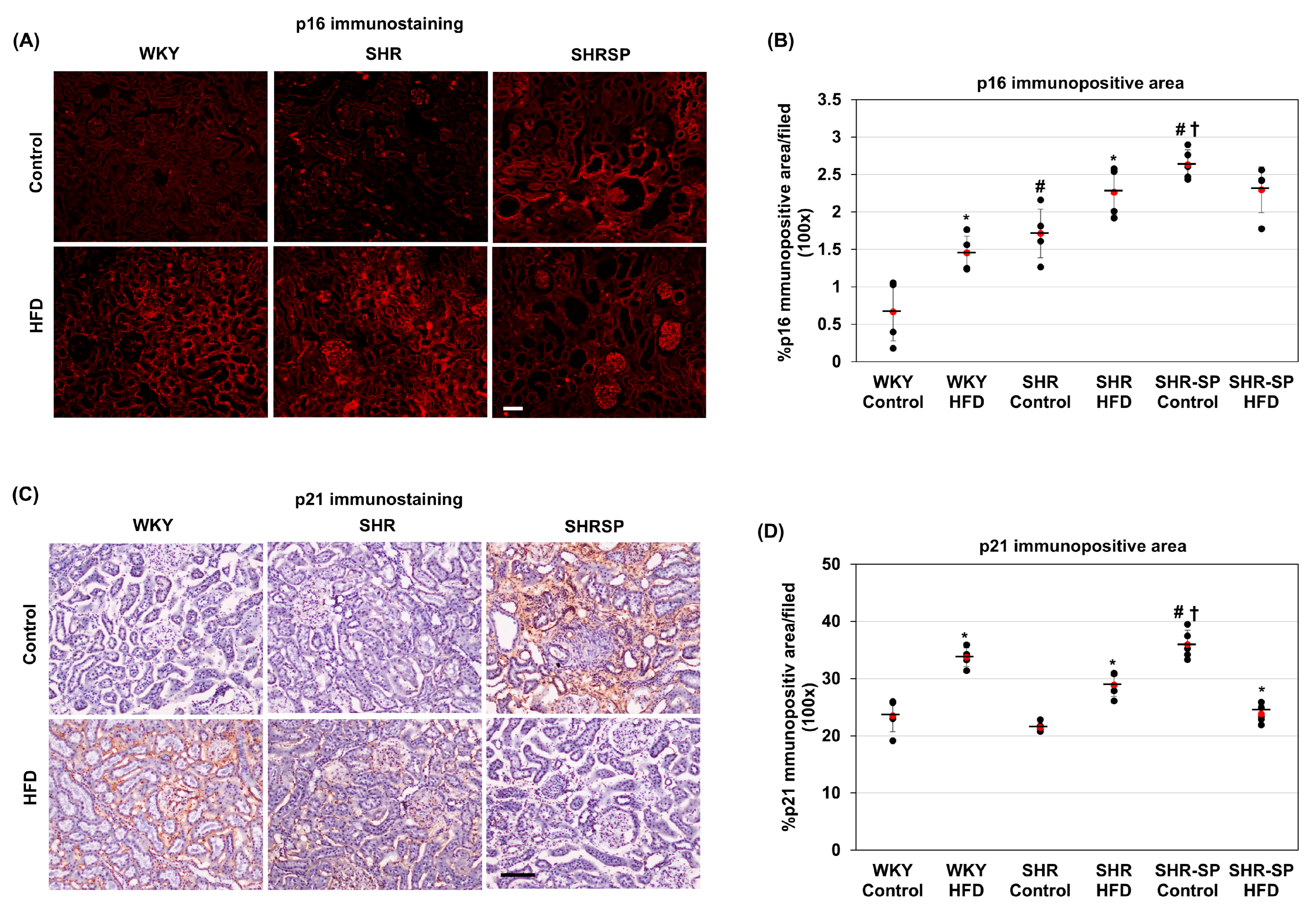
2.4. The Effects of a High-Fat Diet on Senescence-Related Changes in the Kidneys in Normotensive and Hypertensive Rat Models
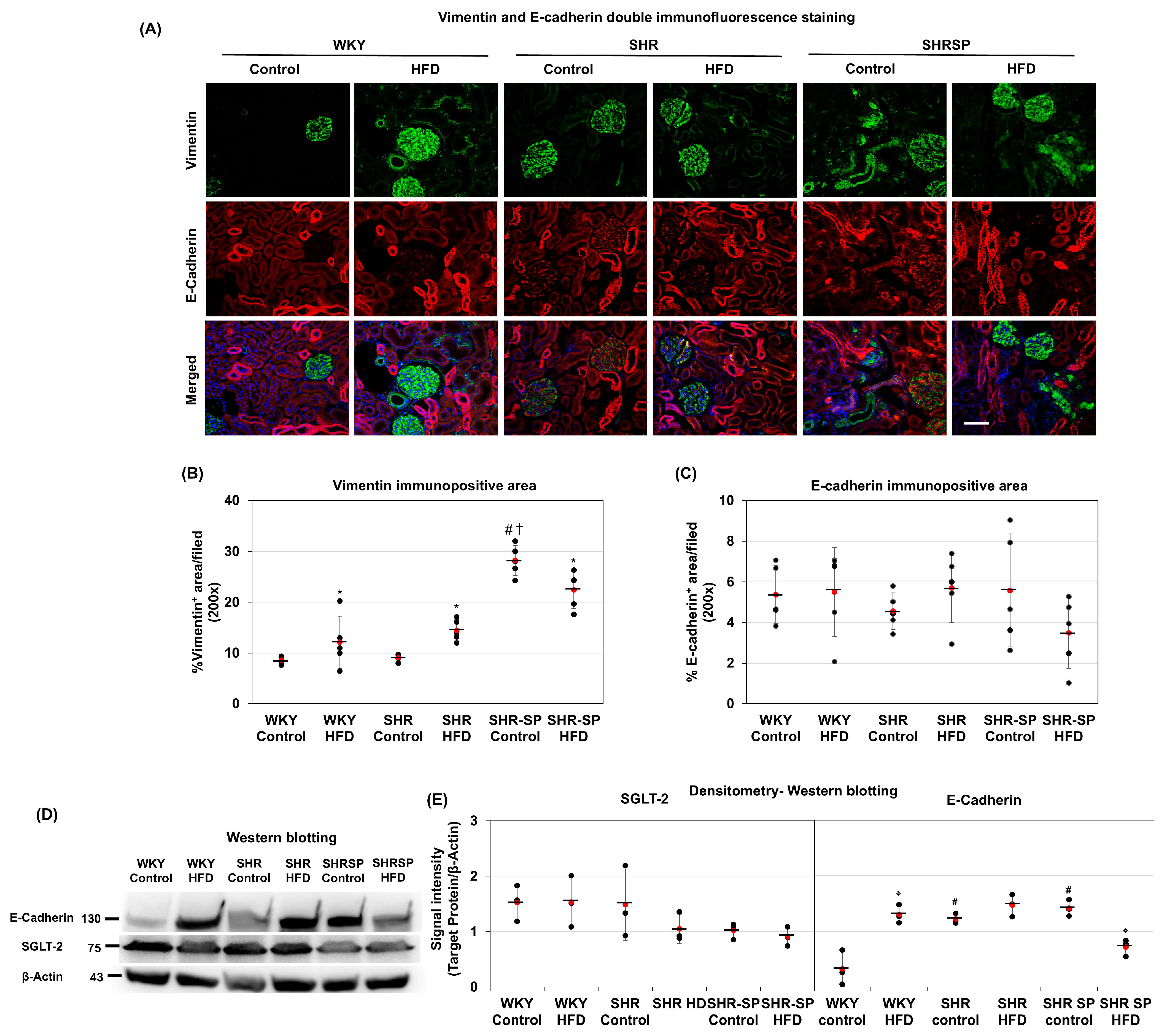
2.5. The Effects of a High-Fat Diet on Changes in Mesenchymal and Epithelial Markers in the Kidneys in Normotensive and Hypertensive Rat Models
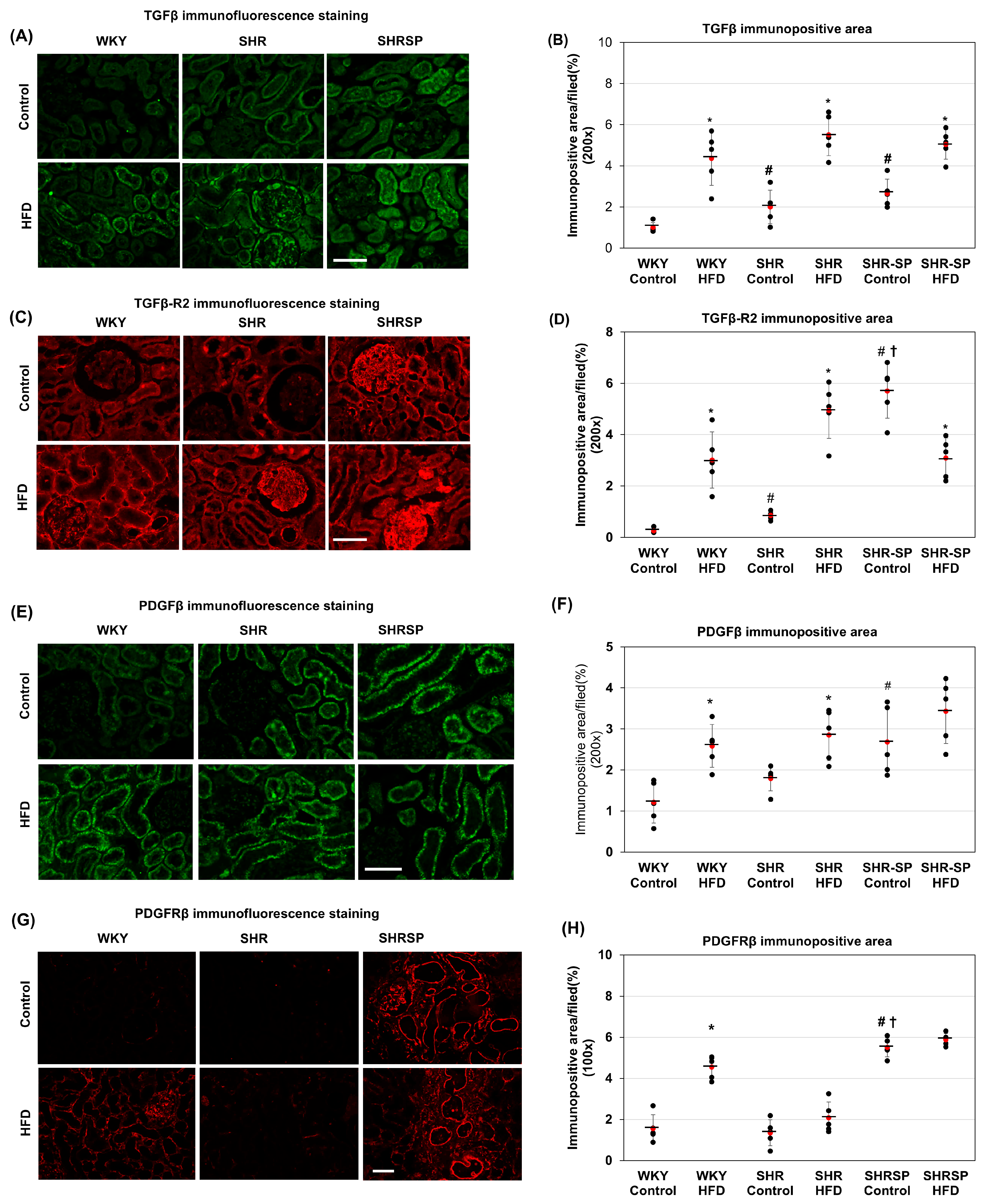
2.6. The Effects of a High-Fat Diet on TGF-β and TGF-βR2 in the Kidneys in Normotensive and Hypertensive Rat Models
2.7. The Effects of a High-Fat Diet on PDGFβ and PDGF-Rβ in the Kidneys of Normotensive and Hypertensive Rat Models
2.8. The Effects of a High-Fat Diet on TGFβ and PDGFβ Signaling in the Kidneys in Normotensive and Hypertensive Rat Models
2.9. A Gene Ontology Analysis of Kidney-Fibrosis-Related Genes in the SHR-SP Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. The Experimental Design
4.3. Tissue Preparation for Staining
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Periodic Acid–Schiff (PAS) Staining
4.6. Azan Staining
4.7. Immunostaining
4.8. Quantification of Staining
4.9. Total RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
4.10. The Western Blot Analysis
4.11. Genetic Analysis of the DNA Fragment Related to Kidney Fibrosis in SHR-SP Rats
4.12. The Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.; Mone, P.; Jankauskas, S.S.; Gambardella, J.; Santulli, G. Chronic kidney disease: Definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J. Clin. Hypertens. 2021, 23, 831–834. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, X.; Zhao, Y.; Gu, M.; Chen, X.; Zhang, H.; Wu, X.; Yu, C.; Niu, J.; Ding, W.; et al. Different stages of chronic kidney disease are associated with physical performance in adults over 60 years. Front. Public Health 2022, 10, 963913. [Google Scholar] [CrossRef]
- Hu, J.; Ke, R.; Teixeira, W.; Dong, Y.; Ding, R.; Yang, J.; Ai, X.; Ye, D.W.; Shang, J. Global, Regional, and National Burden of CKD due to Glomerulonephritis from 1990 to 2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Clin. J. Am. Soc. Nephrol. 2023, 18, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Lanktree, M.B.; Haghighi, A.; di Bari, I.; Song, X.; Pei, Y. Insights into Autosomal Dominant Polycystic Kidney Disease from Genetic Studies. Clin. J. Am. Soc. Nephrol. 2021, 16, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B.; Lusco, M.A.; Najafian, B.; Alpers, C.E. AJKD Atlas of Renal Pathology: Chronic Interstitial Nephritis. Am. J. Kidney Dis. 2017, 70, e1–e2. [Google Scholar] [CrossRef]
- Tsuchimoto, A.; Matsukuma, Y.; Ueki, K.; Tanaka, S.; Masutani, K.; Nakagawa, K.; Mitsuiki, K.; Uesugi, N.; Katafuchi, R.; Tsuruya, K.; et al. Utility of Columbia classification in focal segmental glomerulosclerosis: Renal prognosis and treatment response among the pathological variants. Nephrol. Dial. Transplant. 2020, 35, 1219–1227. [Google Scholar] [CrossRef]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131. [Google Scholar] [CrossRef]
- Nordheim, E.; Geir Jenssen, T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 2021, 10, R151–R159. [Google Scholar] [CrossRef]
- Lichtnekert, J.; Anders, H.J. Lupus nephritis-related chronic kidney disease. Nat. Rev. Rheumatol. 2024, 20, 699–711. [Google Scholar] [CrossRef]
- Said, S.; Hernandez, G.T. The link between chronic kidney disease and cardiovascular disease. J. Nephropathol. 2014, 3, 99–104. [Google Scholar] [CrossRef]
- Zhang, X.; Lerman, L.O. The metabolic syndrome and chronic kidney disease. Transl. Res. 2017, 183, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Koszegi, S.; Molnar, A.; Lenart, L.; Hodrea, J.; Balogh, D.B.; Lakat, T.; Szkibinszkij, E.; Hosszu, A.; Sparding, N.; Genovese, F.; et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J. Physiol. 2019, 597, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshika, M.; Komiyama, Y.; Nishimura, M. The central mechanism underlying hypertension: A review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens. Res. 2011, 34, 1147–1160. [Google Scholar] [CrossRef]
- Bidani, A.K.; Polichnowski, A.J.; Loutzenhiser, R.; Griffin, K.A. Renal microvascular dysfunction, hypertension and CKD progression. Curr. Opin. Nephrol. Hypertens. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Burke, M.; Pabbidi, M.; Farley, J.; Roman, R.J. Molecular Mechanisms of Renal Blood Flow Autoregulation. Curr. Vasc. Pharmacol. 2014, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Ikemiya, Y.; Fukiyama, K. Risk factors of end-stage renal disease and serum creatinine in a community-based mass screening. Kidney Int. 1997, 51, 850–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; do Carmo, J.M.; Aberdein, N.; Zhou, X.; Williams, J.M.; da Silva, A.A.; Hall, J.E. Synergistic Interaction of Hypertension and Diabetes in Promoting Kidney Injury and the Role of Endoplasmic Reticulum Stress. Hypertension 2017, 69, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Kabootari, M.; Habibi Tirtashi, R.; Amouzegar, A.; Masoumi, S.; Azizi, F.; Amouzegar, A. Changes in metabolic syndrome status and risk of chronic kidney disease over a decade of follow-up in the Iranian population. Sci. Rep. 2025, 15, 19041. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Dow, C.A.; Stauffer, B.L.; Greiner, J.J.; De Souza, C.A. Influence of habitual high dietary fat intake on endothelium-dependent vasodilation. Appl. Physiol. Nutr. Metab. 2015, 40, 711–715. [Google Scholar] [CrossRef]
- Hong, N.; Lin, Y.; Ye, Z.; Yang, C.; Huang, Y.; Duan, Q.; Xie, S. The relationship between dyslipidemia and inflammation among adults in east coast China: A cross-sectional study. Front. Immunol. 2022, 13, 937201. [Google Scholar] [CrossRef] [PubMed]
- Saud, A.; Ali, N.A.; Gali, F.; Hadi, N. The role of cytokines, adhesion molecules, and toll-like receptors in atherosclerosis progression: The effect of Atorvastatin. J. Med. Life 2022, 15, 751–756. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Guo, J. Lipotoxic Proximal Tubular Injury: A Primary Event in Diabetic Kidney Disease. Front. Med. 2021, 8, 751529. [Google Scholar] [CrossRef]
- Kim, J.J.; Wilbon, S.S.; Fornoni, A. Podocyte Lipotoxicity in CKD. Kidney360 2021, 2, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, L.; Smarkusz-Zarzecka, J.; Zyśk, B.; Orywal, K.; Mroczko, B.; Cwalina, U. Could Selected Adipokines/Cytokines Serve as Markers of Adipose Tissue Dysfunction? Int. J. Mol. Sci. 2024, 25, 13744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sheikh, A.M.; Tabassum, S.; Iwasa, K.; Shibly, A.Z.; Zhou, X.; Wang, R.; Bhuiya, J.; Abdullah, F.B.; Yano, S.; et al. Effect of high-fat diet on cerebral pathological changes of cerebral small vessel disease in SHR/SP rats. Geroscience 2024, 46, 3779–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Han, D.; Zhao, C.; Yang, F.; Wang, Z.; Gao, Y.; Jin, M.; Tao, R. New insights into the role of Klotho in inflammation and fibrosis: Molecular and cellular mechanisms. Front. Immunol. 2024, 15, 1454142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Huang, S.; Sun, K.; Chen, Y.; Xie, G.; Bao, J.; Fan, Y. Protective effect of compound K against podocyte injury in chronic kidney disease by maintaining mitochondrial homeostasis. Sci. Rep. 2025, 15, 435. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tian, Y.; Zhou, L.; Zhou, D.; Tan, R.J.; Stolz, D.B.; Liu, Y. Tenascin-C Is a Major Component of the Fibrogenic Niche in Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 785–801. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Meligy, F.Y.; Kagawa, S.; Ishii, M.; Betsuyaku, T. High fat diet activates adult mouse lung stem cells and accelerates several aging-induced effects. Stem Cell Res. 2018, 33, 25–35. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Zhou, Y.; Han, W.; Fu, X. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp. Biol. Med. 2016, 241, 1–13. [Google Scholar] [CrossRef]
- Makihara, N.; Arimura, K.; Ago, T.; Tachibana, M.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kuroda, J.; Sugimori, H.; et al. Involvement of platelet-derived growth factor receptor β in fibrosis through extracellular matrix protein production after ischemic stroke. Exp. Neurol. 2015, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.F.; Ngarashi, D.; Koike, M.; Misumi, M.; Ohara, H.; Nabika, T. Evaluation of Pathological Association between Stroke-Related QTL and Salt-Induced Renal Injury in Stroke-Prone Spontaneously Hypertensive Rat. BioMed Res. Int. 2019, 2019, 5049746. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic Kidney Disease: Its Relationship with obesity. Cureus 2022, 14, e30535. [Google Scholar] [CrossRef] [PubMed]
- Gandolgor, T.A.; Ohara, H.; Cui, Z.H.; Hirashima, T.; Ogawa, T.; Saar, K.; Hübner, N.; Watanabe, T.; Isomura, M.; Nabika, T. Two genomic regions of chromosomes 1 and 18 explain most of the stroke susceptibility under salt loading in stroke-prone spontaneously hypertensive rat/Izm. Hypertension 2013, 62, 55–61. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, N.; Harada, Y.; Maruyama, R.; Masuda, J.; Nabika, T. Genetic effects of blood pressure quantitative trait loci on hypertension-related organ damage: Evaluation using multiple congenic strains. Hypertens. Res. 2008, 31, 1773–1779. [Google Scholar] [CrossRef]
- Ono, H.; Ono, Y. Nephrosclerosis and hypertension. Med Clin. N. Am. 1997, 81, 1273–1288. [Google Scholar] [CrossRef]
- Yamori, Y.; Horie, R.; Ohtaka, M.; Nara, Y. Effect of high fat cholesterol diet on cerebrovascular circulation and the heart in stroke-prone SHR. Jpn. Hear. J. 1977, 18, 533–535. [Google Scholar] [CrossRef][Green Version]
- Hajare, A.D.; Dagar, N.; Gaikwad, A.B. Klotho antiaging protein: Molecular mechanisms and therapeutic potential in diseases. Mol. Biomed. 2025, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, J.; Li, M.; Wang, L.; Xu, X.; Li, M. Association between serum Klotho concentration and hypertension in postmenopausal women, a cross-sectional study from NHANES 2013-2016. BMC Geriatr. 2023, 23, 466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Liu, X.; Zhu, X.; Lin, C.; Chen, X.; Huang, Y.; Huang, Z. Association of serum Klotho concentrations with abdominal obesity among middle-aged adults in the US: Evidence from NHANES 2011-2016. Nutrition 2025, 136, 112809. [Google Scholar] [CrossRef]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Suárez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Carro, B.; Cannata-Andía, J.B.; Mora-Fernández, C.; Navarro-González, J.F. Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants 2023, 12, 239. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Q.; Luo, B.; Zhang, L.; Lin, L.; Han, L.; Hao, M.; Li, M.; Yu, L.; Li, M. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur. J. Pharmacol. 2019, 859, 172549. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J. Current Opinion for Hypertension in Renal Fibrosis. In Renal Fibrosis: Mechanisms and Therapies; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 37–47. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Harding, P.; Haney, L.B.; Glass, W.F., 2nd. Regulation of the mesangial cell myofibroblast phenotype by actin polymerization. J. Cell. Physiol. 2003, 195, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Li, S.; Gao, J.; Ding, L.; Wang, C.; Chen, W.; Shan, G.; Zhang, F.; Yu, J.; Xu, G. Tenascin-C Is Increased in Inflammatory Bowel Disease and Is Associated with response to Infliximab Therapy. BioMed Res. Int. 2019, 2019, 1475705. [Google Scholar] [CrossRef] [PubMed]
- Arendshorst, W.J.; Beierwaltes, W.H. Renal and nephron hemodynamics in spontaneously hypertensive rats. Am. J. Physiol. 1979, 236, F246–F251. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.A.; Churchill, P.C.; Picken, M.; Webb, R.C.; Kurtz, T.W.; Bidani, A.K. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am. J. Hypertens. 2001, 14, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Szabó, R.; Börzsei, D.; Hoffmann, A.; Lesi, Z.N.; Gesztelyi, R.; Juhász, B.; Szebeni, G.J.; Osman, J.; Sebestyén, J.; Nagy, A.; et al. Lifestyle-Induced Redox-Sensitive Alterations: Cross-Talk among the RAAS, Antioxidant/Inflammatory Status, and Hypertension. Oxidative Med. Cell. Longev. 2021, 2021, 3080863. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Jurk, D.; Doolittle, M.L.; Kosinsky, R.L.; Monroe, D.G.; LeBrasseur, N.K.; Robbins, P.D.; Niedernhofer, L.J.; Khosla, S.; Passos, J.F. Distinct secretomes in p16- and p21- positive senescent cells across tissues. bioRxiv 2023. bioRxiv:2023.12.05.569858. [Google Scholar] [CrossRef]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. npj Aging 2024, 10, 53. [Google Scholar] [CrossRef]
- Matsuda, S.; Revandkar, A.; Dubash, T.D.; Ravi, A.; Wittner, B.S.; Lin, M.; Morris, R.; Burr, R.; Guo, H.; Seeger, K.; et al. TGF-β in the microenvironment induces a physiologically occurring immune-suppressive senescent state. Cell Rep. 2023, 42, 112129. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol.-Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, H.; Yin, Y.; Li, J.; Tang, Y.; Purkayastha, S.; Li, L.; Cai, D. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat. Med. 2014, 20, 1001–1008. [Google Scholar] [CrossRef]
- Derhaschnig, U.; Shehata, M.; Herkner, H.; Bur, A.; Woisetschläger, C.; Laggner, A.N.; Hirschl, M.M. Increased levels of transforming growth factor-beta1 in essential hypertension. Am. J. Hypertens. 2002, 15, 207–211. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Mitaki, S.; Michikawa, M.; Nagai, A. Alzheimer’s Amyloid β Peptide Induces Angiogenesis in an Alzheimer’s Disease Model Mouse through Placental Growth Factor and Angiopoietin 2 Expressions. Int. J. Mol. Sci. 2023, 24, 4510. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, F.B.; Sheikh, A.M.; Tabassum, S.; Nagai, A.; Yoshino, J.; Kanda, T.; Nabika, T.; Yano, S. A High-Fat Diet Increases Kidney Fibrosis Through Regulating TGF-β and PDGF-β Signaling Pathways in Normotensive and Hypertensive Rat Models. Int. J. Mol. Sci. 2025, 26, 8031. https://doi.org/10.3390/ijms26168031
Abdullah FB, Sheikh AM, Tabassum S, Nagai A, Yoshino J, Kanda T, Nabika T, Yano S. A High-Fat Diet Increases Kidney Fibrosis Through Regulating TGF-β and PDGF-β Signaling Pathways in Normotensive and Hypertensive Rat Models. International Journal of Molecular Sciences. 2025; 26(16):8031. https://doi.org/10.3390/ijms26168031
Chicago/Turabian StyleAbdullah, Fatema Binte, Abdullah Md. Sheikh, Shatera Tabassum, Atsushi Nagai, Jun Yoshino, Takeshi Kanda, Toru Nabika, and Shozo Yano. 2025. "A High-Fat Diet Increases Kidney Fibrosis Through Regulating TGF-β and PDGF-β Signaling Pathways in Normotensive and Hypertensive Rat Models" International Journal of Molecular Sciences 26, no. 16: 8031. https://doi.org/10.3390/ijms26168031
APA StyleAbdullah, F. B., Sheikh, A. M., Tabassum, S., Nagai, A., Yoshino, J., Kanda, T., Nabika, T., & Yano, S. (2025). A High-Fat Diet Increases Kidney Fibrosis Through Regulating TGF-β and PDGF-β Signaling Pathways in Normotensive and Hypertensive Rat Models. International Journal of Molecular Sciences, 26(16), 8031. https://doi.org/10.3390/ijms26168031





