Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen
Abstract
1. Introduction
2. Results
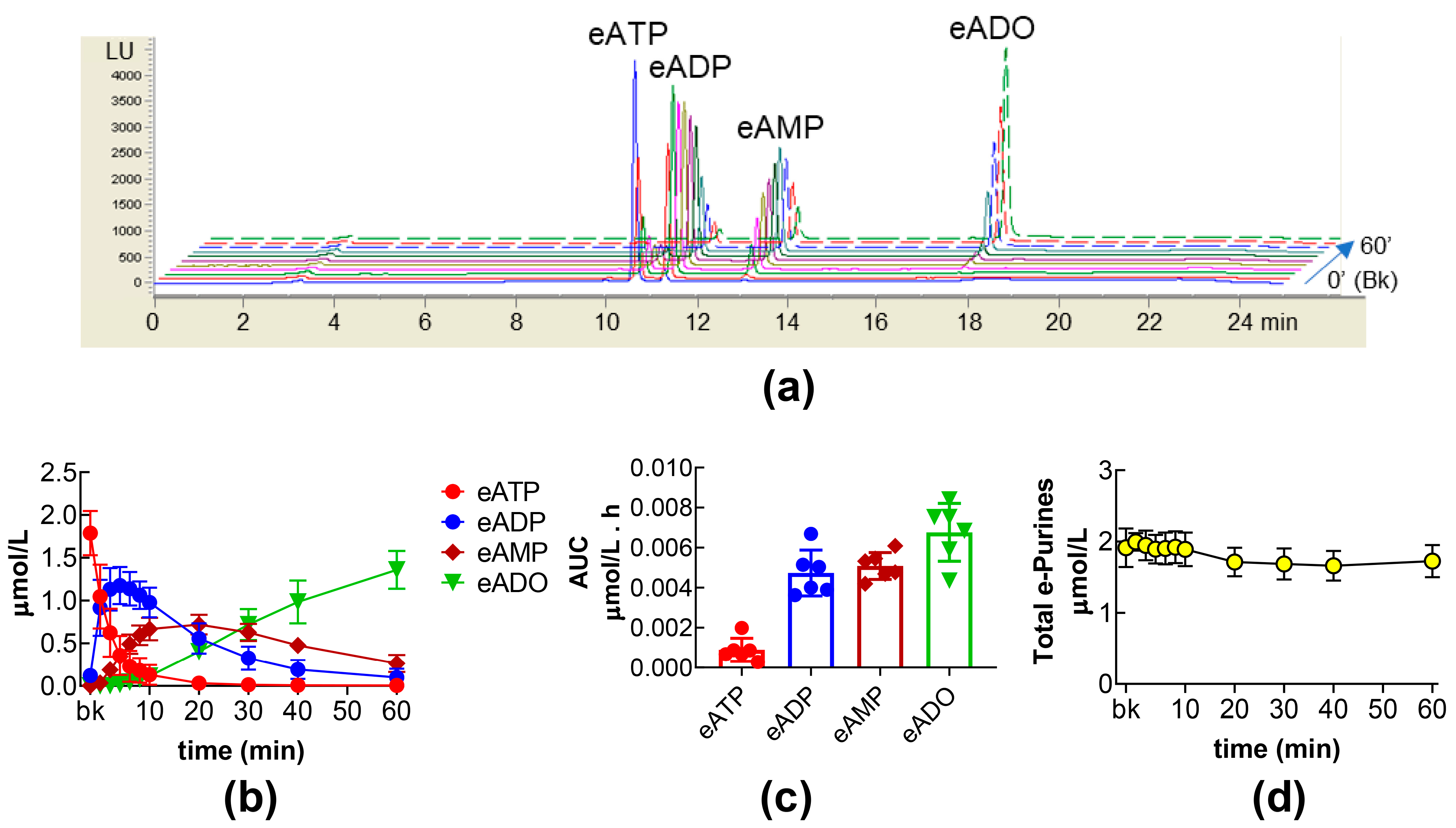
2.1. Time Course of s-NTDs-Mediated eATP Degradation to eADP, eAMP, and eADO in the Bladder Lumen
2.2. Effects of PGE2, PGF2a, PGI2, and PGD2 on eATP Degradation by s-NTDs in the Bladder Lumen
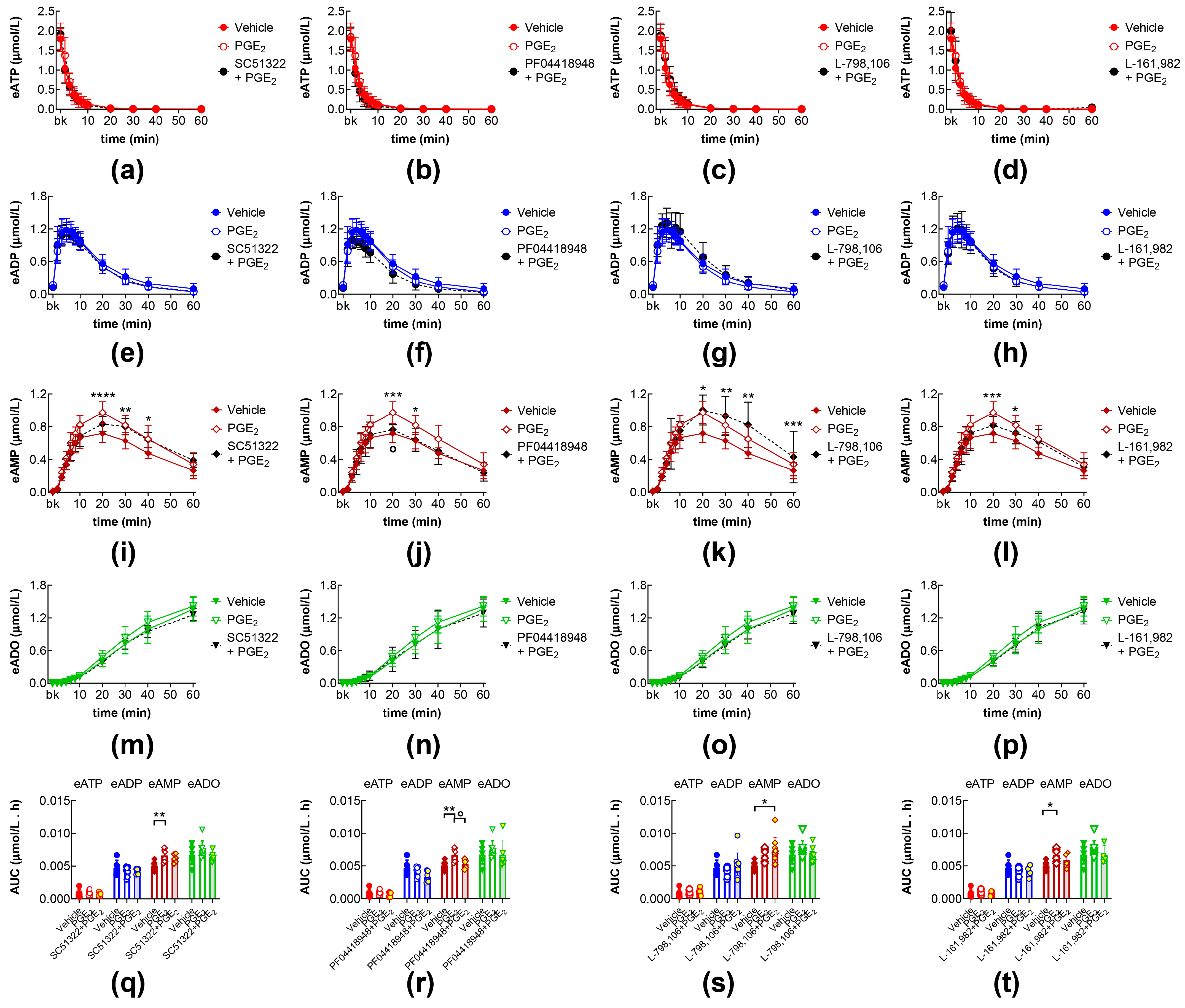
2.3. Effects of PGE2 in the Presence of EP1, EP2, EP3, and EP4 Receptor Antagonists
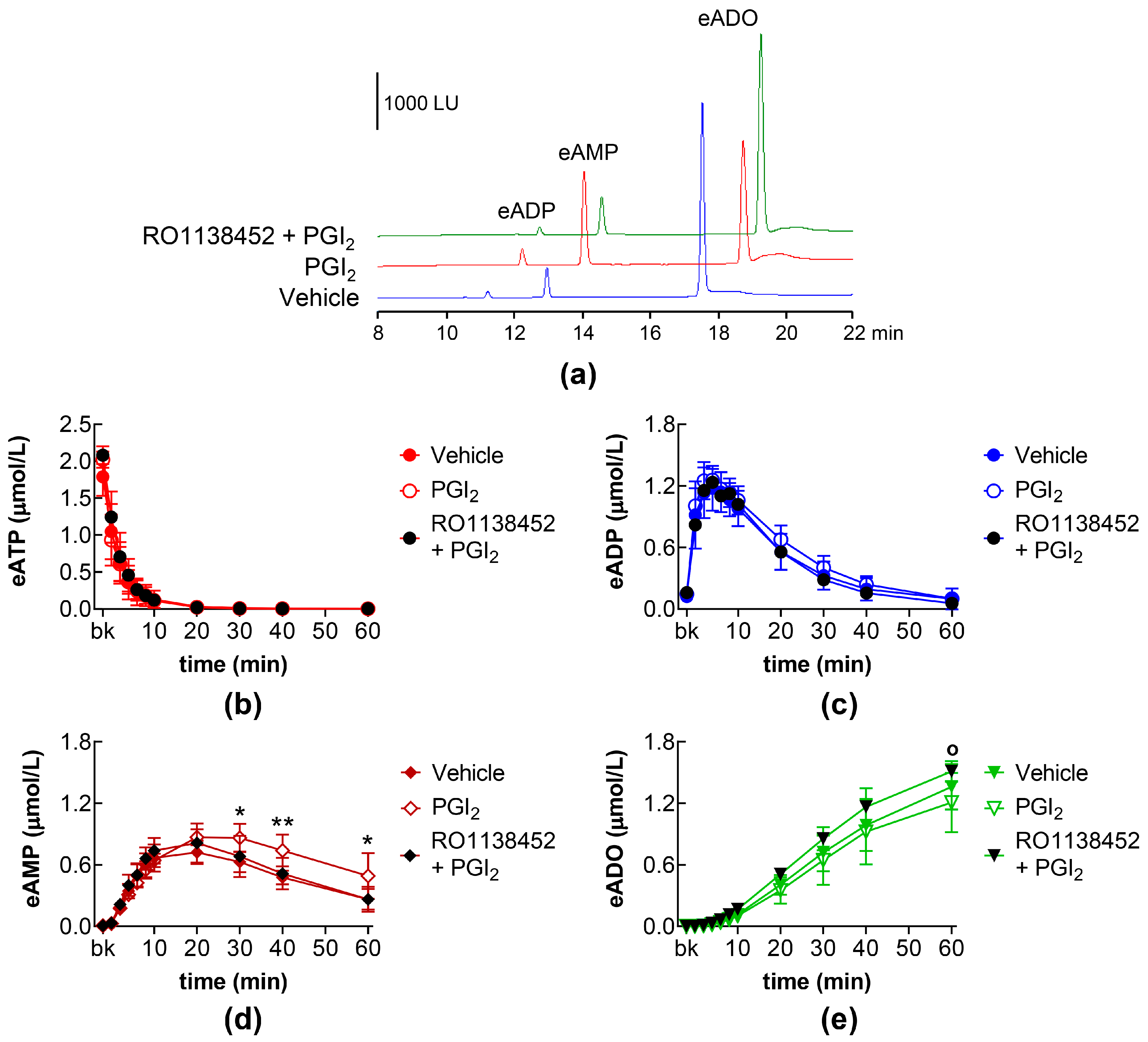
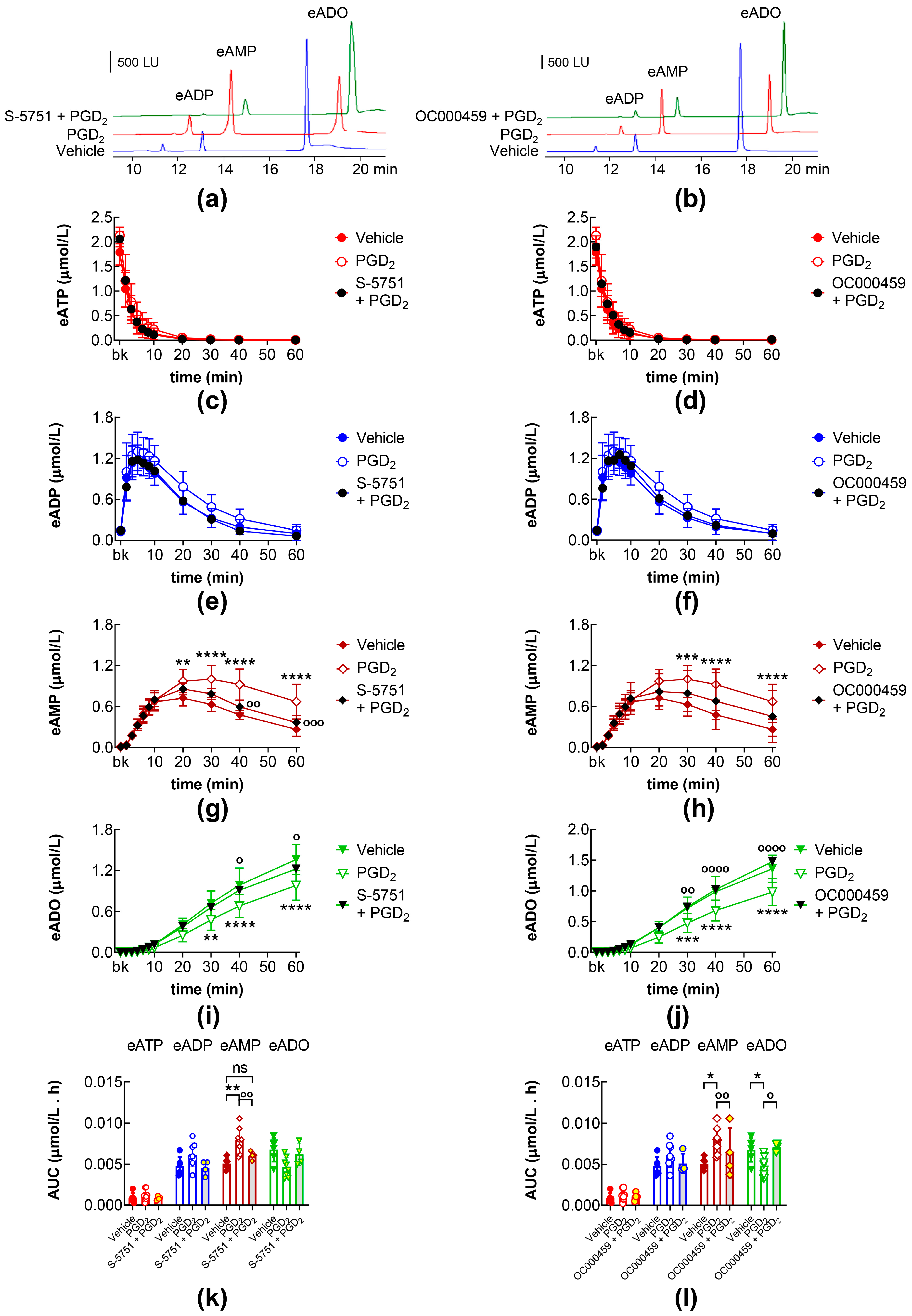
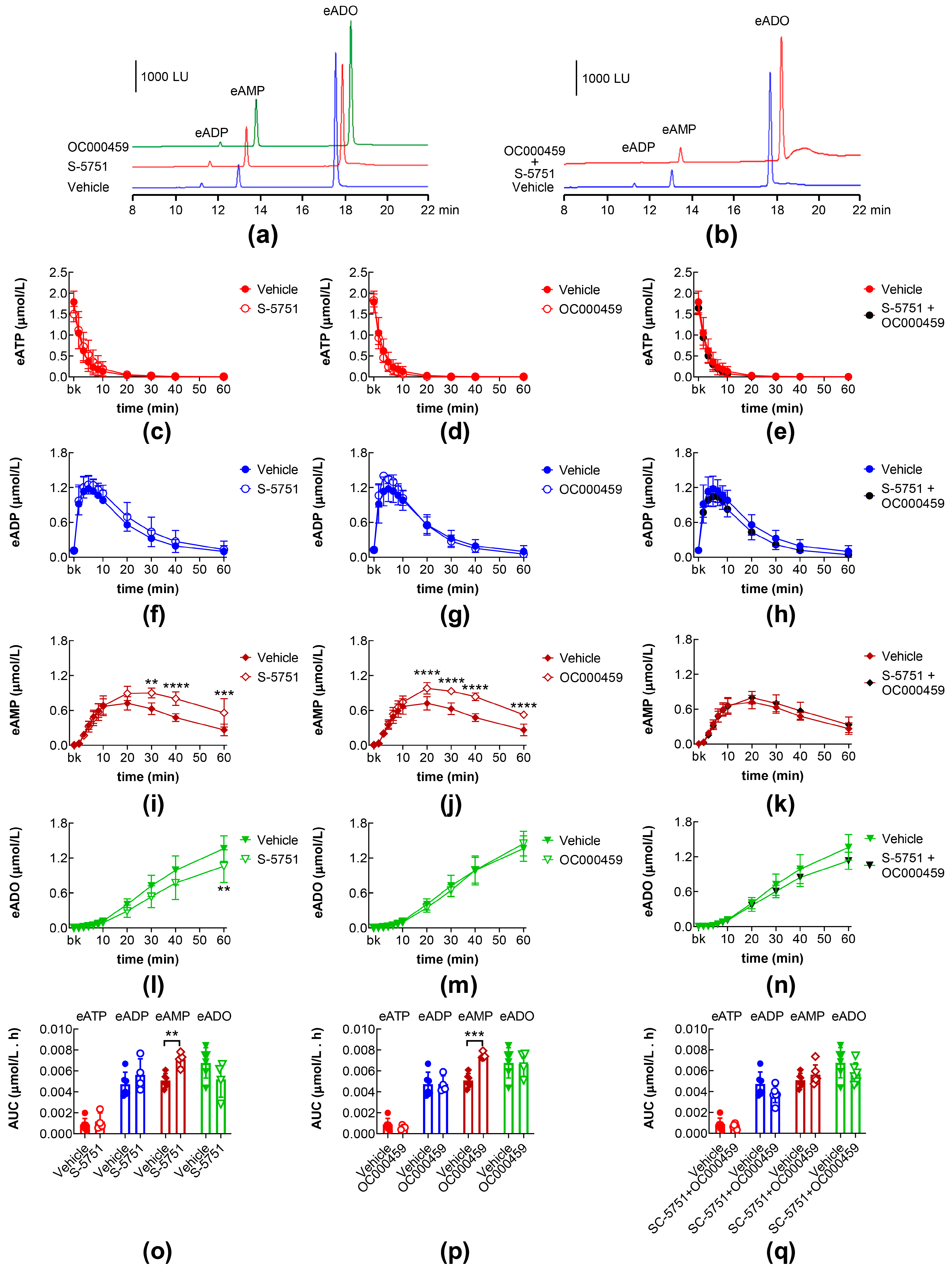
2.4. Effects of PGI2 and PGD2 in the Presence of IP, DP1, and DP2 Receptor Antagonists
2.5. Effects of DP1 and DP2 Receptor Antagonists on Intraluminal eATP Degradation by s-NTDs
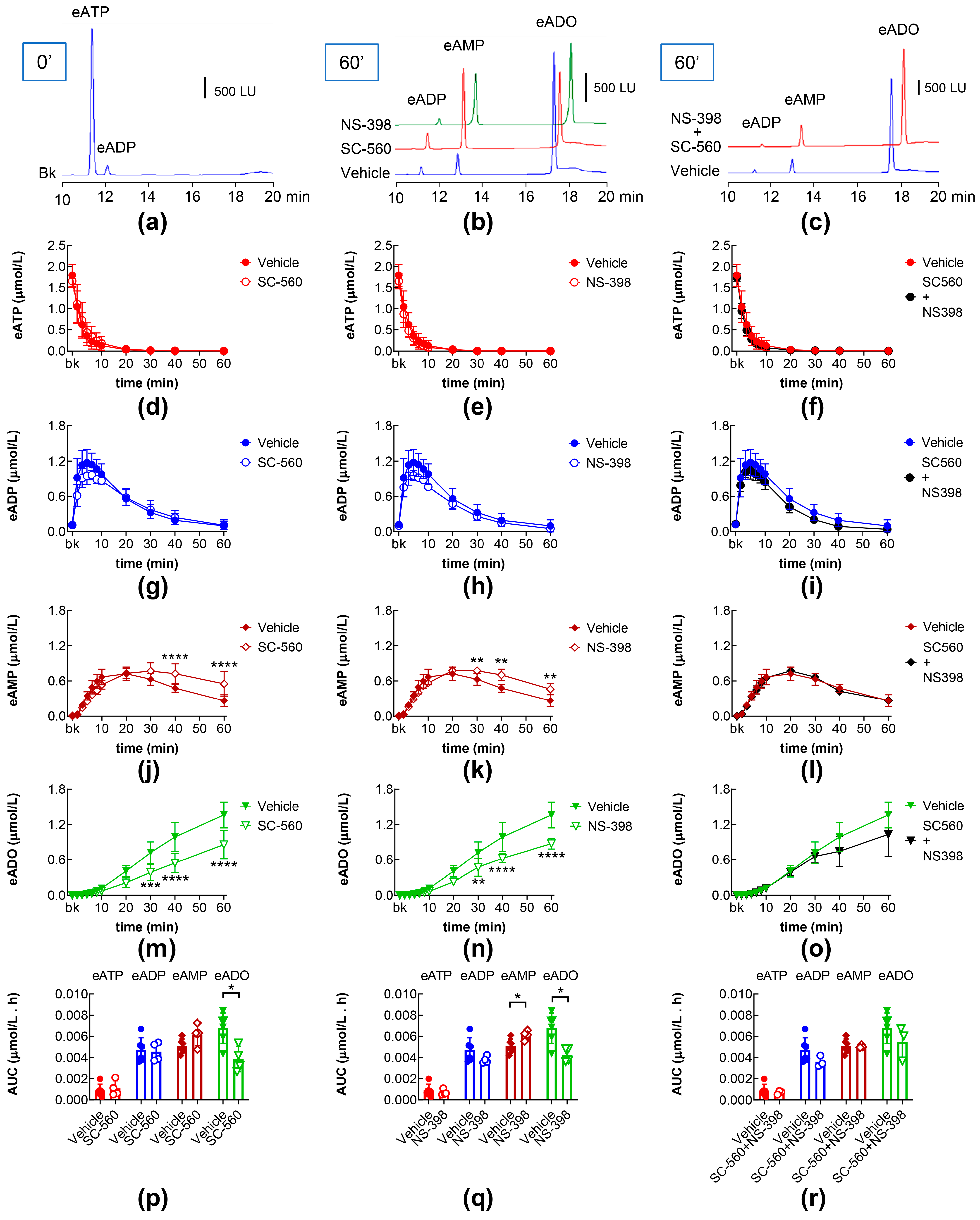
2.6. Effects of COX-1 and COX-2 Inhibition on Intraluminal eATP Degradation by s-NTDs
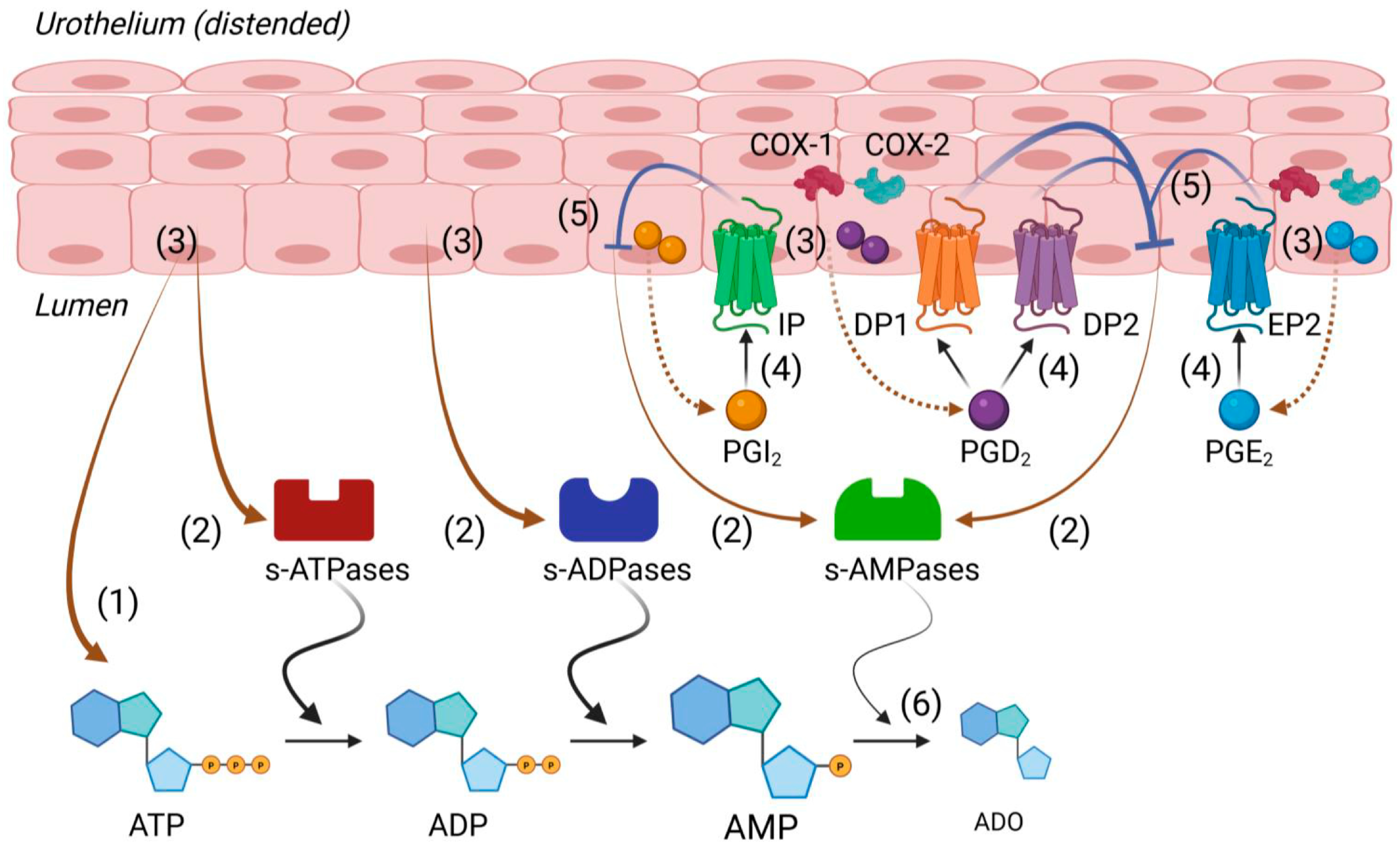
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethical Approval
4.3. Ex Vivo Mucosa-Only Bladder Preparation
4.4. Treatment of Bladder Preparations with Drugs
4.5. Collection of Intraluminal Solutions Containing s-NTDs
4.6. Time-Course of eATP Hydrolysis in Intraluminal Solutions
4.7. HPLC Analysis of eATP, eADP, eAMP, and eADO
4.8. Drugs
4.9. Statistical Analyses of the Results
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durnin, L.; Corrigan, R.D.; Sanders, K.M.; Mutafova-Yambolieva, V.N. A Decentralized (Ex Vivo) Murine Bladder Model with the Detrusor Muscle Removed for Direct Access to the Suburothelium during Bladder Filling. J. Vis. Exp. 2019, 153, e60344. [Google Scholar] [CrossRef]
- Durnin, L.; Hayoz, S.; Corrigan, R.D.; Yanez, A.; Koh, S.D.; Mutafova-Yambolieva, V.N. Urothelial purine release during filling of murine and primate bladders. Am. J. Physiol. Renal. Physiol. 2016, 311, F708–F716. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Dayton, J.; Perrino, B.A.; Mutafova-Yambolieva, V.N. Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases. Front. Physiol. 2022, 13, 918100. [Google Scholar] [CrossRef]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Perrino, B.A.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Urinary ATP Levels Are Controlled by Nucleotidases Released from the Urothelium in a Regulated Manner. Metabolites 2022, 13, 30. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014, 10, 103–155. [Google Scholar] [CrossRef] [PubMed]
- Mutafova-Yambolieva, V.N. Mechanosensitive release of ATP in the urinary bladder mucosa. Purinergic Signal. 2024, 21, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Kondo, M.; Takezawa, K.; Kiuchi, H.; Sekii, Y.; Inagaki, Y.; Soda, T.; Fukuhara, S.; Fujita, K.; Uemura, M.; et al. Intravesical ATP instillation induces urinary frequency because of activation of bladder afferent nerves without inflammatory changes in mice: A promising model for overactive bladder. Biochem. Biophys. Res. Commun. 2018, 506, 498–503. [Google Scholar] [CrossRef]
- Lee, H.; Koh, B.H.; Peri, L.E.; Sanders, K.M.; Koh, S.D. Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRalpha+ interstitial cells. J. Physiol. 2014, 592, 1283–1293. [Google Scholar] [CrossRef]
- Sui, G.; Fry, C.H.; Montgomery, B.; Roberts, M.; Wu, R.; Wu, C. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am. J. Physiol. Renal. Physiol. 2014, 306, F286–F298. [Google Scholar] [CrossRef]
- Stenqvist, J.; Aronsson, P.; Carlsson, T.; Winder, M.; Tobin, G. In vivo paracrine effects of ATP-induced urothelial acetylcholine in the rat urinary bladder. Auton. Neurosci. 2020, 227, 102689. [Google Scholar] [CrossRef]
- Munoz, A.; Smith, C.P.; Boone, T.B.; Somogyi, G.T. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem. Int. 2011, 58, 295–300. [Google Scholar] [CrossRef]
- Nile, C.J.; de Vente, J.; Gillespie, J.I. Stretch independent regulation of prostaglandin E2 production within the isolated guinea-pig lamina propria. BJU Int. 2010, 105, 540–548. [Google Scholar] [CrossRef]
- Winder, M.; Tobin, G.; Zupancic, D.; Romih, R. Signalling molecules in the urothelium. BioMed. Res. Int. 2014, 2014, 297295. [Google Scholar] [CrossRef] [PubMed]
- Truschel, S.T.; Wang, E.; Ruiz, W.G.; Leung, S.M.; Rojas, R.; Lavelle, J.; Zeidel, M.; Stoffer, D.; Apodaca, G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol. Biol. Cell 2002, 13, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.; Lee, J.M.; Ruiz, W.G.; Balestreire, E.M.; von, B.M.; Barrick, S.; Cockayne, D.A.; Birder, L.A.; Apodaca, G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J. Clin. Investig. 2005, 115, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Pandita, R.K.; Andersson, K.E. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J. Urol. 2002, 168, 1230–1234. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fry, C.H. ATP release from freshly isolated guinea-pig bladder urothelial cells: A quantification and study of the mechanisms involved. BJU Int. 2015, 115, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Tsiapakidou, S.; Apostolidis, A.; Pantazis, K.; Grimbizis, G.F.; Mikos, T. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 3143–3155. [Google Scholar] [CrossRef]
- Cheng, Y.; Mansfield, K.J.; Allen, W.; Chess-Williams, R.; Burcher, E.; Moore, K.H. ATP during early bladder stretch is important for urgency in detrusor overactivity patients. BioMed. Res. Int. 2014, 2014, 204604. [Google Scholar] [CrossRef]
- Sun, Y.; Chai, T.C. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am. J. Physiol.-Cell Physiol. 2006, 290, C27–C34. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.A.; Cheng, Y.; Mansfield, K.J.; Parkin, K.; Mukerjee, C.; Moore, K.H. Decreased intravesical adenosine triphosphate in patients with refractory detrusor overactivity and bacteriuria. J. Urol. 2013, 189, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Sharma, G.; Devana, S.K.; Zohmangaihi, D.; Mavuduru, R.S.; Mandal, A.K.; Sharma, A.P.; Bora, G.S. Urinary adenosine triphosphate and nitric oxide levels in patients with underactive bladder: A preliminary study. World J. Urol. 2021, 39, 4421–4425. [Google Scholar] [CrossRef] [PubMed]
- Aresta Branco, M.S.L.; Cruz, A.G.; Peri, L.E.; Mutafova-Yambolieva, V.N. The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 9964. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 7322. [Google Scholar] [CrossRef]
- Yokoyama, O.; Tanaka, I.; Kusukawa, N.; Yamauchi, H.; Ito, H.; Aoki, Y.; Oyama, N.; Miwa, Y.; Akino, H. Antimuscarinics suppress adenosine triphosphate and prostaglandin E2 release from urothelium with potential improvement in detrusor overactivity in rats with cerebral infarction. J. Urol. 2011, 185, 2392–2397. [Google Scholar] [CrossRef]
- Downie, J.W.; Karmazyn, M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J. Pharmacol. Exp. Ther. 1984, 230, 445–449. [Google Scholar] [CrossRef]
- Lecci, A.; Birder, L.A.; Meini, S.; Catalioto, R.M.; Tramontana, M.; Giuliani, S.; Criscuoli, M.; Maggi, C.A. Pharmacological evaluation of the role of cyclooxygenase isoenzymes on the micturition reflex following experimental cystitis in rats. Br. J. Pharmacol. 2000, 130, 331–338. [Google Scholar] [CrossRef]
- Gutierrez Cruz, A.; Borhani Peikani, M.; Beaulac, T.D.; Mutafova-Yambolieva, V.N. Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa. Int. J. Mol. Sci. 2025, 26, 131. [Google Scholar] [CrossRef]
- Rahnama’i, M.S.; van Kerrebroeck, P.E.; de Wachter, S.G.; van Koeveringe, G.A. The role of prostanoids in urinary bladder physiology. Nat. Rev. Urol. 2012, 9, 283–290. [Google Scholar] [CrossRef]
- Abramovitz, M.; Adam, M.; Boie, Y.; Carrière, M.; Denis, D.; Godbout, C.; Lamontagne, S.; Rochette, C.; Sawyer, N.; Tremblay, N.M.; et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta 2000, 1483, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Juteau, H.; Gareau, Y.; Labelle, M.; Sturino, C.F.; Sawyer, N.; Tremblay, N.; Lamontagne, S.; Carrière, M.C.; Denis, D.; Metters, K.M. Structure-activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg. Med. Chem. 2001, 9, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Machwate, M.; Harada, S.; Leu, C.T.; Seedor, G.; Labelle, M.; Gallant, M.; Hutchins, S.; Lachance, N.; Sawyer, N.; Slipetz, D.; et al. Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol. Pharmacol. 2001, 60, 36–41. [Google Scholar] [CrossRef] [PubMed]
- af Forselles, K.J.; Root, J.; Clarke, T.; Davey, D.; Aughton, K.; Dack, K.; Pullen, N. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP2 receptor antagonist. Br. J. Pharmacol. 2011, 164, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, M.; Arimura, A.; Hirano, Y.; Yasui, K.; Suzuki, N.; Deguchi, M.; Abraham, W.M. A prostaglandin D2 receptor antagonist modifies experimental asthma in sheep. Clin. Exp. Allergy 2009, 39, 1404–1414. [Google Scholar] [CrossRef]
- Pettipher, R.; Vinall, S.L.; Xue, L.; Speight, G.; Townsend, E.R.; Gazi, L.; Whelan, C.J.; Armer, R.E.; Payton, M.A.; Hunter, M.G. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J. Pharmacol. Exp. Ther. 2012, 340, 473–482. [Google Scholar] [CrossRef]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef]
- Futaki, N.; Takahashi, S.; Yokoyama, M.; Arai, I.; Higuchi, S.; Otomo, S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins 1994, 47, 55–59. [Google Scholar] [CrossRef]
- Silva-Ramos, M.; Silva, I.; Oliveira, O.; Ferreira, S.; Reis, M.J.; Oliveira, J.C.; Correia-de-Sa, P. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS ONE 2013, 8, e64696. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, E.Y.; Hong, S.H.; Seo, S.I.; Park, Y.H.; Hwang, T.K. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int. J. Urol. 2005, 12, 875–880. [Google Scholar] [CrossRef]
- Yamauchi, H.; Akino, H.; Ito, H.; Aoki, Y.; Nomura, T.; Yokoyama, O. Urinary prostaglandin E2 was increased in patients with suprapontine brain diseases, and associated with overactive bladder syndrome. Urology 2010, 76, E13–E19. [Google Scholar] [CrossRef] [PubMed]
- McLatchie, L.; Sahai, A.; Caldwell, A.; Dasgupta, P.; Fry, C. ATP shows more potential as a urinary biomarker than acetylcholine and PGE2, but its concentration in urine is not a simple function of dilution. Neurourol. Urodyn. 2021, 40, 753–762. [Google Scholar] [CrossRef]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Babou Kammoe, R.B.; Sévigny, J. Extracellular nucleotides in smooth muscle contraction. Biochem. Pharmacol. 2024, 220, 116005. [Google Scholar] [CrossRef]
- Yu, W.; Robson, S.C.; Hill, W.G. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS ONE 2011, 6, e18704. [Google Scholar] [CrossRef]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Differential Influences of Endogenous and Exogenous Sensory Neuropeptides on the ATP Metabolism by Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 15650. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Kwok, B.; Kukadia, P.; McAvera, R.; Corrigan, R.D.; Ward, S.M.; Zhang, Y.; Chen, Q.; Koh, S.D.; Sanders, K.M.; et al. An ex vivo bladder model with detrusor smooth muscle removed to analyse biologically active mediators released from the suburothelium. J. Physiol. 2019, 597, 1467–1485. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Cheng, D.; Mi, Z.; Menshikova, E.V. Characterization of the N6-etheno-bridge method to assess extracellular metabolism of adenine nucleotides: Detection of a possible role for purine nucleoside phosphorylase in adenosine metabolism. Purinergic Signal. 2020, 16, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Levitt, B.; Head, R.J.; Westfall, D.P. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: Application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal. Biochem. 1984, 137, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bobalova, J.; Bobal, P.; Mutafova-Yambolieva, V.N. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Anal. Biochem. 2002, 305, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Stromberga, Z.; Chess-Williams, R.; Moro, C. The five primary prostaglandins stimulate contractions and phasic activity of the urinary bladder urothelium, lamina propria and detrusor. BMC Urol. 2020, 20, 48. [Google Scholar] [CrossRef]
- Phelps, C.; Chess-Williams, R.; Moro, C. Ageing influences detrusor contractions to prostaglandin, angiotensin, histamine and 5-HT (serotonin), independent to the Rho kinase and extracellular calcium pathways. Sci. Rep. 2023, 13, 18062. [Google Scholar] [CrossRef]
- Bradding, P.; Porsbjerg, C.; Côté, A.; Dahlén, S.E.; Hallstrand, T.S.; Brightling, C.E. Airway hyperresponsiveness in asthma: The role of the epithelium. J. Allergy. Clin. Immunol. 2024, 153, 1181–1193. [Google Scholar] [CrossRef]
- Poto, R.; Marone, G.; Galli, S.J.; Varricchi, G. Mast cells: A novel therapeutic avenue for cardiovascular diseases? Cardiovasc. Res. 2024, 120, 681–698. [Google Scholar] [CrossRef]
- Alcedo, K.P.; Bowser, J.L.; Snider, N.T. The elegant complexity of mammalian ecto-5′-nucleotidase (CD73). Trends. Cell Biol. 2021, 31, 829–842. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456. [Google Scholar] [CrossRef] [PubMed]
- Pangrsic, T.; Potokar, M.; Stenovec, M.; Kreft, M.; Fabbretti, E.; Nistri, A.; Pryazhnikov, E.; Khiroug, L.; Giniatullin, R.; Zorec, R. Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 2007, 282, 28749–28758. [Google Scholar] [CrossRef]
- Ochiai, T.; Sasaki, Y.; Yokoyama, C.; Kuwata, H.; Hara, S. Absence of prostacyclin greatly relieves cyclophosphamide-induced cystitis and bladder pain in mice. FASEB J. 2021, 35, e21952. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.N.; Nilsson, K.F.; Wiklund, P.N.; Gustafsson, L.E. Release and inhibitory effects of prostaglandin D2 in guinea pig urinary bladder and the role of urothelium. Biochim. Biophys. Acta. 2014, 1840, 3443–3451. [Google Scholar] [CrossRef]
- Guan, N.N.; Svennersten, K.; de Verdier, P.J.; Wiklund, N.P.; Gustafsson, L.E. Receptors involved in the modulation of guinea pig urinary bladder motility by prostaglandin D2. Br. J. Pharmacol. 2015, 172, 4024–4037. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Andersson, K.E.; Wein, A.J. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 2004, 56, 581–631. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Heim, V.K.; Tarcan, T.; Siroky, M.B. Alteration of urothelial-mediated tone in the ischemic bladder: Role of eicosanoids. Neurourol. Urodyn. 2004, 23, 258–264. [Google Scholar] [CrossRef]
- Masick, J.M.; Levin, R.M.; Hass, M.A. The effect of partial outlet obstruction on prostaglandin generation in the rabbit urinary bladder. Prostaglandins Other Lipid Mediat. 2001, 66, 211–219. [Google Scholar] [CrossRef]
- Jeremy, J.Y.; Mikhailidis, D.P.; Dandona, P. The rat urinary bladder produces prostacyclin as well as other prostaglandins. Prostaglandins Leukot. Med. 1984, 16, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Nagase, K.; Tanase, K.; Aoki, Y.; Akino, H.; Yokoyama, O. Modulation of stretch evoked adenosine triphosphate release from bladder epithelium by prostaglandin E2. J. Urol. 2011, 185, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Alvarez, M.; Yeh, J.; Alvarez-Lugo, L.; Lu, M.; Sukumar, N.; Hill, W.G.; Chai, T.C. Mouse urothelial genes associated with voiding behavior changes after ovariectomy and bladder lipopolysaccharide exposure. Neurourol. Urodyn. 2018, 37, 2398–2405. [Google Scholar] [CrossRef]
- Mengdan, L.; Chen, W.; Jieyu, L.; Peiyu, J.; Fei, W.; Shengnan, L.; Shuhua, X. Low concentration arsenite activated JAK2/STAT3 signal and increased proliferative factor expressions in SV-HUC-1cells after short and long time treatment. Environ. Toxicol. 2017, 32, 2154–2162. [Google Scholar] [CrossRef]
- Porcher, C.; Horowitz, B.; Bayguinov, O.; Ward, S.M.; Sanders, K.M. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology 2002, 122, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.A.; Akkermans, L.M.; Kroese, A.B. Effects of the inflammatory mediator prostaglandin E2 on myenteric neurons in guinea pig ileum. Am. J. Physiol. 1997, 272, G1451–G1456. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Chang, M.; Cho, W.; Zhang, Y.; Redha, R.; Davis, L.; Chang, S.; DuBois, R.N.; Hao, C.M.; Breyer, M. Cloning, expression, and regulation of rabbit cyclooxygenase-2 in renal medullary interstitial cells. Am. J. Physiol. 1997, 273, F18–F26. [Google Scholar] [CrossRef]
- Owusu-Ofori, K.; Learned, M.K.; Mellon, W.S.; Nakada, S.Y. PI3K mediates stretch-induced COX-2 expression during urinary tract obstruction. J. Endourol. 2013, 27, 220–229. [Google Scholar] [CrossRef]
- Jerde, T.J.; Mellon, W.S.; Bjorling, D.E.; Checura, C.M.; Owusu-Ofori, K.; Parrish, J.J.; Nakada, S.Y. Stretch induction of cyclooxygenase-2 expression in human urothelial cells is calcium- and protein kinase C zeta-dependent. Mol. Pharmacol. 2008, 73, 18–26. [Google Scholar] [CrossRef] [PubMed]

| Drug | Type | Concentration (µM) |
|---|---|---|
| PGE2 | EP receptor agonist | 10 |
| PGD2 | DP receptor agonist | 10 |
| PGI2 (Epoprostenol) | IP receptor agonist | 10 |
| PGF2α | FP receptor agonist | 10 |
| SC-560 | COX-1 inhibitor | 0.1 |
| NS-398 | COX-2 inhibitor | 10 |
| S-5751 | DP1 receptor antagonist | 1 |
| OC51322 | EP1 receptor antagonist | 10 |
| RO1138452 | IP receptor antagonist | 10 |
| SC51322 | EP1 receptor antagonist | 1 |
| PF04418948 | EP2 receptor antagonist | 1 |
| L-798,106 | EP3 receptor antagonist | 0.25 |
| L-161,982 | EP4 receptor antagonist | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borhani Peikani, M.; Gutierrez Cruz, A.; Buckley, Z.S.; Mutafova-Yambolieva, V.N. Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen. Int. J. Mol. Sci. 2025, 26, 8023. https://doi.org/10.3390/ijms26168023
Borhani Peikani M, Gutierrez Cruz A, Buckley ZS, Mutafova-Yambolieva VN. Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen. International Journal of Molecular Sciences. 2025; 26(16):8023. https://doi.org/10.3390/ijms26168023
Chicago/Turabian StyleBorhani Peikani, Mahsa, Alejandro Gutierrez Cruz, Zoe S. Buckley, and Violeta N. Mutafova-Yambolieva. 2025. "Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen" International Journal of Molecular Sciences 26, no. 16: 8023. https://doi.org/10.3390/ijms26168023
APA StyleBorhani Peikani, M., Gutierrez Cruz, A., Buckley, Z. S., & Mutafova-Yambolieva, V. N. (2025). Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen. International Journal of Molecular Sciences, 26(16), 8023. https://doi.org/10.3390/ijms26168023






