New Frontiers in 3D Printing Using Biocompatible Polymers
Abstract
1. Introduction
2. Biocompatible Polymers for 3D Printing
2.1. Silk
2.2. Keratin
2.3. Collagen
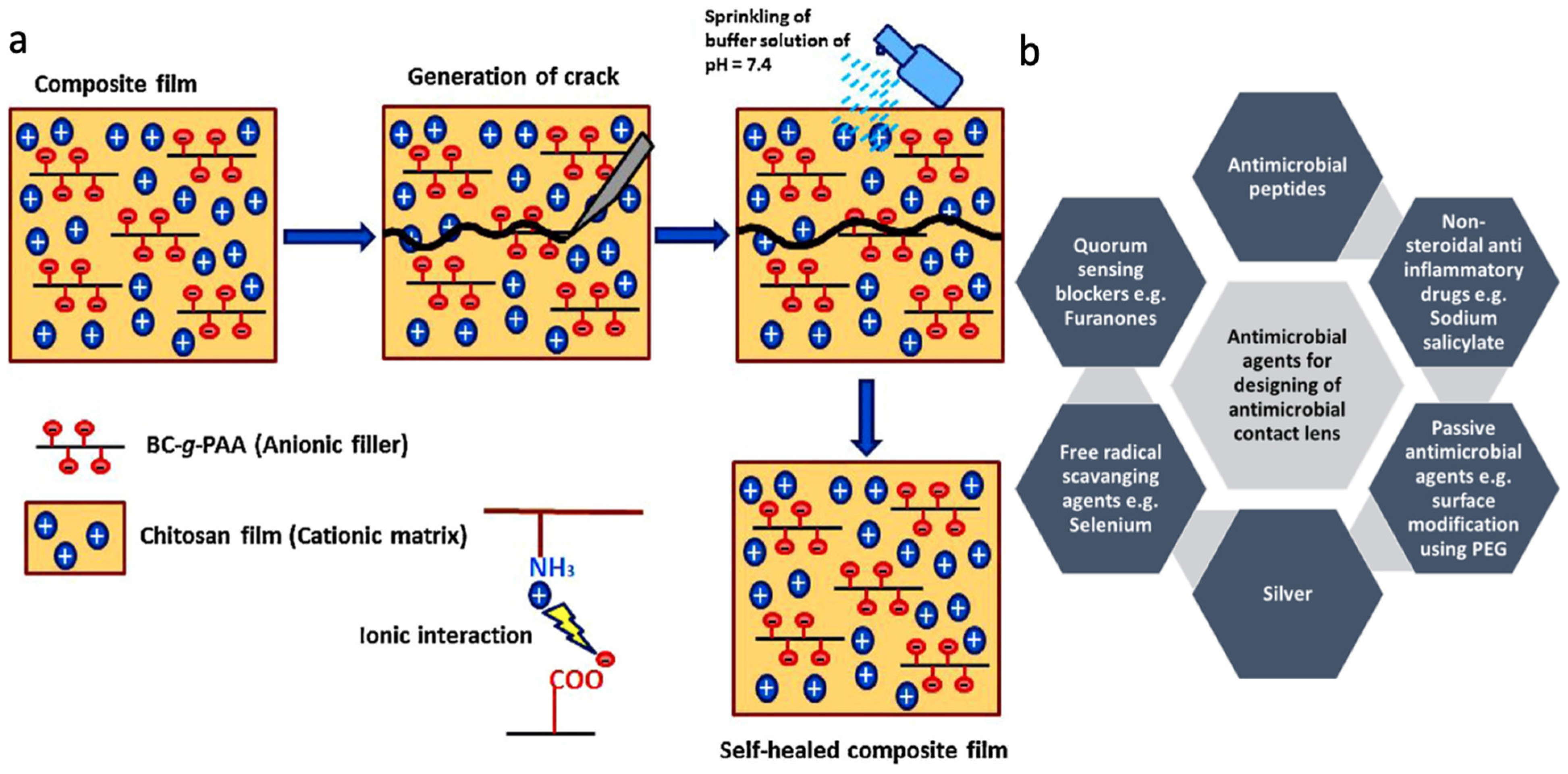
2.4. Chitosan
2.5. Gellan Gum
2.6. Cellulose
2.7. Polycaprolactone
2.8. Polyhydroxyalkanoate
3. Three-Dimensional Printing Theory and Methods
3.1. Printing Development
3.2. Printing Methods
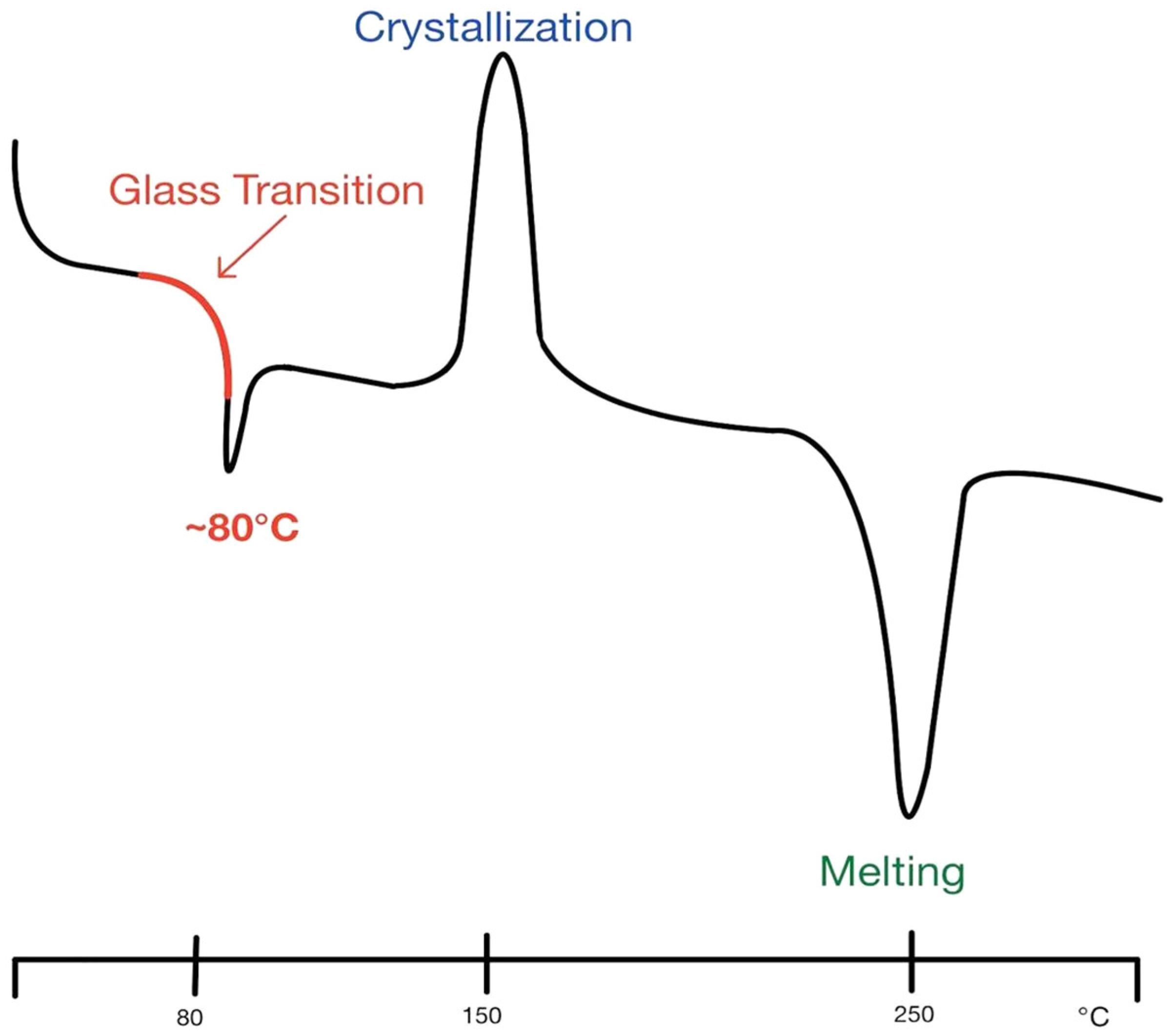
3.3. Printing: Solution Drying
3.4. Post Printing: Crosslinking
4. Three-Dimensional Printing Technologies
4.1. In Situ Direct Printing
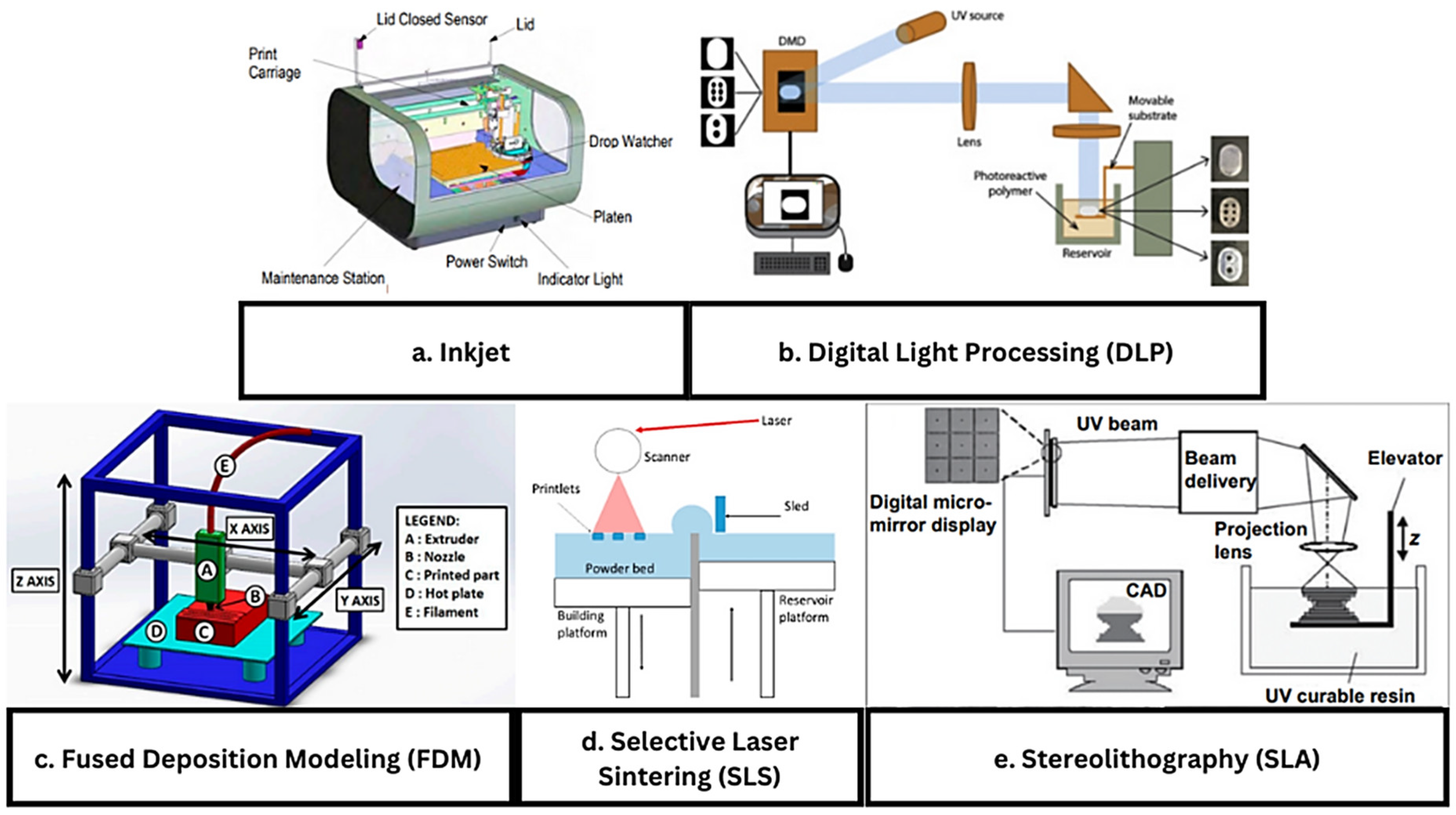
4.2. Inkjet Printers
4.3. Digital Light Processing
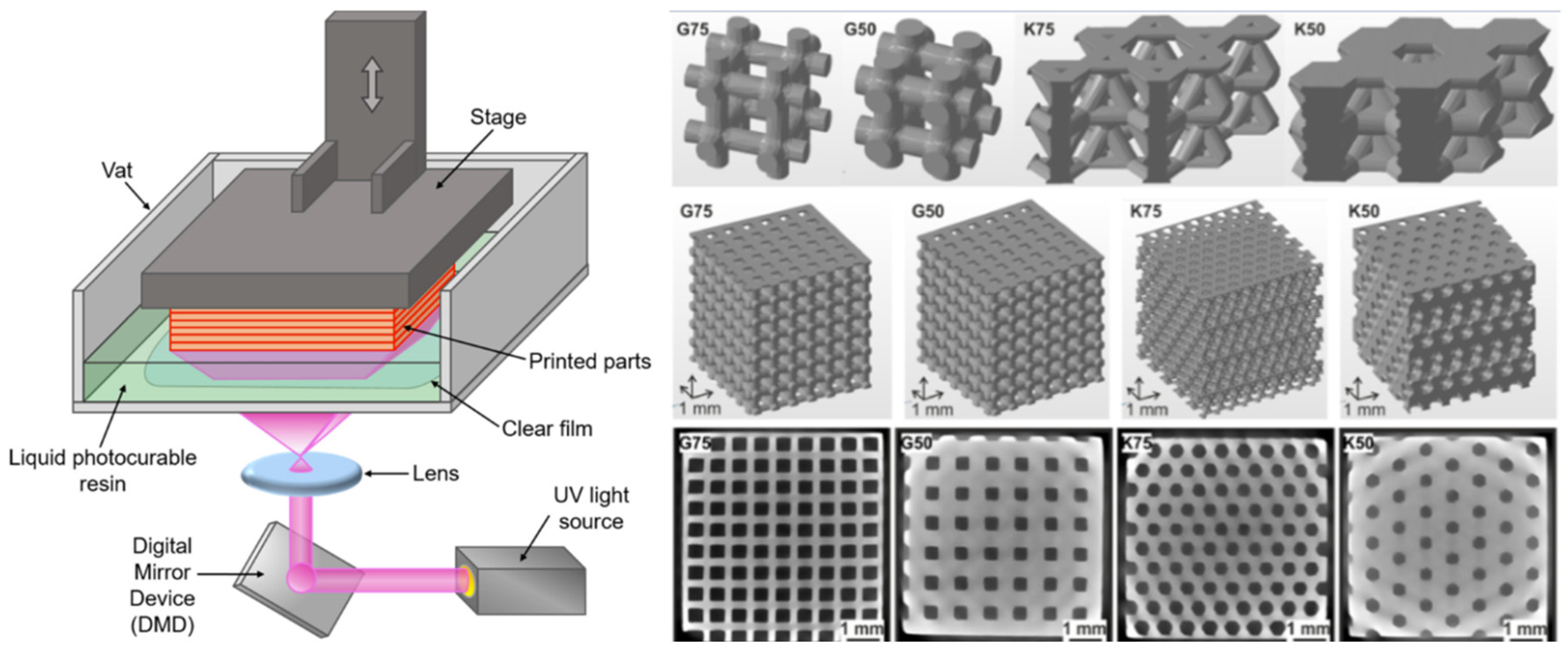
4.4. Fused Deposition Modeling
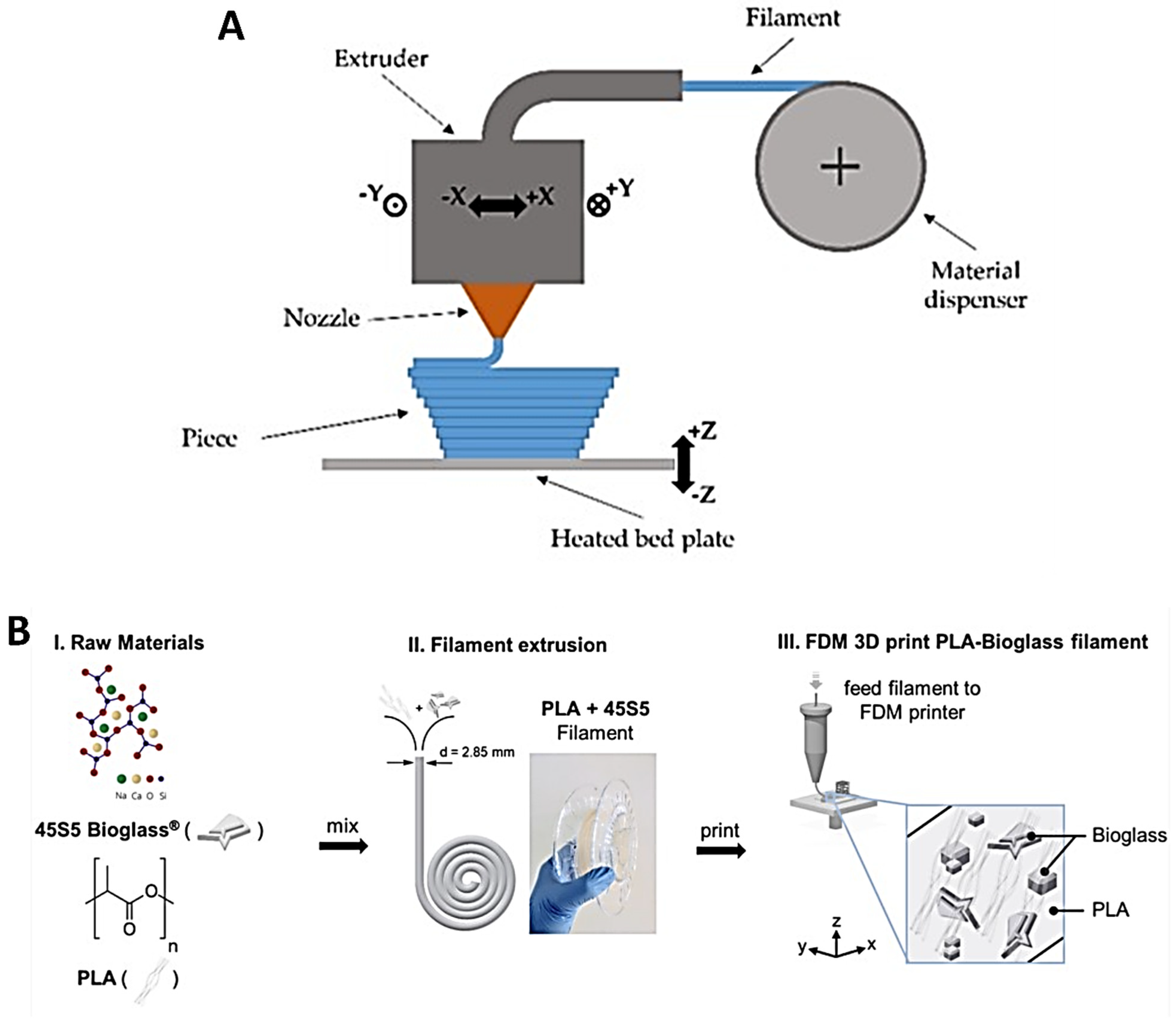
4.5. Selective Laser Sintering
4.6. Stereolithography
4.7. Four-Dimensional Printing
5. Applications
5.1. Extracellular Matrix (ECM) Scaffolds
5.2. Tissue Regeneration
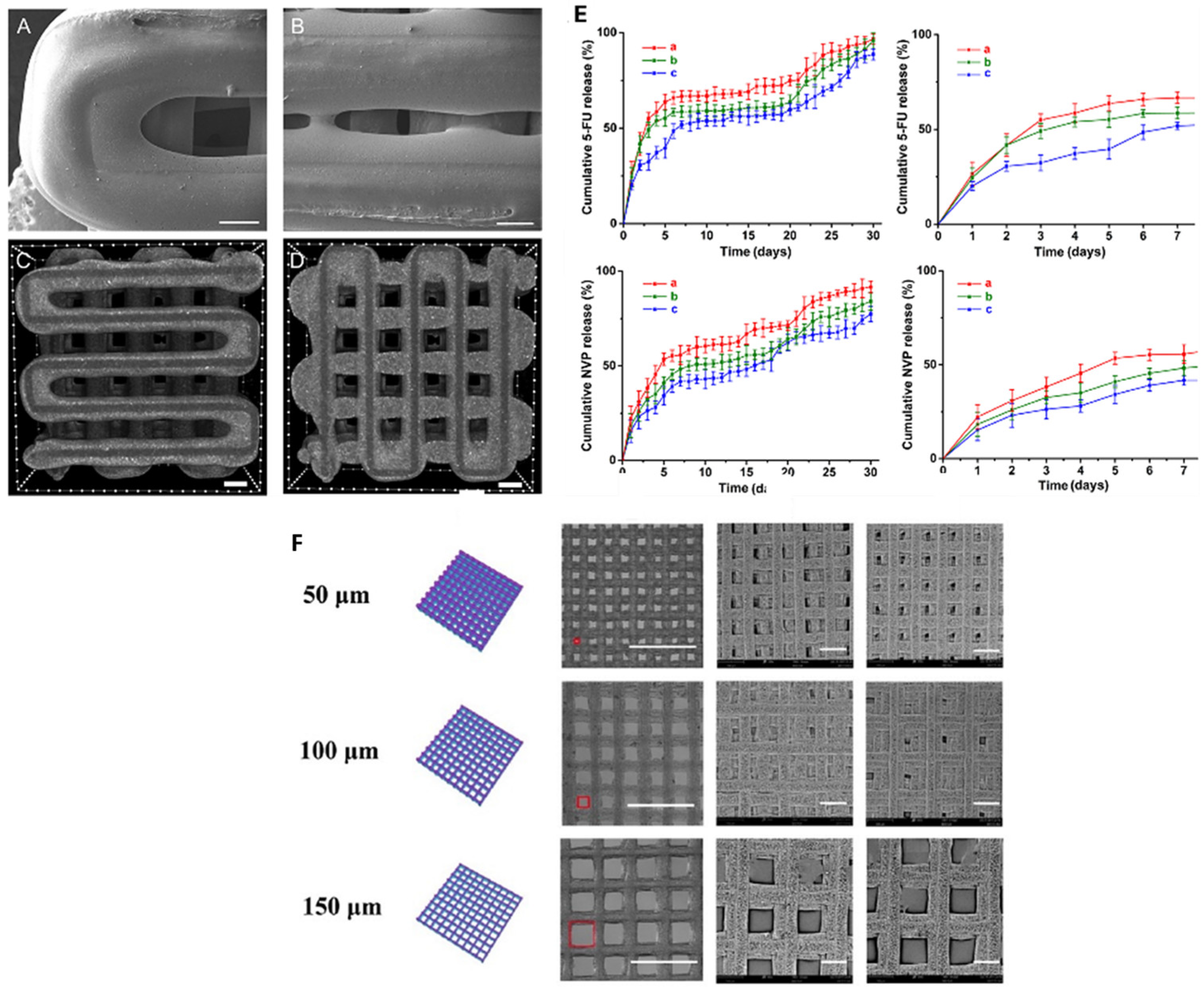
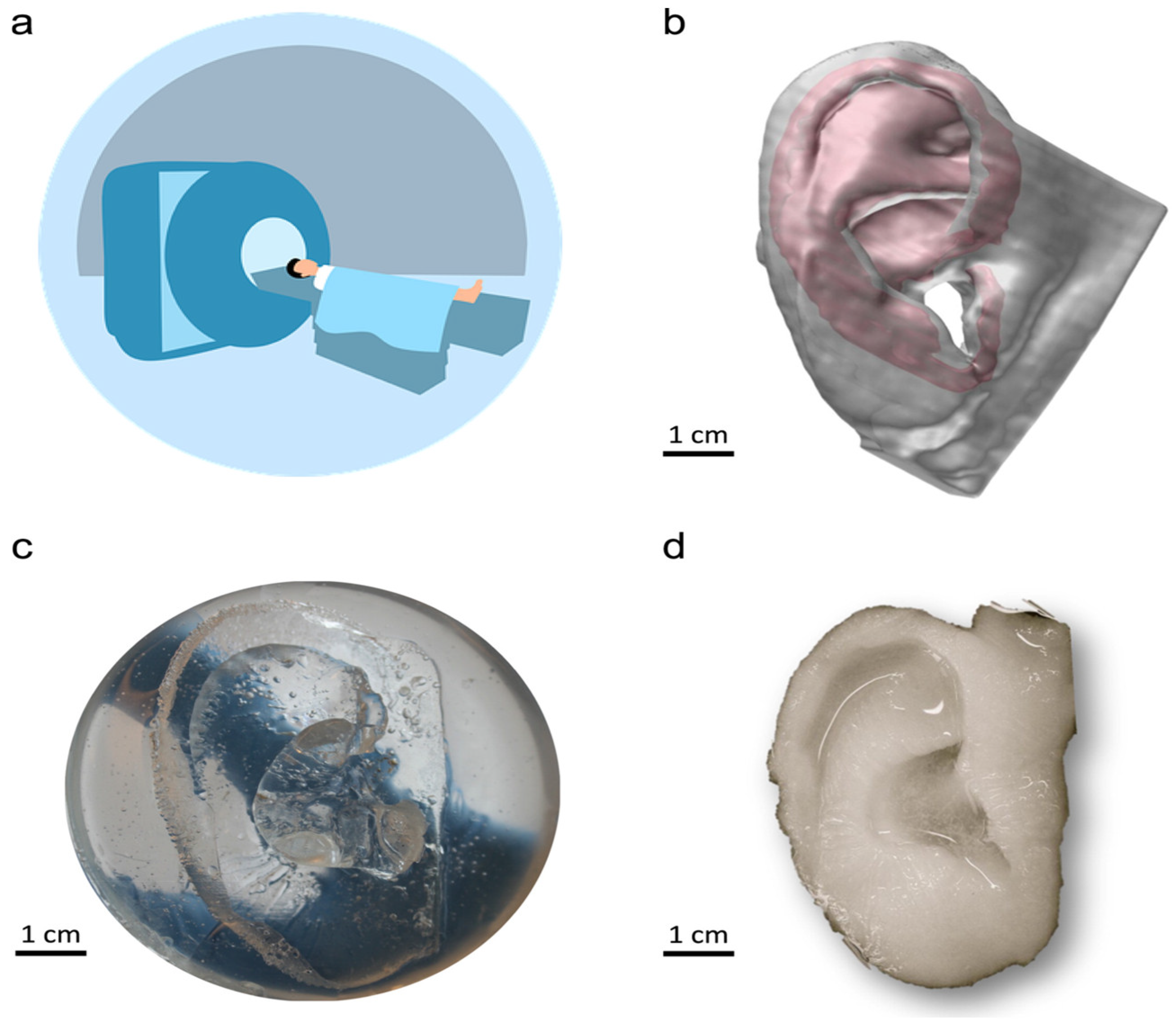
5.3. Drug Delivery
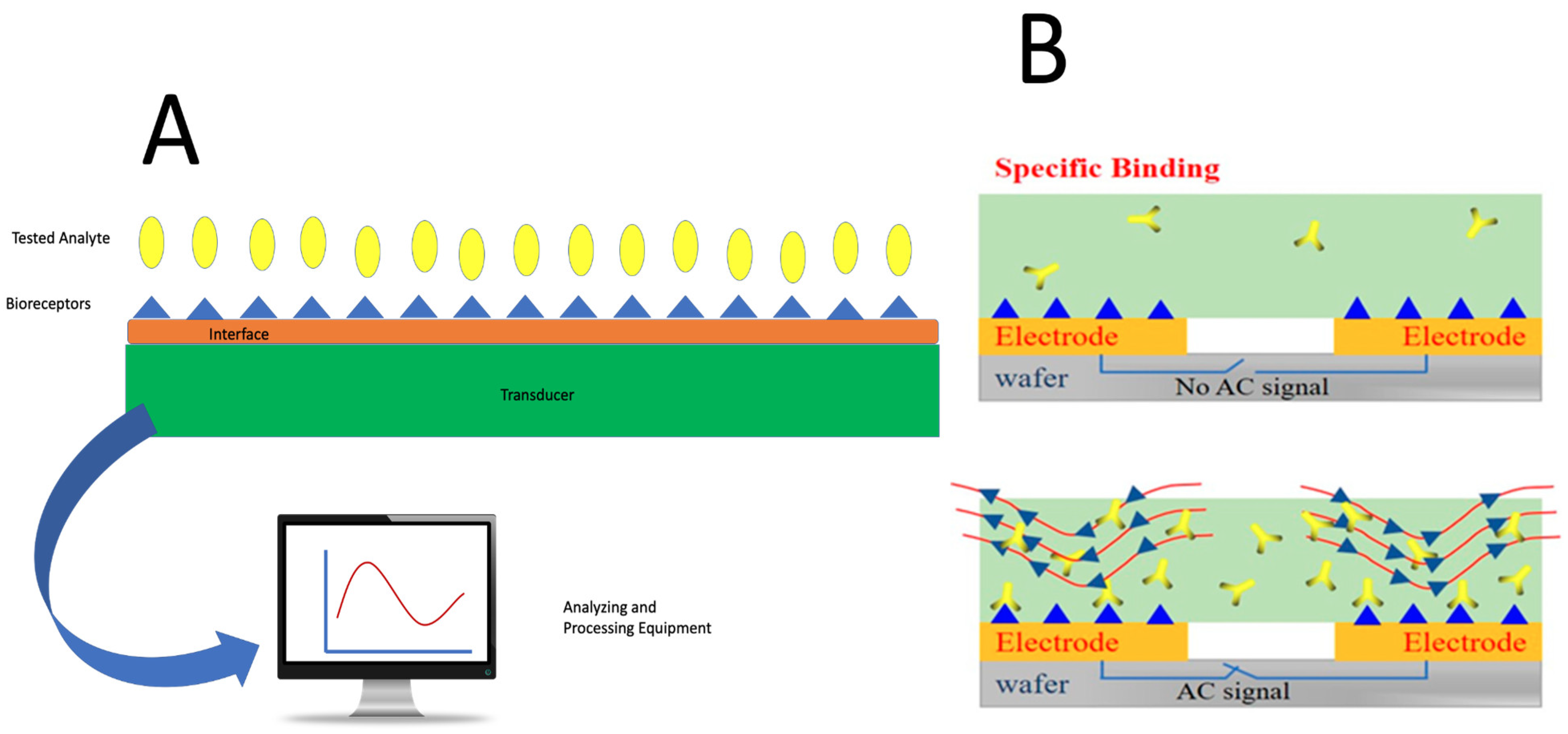
5.4. Biosensor
| 3D Printing Application | Desired Function | Key Factors | Optimal Materials | Refs. |
|---|---|---|---|---|
| ECM Scaffolds | - Create a scaffold to grow new tissue or biomaterial - Must degrade after its function has been fulfilled | - Porous and biocompatible with similar mechanical properties to the body’s ECM - Space for cell proliferation and growth factors | - Alginate - Chitosan - Collagen - Fibronectin - Hyaluronic Acid - Composites - Metals - Ceramics | [106] |
| Tissue Regeneration | - Create new bioengineered and printed tissue from polymers to create biocompatible working tissue | - Tissue must be able to attach to the natural ECM - Must be biocompatible and able to handle blood flow | - Nanomaterials - Mesenchymal Stem Cells | [107] |
| Drug Delivery | - Print and develop stents and other inorganic materials that can elude drugs to treat certain diseases | - Implant must be non-toxic to the body - Must elute the drug at the appropriate order (first, second, zero) | - Metals - Drug-Coated Polymers | [108] |
| Biosensors | - Develop sensors to act as markers for various diseases and bioavailability errors | - Must be able to recognize signals and make affected decisions based on those signals | - Electrically Conductive | [109] |
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Health Resources and Services Administration. Organ Transplant Waitlist; Health Resources and Services Administration: Rockville, MD, USA, 2022.
- Abouna, G.M. Organ shortage crisis: Problems and possible solutions. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2008; pp. 34–38. [Google Scholar]
- Voora, S.; Adey, D.B. Management of kidney transplant recipients by general nephrologists: Core curriculum 2019. Am. J. Kidney Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J. Kidney transplant: New opportunities and challenges. Clevel. Clin. J. Med. 2018, 85, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. J. Br. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Dominguez-Gil, B.; Greer, D.; Manara, A.; Souter, M. Organ donation after circulatory death: Current status and future potential. Intensive Care Med. 2019, 45, 310–321. [Google Scholar] [CrossRef]
- Vijeratnam, S.S.; Candy, B.; Craig, R.; Marshall, A.; Stone, P.; Low, J.T. Palliative care for patients with end-stage liver disease on the liver transplant waiting list: An international systematic review. Dig. Dis. Sci. 2021, 66, 4072–4089. [Google Scholar] [CrossRef]
- Mason, J.; Visintini, S.; Quay, T. An Overview of Clinical Applications of 3-D Printing and Bioprinting; CADTH Issues in Emerging Health Technologies: Ottawa, ON, USA, 2019.
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar] [CrossRef]
- Ayyildiz, O. Customised spectacles using 3-D printing technology. Clin. Exp. Optom. 2018, 101, 747–751. [Google Scholar] [CrossRef]
- Furlow, B. Medical 3-D printing. Radiol. Technol. 2017, 88, 519CT–537CT. [Google Scholar]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D Bioprinting of Cells, Tissues and Organs; Nature Publishing Group: London, UK, 2020; Volume 10, pp. 1–3. [Google Scholar]
- Kotta, S.; Nair, A.; Alsabeelah, N. 3D printing technology in drug delivery: Recent progress and application. Curr. Pharm. Des. 2018, 24, 5039–5048. [Google Scholar] [CrossRef]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Horst, D.J. 3D printing of pharmaceutical drug delivery systems. Arch. Org. Inorg. Chem. Sci. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D printed drug delivery systems based on natural products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Jain, V.; Haider, N.; Jain, K. 3D printing in personalized drug delivery. Curr. Pharm. Des. 2018, 24, 5062–5071. [Google Scholar]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.; Lee, O.J.; Lee, J.S.; Yoon, S.-I. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Mu, X.; Fitzpatrick, V.; Kaplan, D.L. From silk spinning to 3D printing: Polymer manufacturing using directed hierarchical molecular assembly. Adv. Healthc. Mater. 2020, 9, 1901552. [Google Scholar] [CrossRef]
- Mu, X.; Sahoo, J.K.; Cebe, P.; Kaplan, D.L. Photo-crosslinked silk fibroin for 3D printing. Polymers 2020, 12, 2936. [Google Scholar] [CrossRef]
- Tonin, C.; Aluigi, A.; Varesano, A.; Vineis, C. Keratin-based nanofibres. Nanofibers 2010, 1, 139–158. [Google Scholar]
- Williams, J. Photopolymerization and photocrosslinking of polymers. In Photochemistry; Springer: Berlin/Heidelberg, Germany, 1969; pp. 227–250. [Google Scholar]
- Chan, W.W.; Yeo, D.C.L.; Tan, V.; Singh, S.; Choudhury, D.; Naing, M.W. Additive biomanufacturing with collagen inks. Bioengineering 2020, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as bioink for bioprinting: A comprehensive review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ramezani, H.; Sun, M.; Xie, M.; Nie, J.; Lv, S.; Cai, J.; Fu, J.; He, Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020, 8, 5020–5028. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D bioprinting of gellan gum and poly (ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity. Mater. Des. 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A study on degradation behavior of 3D printed gellan gum scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef]
- Chandler, D. 3-D Printing with Cellulose; Massachusetts Institute of Technology: Cambridge, MA, USA, 2017. [Google Scholar]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA/PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Pereira, T.F.; Oliveira, M.F.; Maia, I.A.; Silva, J.V.; Costa, M.F.; Thiré, R.M. 3D Printing of Poly (3-hydroxybutyrate) Porous Structures Using Selective Laser Sintering. In Macromolecular Symposia; Wiley Online Library: Weinheim, Germany, 2012; pp. 64–73. [Google Scholar]
- Mehrpouya, M.; Vahabi, H.; Barletta, M.; Laheurte, P.; Langlois, V. Additive manufacturing of polyhydroxyalkanoates (PHAs) biopolymers: Materials, printing techniques, and applications. Mater. Sci. Eng. C 2021, 127, 112216. [Google Scholar] [CrossRef] [PubMed]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Barui, S.; Panda, A.K.; Naskar, S.; Kuppuraj, R.; Basu, S.; Basu, B. 3D inkjet printing of biomaterials with strength reliability and cytocompatibility: Quantitative process strategy for Ti-6Al-4V. Biomaterials 2019, 213, 119212. [Google Scholar] [CrossRef]
- Ford, S.J.; Routley, M.J.; Phaal, R.; Probert, D.R. The industrial emergence of commercial inkjet printing. Eur. J. Innov. Manag. 2014, 17, 126–143. [Google Scholar] [CrossRef]
- Wallace, J.; Wang, M.O.; Thompson, P.; Busso, M.; Belle, V.; Mammoser, N.; Kim, K.; Fisher, J.P.; Siblani, A.; Xu, Y. Validating continuous digital light processing (cDLP) additive manufacturing accuracy and tissue engineering utility of a dye-initiator package. Biofabrication 2014, 6, 015003. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dehghani, M.; Alsaadi, N.; Nejad, M.G.; Saber-Samandari, S.; Toghraie, D.; Su, C.-H.; Nguyen, H.C. A femoral shape porous scaffold bio-nanocomposite fabricated using 3D printing and freeze-drying technique for orthopedic application. Mater. Chem. Phys. 2022, 275, 125302. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110054. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjic, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
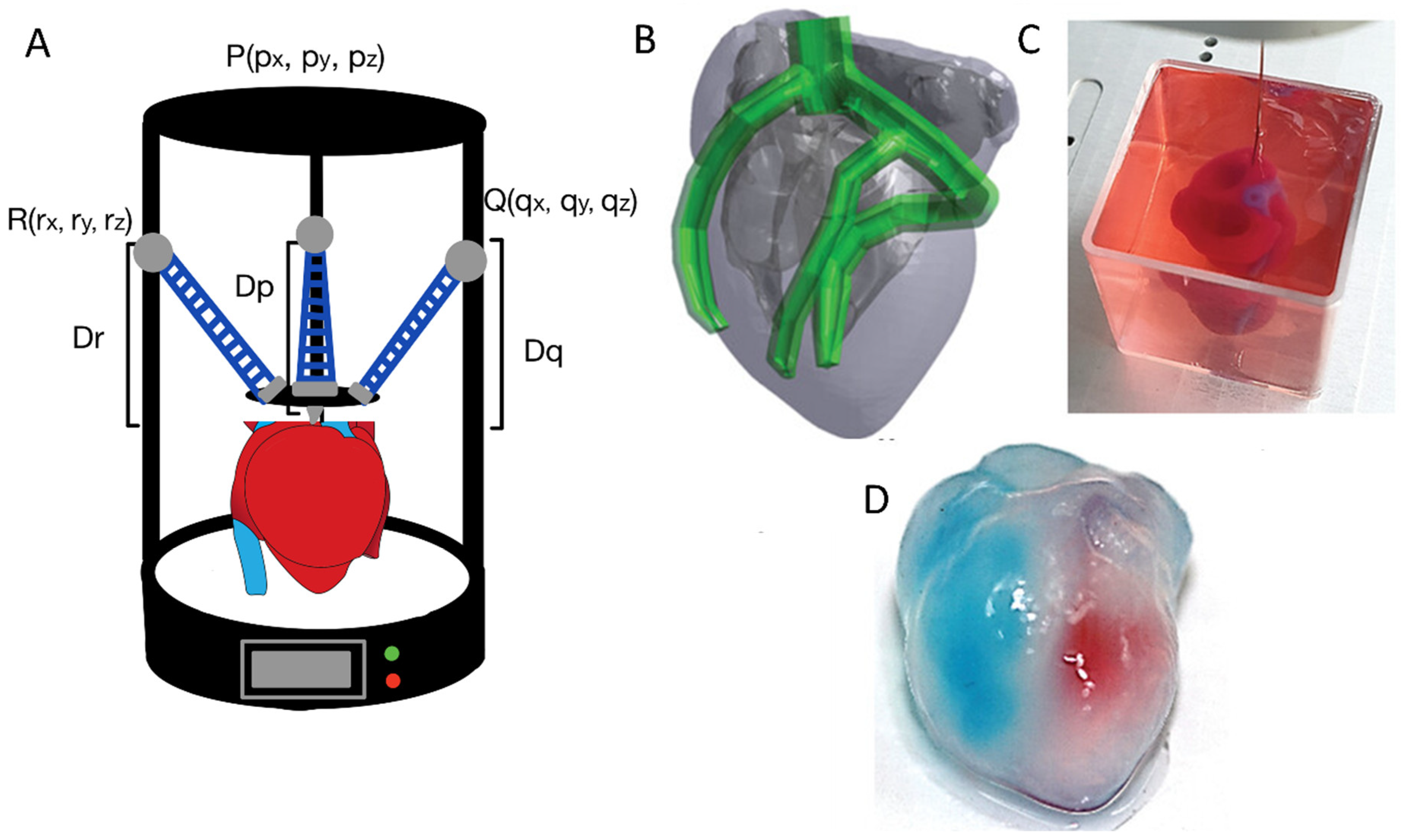
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Porter, M.M.; Ravikumar, N.; Barthelat, F.; Martini, R. 3D-printing and mechanics of bio-inspired articulated and multi-material structures. J. Mech. Behav. Biomed. Mater. 2017, 73, 114–126. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, K.; Kim, D.S.; Choi, K.H. Direct printing of copper conductive micro-tracks by multi-nozzle electrohydrodynamic inkjet printing process. J. Mater. Process. Technol. 2012, 212, 700–706. [Google Scholar] [CrossRef]
- Bell, C. 3D Printing with Delta Printers; Apress: New York, NY, USA, 2015; Volume 1. [Google Scholar]
- Bae, S.-U.; Kim, B.-J. Effects of Cellulose Nanocrystal and Inorganic Nanofillers on the Morphological and Mechanical Properties of Digital Light Processing (DLP) 3D-Printed Photopolymer Composites. Appl. Sci. 2021, 11, 6835. [Google Scholar] [CrossRef]
- Schmidleithner, C.; Malferrari, S.; Palgrave, R.; Bomze, D.; Schwentenwein, M.; Kalaskar, D.M. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed. Mater. 2019, 14, 45018. [Google Scholar] [CrossRef]
- Casavola, C.; Cazzato, A.; Moramarco, V.; Renna, G. Mechanical Behaviour of ABS-Fused Filament Fabrication Compounds under Impact Tensile Loadings. Materials 2019, 12, 1295. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of biomaterials for 3D printing by fused deposition modeling technique: A review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly (lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-based 3D printing of microfluidic devices for chemical and biomedical applications: A topical review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Wang, X.; Laoui, T.; Froyen, L. Lasers and materials in selective laser sintering. Assem. Autom. 2003, 23, 357–371. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef]
- Partee, B.; Hollister, S.J.; Das, S. Fabrication of polycaprolactone bone tissue engineering scaffolds using selective laser sintering. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Anaheim, CA, USA, 13–19 November 2004; pp. 525–535. [Google Scholar]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Ogunsanya, M.; Isichei, J.; Parupelli, S.K.; Desai, S.; Cai, Y. In-situ droplet monitoring of inkjet 3D printing process using image analysis and machine learning models. Procedia Manuf. 2021, 53, 427–434. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Sun, C.; Fang, N.; Wu, D.M.; Zhang, X. Projection micro-stereolithography using digital micro-mirror dynamic mask. Sens. Actuators A Phys. 2005, 121, 113–120. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications, and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection micro stereolithography based 3D printing and its applications. Int. J. Extrem. Manuf. 2020, 2, 022004. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D Printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Li, X.; Sarkar, K.; Polak, J.M.; et al. 4D Printing Smart Biomaterials for Tissue Engineering. Mater. Today 2017, 20, 577–591. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Zengjie, F.; Suthiwanich, K.; Lorestani, F.; Orive, G.; Ostrovidov, S.; Khademhosseini, A. Advances and Future Perspectives in 4D Bioprinting. Biotechnol. J. 2018, 13, e1800148. [Google Scholar] [CrossRef]
- Raei, F.; Abdi, A.; Mashayekhan, S. 4D bioprinting: Materials, mechanisms, and mathematical modeling for next-generation tissue engineering. Bioprinting 2025, 50, e00428. [Google Scholar] [CrossRef]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D printing: Advancement in biogenerative engineering to combat shortage of organs and bioapplicable materials. Regen. Eng. Transl. Med. 2021, 8, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef]
- Jiang, W.; Mei, H.; Zhao, S. Applications of 3D Bio-Printing in Tissue Engineering and Biomedicine. J. Biomed. Nanotechnol. 2021, 17, 989–1006. [Google Scholar] [CrossRef]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef]
- Holmes, B.; Bulusu, K.; Plesniak, M.; Zhang, L.G. A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 2016, 27, 064001. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Elizondo, M.E.; Koons, G.L.; Diba, M.; Chim, L.K.; Cosgriff-Hernandez, E.; Melchiorri, A.J.; Mikos, A.G. Fiber engraving for bioink bioprinting within 3D printed tissue engineering scaffolds. Bioprinting 2020, 18, e00076. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-jet 3D printed drug delivery implants to inhibit growth and metastasis of orthotopic breast cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef]
- Domsta, V.; Seidlitz, A. 3D-Printing of drug-eluting implants: An overview of the current developments described in the literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Beohar, N.; Davidson, C.J.; Kip, K.E.; Goodreau, L.; Vlachos, H.A.; Meyers, S.N.; Benzuly, K.H.; Flaherty, J.D.; Ricciardi, M.J.; Bennett, C.L. Outcomes and complications associated with off-label and untested use of drug-eluting stents. Jama 2007, 297, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Darwish, L.R.; El-Wakad, M.T.; Farag, M.M. Towards an ultra-affordable three-dimensional bioprinter: A heated inductive-enabled syringe pump extrusion multifunction module for open-source fused deposition modeling three-dimensional printers. J. Manuf. Sci. Eng. 2021, 143, 125001. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and methods of biosensor interfaces with stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Wu, Y.; Mao, Z. Two-dimensional hexagonal NiCo2O4 Nanoplates@ PEDOT/RGO nanocomposite: A design and construction high selective H2O2 sensing interface. J. Electrochem. Soc. 2020, 167, 067519. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Peng, Z.; Song, M.; Jin, L. Rapid and sensitive detection of bisphenol a based on self-assembly. Micromachines 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Wang, Z.; Zheng, Y.; Xu, J.; Zhao, J.; Pan, H. Three-Dimensional Printing of Silk Fibroin/Nanohydroxyapatite Scaffolds for Bone Regeneration in Rat Calvarial Defects. J. Biol. Eng. 2024, 18, 12. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. Three-Dimensional Printed Gelatin/Hydroxyapatite Scaffolds for Stem Cell Chondrogenic Differentiation and Articular Cartilage Repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Zhanbassynova, A.; Mukasheva, F.; Abilev, M.; Berillo, D.; Trifonov, A.; Akilbekova, D. Impact of Hydroxyapatite on Gelatin/Oxidized Alginate 3D-Printed Cryogel Scaffolds. Gels 2024, 10, 406. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of nanotechnology in 3D printed tissue engineering scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Heger, Z.; Zitka, J.; Cernei, N.; Krizkova, S.; Sztalmachova, M.; Kopel, P.; Masarik, M.; Hodek, P.; Zitka, O.; Adam, V. 3D-printed biosensor with poly (dimethylsiloxane) reservoir for magnetic separation and quantum dots-based immunolabeling of metallothionein. Electrophoresis 2015, 36, 1256–1264. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Hassana, B.O.; Guessasma, S.; Belhabib, S.; Nouri, H. Explaining the Difference between Real Part and Virtual Design of 3D Printed Porous Polymer at the Microstructural Level. Macromol. Mater. Eng. 2016, 301, 566–576. [Google Scholar] [CrossRef]
- Yaneva, A.; Shopova, D.; Bakova, D.; Mihaylova, A.; Kasnakova, P.; Hristozova, M.; Semerdjieva, M. The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life. Bioengineering 2023, 10, 910. [Google Scholar] [CrossRef] [PubMed]
| Material Class | Materials | Biopolymer Composite | Printability | Mechanical Properties | Cytocompatibility | Applications |
|---|---|---|---|---|---|---|
| Proteins | Silk fibroin (SF) | e.g., SF with methacrylate groups, SF hydrogels | Photo-crosslinkable; UV-curable; good print resolution [27,28] | High tensile strength, elasticity [27] | Excellent biocompatibility, promotes cell adhesion [28] | Porous materials, tissue scaffolds printed via UV crosslinking |
| Keratin | e.g., Keratin–PLA–Chitosan, Keratin–Cellulose, Keratin–glycerol | Printable via SLA-based UV crosslinking; blendable with PLA, chitosan [29,30] | Moderate mechanical strength; flexible | Good cytocompatibility; supports regeneration [29] | Tissue scaffolds, drug delivery, regenerative medicine; SLA-based UV crosslinking | |
| Collagen | Blended with supportive hydrogels for 3D printing | Direct ink writing; requires additives for printability [31,32] | Moderate mechanical strength, biodegradable [31] | Excellent biocompatibility, antimicrobial [32] | Cell scaffolds, tissue/organ components; printed in support baths | |
| Polysaccharides | Chitosan | Requires additives; printed via direct ink writing into heated water | Printable in support baths; blends with hydrogels [33,34] | Low mechanical strength alone; often combined for reinforcement [33] | Excellent cytocompatibility, native ECM component [34] | Tissue scaffolding, biosensor interfaces |
| Gellan gum | Crosslinked with chemicals or UV | UV crosslinkable; printable hydrogels [35,36] | Soft mechanical properties; tunable via crosslinking [35] | Good cytocompatibility [36] | Wound dressings, artificial cartilage, bone osteogenesis | |
| Cellulose (CNF, CNC, BNC) | As additive or modified cellulose acetate | Printable as additive or modified derivatives [37,38] | Variable mechanical properties; generally high strength [37] | Biocompatible; supports tissue growth [38] | Biosensors, tissue scaffolding, wound dressing, artificial skin | |
| Synthetic Biocompatible Polymers | Polycaprolactone (PCL) | e.g., PCL + hydroxyapatite | Good printability with the composites [39,40] | Improved mechanical properties [39,40] | Broad applications in bone tissue engineering [40] | Bone scaffolds, trachea substitutes |
| Polyhydroxyalkanoates (PHA) | e.g., PHA + palm fibers | Porous scaffolds printed by SLS [41] | Good mechanical properties [42] | Biocompatible [42] | Drug delivery, vessel stents, tissue engineering; FDM and SLS printing |
| Printing Type | Printing Method | Advantages | Disadvantages | Biomaterials | Refs. |
|---|---|---|---|---|---|
| Inkjet | Layer-by-layer deposition | - Multiple print heads - Smooth finish - Spread area printing - No post processing | - Low material properties - Limited materials | - Silk (composite) - Cellulose - Gellan gum | [44,52,67] |
| Digital Light Processing | Photopolymerization (resin curing) | - Many materials - Quick printing - High accuracy and quality | - Post curing required - Lower structural properties - Necessary post processing | - Silk (composite) - Gellan gum | [26,46,52,68] |
| Fused Deposition Modeling | Extrusion | - Low cost - Material variety - Basic printing style | - Lower mechanical properties - Necessary post processing - Frequent machine issues - Long print time | - PCL - PHA | [58,59,60,69] |
| Selective Laser Sintering | Powder sintering | - No supports - High material properties - Relatively lower cost - Fast print time | - Necessary post processing - Low material availability - Brittle | - PCL - PHA - Cellulose | [41,62,63,64,70] |
| Stereolithography | Photopolymerization (resin curing) | - Smooth finish - Material variety - Quick printing - High accuracy | - Higher cost - Post curing required - Lower structural properties | - PCL - PHA - Cellulose - Gellan gum | [65,66,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poluri, N.; Carter, J.; Grasso, J.; Miller, W.; Leinbach, M.; Durant, F.; Benbrook, R.; John, A.; Wang, A.; Hu, X. New Frontiers in 3D Printing Using Biocompatible Polymers. Int. J. Mol. Sci. 2025, 26, 8016. https://doi.org/10.3390/ijms26168016
Poluri N, Carter J, Grasso J, Miller W, Leinbach M, Durant F, Benbrook R, John A, Wang A, Hu X. New Frontiers in 3D Printing Using Biocompatible Polymers. International Journal of Molecular Sciences. 2025; 26(16):8016. https://doi.org/10.3390/ijms26168016
Chicago/Turabian StylePoluri, Nagireddy, Jacob Carter, John Grasso, Walter Miller, Matthew Leinbach, Frederick Durant, Riley Benbrook, Assa John, Allan Wang, and Xiao Hu. 2025. "New Frontiers in 3D Printing Using Biocompatible Polymers" International Journal of Molecular Sciences 26, no. 16: 8016. https://doi.org/10.3390/ijms26168016
APA StylePoluri, N., Carter, J., Grasso, J., Miller, W., Leinbach, M., Durant, F., Benbrook, R., John, A., Wang, A., & Hu, X. (2025). New Frontiers in 3D Printing Using Biocompatible Polymers. International Journal of Molecular Sciences, 26(16), 8016. https://doi.org/10.3390/ijms26168016







