Physical Activity, Exerkines, and Their Role in Cancer Cachexia
Abstract
1. Introduction
2. Cancer Cachexia: Mechanisms and Clinical Implications
2.1. Defining Cancer Cachexia
2.2. Pathophysiology of Cachexia: Molecular Mechanisms
2.2.1. Pro-Inflammatory Cytokine Network
2.2.2. Key Proteolytic Systems in Muscle Wasting
2.2.3. Mitochondrial Dysfunction
2.2.4. Anabolic Resistance
2.2.5. Neuroendocrine Dysregulation and Anorexia
2.2.6. Ghrelin Biology, Resistance, and Therapeutic Modulation
2.2.7. Neuromuscular Junction (NMJ) Instability
2.2.8. Gut Barrier Dysfunction, Dysbiosis, and Systemic Inflammation
2.3. The Interrelationship Between Muscle and Bone in Cancer Cachexia
2.3.1. Bone Metabolism in Cachexia
2.3.2. Muscle–Bone Crosstalk in the Cancer Cachexia
2.4. Adipose Tissue Dysfunction in Cancer Cachexia
2.5. Altered Adipokine Secretion
3. Physical Activity in Cancer Cachexia
3.1. Exercise Intervention in Cancer Cachexia
3.2. Molecular Mechanisms of Action of Physical Exercise in Cancer Cachexia
4. Exerkines: Molecular Mediators of Exercise-Induced Systemic Adaptations
4.1. Protein and Peptide Exerkines
4.1.1. IL-6
4.1.2. Leukemia Inhibitory Factor
4.1.3. Myostatin
4.1.4. Activin A
4.1.5. Follistatin
4.1.6. Decorin
4.1.7. IGF-1
4.1.8. IL-15
4.1.9. Irisin
4.1.10. Fibrinogen C Domain Containing 1
4.1.11. Apelin
4.1.12. FGF21
4.1.13. Growth/Differentiation Factor 15
4.1.14. Osteocalcin
4.1.15. Brain-Derived Neurotrophic Factor
4.1.16. Angiopoietin-Like 4
4.2. Metabolic Exerkines
4.2.1. Lactate
4.2.2. Succinate
4.2.3. β-Aminoisobutyric Acid
4.2.4. Kynurenine Pathway Metabolites
4.2.5. 12,13-diHOME
4.3. Extracellular Vesicles and RNA-Based Exerkines
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neshan, M.; Tsilimigras, D.I.; Han, X.; Zhu, H.; Pawlik, T.M. Molecular Mechanisms of Cachexia: A Review. Cells 2024, 13, 252. [Google Scholar] [CrossRef]
- Poisson, J.; Martinez-Tapia, C.; Heitz, D.; Geiss, R.; Albrand, G.; Falandry, C.; Gisselbrecht, M.; Couderc, A.-L.; Boulahssass, R.; Liuu, E.; et al. Prevalence and prognostic impact of cachexia among older patients with cancer: A nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle 2021, 12, 1477–1488. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Rupert, J.; Bonetto, A.; Narasimhan, A.; Liu, Y.; O’Connell, T.; Koniaris, L.; Zimmers, T. IL-6 Trans-Signaling and Crosstalk Among Tumor, Muscle and Fat Mediate Pancreatic Cancer Cachexia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Dizdar, Ö.; Kılıçkap, S. Global Epidemiology of Gastrointestinal Cancers. In Textbook of Gastrointestinal Oncology; Yalcin, S., Philip, P.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–12. [Google Scholar]
- Mamun, T.I.; Younus, S.; Rahman, M.H. Gastric cancer-Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat. Res. Commun. 2024, 41, 100845. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Boocock, E.; Grande, A.J.; Maddocks, M. Exercise-based interventions for cancer cachexia: A systematic review of randomised and non-randomised controlled trials. Asia Pac. J. Oncol. Nurs. 2023, 10 (Suppl. S1), 100335. [Google Scholar] [CrossRef] [PubMed]
- Bowers, M.; Petrasso, C.; McLuskie, A.; Bayly, J.; Laird, B.J.A.; Higginson, I.J.; Maddocks, M. Multicomponent Interventions for Adults With Cancer Cachexia: A Systematic Review. J. Cachexia Sarcopenia Muscle 2025, 16, e13716. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Rubio-Zarapuz, A.; Martinez-Guardado, I.; Navarro-Jimenez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 4604. [Google Scholar] [CrossRef]
- Bertocchi, E.; Frigo, F.; Buonaccorso, L.; Venturelli, F.; Bassi, M.C.; Tanzi, S. Cancer cachexia: A scoping review on non-pharmacological interventions. Asia Pac. J. Oncol. Nurs. 2024, 11, 100438. [Google Scholar] [CrossRef]
- Constantina, C.; Mary, E.; George, O.; Konstantinos, F.; Christiana, K.; Nicos, M.; Andreas, C. Nonpharmacological Management of Cancer-Related Cachexia: A Systematic Review. Semin. Oncol. Nurs. 2025, 41, 151803. [Google Scholar] [CrossRef]
- Maddocks, M.; Murton, A.J.; Wilcock, A. Therapeutic exercise in cancer cachexia. Crit. Rev. Oncog. 2012, 17, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Horawski, J.L.; Fleszar-Pavlovic, S.E.; Lopez-Pentecost, M.; Crane, T.E.; Wheeler, M.G.; Kholodovsky, E.; Best, T.M. The role of resistance training in mitigating cancer-induced cachexia: A systematic review. Sports Med. Health Sci. 2025, in press. [Google Scholar] [CrossRef]
- Tsitkanou, S.; Murach, K.A.; Washington, T.A.; Greene, N.P. Exercise Counteracts the Deleterious Effects of Cancer Cachexia. Cancers 2022, 14, 2512. [Google Scholar] [CrossRef]
- Grande, A.J.; Silva, V.; Sawaris Neto, L.; Teixeira Basmage, J.P.; Peccin, M.S.; Maddocks, M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2021, 3, CD010804. [Google Scholar] [CrossRef]
- Mavropalias, G.; Sim, M.; Taaffe, D.R.; Galvao, D.A.; Spry, N.; Kraemer, W.J.; Hakkinen, K.; Newton, R.U. Exercise medicine for cancer cachexia: Targeted exercise to counteract mechanisms and treatment side effects. J. Cancer Res. Clin. Oncol. 2022, 148, 1389–1406. [Google Scholar] [CrossRef]
- Ahmadi Hekmatikar, A.; Nelson, A.; Petersen, A. Highlighting the idea of exerkines in the management of cancer patients with cachexia: Novel insights and a critical review. BMC Cancer 2023, 23, 889. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Brown, L.R.; Laird, B.J.A.; Wigmore, S.J.; Skipworth, R.J.E. Understanding Cancer Cachexia and Its Implications in Upper Gastrointestinal Cancers. Curr. Treat. Options Oncol. 2022, 23, 1732–1747. [Google Scholar] [CrossRef]
- Akezaki, Y.; Kikuuchi, M.; Hamada, K.; Ookura, M. Incidence of cachexia in patients with advanced gastrointestinal cancer at the beginning of rehabilitation intervention. J. Phys. Ther. Sci. 2020, 32, 16–19. [Google Scholar] [CrossRef]
- Valaire, R.; Garden, F.; Razmovski-Naumovski, V. Are measures and related symptoms of cachexia recorded as outcomes in gastrointestinal cancer chemotherapy clinical trials? J. Cachexia Sarcopenia Muscle 2024, 15, 1146–1156. [Google Scholar] [CrossRef]
- Tao, Z.; Chen, Z.; Gao, Y.; Quan, M. Influence of cachexia on immunotherapy efficacy and prognosis for malignant tumors of the digestive system. Cancer Rep. 2024, 7, e2100. [Google Scholar] [CrossRef] [PubMed]
- Yule, M.S.; Brown, L.R.; Waller, R.; Wigmore, S.J. Cancer cachexia. BMJ 2024, 387, e080040. [Google Scholar] [CrossRef] [PubMed]
- Anandavadivelan, P.; Lagergren, P. Cachexia in patients with oesophageal cancer. Nat. Rev. Clin. Oncol. 2016, 13, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.X.; Ma, J.L.; Zhang, J.Q.; Yan, L.L.; Zhou, Y.; Mao, X.L.; Li, S.W.; Zhou, X.B. Metabolic reprogramming and immunological changes in the microenvironment of esophageal cancer: Future directions and prospects. Front. Immunol. 2025, 16, 1524801. [Google Scholar] [CrossRef]
- Li, L.; Ling, Z.Q. Mechanisms of cancer cachexia and targeted therapeutic strategies. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189208. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Peña, M.M.O.; Davis, J.M.; White, J.P.; Carson, J.A. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc Min/+ mouse. Pflügers Arch.—Eur. J. Physiol. 2009, 457, 989–1001. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cancer cachexia. Curr. Opin. Gastroenterol. 2010, 26, 146–151. [Google Scholar] [CrossRef]
- Tisdale, M.J. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J. Support. Oncol. 2005, 3, 209–217. [Google Scholar]
- Stovroff, M.C.; Fraker, D.L.; Swedenborg, J.A.; Norton, J.A. Cachectin/tumor necrosis factor: A possible mediator of cancer anorexia in the rat. Cancer Res. 1988, 48, 4567–4572. [Google Scholar]
- Chen, J.L.; Colgan, T.D.; Walton, K.L.; Gregorevic, P.; Harrison, C.A. The TGF-β signalling network in muscle development, adaptation and disease. In Growth Factors and Cytokines in Skeletal Muscle Development, Growth, Regeneration and Disease; Advances in Experimental Medicine and Biology (AEMB, Volume 900); Springer: Cham, Switzerland, 2016; pp. 97–131. [Google Scholar]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Murphy, K.T.; Chee, A.; Gleeson, B.G.; Naim, T.; Swiderski, K.; Koopman, R.; Lynch, G.S. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2011, 301, R716–R726. [Google Scholar] [CrossRef] [PubMed]
- Padrao, A.I.; Moreira-Goncalves, D.; Oliveira, P.A.; Teixeira, C.; Faustino-Rocha, A.I.; Helguero, L.; Vitorino, R.; Santos, L.L.; Amado, F.; Duarte, J.A. Endurance training prevents TWEAK but not myostatin-mediated cardiac remodelling in cancer cachexia. Arch. Biochem. Biophys. 2015, 567, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Klimek, M.E.B.; Aydogdu, T.; Link, M.J.; Pons, M.; Koniaris, L.G.; Zimmers, T.A. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem. Biophys. Res. Commun. 2010, 391, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Sanborn, M.A.; Vijeth, S.; Gajwani, P.; Wang, X.; Jung, D.; Valyi-Nagy, T.; Chakraborty, S.; Mancinelli, G.; Toth, P.T.; et al. Skeletal muscle endothelial dysfunction through the activin A–PGC1α axis drives progression of cancer cachexia. Nat. Cancer 2025. [Google Scholar] [CrossRef]
- Winkles, J.A. The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat. Rev. Drug Discov. 2008, 7, 411–425. [Google Scholar] [CrossRef]
- Tajrishi, M.M.; Zheng, T.S.; Burkly, L.C.; Kumar, A. The TWEAK-Fn14 pathway: A potent regulator of skeletal muscle biology in health and disease. Cytokine Growth Factor. Rev. 2014, 25, 215–225. [Google Scholar] [CrossRef]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of Cancer Cachexia: Attempting to Develop New Pharmacological Agents for New Effective Therapeutic Options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef]
- Tomaz da Silva, M.; Roy, A.; Vuong, A.T.; Joshi, A.S.; Josphien, C.; Trivedi, M.V.; Hindi, S.M.; Narkar, V.A.; Kumar, A. The TWEAK/Fn14 signaling mediates skeletal muscle wasting during cancer cachexia. iScience 2025, 28, 112714. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.J.; Murphy, K.T.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G.; et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Ahmed, A.; Lai, H.C.; Cheng, W.C.; Yang, J.C.; Chang, W.C.; Chen, L.M.; Shan, Y.S.; Ma, W.L. Review of the endocrine organ-like tumor hypothesis of cancer cachexia in pancreatic ductal adenocarcinoma. Front. Oncol. 2022, 12, 1057930. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ren, Y.; Zhou, Z.; Yang, J.; Shi, X.; Cai, Y.; Arreola, A.X.; Luo, W.; Fung, K.-M.; Xu, C.; et al. The crosstalk between macrophages and cancer cells potentiates pancreatic cancer cachexia. Cancer Cell 2024, 42, 885–903.e4. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Wang, Y.F.; An, Z.Y.; Lin, D.H.; Jin, W.L. Targeting cancer cachexia: Molecular mechanisms and clinical study. MedComm 2022, 3, e164. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Song, N.Y. The role of myokines in cancer: Crosstalk between skeletal muscle and tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef]
- Kwak, K.S.; Zhou, X.; Solomon, V.; Baracos, V.E.; Davis, J.; Bannon, A.W.; Boyle, W.J.; Lacey, D.L.; Han, H.Q. Regulation of protein catabolism by muscle-specific and cytokine-inducible ubiquitin ligase E3alpha-II during cancer cachexia. Cancer Res. 2004, 64, 8193–8198. [Google Scholar] [CrossRef]
- Aniort, J.; Stella, A.; Philipponnet, C.; Poyet, A.; Polge, C.; Claustre, A.; Combaret, L.; Béchet, D.; Attaix, D.; Boisgard, S. Muscle wasting in patients with end-stage renal disease or early-stage lung cancer: Common mechanisms at work. J. Cachexia Sarcopenia Muscle 2019, 10, 323–337. [Google Scholar] [CrossRef]
- Fu, T.M.; Shen, C.; Li, Q.; Zhang, P.; Wu, H. Mechanism of ubiquitin transfer promoted by TRAF6. Proc. Natl. Acad. Sci. USA 2018, 115, 1783–1788. [Google Scholar] [CrossRef]
- Paul, P.K.; Gupta, S.K.; Bhatnagar, S.; Panguluri, S.K.; Darnay, B.G.; Choi, Y.; Kumar, A. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J. Cell Biol. 2010, 191, 1395–1411. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Ye, Z.Y.; Qian, Z.Y.; Xu, X.D.; Hu, J.F. Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of gastric cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 81. [Google Scholar] [CrossRef] [PubMed]
- Peris-Moreno, D.; Cussonneau, L.; Combaret, L.; Polge, C.; Taillandier, D. Ubiquitin Ligases at the Heart of Skeletal Muscle Atrophy Control. Molecules 2021, 26, 407. [Google Scholar] [CrossRef] [PubMed]
- Segatto, M.; Fittipaldi, R.; Pin, F.; Sartori, R.; Dae Ko, K.; Zare, H.; Fenizia, C.; Zanchettin, G.; Pierobon, E.S.; Hatakeyama, S.; et al. Epigenetic targeting of bromodomain protein BRD4 counteracts cancer cachexia and prolongs survival. Nat. Commun. 2017, 8, 1707. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxidative Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef]
- Carson, J.A.; Hardee, J.P.; VanderVeen, B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin. Cell Dev. Biol. 2016, 54, 53–67. [Google Scholar] [CrossRef]
- McLean, J.B.; Moylan, J.S.; Andrade, F.H. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front. Physiol. 2014, 5, 503. [Google Scholar] [CrossRef]
- Hardee, J.P.; Montalvo, R.N.; Carson, J.A. Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal Muscle Oxidative Metabolism. Oxidative Med. Cell. Longev. 2017, 2017, 8018197. [Google Scholar] [CrossRef]
- Laviano, A.; Seelaender, M.; Rianda, S.; Silverio, R.; Rossi Fanelli, F. Neuroinflammation: A contributing factor to the pathogenesis of cancer cachexia. Crit. Rev. Oncog. 2012, 17, 247–251. [Google Scholar] [CrossRef]
- Le Thuc, O.; Stobbe, K.; Cansell, C.; Nahon, J.L.; Blondeau, N.; Rovère, C. Hypothalamic Inflammation and Energy Balance Disruptions: Spotlight on Chemokines. Front. Endocrinol. 2017, 8, 197. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef]
- Olson, B.; Diba, P.; Korzun, T.; Marks, D.L. Neural Mechanisms of Cancer Cachexia. Cancers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Z.; An, Z.; Jin, W. Cancer cachexia: Focus on cachexia factors and inter-organ communication. Chin. Med. J. 2024, 137, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Kanter, N.G.; Cohen-Woods, S.; Balfour, D.A.; Burt, M.G.; Waterman, A.L.; Koczwara, B. Hypothalamic-Pituitary-Adrenal Axis Dysfunction in People With Cancer: A Systematic Review. Cancer Med. 2024, 13, e70366. [Google Scholar] [CrossRef]
- Jiao, Z.T.; Luo, Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients 2022, 14, 4191. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.M.; Gill, D.A.; Davies, R.; Loveridge, N.; Houston, P.A.; Robinson, I.C.; Wells, T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004, 145, 234–242. [Google Scholar] [CrossRef]
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLoS ONE 2017, 12, e0173113. [Google Scholar] [CrossRef]
- Chen, J.A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.P.; Garcia, J.M. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Reano, S.; Ferrara, M.; Angelino, E.; Gnocchi, V.F.; Prodam, F.; Ronchi, G.; Fagoonee, S.; Fornaro, M.; et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Investig. 2013, 123, 611–622. [Google Scholar] [CrossRef]
- Shao, T.; Verma, H.K.; Pande, B.; Costanzo, V.; Ye, W.; Cai, Y.; Bhaskar, L. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front. Physiol. 2021, 12, 751374. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, N.; Feki, M.; Bragazzi, N.L.; Knechtle, B.; Hill, L.; Nikolaidis, P.T.; Bouassida, A. Ghrelin Response to Acute and Chronic Exercise: Insights and Implications from a Systematic Review of the Literature. Sports Med. 2021, 51, 2389–2410. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Castorena, C.M.; Osborne-Lawrence, S.; Vijayaraghavan, P.; Metzger, N.P.; Elmquist, J.K.; Zigman, J.M. Ghrelin mediates exercise endurance and the feeding response post-exercise. Mol. Metab. 2018, 9, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Lovell, A.J.; Hoecht, E.M.; Hucik, B.; Cervone, D.T.; Dyck, D.J. The effects of diet and chronic exercise on skeletal muscle ghrelin response. Metabol. Open 2022, 14, 100182. [Google Scholar] [CrossRef]
- Fuoco, D.; Kilgour, R.D.; Vigano, A. A hypothesis for a possible synergy between ghrelin and exercise in patients with cachexia: Biochemical and physiological bases. Med. Hypotheses 2015, 85, 927–933. [Google Scholar] [CrossRef]
- Neary, N.M.; Small, C.J.; Wren, A.M.; Lee, J.L.; Druce, M.R.; Palmieri, C.; Frost, G.S.; Ghatei, M.A.; Coombes, R.C.; Bloom, S.R. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2004, 89, 2832–2836. [Google Scholar] [CrossRef]
- Hiura, Y.; Takiguchi, S.; Yamamoto, K.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Fujiwara, Y.; Mori, M.; et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: A prospective, randomized, placebo-controlled phase 2 study. Cancer 2012, 118, 4785–4794. [Google Scholar] [CrossRef]
- Yeom, E.; Yu, K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp. Mol. Med. 2022, 54, 426–432. [Google Scholar] [CrossRef]
- Matsumoto, T.; Cho, S.; Nakasya, A.; Nagai, H.; Satake, H.; Yasui, H. Early administration of anamorelin improves cancer cachexia in gastrointestinal cancer patients: An observational study. Sci. Rep. 2024, 14, 30017. [Google Scholar] [CrossRef]
- Hamauchi, S.; Furuse, J.; Takano, T.; Munemoto, Y.; Furuya, K.; Baba, H.; Takeuchi, M.; Choda, Y.; Higashiguchi, T.; Naito, T. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019, 125, 4294–4302. [Google Scholar] [CrossRef]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H. Anamorelin (ONO-7643) for the treatment of patients with non–small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018, 124, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Uchino, J.; Kojima, T.; Matano, Y.; Minato, K.; Tanaka, K.; Mizukami, T.; Atagi, S.; Higashiguchi, T.; Muro, K. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in patients with cancer cachexia and low body mass index. Cancer 2022, 128, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Friend, J.; Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: A multicenter, randomized, double-blind, crossover, pilot study. Support. Care Cancer 2013, 21, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef]
- Lu, X.; Huang, L.; Huang, Z.; Feng, D.; Clark, R.J.; Chen, C. LEAP-2: An Emerging Endogenous Ghrelin Receptor Antagonist in the Pathophysiology of Obesity. Front. Endocrinol. 2021, 12, 717544. [Google Scholar] [CrossRef]
- Varshney, S.; Shankar, K.; Kerr, H.L.; Anderson, L.J.; Gupta, D.; Metzger, N.P.; Singh, O.; Ogden, S.B.; Paul, S.; Piñon, F.; et al. The LEAP2 Response to Cancer-Related Anorexia-Cachexia Syndrome in Male Mice and Patients. Endocrinology 2024, 165, bqae132. [Google Scholar] [CrossRef]
- Oneda, E.; Manno, A.; Noventa, S.; Libertini, M.; Cherri, S.; Zaniboni, A. Role of diet, physical activity and new drugs in the primary management of cancer cachexia in gastrointestinal tumors—A comprehensive review. Front. Oncol. 2025, 15, 1600425. [Google Scholar] [CrossRef]
- Sartori, R.; Hagg, A.; Zampieri, S.; Armani, A.; Winbanks, C.E.; Viana, L.R.; Haidar, M.; Watt, K.I.; Qian, H.; Pezzini, C.; et al. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci. Transl. Med. 2021, 13, eaay9592. [Google Scholar] [CrossRef]
- Daou, N.; Hassani, M.; Matos, E.; De Castro, G.S.; Galvao Figueredo Costa, R.; Seelaender, M.; Moresi, V.; Rocchi, M.; Adamo, S.; Li, Z.; et al. Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation. Int. J. Mol. Sci. 2020, 21, 1092. [Google Scholar] [CrossRef]
- Boehm, I.; Miller, J.; Wishart, T.M.; Wigmore, S.J.; Skipworth, R.J.; Jones, R.A.; Gillingwater, T.H. Neuromuscular junctions are stable in patients with cancer cachexia. J. Clin. Investig. 2020, 130, 1461–1465. [Google Scholar] [CrossRef]
- Klein, G.L.; Petschow, B.W.; Shaw, A.L.; Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: A new therapeutic target. Curr. Opin. Support. Palliat. Care 2013, 7, 361–367. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Caro, P.L.; de Matos-Neto, E.M.; Lima, J.; Radloff, K.; Alves, M.J.; Camargo, R.G.; Pessoa, A.F.M.; Simoes, E.; Gama, P.; et al. Cancer cachexia induces morphological and inflammatory changes in the intestinal mucosa. J. Cachexia Sarcopenia Muscle 2019, 10, 1116–1127. [Google Scholar] [CrossRef]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Hul, M.V.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef] [PubMed]
- Puppa, M.J.; White, J.P.; Sato, S.; Cairns, M.; Baynes, J.W.; Carson, J.A. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim. Biophys. Acta 2011, 1812, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Villani, A.; Potenza, A.; Favaro, E.; Finocchiaro, C.; Perri, F.; Pazienza, V. Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. Int. J. Mol. Sci. 2023, 24, 1849. [Google Scholar] [CrossRef]
- Pin, F.; Prideaux, M.; Huot, J.R.; Essex, A.L.; Plotkin, L.I.; Bonetto, A.; Bonewald, L.F. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021, 520, 80–90. [Google Scholar] [CrossRef]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef]
- Kaji, H. Bone-muscle interactions. Osteoporos. Sarcopenia 2025, 11 (Suppl. 2), 32–39. [Google Scholar] [CrossRef]
- Anastasilaki, E.; Paccou, J.; Gkastaris, K.; Anastasilakis, A.D. Glucocorticoid-induced osteoporosis: An overview with focus on its prevention and management. Hormones 2023, 22, 611–622. [Google Scholar] [CrossRef]
- Zwickl, H.; Zwickl-Traxler, E.; Haushofer, A.; Seier, J.; Podar, K.; Weber, M.; Hackner, K.; Jacobi, N.; Pecherstorfer, M.; Vallet, S. Effect of cachexia on bone turnover in cancer patients: A case-control study. BMC Cancer 2021, 21, 744. [Google Scholar] [CrossRef]
- Bonetto, A.; Kays, J.K.; Parker, V.A.; Matthews, R.R.; Barreto, R.; Puppa, M.J.; Kang, K.S.; Carson, J.A.; Guise, T.A.; Mohammad, K.S.; et al. Differential Bone Loss in Mouse Models of Colon Cancer Cachexia. Front. Physiol. 2016, 7, 679. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef]
- Pin, F.; Jones, A.J.; Huot, J.R.; Narasimhan, A.; Zimmers, T.A.; Bonewald, L.F.; Bonetto, A. RANKL Blockade Reduces Cachexia and Bone Loss Induced by Non-Metastatic Ovarian Cancer in Mice. J. Bone Miner. Res. 2022, 37, 381–396. [Google Scholar] [CrossRef]
- Sims, N.A. Influences of the IL-6 cytokine family on bone structure and function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef] [PubMed]
- Anloague, A.; Delgado-Calle, J. Osteocytes: New Kids on the Block for Cancer in Bone Therapy. Cancers 2023, 15, 2645. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.A.; Cardenas, E.R.; Jiang, J.X. Osteocytes and Bone Metastasis. Front. Endocrinol. 2020, 11, 567844. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, D.; Hong, Z. Sarcopenia and cachexia: Molecular mechanisms and therapeutic interventions. MedComm 2025, 6, e70030. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- Yuan, S.; Wan, Z.-H.; Cheng, S.-L.; Michaëlsson, K.; Larsson, S.C. Insulin-like Growth Factor-1, Bone Mineral Density, and Fracture: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, 1552–1558. [Google Scholar] [CrossRef]
- Pin, F.; Bonewald, L.F.; Bonetto, A. Role of myokines and osteokines in cancer cachexia. Exp. Biol. Med. 2021, 246, 2118–2127. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; Shi, X.; Zhang, W.; Pennington, C.; Thakore, H.; Haque, M.; Kang, B.; Isales, C.M.; Fulzele, S.; Wenger, K.H. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 2007, 40, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2017, 96, 38–44. [Google Scholar] [CrossRef]
- Tsourdi, E. RANKL blockade for cancer cachexia; A new therapeutic opportunity? J. Bone Miner. Res. 2022, 37, 379–380. [Google Scholar] [CrossRef]
- Pauk, M.; Saito, H.; Hesse, E.; Taipaleenmäki, H. Muscle and Bone Defects in Metastatic Disease. Curr. Osteoporos. Rep. 2022, 20, 273–289. [Google Scholar] [CrossRef]
- Booth, F.W.; Ruegsegger, G.N.; Olver, T.D. Exercise Has a Bone to Pick with Skeletal Muscle. Cell Metab. 2016, 23, 961–962. [Google Scholar] [CrossRef]
- Tamayo-Torres, E.; Garrido, A.; de Cabo, R.; Carretero, J.; Gomez-Cabrera, M.C. Molecular mechanisms of cancer cachexia. Role of exercise training. Mol. Aspects Med. 2024, 99, 101293. [Google Scholar] [CrossRef]
- Daas, S.I.; Rizeq, B.R.; Nasrallah, G.K. Adipose tissue dysfunction in cancer cachexia. J. Cell. Physiol. 2019, 234, 13–22. [Google Scholar] [CrossRef]
- Mannelli, M.; Gamberi, T.; Magherini, F.; Fiaschi, T. The Adipokines in Cancer Cachexia. Int. J. Mol. Sci. 2020, 21, 4860. [Google Scholar] [CrossRef]
- Batista Júnior, M.L.; Henriques, F. Adipose Tissue Remodeling during Cancer Cachexia. In Muscle Cells—Recent Advances and Future Perspectives; Valarmathi, M.T., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Batista, M.L., Jr.; Henriques, F.S.; Neves, R.X.; Olivan, M.R.; Matos-Neto, E.M.; Alcântara, P.S.; Maximiano, L.F.; Otoch, J.P.; Alves, M.J.; Seelaender, M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 2016, 7, 37–47. [Google Scholar] [CrossRef]
- Geppert, J.; Rohm, M. Cancer cachexia: Biomarkers and the influence of age. Mol. Oncol. 2024, 18, 2070–2086. [Google Scholar] [CrossRef]
- Tsoli, M.; Moore, M.; Burg, D.; Painter, A.; Taylor, R.; Lockie, S.H.; Turner, N.; Warren, A.; Cooney, G.; Oldfield, B. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 2012, 72, 4372–4382. [Google Scholar] [CrossRef]
- Mota, I.N.R.; Satari, S.; Marques, I.S.; Santos, J.M.O.; Medeiros, R. Adipose tissue rearrangement in cancer cachexia: The involvement of β3-adrenergic receptor associated pathways. Biochim. Biophys. Acta—Rev. Cancer 2024, 1879, 189103. [Google Scholar] [CrossRef] [PubMed]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Rohm, M.; Schäfer, M.; Laurent, V.; Üstünel, B.E.; Niopek, K.; Algire, C.; Hautzinger, O.; Sijmonsma, T.P.; Zota, A.; Medrikova, D. An AMP-activated protein kinase–stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 2016, 22, 1120–1130. [Google Scholar] [CrossRef]
- Xie, H.; Heier, C.; Meng, X.; Bakiri, L.; Pototschnig, I.; Tang, Z.; Schauer, S.; Baumgartner, V.J.; Grabner, G.F.; Schabbauer, G.; et al. An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia. Proc. Natl. Acad. Sci. USA 2022, 119, e2112840119. [Google Scholar] [CrossRef]
- Bos, S.A.; Gill, C.M.; Martinez-Salazar, E.L.; Torriani, M.; Bredella, M.A. Preliminary investigation of brown adipose tissue assessed by PET/CT and cancer activity. Skelet. Radiol. 2019, 48, 413–419. [Google Scholar] [CrossRef]
- Shellock, F.G.; Riedinger, M.S.; Fishbein, M.C. Brown adipose tissue in cancer patients: Possible cause of cancer-induced cachexia. J. Cancer Res. Clin. Oncol. 1986, 111, 82–85. [Google Scholar] [CrossRef]
- Becker, A.S.; Zellweger, C.; Bacanovic, S.; Franckenberg, S.; Nagel, H.W.; Frick, L.; Schawkat, K.; Eberhard, M.; Bluthgen, C.; Volbracht, J.; et al. Brown fat does not cause cachexia in cancer patients: A large retrospective longitudinal FDG-PET/CT cohort study. PLoS ONE 2020, 15, e0239990. [Google Scholar] [CrossRef]
- Eljalby, M.; Huang, X.; Becher, T.; Wibmer, A.G.; Jiang, C.S.; Vaughan, R.; Schöder, H.; Cohen, P. Brown adipose tissue is not associated with cachexia or increased mortality in a retrospective study of patients with cancer. Am. J. Physiol.—Endocrinol. Metab. 2023, 324, E144–E153. [Google Scholar] [CrossRef]
- Panagiotou, G.; Babazadeh, D.; Mazza, D.F.; Azghadi, S.; Cawood, J.M.; Rosenberg, A.S.; Imamura, F.; Forouhi, N.G.; Chaudhari, A.J.; Abdelhafez, Y.G.; et al. Brown adipose tissue is associated with reduced weight loss and risk of cancer cachexia: A retrospective cohort study. Clin. Nutr. 2025, 45, 262–269. [Google Scholar] [CrossRef]
- Chu, K.; Bos, S.A.; Gill, C.M.; Torriani, M.; Bredella, M.A. Brown adipose tissue and cancer progression. Skelet. Radiol. 2020, 49, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Silvério, R.; Lira, F.S.; Oyama, L.M.; Oller do Nascimento, C.M.; Otoch, J.P.; Alcântara, P.S.M.; Batista, M.L.; Seelaender, M. Lipases and lipid droplet-associated protein expression in subcutaneous white adipose tissue of cachectic patients with cancer. Lipids Health Dis. 2017, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Terashima, M.; Takagane, A.; Oyama, K.; Fujiwara, H.; Wakabayashi, G. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int. J. Clin. Oncol. 2009, 14, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Woo, Y.C.; Wang, Y.; Yeung, C.Y.; Xu, A.; Lam, K.S.L. Obesity, adipokines and cancer: An update. Clin. Endocrinol. 2015, 83, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kerem, M.; Ferahkose, Z.; Yilmaz, U.T.; Pasaoglu, H.; Ofluoglu, E.; Bedirli, A.; Salman, B.; Sahin, T.T.; Akin, M. Adipokines and ghrelin in gastric cancer cachexia. World J. Gastroenterol. 2008, 14, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Krzystek-Korpacka, M.; Markocka-Maczka, K.; Diakowski, W.; Matusiewicz, M.; Grabowski, K. Circulating leptin and inflammatory response in esophageal cancer, esophageal cancer-related cachexia–anorexia syndrome (CAS) and non-malignant CAS of the alimentary tract. Cytokine 2010, 51, 132–137. [Google Scholar] [CrossRef]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 severity in obesity: Leptin and inflammatory cytokine interplay in the link between high morbidity and mortality. Front. Immunol. 2021, 12, 649359. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.; Petri, W.A. Leptin regulation of immune responses. Trends Mol. Med. 2016, 22, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.; Digiovanni, L.; Good, J.; Salvatore, D.; Fenderson, D.; Domchek, S.; Stopfer, J.; Galantino, M.L.; Bryan, C.; Hwang, W.-T. Exercise-induced dose-response alterations in adiponectin and leptin levels are dependent on body fat changes in women at risk for breast cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Diakowska, D.; Markocka-Mączka, K.; Szelachowski, P.; Grabowski, K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis. Markers 2014, 2014, 619649. [Google Scholar] [CrossRef]
- Wei, T.; Ye, P.; Peng, X.; Wu, L.L.; Yu, G.Y. Circulating adiponectin levels in various malignancies: An updated meta-analysis of 107 studies. Oncotarget 2016, 7, 48671–48691. [Google Scholar] [CrossRef]
- Balstad, T.R.; Brunelli, C.; Pettersen, C.H.; Schønberg, S.A.; Skorpen, F.; Fallon, M.; Kaasa, S.; Bye, A.; Laird, B.J.A.; Stene, G.B.; et al. Power Comparisons and Clinical Meaning of Outcome Measures in Assessing Treatment Effect in Cancer Cachexia: Secondary Analysis From a Randomized Pilot Multimodal Intervention Trial. Front. Nutr. 2021, 7, 602775. [Google Scholar] [CrossRef]
- Langer, H.T.; Ramsamooj, S.; Dantas, E.; Murthy, A.; Ahmed, M.; Ahmed, T.; Hwang, S.K.; Grover, R.; Pozovskiy, R.; Liang, R.J.; et al. Restoring adiponectin via rosiglitazone ameliorates tissue wasting in mice with lung cancer. Acta. Physiol. 2024, 240, e14167. [Google Scholar] [CrossRef]
- Massart, I.S.; Kouakou, A.N.; Pelet, N.; Lause, P.; Schakman, O.; Loumaye, A.; Abou-Samra, M.; Deldicque, L.; Bindels, L.B.; Brichard, S.M.; et al. Administration of adiponectin receptor agonist AdipoRon relieves cancer cachexia by mitigating inflammation in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2024, 15, 919–933. [Google Scholar] [CrossRef]
- Li, Y.; Onodera, T.; Scherer, P.E. Adiponectin. Trends Endocrinol. Metab. 2024, 35, 674–675. [Google Scholar] [CrossRef]
- Otu, L.I.; Otu, A. Adiponectin and the Control of Metabolic Dysfunction: Is Exercise the Magic Bullet? Front. Physiol. 2021, 12, 651732. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport. Health Sci. 2023, 12, 147–157. [Google Scholar] [CrossRef]
- Polito, R.; Monda, V.; Nigro, E.; Messina, A.; Di Maio, G.; Giuliano, M.T.; Orrù, S.; Imperlini, E.; Calcagno, G.; Mosca, L.; et al. The Important Role of Adiponectin and Orexin-A, Two Key Proteins Improving Healthy Status: Focus on Physical Activity. Front. Physiol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Karapanagiotou, E.M.; Tsochatzis, E.A.; Dilana, K.D.; Tourkantonis, I.; Gratsias, I.; Syrigos, K.N. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 2008, 61, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, G.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Antonopoulos, C.; Tachmatzidis, D.; Didangelos, T.; Lambadiari, V.; Kadoglou, N.P. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: A systematic review and meta-analysis. Cytokine 2023, 164, 156157. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M. Interplay of adipokines and myokines in cancer pathophysiology: Emerging therapeutic implications. World J. Exp. Med. 2013, 3, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Saeteaw, M.; Sanguanboonyaphong, P.; Yoodee, J.; Craft, K.; Sawangjit, R.; Ngamphaiboon, N.; Shantavasinkul, P.C.; Subongkot, S.; Chaiyakunapruk, N. Efficacy and safety of pharmacological cachexia interventions: Systematic review and network meta-analysis. BMJ Support. Palliat. Care 2021, 11, 75–85. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Ehrman, J.K.; Gordon, P.M.; Visich, P.; Keteyian, S.J. Clinical Exercise Physiology: Exercise Management for Chronic Diseases and Special Populations; Human kinetics: Champaign, IL, USA, 2022. [Google Scholar]
- Kamel, F.H.; Basha, M.A.; Alsharidah, A.S.; Salama, A.B. Resistance Training Impact on Mobility, Muscle Strength and Lean Mass in Pancreatic Cancer Cachexia: A Randomized Controlled Trial. Clin. Rehabil. 2020, 34, 1391–1399. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Stene, G.B.; Baracos, V.; Bye, A.; Dajani, O.; Hendifar, A.E.; Strasser, F.; Chasen, M.R. Results from a randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to attenuate cachexia in patients with advanced cancer undergoing chemotherapy. J. Clin. Oncol. 2024, 42 (Suppl. 17), LBA12007. [Google Scholar] [CrossRef]
- De Lazzari, N.; Gotte, M.; Kasper, S.; Meier, E.; Schuler, M.; Pogorzelski, M.; Siveke, J.T.; Tewes, M. P-move: A randomized control trial of exercise in patients with advanced pancreatic or biliary tract cancer (aPBC) receiving beyond first-line chemotherapy. Support. Care Cancer 2024, 32, 437. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2019, 13, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Puppa, M.J.; White, J.P.; Velázquez, K.T.; Baltgalvis, K.A.; Sato, S.; Baynes, J.W.; Carson, J.A. The effect of exercise on IL-6-induced cachexia in the ApcMin/+ mouse. J. Cachexia Sarcopenia Muscle 2012, 3, 117–137. [Google Scholar] [CrossRef]
- Lambert, C.P. Resistance exercise to mitigate cancer cachexia: Molecular mechanisms and practical applications. J. Cancer Ther. 2022, 13, 497–506. [Google Scholar] [CrossRef]
- Morinaga, M.; Sako, N.; Isobe, M.; Lee-Hotta, S.; Sugiura, H.; Kametaka, S. Aerobic Exercise Ameliorates Cancer Cachexia-Induced Muscle Wasting through Adiponectin Signaling. Int. J. Mol. Sci. 2021, 22, 3110. [Google Scholar] [CrossRef]
- Pin, F.; Busquets, S.; Toledo, M.; Camperi, A.; Lopez-Soriano, F.J.; Costelli, P.; Argilés, J.M.; Penna, F. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget 2015, 6, 43202. [Google Scholar] [CrossRef]
- Khamoui, A.V.; Park, B.-S.; Kim, D.-H.; Yeh, M.-C.; Oh, S.-L.; Elam, M.L.; Jo, E.; Arjmandi, B.H.; Salazar, G.; Grant, S.C.; et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism 2016, 65, 685–698. [Google Scholar] [CrossRef]
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined exercise training positively affects muscle wasting in tumor-bearing mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef]
- Ballarò, R.; Penna, F.; Pin, F.; Gómez-Cabrera, M.C.; Viña, J.; Costelli, P. Moderate Exercise Improves Experimental Cancer Cachexia by Modulating the Redox Homeostasis. Cancers 2019, 11, 285. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Schwartz, A.L.; Matthews, C.E.; Courneya, K.S.; Schmitz, K.H. Implementing the exercise guidelines for cancer survivors. J. Support. Oncol. 2012, 10, 171. [Google Scholar] [CrossRef]
- Bland, K.A. Evaluating the Role of Exercise as a Management Strategy to Counteract the Burden of Cancer Cachexia. Ph.D. Thesis, Australian Catholic University, Melbourne, Australia, 2023. [Google Scholar]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Halle, J.L.; Counts, B.R.; Carson, J.A. Exercise as a therapy for cancer-induced muscle wasting. Sports Med. Health Sci. 2020, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.; Buss, L.A.; Draper, N.; Currie, M.J. Exercise in People With Cancer: A Spotlight on Energy Regulation and Cachexia. Front. Physiol. 2022, 13, 836804. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachexia Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Steindorf, K.; Clauss, D.; Wiskemann, J.; Schmidt, M.E. Physical Activity and Gastrointestinal Cancers: Primary and Tertiary Preventive Effects and Possible Biological Mechanisms. Sports 2015, 3, 145–158. [Google Scholar] [CrossRef]
- Wiskemann, J.; Clauss, D.; Tjaden, C.; Hackert, T.; Schneider, L.; Ulrich, C.M.; Steindorf, K. Progressive Resistance Training to Impact Physical Fitness and Body Weight in Pancreatic Cancer Patients: A Randomized Controlled Trial. Pancreas 2019, 48, 257–266. [Google Scholar] [CrossRef]
- Zhou, X.; Li, S.; Wang, L.; Wang, J.; Zhang, P.; Chen, X. The emerging role of exercise preconditioning in preventing skeletal muscle atrophy. Front. Physiol. 2025, 16, 1559594. [Google Scholar] [CrossRef]
- Hesketh, S.J. Advancing cancer cachexia diagnosis with -omics technology and exercise as molecular medicine. Sports Med. Health Sci. 2024, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Yamashita, A.S.; Rosa, J.C.; Koyama, C.H.; Caperuto, E.C.; Batista, M.L., Jr.; Seelaender, M.C. Exercise training decreases adipose tissue inflammation in cachectic rats. Horm. Metab. Res. 2012, 44, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Q.; Liu, L.; Cheng, Y.; Han, Y.; Chen, X.; Lin, J.; Li, Z.; Liu, H.; Zhang, X.; et al. Swimming Attenuates Muscle Wasting and Mediates Multiple Signaling Pathways and Metabolites in CT-26 Bearing Mice. Front. Mol. Biosci. 2021, 8, 812681. [Google Scholar]
- Molanouri Shamsi, M.; Chekachak, S.; Soudi, S.; Quinn, L.S.; Ranjbar, K.; Chenari, J.; Yazdi, M.H.; Mahdavi, M. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine 2017, 90, 100–108. [Google Scholar] [CrossRef]
- Bordignon, C.; Dos Santos, B.S.; Rosa, D.D. Impact of Cancer Cachexia on Cardiac and Skeletal Muscle: Role of Exercise Training. Cancers 2022, 14, 342. [Google Scholar] [CrossRef]
- Tsitkanou, S.; Koopmans, P.; Peterson, C.; Cabrera, A.R.; Muhyudin, R.; Morena, F.; Khadgi, S.; Schrems, E.R.; Washington, T.A.; Murach, K.A.; et al. Myocellular adaptations to short-term weighted wheel-running exercise are largely conserved during C26-tumour induction in male and female mice. Exp. Physiol. 2025. [Google Scholar] [CrossRef]
- Sato, S.; Gao, S.; Puppa, M.J.; Kostek, M.C.; Wilson, L.B.; Carson, J.A. High-Frequency Stimulation on Skeletal Muscle Maintenance in Female Cachectic Mice. Med. Sci. Sports Exerc. 2019, 51, 1828–1837. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugimoto, K.; Fujimoto, T.; Xie, K.; Takahashi, T.; Akasaka, H.; Kurinami, H.; Yasunobe, Y.; Matsumoto, T.; Fujino, H. Preventive effects of low-intensity exercise on cancer cachexia–induced muscle atrophy. FASEB J. 2019, 33, 7852–7862. [Google Scholar] [CrossRef]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Peres, S.B.; Batista, M.L., Jr. Exercise Training as Therapeutic Approach in Cancer Cachexia: A Review of Potential Anti-inflammatory Effect on Muscle Wasting. Front. Physiol. 2020, 11, 570170. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed. Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.L.; Wang, R.; Wang, X.Q.; Zhang, H. Exercise for osteoporosis: A literature review of pathology and mechanism. Front. Immunol. 2022, 13, 1005665. [Google Scholar] [CrossRef]
- Alves, C.R.; das Neves, W.; de Almeida, N.R.; Eichelberger, E.J.; Jannig, P.R.; Voltarelli, V.A.; Tobias, G.C.; Bechara, L.R.; de Paula Faria, D.; Alves, M.J. Exercise training reverses cancer-induced oxidative stress and decrease in muscle COPS2/TRIP15/ALIEN. Mol. Metab. 2020, 39, 101012. [Google Scholar] [CrossRef]
- Powers, S.K.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef]
- Linke, A.; Adams, V.; Schulze, P.C.; Erbs, S.; Gielen, S.; Fiehn, E.; Möbius-Winkler, S.; Schubert, A.; Schuler, G.; Hambrecht, R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation 2005, 111, 1763–1770. [Google Scholar] [CrossRef]
- Brinkmann, C.; Chung, N.; Schmidt, U.; Kreutz, T.; Lenzen, E.; Schiffer, T.; Geisler, S.; Graf, C.; Montiel-Garcia, G.; Renner, R.; et al. Training alters the skeletal muscle antioxidative capacity in non-insulin-dependent type 2 diabetic men. Scand. J. Med. Sci. Sports 2012, 22, 462–470. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef]
- Gomes, M.J.; Pagan, L.U.; Lima, A.R.R.; Reyes, D.R.A.; Martinez, P.F.; Damatto, F.C.; Pontes, T.H.D.; Rodrigues, E.A.; Souza, L.M.; Tosta, I.F.; et al. Effects of aerobic and resistance exercise on cardiac remodelling and skeletal muscle oxidative stress of infarcted rats. J. Cell Mol. Med. 2020, 24, 5352–5362. [Google Scholar] [CrossRef]
- Scheffer, D.L.; Silva, L.A.; Tromm, C.B.; da Rosa, G.L.; Silveira, P.C.; de Souza, C.T.; Latini, A.; Pinho, R.A. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl. Physiol. Nutr. Metab. 2012, 37, 1239–1246. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The antioxidant effect of exercise: A systematic review and meta-analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and Exercise against Muscle Wasting during Cancer Cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef]
- Ballarò, R.; Beltrà, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef]
- Longobucco, Y.; Masini, A.; Marini, S.; Barone, G.; Fimognari, C.; Bragonzoni, L.; Dallolio, L.; Maffei, F. Exercise and Oxidative Stress Biomarkers among Adult with Cancer: A Systematic Review. Oxidative Med. Cell. Longev. 2022, 2022, 2097318. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Calcaterra, G.; Casciani, F.; Pecorelli, S.; Mehta, J.L. ‘Exerkines’: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines 2024, 12, 1975. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Noble, E.E.; Call, J.A. The role of exerkines on brain mitochondria: A mini-review. J. Appl. Physiol. 2023, 134, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Smith, B.J.; Volpe, S.L.; Shen, C.-L. Exerkines, Nutrition, and Systemic Metabolism. Nutrients 2024, 16, 410. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Keller, P.; Keller, C.; Fischer, C.; Hiscock, N.; van Hall, G.; Plomgaard, P.; Febbraio, M.A. Muscle-derived interleukin-6: Lipolytic, anti-inflammatory and immune regulatory effects. Pflügers Arch. 2003, 446, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Walzik, D.; Wences-Chirino, T.; Zimmer, P.; Joisten, N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Zhang, L.-D.; Luo, X.; Wu, L.-L.; Chen, Z.-W.; Wei, G.-H.; Zhang, K.-Q.; Du, Z.-A.; Li, R.-Z.; So, K.-F.; et al. All roads lead to Rome—A review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1210–1227. [Google Scholar]
- Zhou, N.; Gong, L.; Zhang, E.; Wang, X. Exploring exercise-driven exerkines: Unraveling the regulation of metabolism and inflammation. PeerJ 2024, 12, e17267. [Google Scholar] [CrossRef]
- Katz, D.H.; Lindholm, M.E.; Ashley, E.A. Charting the Molecular Terrain of Exercise: Energetics, Exerkines, and the Future of Multiomic Mapping. Physiology 2025, 40, 185–202. [Google Scholar] [CrossRef]
- Goj, T.; Hoene, M.; Fritsche, L.; Schneeweiss, P.; Machann, J.; Petrera, A.; Hauck, S.M.; Fritsche, A.; Birkenfeld, A.L.; Peter, A.; et al. The Acute Cytokine Response to 30-Minute Exercise Bouts Before and After 8-Week Endurance Training in Individuals With Obesity. J. Clin. Endocrinol. Metab. 2022, 108, 865–875. [Google Scholar] [CrossRef]
- Nielsen, S.; Akerstrom, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef] [PubMed]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef] [PubMed]
- Ercan, Z.; Deniz, G.; Yentur, S.B.; Arikan, F.B.; Karatas, A.; Alkan, G.; Koca, S.S. Effects of acute aerobic exercise on cytokines, klotho, irisin, and vascular endothelial growth factor responses in rheumatoid arthritis patients. Ir. J. Med. Sci. 2023, 192, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; She, Y.; Yu, M.; Min, W.; Shang, W.; Zhang, Z. Adipose-Muscle crosstalk in age-related metabolic disorders: The emerging roles of adipo-myokines. Ageing Res. Rev. 2023, 84, 101829. [Google Scholar] [CrossRef]
- Brenner, D.R.; Ruan, Y.; Adams, S.C.; Courneya, K.S.; Friedenreich, C.M. The impact of exercise on growth factors (VEGF and FGF2): Results from a 12-month randomized intervention trial. Eur. Rev. Aging Phys. Act. 2019, 16, 8. [Google Scholar] [CrossRef]
- Mathes, S.; Fahrner, A.; Ghoshdastider, U.; Rüdiger, H.A.; Leunig, M.; Wolfrum, C.; Krützfeldt, J. FGF-2–dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021013118. [Google Scholar] [CrossRef]
- Bettariga, F.; Taaffe, D.R.; Galvão, D.A.; Lopez, P.; Bishop, C.; Markarian, A.M.; Natalucci, V.; Kim, J.-S.; Newton, R.U. Exercise training mode effects on myokine expression in healthy adults: A systematic review with meta-analysis. J. Sport. Health Sci. 2024, 13, 764–779. [Google Scholar] [CrossRef]
- Rodriguez, A.; Becerril, S.; Hernandez-Pardos, A.W.; Fruhbeck, G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.M.T.; Hameed, A.T.A.Z.; Marsool, M.D.M.; Jain, H.; Prajjwal, P.; Khazmi, I.; Nazzal, R.S.; AL-Najati, H.M.H.; Al-Zuhairi, B.H.Y.K.; Razzaq, M. Exercise-Induced cytokines, diet, and inflammation and their role in adipose tissue metabolism. Health Sci. Rep. 2024, 7, e70034. [Google Scholar] [CrossRef]
- Mu, W.-J.; Zhu, J.-Y.; Chen, M.; Guo, L. Exercise-mediated browning of white adipose tissue: Its significance, mechanism and effectiveness. Int. J. Mol. Sci. 2021, 22, 11512. [Google Scholar] [CrossRef]
- Dumond Bourie, A.; Potier, J.-B.; Pinget, M.; Bouzakri, K. Myokines: Crosstalk and consequences on liver physiopathology. Nutrients 2023, 15, 1729. [Google Scholar] [CrossRef]
- Jin, L.; Diaz-Canestro, C.; Wang, Y.; Tse, M.A.; Xu, A. Exerkines and cardiometabolic benefits of exercise: From bench to clinic. EMBO Mol. Med. 2024, 16, 432–444. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, T.; Yu, J.; Li, S.; Gong, L.; Zhang, Y. FGF21: A Sharp Weapon in the Process of Exercise to Improve NAFLD. FBL 2023, 28, 351. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Kornel, A.; Den Hartogh, D.J.; Klentrou, P.; Tsiani, E. Role of the myokine irisin on bone homeostasis: Review of the current evidence. Int. J. Mol. Sci. 2021, 22, 9136. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Smargiassi, A.; Peng, G.; Brotto, M.; Robling, A.G.; Bonewald, L.F. L BAIBA Synergizes with Sub Optimal Mechanical Loading to Promote New Bone Formation. J. Bone Miner. Res. Plus 2023, 7, e10746. [Google Scholar] [CrossRef] [PubMed]
- Shimonty, A.; Bonewald, L.F.; Huot, J.R. Metabolic Health and Disease: A Role of Osteokines? Calcif. Tissue Int. 2023, 113, 21–38. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenia, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Z.; Herold, F.; Ludyga, S.; Kuang, J.; Chen, Y.; Liu, Z.; Erickson, K.I.; Goodpaster, B.H.; Cheval, B. Physical activity, cathepsin B, and cognitive health. Trends Mol. Med. 2025, 31, 595–609. [Google Scholar] [CrossRef]
- Schön, M.; Kovaničová, Z.; Košutzká, Z.; Nemec, M.; Tomková, M.; Jacková, L.; Máderová, D.; Slobodová, L.; Valkovič, P.; Ukropec, J.; et al. Effects of running on adiponectin, insulin and cytokines in cerebrospinal fluid in healthy young individuals. Sci. Rep. 2019, 9, 1959. [Google Scholar] [CrossRef]
- Leiter, O.; Lowe, J.; Brici, D.; Walker, T.L. Exerkines and brain rejuvenation. Alzheimer’s Dement. 2024, 20, e083404. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhu, B.; Luo, A.L.; Yang, L.; Yang, C. The Role of Cardiokines in Heart Diseases: Beneficial or Detrimental? Biomed. Res. Int. 2018, 2018, 8207058. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Mi, C.; Chen, K.; Ji, Y.; Wang, L.; Zhang, J.; Cheng, K. Exercise protects the heart against myocardial infarction through upregulation of miR-1192. Biochem. Biophys. Res. Commun. 2020, 521, 1061–1069. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; González, A.; García-Hermoso, A.; Amézqueta, I.L.; Izquierdo, M.; Díez, J. Revisiting skeletal myopathy and exercise training in heart failure: Emerging role of myokines. Metabolism 2023, 138, 155348. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2020, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Goussetis, E.; Spiropoulos, A.; Tsironi, M.; Skenderi, K.; Margeli, A.; Graphakos, S.; Baltopoulos, P.; Papassotiriou, I. Spartathlon, a 246 kilometer foot race: Effects of acute inflammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol. Dis. 2009, 42, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Song, M.; Tang, Y.; Cao, K.; Qi, L.; Xie, K. Unveiling the role of interleukin-6 in pancreatic cancer occurrence and progression. Front. Endocrinol. 2024, 15, 1408312. [Google Scholar] [CrossRef]
- Poulia, K.A.; Sarantis, P.; Antoniadou, D.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic Cancer and Cachexia-Metabolic Mechanisms and Novel Insights. Nutrients 2020, 12, 1543. [Google Scholar] [CrossRef]
- van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin. Sci. 2020, 134, 2091–2115. [Google Scholar] [CrossRef]
- Croft, L.; Bartlett, J.D.; MacLaren, D.P.; Reilly, T.; Evans, L.; Mattey, D.L.; Nixon, N.B.; Drust, B.; Morton, J.P. High-intensity interval training attenuates the exercise-induced increase in plasma IL-6 in response to acute exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 1098–1107. [Google Scholar] [CrossRef]
- Zheng, G.; Qiu, P.; Xia, R.; Lin, H.; Ye, B.; Tao, J.; Chen, L. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Front. Aging Neurosci. 2019, 11, 98. [Google Scholar] [CrossRef]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabák, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical activity and inflammatory markers over 10 years: Follow-up in men and women from the Whitehall II cohort study. Circulation 2012, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Brüning, J.; et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef]
- Wang, J.; Chang, C.Y.; Yang, X.; Zhou, F.; Liu, J.; Feng, Z.; Hu, W. Leukemia inhibitory factor, a double-edged sword with therapeutic implications in human diseases. Mol. Ther. 2023, 31, 331–343. [Google Scholar] [CrossRef]
- Zeng, R.; Tong, C.; Xiong, X. The Molecular Basis and Therapeutic Potential of Leukemia Inhibitory Factor in Cancer Cachexia. Cancers 2022, 14, 2955. [Google Scholar] [CrossRef]
- Seto, D.N.; Kandarian, S.C.; Jackman, R.W. A Key Role for Leukemia Inhibitory Factor in C26 Cancer Cachexia. J. Biol. Chem. 2015, 290, 19976–19986. [Google Scholar] [CrossRef]
- Broholm, C.; Pedersen, B.K. Leukaemia inhibitory factor--an exercise-induced myokine. Exerc. Immunol. Rev. 2010, 16, 77–85. [Google Scholar] [PubMed]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Scheele, C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 2011, 111, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Broholm, C.; Brandt, C.; Schultz, N.S.; Nielsen, A.R.; Pedersen, B.K.; Scheele, C. Deficient leukemia inhibitory factor signaling in muscle precursor cells from patients with type 2 diabetes. Am. J. Physiol.—Endocrinol. Metab. 2012, 303, E283–E292. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Chang, C.-Y.; Zhou, F.; Liu, J.; Xu, H.; Ibrahim, M.; Gomez, M.; Guo, G.L.; Liu, H.; et al. Leukemia inhibitory factor suppresses hepatic de novo lipogenesis and induces cachexia in mice. Nat. Commun. 2024, 15, 627. [Google Scholar] [CrossRef]
- Agca, S.; Kir, S. The role of interleukin-6 family cytokines in cancer cachexia. FEBS J. 2024, 291, 4009–4023. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2022, 106, 181–234. [Google Scholar]
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef]
- Parfenova, O.K.; Kukes, V.G.; Grishin, D.V. Follistatin-Like Proteins: Structure, Functions and Biomedical Importance. Biomedicines 2021, 9, 999. [Google Scholar] [CrossRef]
- Suh, J.; Lee, Y.S. Myostatin Inhibitors: Panacea or Predicament for Musculoskeletal Disorders? J. Bone Metab. 2020, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lehar, A.; Meir, J.U.; Koch, C.; Morgan, A.; Warren, L.E.; Rydzik, R.; Youngstrom, D.W.; Chandok, H.; George, J.; et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl. Acad. Sci. USA 2020, 117, 23942–23951. [Google Scholar] [CrossRef] [PubMed]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, I.; Sanjaya, A.; Lesmana, R.; Yen, P.M.; Goenawan, H. Hippo pathway effectors YAP and TAZ and their association with skeletal muscle ageing. J. Physiol. Biochem. 2021, 77, 63–73. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Watanabe, A.; Shiratori, T.; Kaku, R.; Ueda, K.; Okamoto, K.; Kataoka, Y.; Ohshio, Y.; Hanaoka, J. Myostatin expression in lung cancer induces sarcopenia and promotes cancer progression. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 232–239. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Cardaci, T.D.; Machek, S.B.; Wilburn, D.T.; Heileson, J.L. Resistance Exercise-Induced Increases in Muscle Myostatin mRNA and Protein Expression Are Subsequently Decreased in Circulation in the Presence of Increased Levels of the Extracellular Matrix Stabilizing Protein Decorin. J. Sports Sci. Med. 2022, 21, 616–624. [Google Scholar] [CrossRef]
- Khalafi, M.; Aria, B.; Symonds, M.E.; Rosenkranz, S.K. The effects of resistance training on myostatin and follistatin in adults: A systematic review and meta-analysis. Physiol. Behav. 2023, 269, 114272. [Google Scholar] [CrossRef]
- Ayaz, E.Y.; Dincer, B.; Cinbaz, G.; Karacan, E.; Benli, R.K.; Mete, E.; Bilgiç, H.; Mesci, B. The Effect of Exercise on Spexin and Follistatin in Elderly Individuals. J. Cachexia Sarcopenia Muscle 2025, 16, e13692. [Google Scholar] [CrossRef]
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology 2011, 152, 164–171. [Google Scholar] [CrossRef]
- Korzun, T.; Moses, A.S.; Kim, J.; Patel, S.; Schumann, C.; Levasseur, P.R.; Diba, P.; Olson, B.; Rebola, K.G.O.; Norgard, M.; et al. Nanoparticle-Based Follistatin Messenger RNA Therapy for Reprogramming Metastatic Ovarian Cancer and Ameliorating Cancer-Associated Cachexia. Small 2022, 18, e2204436. [Google Scholar] [CrossRef]
- Ataeinosrat, A.; Saeidi, A.; Abednatanzi, H.; Rahmani, H.; Daloii, A.A.; Pashaei, Z.; Hojati, V.; Basati, G.; Mossayebi, A.; Laher, I.; et al. Intensity Dependent Effects of Interval Resistance Training on Myokines and Cardiovascular Risk Factors in Males With Obesity. Front. Endocrinol. 2022, 13, 895512. [Google Scholar] [CrossRef] [PubMed]
- Broniec, M.N.; Norland, K.; Thomas, J.; Wang, X.; Harris, R.A. The decorin and myostatin response to acute whole body vibration: Impact of adiposity, sex, and race. Int. J. Obes. 2024, 48, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kishioka, Y.; Wakamatsu, J.-I.; Hattori, A.; Hennebry, A.; Berry, C.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin Binds Myostatin and Modulates Its Activity to Muscle Cells. Biochem. Biophys. Res. Commun. 2006, 340, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mondal, D.K.; Ulas, M.; Neill, T.; Iozzo, R.V. Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways. Am. J. Physiol. Cell Physiol. 2022, 322, C554–C566. [Google Scholar] [CrossRef]
- Appunni, S.; Saxena, A.; Ramamoorthy, V.; Zhang, Y.; Doke, M.; Nair, S.S.; Khosla, A.A.; Rubens, M. Decorin: Matrix-based pan-cancer tumor suppressor. Mol. Cell Biochem. 2025, 480, 3569–3591. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Yoshio, S.; Sakamoto, Y.; Hashida, R.; Koya, S.; Hirota, K.; Nakano, D.; Yamamura, S.; Niizeki, T.; Matsuse, H.; et al. Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma. J. Clin. Med. 2020, 9, 936. [Google Scholar] [CrossRef]
- Xu, W.; Neill, T.; Yang, Y.; Hu, Z.; Cleveland, E.; Wu, Y.; Hutten, R.; Xiao, X.; Stock, S.R.; Shevrin, D.; et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther. 2015, 22, 247–256. [Google Scholar] [CrossRef]
- Adams, G.R. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J. Appl. Physiol. 2002, 93, 1159–1167. [Google Scholar] [CrossRef]
- Frystyk, J. Exercise and the Growth Hormone-Insulin-Like Growth Factor Axis. Med. Sci. Sports Exerc. 2010, 42, 58–66. [Google Scholar] [CrossRef]
- Philippou, A.; Maridaki, M.; Halapas, A.; Koutsilieris, M. The role of the insulin-like growth factor 1 (IGF-1) in skeletal muscle physiology. In Vivo 2007, 21, 45–54. [Google Scholar]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Moore, D.R.; McKay, B.R.; Tarnopolsky, M.A.; Parise, G. Blunted satellite cell response is associated with dysregulated IGF-1 expression after exercise with age. Eur. J. Appl. Physiol. 2018, 118, 2225–2231. [Google Scholar] [CrossRef]
- Philippou, A.; Papageorgiou, E.; Bogdanis, G.; Halapas, A.; Sourla, A.; Maridaki, M.; Pissimissis, N.; Koutsilieris, M. Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: Characterization of the MGF E peptide actions in vitro. In Vivo 2009, 23, 567–575. [Google Scholar] [PubMed]
- Yang, S.; Alnaqeeb, M.; Simpson, H.; Goldspink, G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J. Muscle Res. Cell Motil. 1996, 17, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Rosen, C.J. The insulin-like growth factor system in bone: Basic and clinical implications. Endocrinol. Metab. Clin. N. Am. 2012, 41, 323–333. [Google Scholar] [CrossRef] [PubMed]
- de Alcantara Borba, D.; da Silva Alves, E.; Rosa, J.P.P.; Facundo, L.A.; Costa, C.M.A.; Silva, A.C.; Narciso, F.V.; Silva, A.; de Mello, M.T. Can IGF-1 Serum Levels Really be Changed by Acute Physical Exercise? A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2020, 17, 575–584. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Hyoung Park, J.; Kaipparettu, B.A.; Putluri, N.; et al. Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab. 2019, 30, 261–273.e6. [Google Scholar] [CrossRef]
- Dreher, S.I.; Grubba, P.; von Toerne, C.; Moruzzi, A.; Maurer, J.; Goj, T.; Birkenfeld, A.L.; Peter, A.; Loskill, P.; Hauck, S.M.; et al. IGF1 promotes human myotube differentiation toward a mature metabolic and contractile phenotype. Am. J. Physiol.—Cell Physiol. 2024, 326, C1462–C1481. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Costelli, P.; Muscaritoli, M.; Bossola, M.; Penna, F.; Reffo, P.; Bonetto, A.; Busquets, S.; Bonelli, G.; Lopez-Soriano, F.J.; Doglietto, G.B.; et al. IGF-1 is downregulated in experimental cancer cachexia. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2006, 291, R674–R683. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; von Haehling, S.; Doehner, W.; Palus, S.; Anker, S.D.; Springer, J. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Maleki, A.H.; Symonds, M.E.; Sakhaei, M.H.; Rosenkranz, S.K.; Ehsanifar, M.; Korivi, M.; Liu, Y. Interleukin-15 responses to acute and chronic exercise in adults: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1288537. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Pedersen, B.K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.Y.; Oh, S.L.; Kim, Y.A.; So, B.; Seong, J.K.; Song, W. Effect of treadmill exercise on interleukin-15 expression and glucose tolerance in zucker diabetic Fatty rats. Diabetes Metab. J. 2013, 37, 358–364. [Google Scholar] [CrossRef]
- Martínez-Hernández, P.L.; Hernanz-Macías, Á.; Gómez-Candela, C.; Grande-Aragón, C.; Feliu-Batlle, J.; Castro-Carpeño, J.; Martínez-Muñoz, I.; Zurita-Rosa, L.; Villarino-Sanz, M.; Prados-Sánchez, C.; et al. Serum interleukin-15 levels in cancer patients with cachexia. Oncol. Rep. 2012, 28, 1443–1452. [Google Scholar] [CrossRef]
- Carbó, N.; López-Soriano, J.; Costelli, P.; Busquets, S.; Alvarez, B.; Baccino, F.M.; Quinn, L.S.; López-Soriano, F.J.; Argilés, J.M. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br. J. Cancer 2000, 83, 526–531. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, Y.; Qin, M.; Yi, X. Interleukin 15: A new intermediary in the effects of exercise and training on skeletal muscle and bone function. J. Cell Mol. Med. 2024, 28, e70136. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Us Altay, D.; Keha, E.E.; Ozer Yaman, S.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the expression of irisin and some cachectic factors in mice with experimentally induced gastric cancer. Qjm 2016, 109, 785–790. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2019, 178, 507–508. [Google Scholar] [CrossRef]
- Graca, F.A.; Rai, M.; Hunt, L.C.; Stephan, A.; Wang, Y.-D.; Gordon, B.; Wang, R.; Quarato, G.; Xu, B.; Fan, Y.; et al. The myokine Fibcd1 is an endogenous determinant of myofiber size and mitigates cancer-induced myofiber atrophy. Nat. Commun. 2022, 13, 2370. [Google Scholar] [CrossRef]
- Luo, J.; Liu, W.; Feng, F.; Chen, L. Apelin/APJ system: A novel therapeutic target for locomotor system diseases. Eur. J. Pharmacol. 2021, 906, 174286. [Google Scholar] [CrossRef] [PubMed]
- Ligetvári, R.; Szokodi, I.; Far, G.; Csöndör, É.; Móra, Á.; Komka, Z.; Tóth, M.; Oláh, A.; Ács, P. Apelin as a Potential Regulator of Peak Athletic Performance. Int. J. Mol. Sci. 2023, 24, 8195. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Zhao, L.; Chen, Y.; Chen, K.; Chae, S.A.; de Avila, J.M.; Wang, H.; Zhu, M.J.; Jiang, Z.; Du, M. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci. Adv. 2020, 6, eaaz0359. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, M.S. FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases. Diabetes Metab. J. 2014, 38, 245–251. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Meza-Arana, C.E.; Brito-Cordova, G.; Gomez-Perez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Jin, L.; Geng, L.; Ying, L.; Lingling, S.; Ye, K.; Yang, R.; Liu, Y.; Wang, Y.; Cai, Y.; Jiang, X.; et al. FGF21-Sirtuin 3 Axis Confers the Protective Effects of Exercise Against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity. Circulation 2022, 146, 1537–1557. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Zong, Y.; He, Y.; Yang, G.; Xiao, Y.; Wang, S. The effects of exercise on FGF21 in adults: A systematic review and meta-analysis. PeerJ 2024, 12, e17615. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.; Ost, M.; Otten, L.; Herpich, C.; Coleman, V.; Endres, A.-S.; Klaus, S.; Müller-Werdan, U.; Norman, K. Higher serum levels of fibroblast growth factor 21 in old patients with cachexia. Nutrition 2019, 63, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T.; et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 2021, 12, 1041. [Google Scholar] [CrossRef]
- Klein, A.B.; Kleinert, M.; Richter, E.A.; Clemmensen, C. GDF15 in Appetite and Exercise: Essential Player or Coincidental Bystander? Endocrinology 2022, 163, bqab242. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, J.; Ding, F.; Ma, L. Role of growth differentiation factor 15 in cancer cachexia (Review). Oncol. Lett. 2023, 26, 462. [Google Scholar] [CrossRef]
- Ramos, C.C.; Pires, J.; Gonzalez, E.; Garcia-Vallicrosa, C.; Reis, C.A.; Falcon-Perez, J.M.; Freitas, D. Extracellular vesicles in tumor-adipose tissue crosstalk: Key drivers and therapeutic targets in cancer cachexia. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 371–396. [Google Scholar] [CrossRef]
- Greenhill, C. Osteocalcin in the adaptation to exercise. Nat. Rev. Endocrinol. 2016, 12, 434. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Onorato, F.; Mastrogregori, A.; Rossi, D.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Role of Physical Activity in Bone-Muscle Crosstalk: Biological Aspects and Clinical Implications. J. Funct. Morphol. Kinesiol. 2021, 6, 55. [Google Scholar] [CrossRef]
- Mohammad Rahimi, G.R.; Niyazi, A.; Alaee, S. The effect of exercise training on osteocalcin, adipocytokines, and insulin resistance: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2021, 32, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.R.; Nederveen, J.P.; Fortino, S.A.; Snijders, T.; Joanisse, S.; Kumbhare, D.A.; Parise, G. Brain-derived neurotrophic factor is associated with human muscle satellite cell differentiation in response to muscle-damaging exercise. Appl. Physiol. Nutr. Metab. 2020, 45, 581–590. [Google Scholar] [CrossRef]
- Yu, T.; Chang, Y.; Gao, X.L.; Li, H.; Zhao, P. Dynamic Expression and the Role of BDNF in Exercise-induced Skeletal Muscle Regeneration. Int. J. Sports Med. 2017, 38, 959–966. [Google Scholar] [CrossRef]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerstrom, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Chan, W.S.; Ng, C.F.; Pang, B.P.S.; Hang, M.; Tse, M.C.L.; Iu, E.C.Y.; Ooi, X.C.; Yang, X.; Kim, J.K.; Lee, C.W.; et al. Exercise-induced BDNF promotes PPARδ-dependent reprogramming of lipid metabolism in skeletal muscle during exercise recovery. Sci. Signal 2024, 17, eadh2783. [Google Scholar] [CrossRef]
- Chang, H.; Kwon, O.; Shin, M.-S.; Kang, G.M.; Leem, Y.H.; Lee, C.H.; Kim, S.J.; Roh, E.; Kim, H.-K.; Youn, B.-S.; et al. Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation. J. Appl. Physiol. 2018, 125, 715–722. [Google Scholar] [CrossRef]
- Ingerslev, B.; Schiøler Hansen, J.; Hoffmann, C.; Clemmesen, J.; Secher, N.; Scheler, M.; Angelis, M.; Häring, H.; Pedersen, B.; Weigert, C.; et al. Angiopoietin-like Protein 4 Is an Exercise-induced Hepatokine in Humans, Regulated by Glucagon and cAMP. Mol. Metab. 2017, 6, 1286–1295. [Google Scholar] [CrossRef]
- Neto, N.I.P.; Boldarine, V.T.; Hachul, A.C.L.; Oyama, L.M.; Lima, J.D.C.C.; Fernandez, E.S.; Otoch, J.P.; de Alcântara, P.S.M.; Tokeshi, F.; Seelaender, M.C.L.; et al. Association between ANGPTL-4 and the proinflammatory process in cancer cachexia patients. Oncotarget 2019, 10, 6444–6455. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Qiu, Y.; Sun, D.; Liu, Y.; Li, Z.; Zhou, B.; Zhang, H.; Xiao, Y.; Wu, G.; et al. Single-cell sequencing unveils key contributions of immune cell populations in cancer-associated adipose wasting. Cell Discov. 2022, 8, 122. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, S.E.; Lee, P.; Lee, J.H.; Jung, K.H.; Hong, S.S. Potential role of ANGPTL4 in cancer progression, metastasis, and metabolism: A brief review. BMB Rep. 2024, 57, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Foo, B.J.W.; Kwok, K.W.; Sakamoto, N.; Mukae, H.; Izumikawa, K.; Mandard, S.; Quenot, J.P.; Lagrost, L.; Teh, W.K.; et al. Antibody Treatment against Angiopoietin-Like 4 Reduces Pulmonary Edema and Injury in Secondary Pneumococcal Pneumonia. mBio 2019, 10, e02469-18. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; He, Z.; Chen, Y.; Dai, L. Dual role of ANGPTL4 in inflammation. Inflamm. Res. 2023, 72, 1303–1313. [Google Scholar] [CrossRef]
- Mandadzhiev, N. The contemporary role of lactate in exercise physiology and exercise prescription—A review of the literature. Folia Medica 2025, 67, e144693. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Cui, Q.; Guo, B.; Ding, W.; Liu, J.; Quan, L.; Li, X.; Xie, P.; Jin, L.; et al. Activation of GPR81 by lactate drives tumour-induced cachexia. Nat. Metab. 2024, 6, 708–723. [Google Scholar] [CrossRef]
- Maurer, J.; Hoene, M.; Weigert, C. Signals from the Circle: Tricarboxylic Acid Cycle Intermediates as Myometabokines. Metabolites 2021, 11, 474. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, D.; Wang, H.; Huang, L.; Gao, X.; Maitiabula, G.; Zhang, L.; Wang, X. Succinate Regulates Exercise-Induced Muscle Remodelling by Boosting Satellite Cell Differentiation Through Succinate Receptor 1. J. Cachexia Sarcopenia Muscle 2025, 16, e13670. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Walzik, D.; Metcalfe, A.J.; Bloch, W.; Zimmer, P. Physical Exercise as Kynurenine Pathway Modulator in Chronic Diseases: Implications for Immune and Energy Homeostasis. Int. J. Tryptophan Res. 2020, 13, 1178646920938688. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Schmidt, M.E.; Prentzell, M.T.; Berdel, B.; Wiskemann, J.; Kellner, K.H.; Debus, J.; Ulrich, C.; Opitz, C.A.; Steindorf, K. Resistance Exercise Reduces Kynurenine Pathway Metabolites in Breast Cancer Patients Undergoing Radiotherapy. Front. Oncol. 2019, 9, 962. [Google Scholar] [CrossRef]
- Saran, T.; Turska, M.; Kocki, T.; Zawadka, M.; Zieliński, G.; Turski, W.A.; Gawda, P. Effect of 4-week physical exercises on tryptophan, kynurenine and kynurenic acid content in human sweat. Sci. Rep. 2021, 11, 11092. [Google Scholar] [CrossRef]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.S.; Chiang, N.Y.; Darlington, L.G.; Williams, R.O. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef]
- Yin, X.; Chen, Y.; Ruze, R.; Xu, R.; Song, J.; Wang, C.; Xu, Q. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Signal Transduct. Target. Ther. 2022, 7, 324. [Google Scholar] [CrossRef]
- Gurup, A.; Yakal, S.; Tarçın, G.; Şahinler Ayla, S.; Turan, H.; Toprak, M.S.; Gungor, Z.B.; Ercan, O. Effect of acute exercise on 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) levels in obese male adolescents. Clin. Endocrinol. 2023, 99, 174–181. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Chen, Y. Recruitment of Thermogenic Fat: Trigger of Fat Burning. Front. Endocrinol. 2021, 12, 696505. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J. Longterm exercise-derived exosomal miR-342-5p: A novel exerkine for cardioprotection. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef]
- Doncheva, A.I.; Romero, S.; Ramirez-Garrastacho, M.; Lee, S.; Kolnes, K.J.; Tangen, D.S.; Olsen, T.; Drevon, C.A.; Llorente, A.; Dalen, K.T. Extracellular vesicles and microRNAs are altered in response to exercise, insulin sensitivity and overweight. Acta Physiol. 2022, 236, e13862. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S. Extracellular vesicles in cancer cachexia: Deciphering pathogenic roles and exploring therapeutic horizons. J. Transl. Med. 2024, 22, 506. [Google Scholar] [CrossRef]
- Hu, W.; Xiong, H.; Ru, Z.; Zhao, Y.; Zhou, Y.; Xie, K.; Xiao, W.; Xiong, Z.; Wang, C.; Yuan, C.; et al. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 2021, 12, 134. [Google Scholar] [CrossRef]
- Marzan, A.L.; Chitti, S.V. Unravelling the Role of Cancer Cell-Derived Extracellular Vesicles in Muscle Atrophy, Lipolysis, and Cancer-Associated Cachexia. Cells 2023, 12, 2598. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, V.; Barone, R.; Trovato, E.; D’Amico, D.; Macaluso, F.; Campanella, C.; Marino Gammazza, A.; Muccilli, V.; Cunsolo, V.; Cancemi, P.; et al. Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia. Cells 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yang, Y.; Wu, Z.; Tan, R.; Pham, T.T.; Yeo, E.Y.M.; Pirisinu, M.; Jayasinghe, M.K.; Pham, T.C.; Liang, K.; et al. Red blood cell extracellular vesicles deliver therapeutic siRNAs to skeletal muscles for treatment of cancer cachexia. Mol. Ther. 2023, 31, 1418–1436. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Bland, K.A.; Krishnasamy, M.; Parr, E.B.; Mulder, S.; Martin, P.; van Loon, L.J.C.; Cormie, P.; Michael, N.; Zopf, E.M. “I want to get myself as fit as I can and not die just yet”—Perceptions of exercise in people with advanced cancer and cachexia: A qualitative study. BMC Palliat. Care 2022, 21, 75. [Google Scholar] [CrossRef]
- Moreira, V.M.; da Silva Franco, C.C.; Prates, K.V.; Gomes, R.M.; de Moraes, A.M.P.; Ribeiro, T.A.; Martins, I.P.; Previate, C.; Pavanello, A.; Matiusso, C.C.I.; et al. Aerobic Exercise Training Attenuates Tumor Growth and Reduces Insulin Secretion in Walker 256 Tumor-Bearing Rats. Front. Physiol. 2018, 9, 465. [Google Scholar] [CrossRef]
- Niels, T.; Tomanek, A.; Freitag, N.; Schumann, M. Can Exercise Counteract Cancer Cachexia? A Systematic Literature Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19, 1534735420940414. [Google Scholar] [CrossRef]
- Orange, S.T.; Leslie, J.; Ross, M.; Mann, D.A.; Wackerhage, H. The exercise IL-6 enigma in cancer. Trends Endocrinol. Metab. 2023, 34, 749–763. [Google Scholar] [CrossRef]
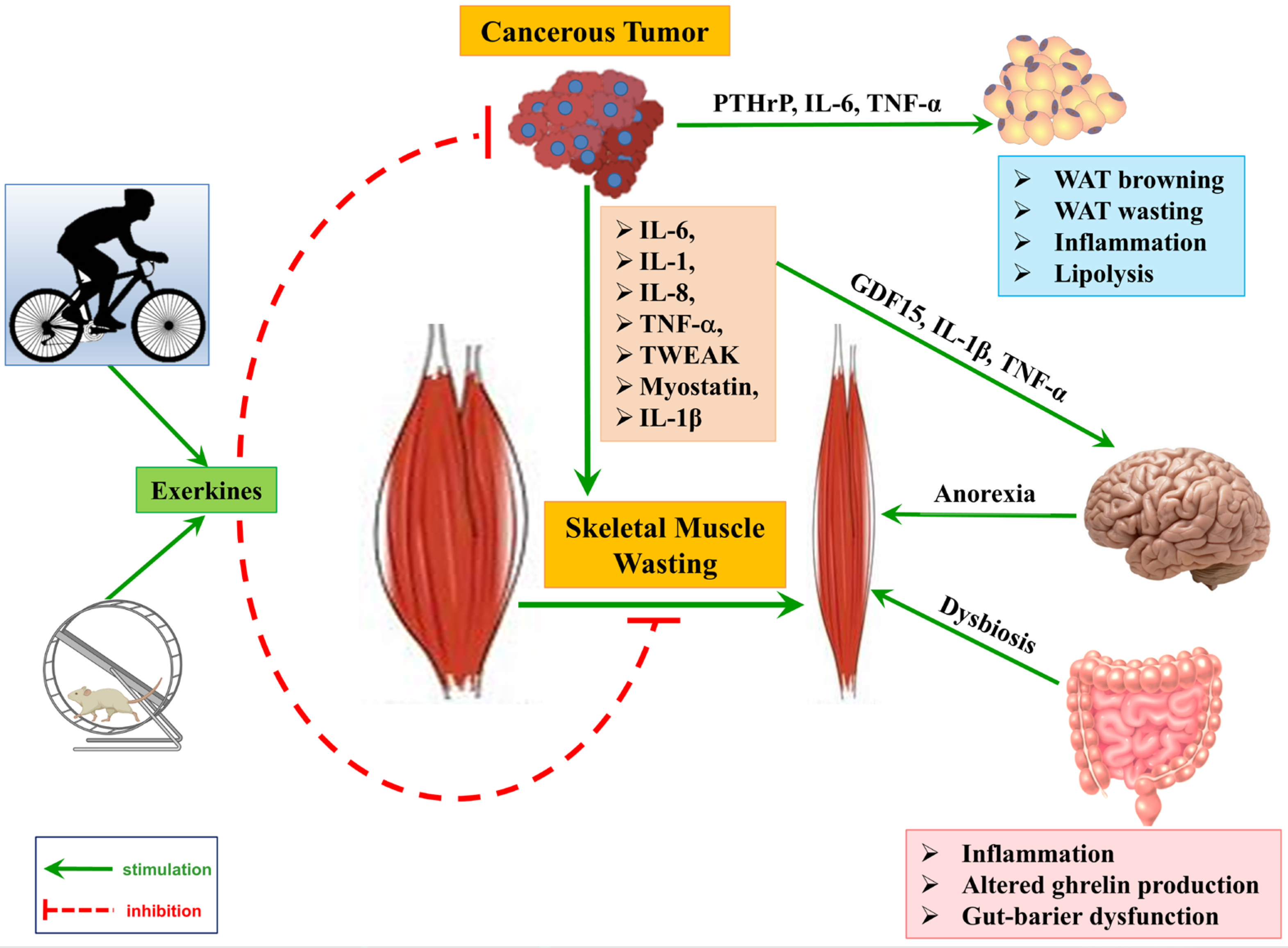
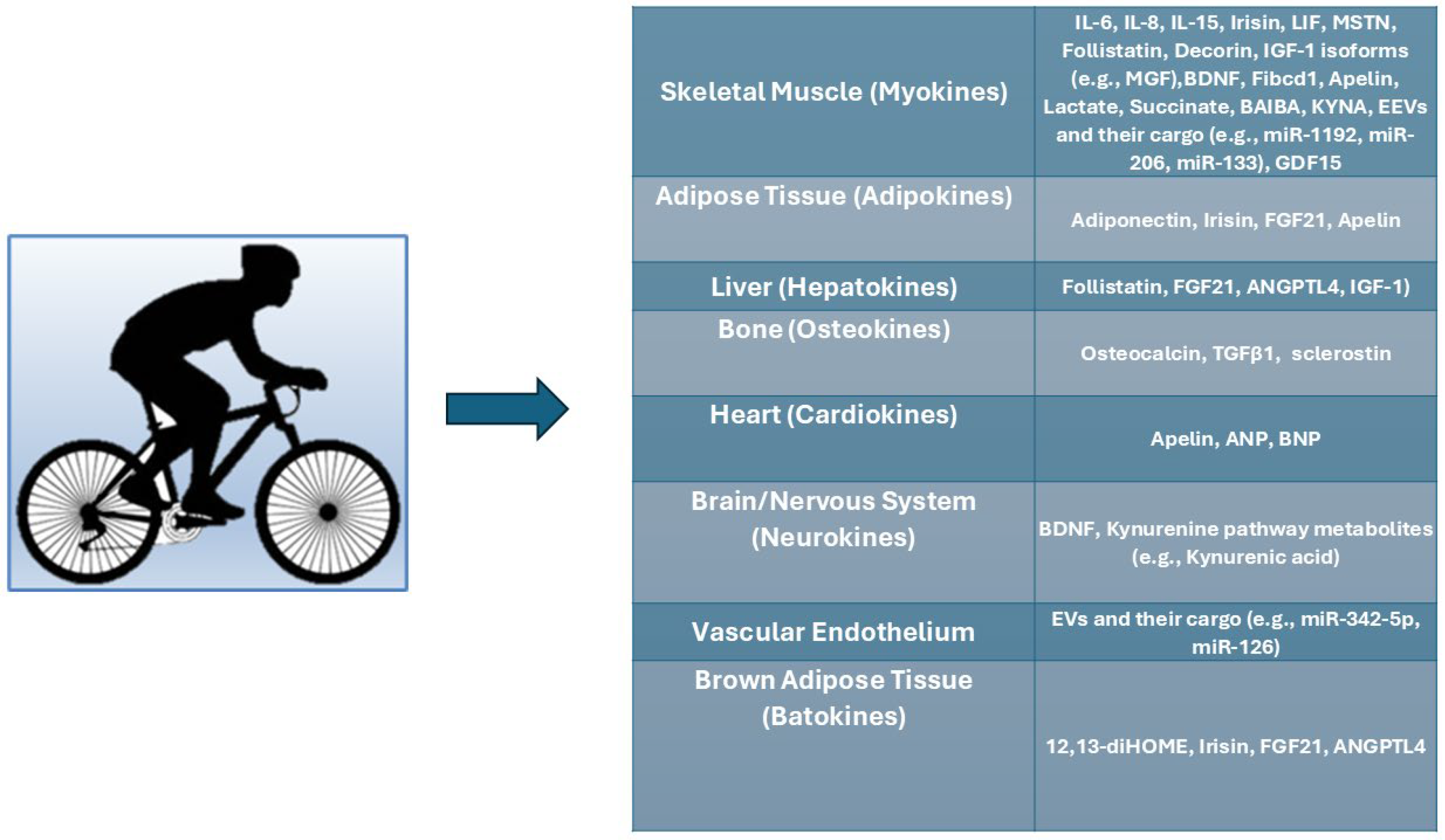
| Characteristic | Description | Underlying Mechanism |
|---|---|---|
| Weight Loss | Unintentional and progressive body weight loss. | Negative energy balance; increased resting energy expenditure; tumor metabolism. Not reversed by nutritional support alone. |
| Muscle Wasting (Skeletal & Cardiac) | Significant, involuntary loss of skeletal muscle mass; affects locomotion, respiratory muscles, and myocardium. | Cytokine excess; altered protein metabolism (proteolysis, reduced synthesis); ubiquitin-proteasome system (UPS) activation; calpain activation. Not reversed by nutritional support alone. |
| Fat Loss | Often accompanies muscle loss, but can occur independently. | Increased lipolysis; adipose tissue browning; tumor-secreted microRNAs. Not reversed by nutritional support alone |
| Anorexia | Lack of hunger, disinterest in eating; distinct from anorexia nervosa. | Inflammatory mediators (cytokines) inducing satiety; digestive factors (nausea, dysgeusia). Limited improvement with nutritional interventions alone. |
| Fatigue & Weakness | Extreme exhaustion, poor tolerance to activity; impacts daily tasks and self-care. | Muscle loss; altered energy metabolism; increased symptom burden (pain, sleep disturbances). A multimodal approach is needed. |
| Systemic Inflammation | Chronic, widespread inflammation throughout the body. | Host cytokines (TNF-α, IL-6, IL-1β); tumor-derived factors; acute phase response. Central driver, often self-perpetuating. |
| Insulin Resistance | Muscles and fat cells do not respond properly to insulin. | Tumor-secreted factors (e.g., ImpL2); impaired anabolic response. Contributes to muscle loss. |
| Increased Protein Turnover | Proteins break down too rapidly to be replaced. | Catabolic state; reduced anabolic hormones (IGF-1, testosterone, ghrelin). Leads to net protein loss |
| Quality of Life Reduction | Increased severity of pain, dry mouth, vomiting, dysgeusia, early satiety, sleep disturbances, anxiety. | Significantly impaired. Direct and indirect effects of the syndrome. |
| Reduced Therapy Tolerance | Patients too weak for effective chemotherapies and radiotherapies. | Overall debilitation and poor clinical parameters. Major clinical dilemma. |
| Increased Mortality | Accounts for 20–25% of all cancer-related deaths; powerful predictor of poor survival. | Impaired vital organ function (heart, respiratory muscles); systemic decline. |
| Exerkine | Primary Source(s) | Exercise Response (Acute/Chronic) | Cachexia-Relevant Findings | Mechanisms/Pathways | Context-Dependent Notes |
|---|---|---|---|---|---|
| IL-6 | Skeletal muscle (myokine); also immune cells | Acute ↑ markedly; chronic training lowers basal IL-6 | Chronic tumor-driven IL-6/trans-signaling promotes wasting; exercise-induced IL-6 supports metabolism | JAK/STAT3; classical vs. trans-signaling; immune cell redistribution | Beneficial when transient post-exercise; harmful when persistently elevated from tumors/systemic inflammation |
| IL-8 | Skeletal muscle; immune cells | Acute ↑ | Indirect; pro-angiogenic/immune roles; limited direct cachexia data | Chemokine signaling | General inflammation vs. reparative angiogenesis may diverge by context |
| IL-10 | Immune cells; possibly muscle-modulated | Acute ↑ with exercise-induced anti-inflammatory milieu | Anti-inflammatory; limited direct cachexia data | Cytokine anti-inflammatory signaling | Anti-inflammatory post-exercise; systemic deficiency/excess may alter outcomes |
| IL-15 | Skeletal muscle (myokine) | Acute ↑; training effects vary | Lower serum in cachectic cancer reported in some cohorts; IL-15 antagonizes tumor-induced muscle protein loss in rats | Anabolic/anti-atrophy; glucose handling | Local muscle effects beneficial; systemic disease may blunt signaling |
| IGF-1 (hepatic + muscle isoforms) | Liver (endocrine); skeletal muscle isoforms (autocrine/paracrine) | Acute ↑ (circulating) variably; muscle IGF-1Ea/Ec upregulated post-exercise | IGF-1 suppressed in experimental cachexia; IGF-1 treatment attenuates lean mass loss in rats | PI3K/Akt/mTOR; inhibits FoxO-E3 ligases; supports satellite cells | Exercise restores local IGF-1 pools; systemic deficiency in cachexia limits anabolism |
| Myostatin (GDF8) | Skeletal muscle; also tumors | Decreases with resistance/endurance training | Often elevated in cachexia; tumor-derived myostatin correlates with muscle loss and worse survival | ActRIIB/Smad2/3; inhibits protein synthesis, promotes proteolysis | Exercise-lowered myostatin is beneficial; tumor or chronic elevation is catabolic |
| Follistatin | Liver and skeletal muscle (hepatokine/myokine) | Plasma ↑ after exercise; training ↑ | Reduced in cachexia; neutralizes myostatin/activin A; FST mRNA therapy preserves muscle and survival in models | Binds myostatin/activin; activates Akt-mTOR | Generally protective; deficit favors wasting |
| Decorin | Skeletal muscle (ECM proteoglycan/myokine) | ↑ after resistance, HIIT; released from contracting myotubes | Binds myostatin; anti-inflammatory; onco-suppressive; Ad.dcn mitigates cachexia in mice | Sequesters TGF-β/myostatin; modulates RTKs; reduces E3 ligases | Exercise-induced decorin supports hypertrophy; deficits may permit atrophy |
| Irisin (FNDC5) | Skeletal muscle; adipose expression changes | ↑ with acute/chronic exercise | Conflicting in cancer: higher with cachexia in some data; lower in specific cancers; increases bone mass in mice | Browning; αV-integrin signaling in bone; metabolic modulation | Exercise-driven pulses beneficial; tumor/systemic disease may dysregulate levels |
| Fibcd1 | Skeletal muscle (cleaved ectodomain) | Exercise-regulated myokine (preclinical) | Recombinant Fibcd1 reduces cancer-induced myofiber atrophy in mice | Myofiber size regulation (receptor engagement on muscle) | Therapeutic replacement shows benefit without stimulating tumor growth (preclinical) |
| Apelin | Skeletal muscle; endothelium; multiple organs | ↑ with exercise (muscle/endothelium) | Higher serum in gastroesophageal cancer (esp. cachectic) without severity correlation | APJ signaling; improves muscle metabolism | Generally beneficial post-exercise; tumor/systemic elevations may be maladaptive |
| FGF21 | Liver, adipose, muscle | Acute ↑; chronic training may ↓ in metabolic disease | Elevated in cachectic patients; muscle overexpression induces autophagy and muscle loss in mice | FGFR1/β-Klotho; mitochondrial integrity; adipose receptor upregulation with exercise | Physiological pulses adaptive; chronic elevation linked to atrophy |
| GDF15 | Stress-responsive, multiple tissues | Acute intense exercise ↑ transiently | Elevated in cancer cachexia; suppresses appetite and directly induces muscle wasting; neutralization reverses weight loss in models | MAP3K11; Bcl-2/caspase-3; brain appetite centers | Transient exercise spikes vs. chronic tumor-driven elevation (harmful) |
| Osteocalcin (OC) | Bone (osteoblasts) | ↑ after acute and chronic exercise | No direct data in cancer cachexia; supports muscle function; osteocalcin-IL-6 axis during exercise | UcOC endocrine effects; bone-muscle crosstalk | Beneficial exercise hormone; cachexia role unknown |
| BDNF | Skeletal muscle and brain | ↑ with exercise (esp. resistance) | No direct cachexia data; supports muscle regeneration and metabolism | Satellite cell activation; AMPK–PGC1α | Likely beneficial with training; deficits may impair repair |
| Cathepsin B | Skeletal muscle | ↑ with exercise; crosses BBB (preclinical) | Neurogenic benefits; cachexia link indirect | Neurogenesis support | Physiological signals beneficial; chronic disease context uncertain |
| Natriuretic peptides (ANP/BNP) | Heart (cardiokines) | ↑ with cardiac load | Mobilize fat; unclear direct cachexia effects | Lipolysis; BAT activation | Exercise cardiometabolic benefits; disease elevations may reflect cardiac stress |
| ANGPTL4 | Liver (systemic during exercise), muscle (local) | Acute ↑ (hepatic dominates systemic) | Chronically elevated in tumors; associates with inflammation, fat wasting; neutralization beneficial in models | Lipid trafficking; endothelial permeability; inflammatory signaling | Short-lived exercise elevations coordinate fuel use; chronic tumor secretion is catabolic |
| Adiponectin (adipokine) | Adipose tissue | Chronic training ↑; context dependent | AdipoRon (AdipoR agonist) and rosiglitazone-mediated restoration ameliorate cachexia in mice | Anti-inflammatory; metabolic remodeling | Exercise-raised adiponectin generally protective; tumor context varies |
| Leukemia Inhibitory Factor (LIF) | Skeletal muscle, immune cells, tumor cells | ↑ in some cancers; exercise regulation unclear but animal data suggest muscle LIF release during intense contraction | Elevated in certain tumor types contributes to muscle wasting; in muscle, acute release may promote satellite cell activation | JAK/STAT3 signaling; inflammation and muscle regeneration pathways | Beneficial acute myokine role in regeneration; chronic tumor-driven secretion linked to cachexia |
| Exerkine | Primary Source(s) | Exercise Response (Acute/Chronic) | Cachexia-Relevant Findings | Mechanisms/Pathways | Context-Dependent Notes |
|---|---|---|---|---|---|
| Lactate (“lactomone”) | Working muscle | Acute ↑ with intensity | Signals metabolism; affects adipose lipolysis and brain appetite centers; direct cachexia data limited | Receptor-mediated signaling; Cori cycle; metabolic reprogramming | Physiological spikes beneficial; chronic tumor lactate may be maladaptive |
| Succinate (TCA dicarboxylate) | Muscle metabolism | Acute ↑; myometabokine | Regulates myokine secretion; direct cachexia data limited | Receptor (SUCNR1) signaling; paracrine crosstalk | Adaptive during exercise; disease-state accumulation may differ |
| BAIBA (β-aminoisobutyric acid) | Muscle (via PGC-1α) | ↑ with training | Protects bone cells from oxidative stress; metabolic benefits; cachexia data limited | Browning; oxidative stress defense | Likely beneficial in physiological ranges |
| 12,13-diHOME (lipokine) | Brown/white adipose; muscle-adipose axis | Acute ↑ after exercise | Promotes fatty acid uptake/oxidation; direct cachexia evidence limited | Lipid transport/oxidation | Supports exercise fuel handling; role in cachexia unknown |
| Kynurenine pathway (Kyn → KYNA vs. QUIN) | Muscle PGC-1α1 drives KATs; systemic tryptophan metabolism | Exercise shifts Kyn → KYNA (less neurotoxic) | Modulates stress/inflammation; muscle-brain axis; cachexia link indirect | KAT enzymes; PGC-1α1 program | Exercise-induced KYNA shift likely protective; chronic inflammation favors QUIN |
| EV/RNA Exerkine | Source Tissue/Cell | Exercise Response | Proposed Targets/Actions | Cachexia-Relevant Findings | Context-Dependent Notes |
|---|---|---|---|---|---|
| Endothelial EV miR-342-5p | Endothelium | ↑ with exercise | Cardiovascular protection; vascular adaptation | Anti-inflammatory/vascular benefits; indirect for cachexia | Physiological increases protective; tumor EV milieu may oppose |
| miR-1192 (exercise-induced) | Circulating (mouse) | ↑ after exercise | Cardioprotection | Indirect; systemic resilience | Exercise protective; disease context unknown |
| miR-126 (endothelial) | Endothelium | ↑ with exercise | Vascular homeostasis; angiogenesis | Indirect; may support perfusion | Exercise protective; tumor EVs may disrupt |
| Muscle-enriched miRs (miR-206, miR-133) | Skeletal muscle | Dynamic changes post-exercise | Myogenesis, regeneration | Potential to counteract atrophy; direct data limited | Training likely beneficial; chronic illness may blunt response |
| Inflammation-responsive miRs (miR-146a, miR-221, miR-21, miR-10b-5p, miR-222-3p, miR-30a-5p) | Circulating/various | Acute decreases/increases depending on miR and timing | Immune modulation; endothelial function; remodeling | Reflect inflammatory tone; potential biomarkers | Patterns differ with intensity/timing; tumor EV cargo may be opposite |
| HSP60 (exercise-upregulated; EV cargo candidate) | Skeletal muscle | ↑ with endurance exercise | Mitochondrial stress signaling; therapy prototype (physiactisome) | Conceptually cytoprotective; therapeutic EV engineered from exercise factor | Endogenous signals beneficial; pharmacologic delivery under study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, J.; Szlachcic, A.; Ptak-Belowska, A.; Brzozowski, T. Physical Activity, Exerkines, and Their Role in Cancer Cachexia. Int. J. Mol. Sci. 2025, 26, 8011. https://doi.org/10.3390/ijms26168011
Bilski J, Szlachcic A, Ptak-Belowska A, Brzozowski T. Physical Activity, Exerkines, and Their Role in Cancer Cachexia. International Journal of Molecular Sciences. 2025; 26(16):8011. https://doi.org/10.3390/ijms26168011
Chicago/Turabian StyleBilski, Jan, Aleksandra Szlachcic, Agata Ptak-Belowska, and Tomasz Brzozowski. 2025. "Physical Activity, Exerkines, and Their Role in Cancer Cachexia" International Journal of Molecular Sciences 26, no. 16: 8011. https://doi.org/10.3390/ijms26168011
APA StyleBilski, J., Szlachcic, A., Ptak-Belowska, A., & Brzozowski, T. (2025). Physical Activity, Exerkines, and Their Role in Cancer Cachexia. International Journal of Molecular Sciences, 26(16), 8011. https://doi.org/10.3390/ijms26168011






