Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review
Abstract
1. Introduction
2. Liver Sinusoidal Endothelial Cells: Anatomy and Role
3. Deep Lens on LSECs’ Immune Modulation
4. LSECs’ Contribution to Fibrosis and Cirrhosis
5. LSECs’ Contribution to “Pro-Carcinogenetic” Cirrhosis
6. LSECs’ Potential Treatment Approaches
7. Three-Dimensional Human Model
8. Present Obstacles and Prospective Pathways
9. Conclusions
Funding
Conflicts of Interest
References
- Puri, M.; Sonawane, S. Liver Sinusoidal Endothelial Cells in the Regulation of Immune Responses and Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease. Int. J. Mol. Sci. 2025, 26, 3988. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Shan, W.; Zhou, J.; You, Y. Liver sinusoidal endothelial cells in hepatic fibrosis: Opportunities for future strategies. Biochem. Biophys. Res. Commun. 2025, 766, 151881. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Cao, Y.Y.; Gao, X.; Targher, G.; Byrne, C.D.; Sun, D.Q.; Zheng, M.H. MAFLD as part of systemic metabolic dysregulation. Hepatol. Int. 2024, 18, 834–847. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Sergi, C.M. NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review. Int. J. Mol. Sci. 2024, 25, 8462. [Google Scholar] [CrossRef]
- Dai, Q.; Ain, Q.; Seth, N.; Rooney, M.; Zipprich, A. Liver sinusoidal endothelial cells: Friend or foe in metabolic dysfunction- associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis. Dig. Liver Dis. 2025, 57, 493–503. [Google Scholar] [CrossRef]
- Mo, H.; Yue, P.; Li, Q.; Tan, Y.; Yan, X.; Liu, X.; Xu, Y.; Luo, Y.; Palihati, S.; Yi, C.; et al. The role of liver sinusoidal endothelial cells in metabolic dysfunction-associated steatotic liver diseases and liver cancer: Mechanisms and potential therapies. Angiogenesis 2025, 28, 14. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, J.; Wu, H.; Kong, X. Innate immune regulation in inflammation resolution and liver regeneration in drug-induced liver injury. Arch. Toxicol. 2025, 99, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.P.; Ge, J.Y.; Song, Y.M.; Yu, X.Q.; Chen, W.H.; Chen, Y.Y.; Ye, D.; Zheng, Y.W. A novel efficient strategy to generate liver sinusoidal endothelial cells from human pluripotent stem cells. Sci. Rep. 2024, 14, 13831. [Google Scholar] [CrossRef]
- Li, F.; Yuan, R.; Zhang, J.; Su, B.; Qi, X. Advances in nanotechnology for the diagnosis and management of metabolic dysfunction-associated steatotic liver disease. Asian J. Pharm. Sci. 2025, 20, 101025. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Gopal, T.; Kandy, R.R.K.; Boopathy, L.K.; Perumal, S.K.; Ganesan, M.; Rasineni, K.; Donohue, T.M., Jr.; Osna, N.A.; Kharbanda, K.K. Mitochondrial Dysfunction-Associated Mechanisms in the Development of Chronic Liver Diseases. Biology 2023, 12, 1311. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Lin, S.; Geller, D.A.; Yan, Y. The microenvironment in the development of MASLD-MASH-HCC and associated therapeutic in MASH-HCC. Front. Immunol. 2025, 16, 1569915. [Google Scholar] [CrossRef]
- Saleh, R.O.; Hamad, H.A.; Najim, M.A.; Menon, S.V.; Kaur, M.; Sivaprasad, G.V.; Abohassan, M.; Juan, W.T.; Husseen, B.; Mustafa, Y.F. Exosome-mediated Transfer of lncRNA in Liver Associated Diseases; Uncovered Truths. Cell Biochem. Biophys. 2025, 83, 1465–1481. [Google Scholar] [CrossRef]
- van Riet, S.; Julien, A.; Atanasov, A.; Nordling, A.; Ingelman-Sundberg, M. The role of sinusoidal endothelial cells and TIMP1 in the regulation of fibrosis in a novel human liver 3D NASH model. Hepatol. Commun. 2024, 8, e0374. [Google Scholar] [CrossRef]
- Hardwick, J.P.; Song, B.J.; Rote, P.; Leahy, C.; Lee, Y.K.; Wolf, A.R.; Diegisser, D.; Garcia, V. The CYP4/20-HETE/GPR75 axis in the progression metabolic dysfunction-associated steatosis liver disease (MASLD) to chronic liver disease. Front. Physiol. 2024, 15, 1497297. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.N.; Khammash, H.A.; Miranda, A.M.; Rutt, L.N.; Twardy, S.M.; Anton, P.E.; Campbell, M.L.; Garza-Ortiz, C.; Orlicky, D.J.; Pelanda, R.; et al. Liver sinusoidal endothelial cells regulate the balance between hepatic immunosuppression and immunosurveillance. Front. Immunol. 2024, 15, 1497788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, L.; Yi, W.; Wang, M.; Xu, F.; Liu, H.; Nie, J.; Pan, Y.H.; Dang, S.; Zhang, W. ADAMTS18-fibronectin interaction regulates the morphology of liver sinusoidal endothelial cells. iScience 2024, 27, 110273. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Baek, E.B.; Hong, E.J.; Kang, J.H.; Park, S.; Park, S.; Hong, E.J.; Cho, Y.E.; Ko, J.W.; Won, Y.S.; et al. TXNIP in liver sinusoidal endothelial cells ameliorates alcohol-associated liver disease via nitric oxide production. Int. J. Biol. Sci. 2024, 20, 606–620. [Google Scholar] [CrossRef]
- Velliou, R.I.; Legaki, A.I.; Nikolakopoulou, P.; Vlachogiannis, N.I.; Chatzigeorgiou, A. Liver endothelial cells in NAFLD and transition to NASH and HCC. Cell. Mol. Life Sci. 2023, 80, 314. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Xia, Y.; Wang, X.; Wang, Z. Research Progress on the Immune Function of Liver Sinusoidal Endothelial Cells in Sepsis. Cells 2025, 14, 373. [Google Scholar] [CrossRef]
- Zhao, X.; Amevor, F.K.; Xue, X.; Wang, C.; Cui, Z.; Dai, S.; Peng, C.; Li, Y. Remodeling the hepatic fibrotic microenvironment with emerging nanotherapeutics: A comprehensive review. J. Nanobiotechnol. 2023, 21, 121, Erratum in J. Nanobiotechnol. 2023, 21, 152. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Sun, L.; Zhou, L.; Niu, Y.; Liang, K.; Wu, B.; Zhao, P.; Liu, Z.; Zhou, X.; et al. hESCs-derived Organoids Achieve Liver Zonation Features through LSEC Modulation. Adv. Sci. 2025, 12, e2411667, Erratum in Adv. Sci. 2025, 12, e11802. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zuo, B.; He, Y. Liver sinusoidal endothelial cells as potential drivers of liver fibrosis (Review). Mol. Med. Rep. 2024, 29, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Z.; Gu, X.; Ji, L. Diagnosis, toxicological mechanism, and detoxification for hepatotoxicity induced by pyrrolizidine alkaloids from herbal medicines or other plants. Crit. Rev. Toxicol. 2024, 54, 123–133. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; He, W.; Dong, H.; Guo, Y.; Yuan, G.; Shi, X.; Wang, D.; Lu, F. Role of liver sinusoidal endothelial cell in metabolic dysfunction-associated fatty liver disease. Cell Commun. Signal. 2024, 22, 346. [Google Scholar] [CrossRef]
- Borah, D.; Blacharczyk, O.; Szafranska, K.; Czyzynska-Cichon, I.; Metwally, S.; Szymanowski, K.; Hubner, W.; Kotlinowski, J.; Dobosz, E.; McCourt, P.; et al. Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation. Cells 2025, 14, 621. [Google Scholar] [CrossRef]
- Antwi, M.B.; Dumitriu, G.; Simon-Santamaria, J.; Romano, J.S.; Li, R.; Smedsrod, B.; Vik, A.; Eskild, W.; Sorensen, K.K. Liver sinusoidal endothelial cells show reduced scavenger function and downregulation of Fc gamma receptor IIb, yet maintain a preserved fenestration in the Glmpgt/gt mouse model of slowly progressing liver fibrosis. PLoS ONE 2023, 18, e0293526. [Google Scholar] [CrossRef]
- Bochimoto, H.; Ishihara, Y.; Zin, N.K.M.; Iwata, H.; Kondoh, D.; Obara, H.; Matsuno, N. Ultrastructural changes in porcine liver sinusoidal endothelial cells of machine perfused liver donated after cardiac death. World J. Gastroenterol. 2022, 28, 2100–2111. [Google Scholar] [CrossRef]
- Li, H. Intercellular crosstalk of liver sinusoidal endothelial cells in liver fibrosis, cirrhosis and hepatocellular carcinoma. Dig. Liver Dis. 2022, 54, 598–613. [Google Scholar] [CrossRef]
- Szafranska, K.; Kruse, L.D.; Holte, C.F.; McCourt, P.; Zapotoczny, B. The wHole Story About Fenestrations in LSEC. Front. Physiol. 2021, 12, 735573. [Google Scholar] [CrossRef]
- Henriques-Pons, A.; Vacani-Martins, N.; Dos Santos, C.L.P.; Meuser-Batista, M. The liver’s dilemma: Sensing real danger in a sea of PAMPs: The (arterial) sinusoidal segment theory. Front. Immunol. 2024, 15, 1503063. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Q.; Xia, N.; Wang, J.; Zhao, W.; Jin, M.; Lu, Q.; Hu, J.; Zhang, R.; Zhang, L.; et al. Immune-endothelial cell crosstalk in hepatic endothelial injury of liver fibrotic mice. Eur. J. Pharmacol. 2025, 1000, 177730. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zhang, X.; Zhou, D.; Zhou, Y.; Pan, Q.; Chai, J.; Gao, J. Disruption of Hepatic Sinusoidal Homeostasis Leads to Hepatopulmonary Syndrome. J. Cell. Mol. Med. 2025, 29, e70585. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease. Biomedicines 2025, 13, 683. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, H.; Wang, H. Extracellular Vesicles in Non-alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Front. Physiol. 2021, 12, 707429. [Google Scholar] [CrossRef]
- Feng, H.; Ou, B.; Dong, W.; Thasler, W.E. Preparation and Culture of Human Liver Resident Immune Cells. Curr. Protoc. Cell Biol. 2018, 80, e50. [Google Scholar] [CrossRef]
- Yang, R.; Yang, L.; Zhang, N.; Wan, Y.; Chen, S.; Xiao, Y.; Liang, X.; Yang, S.; Zhong, Y.; Huang, D.; et al. Targeted delivery of polymeric NO-donor micelles to hepatic stellate cells for restoration of liver function and inhibition of hepatic fibrosis. J. Control. Release 2025, 379, 466–477. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, Q.; Fu, J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy 2024, 20, 221–241. [Google Scholar] [CrossRef]
- Nesci, A.; Ruggieri, V.; Manilla, V.; Spinelli, I.; Santoro, L.; Di Giorgio, A.; Santoliquido, A.; Ponziani, F.R. Endothelial Dysfunction and Liver Cirrhosis: Unraveling of a Complex Relationship. Int. J. Mol. Sci. 2024, 25, 12859. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Islam, S.; Caporarello, N.; Kostallari, E.; Dielis, K.; Tschumperlin, D.J.; Hirsova, P.; Ibrahim, S.H. Liver sinusoidal endothelial cell expressed vascular cell adhesion molecule 1 promotes liver fibrosis. Front. Immunol. 2022, 13, 983255. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Liao, Y.P.; Luo, L.; Xia, T.; Nel, A.E. Use of a Liver-Targeting Immune-Tolerogenic mRNA Lipid Nanoparticle Platform to Treat Peanut-Induced Anaphylaxis by Single- and Multiple-Epitope Nucleotide Sequence Delivery. ACS Nano 2023, 17, 4942–4957. [Google Scholar] [CrossRef]
- Abad-Jorda, L.; Martinez-Alcocer, A.; Guixe-Muntet, S.; Hunt, N.J.; Westwood, L.J.; Lozano, J.J.; Gallego-Duran, R.; Cogger, V.C.; Fernandez-Iglesias, A.; Gracia-Sancho, J. miR-27b-3p modulates liver sinusoidal endothelium dedifferentiation in chronic liver disease. Hepatol. Commun. 2025, 9, e0700. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Mu, W.; Fan, J.; Shen, J. beta-arrestin2 promotes angiogenesis of liver sinusoidal endothelial cells through the VEGF/VEGFR2 pathway to aggravate cirrhosis. Toxicol. Lett. 2024, 401, 1–12. [Google Scholar] [CrossRef]
- Vasuri, F.; Germinario, G.; Ciavarella, C.; Carroli, M.; Motta, I.; Valente, S.; Cescon, M.; D’Errico, A.; Pasquinelli, G.; Ravaioli, M. Trophism and Homeostasis of Liver Sinusoidal Endothelial Graft Cells during Preservation, with and without Hypothermic Oxygenated Perfusion. Biology 2022, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Liu, X.; Liao, Y.P.; Chang, C.H.; Mei, K.C.; Jiang, J.; Tseng, S.; Gochman, G.; Huang, M.; et al. Antigen- and Epitope-Delivering Nanoparticles Targeting Liver Induce Comparable Immunotolerance in Allergic Airway Disease and Anaphylaxis as Nanoparticle-Delivering Pharmaceuticals. ACS Nano 2021, 15, 1608–1626. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Reinke, P.; Volk, H.D.; Lv, Y.; Wu, R. Mechanisms of Immune Tolerance in Liver Transplantation-Crosstalk Between Alloreactive T Cells and Liver Cells With Therapeutic Prospects. Front. Immunol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Liu, X.; Kumar, S.; Gochman, G.; Ji, Y.; Liao, Y.P.; Chang, C.H.; Situ, W.; Lu, J.; et al. Use of Polymeric Nanoparticle Platform Targeting the Liver To Induce Treg-Mediated Antigen-Specific Immune Tolerance in a Pulmonary Allergen Sensitization Model. ACS Nano 2019, 13, 4778–4794. [Google Scholar] [CrossRef]
- van Son, K.C.; Verschuren, L.; Hanemaaijer, R.; Reeves, H.; Takkenberg, R.B.; Drenth, J.P.H.; Tushuizen, M.E.; Holleboom, A.G. Non-Parenchymal Cells and the Extracellular Matrix in Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease. Cancers 2023, 15, 1308. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, C.; Li, X.; Ji, X.; Hu, X.; Wang, X.; Zhang, J. Neutrophil extracellular traps-induced pyroptosis of liver sinusoidal endothelial cells exacerbates intrahepatic coagulation in cholestatic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167700. [Google Scholar] [CrossRef]
- Heldens, A.; Casteleyn, C.; Onghena, L.; Antwi, M.; Neyt, S.; Descamps, B.; Vanhove, C.; Verhelst, X.; Raevens, S.; Van Vlierberghe, H.; et al. The pan-PPAR agonist lanifibranor reduces portal pressure independent of fibrosis reduction through the splanchnic vasculature. Biomed. Pharmacother. 2025, 183, 117826. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Kotlinowski, J.; Blacharczyk, O.; Giergiel, M.; Szymanowski, K.; Metwally, S.; Wojnar-Lason, K.; Dobosz, E.; Koziel, J.; Lekka, M.; et al. Early and late phases of liver sinusoidal endothelial cell (LSEC) defenestration in mouse model of systemic inflammation. Cell. Mol. Biol. Lett. 2024, 29, 139. [Google Scholar] [CrossRef]
- Verhaegh, P.; Wisse, E.; de Munck, T.; Greve, J.W.; Verheij, J.; Riedl, R.; Duimel, H.; Masclee, A.; Jonkers, D.; Koek, G. Electron microscopic observations in perfusion-fixed human non-alcoholic fatty liver disease biopsies. Pathology 2021, 53, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Baiocchini, A.; Del Nonno, F.; Taibi, C.; Visco-Comandini, U.; D’Offizi, G.; Piacentini, M.; Falasca, L. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci. Rep. 2019, 9, 8760. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Zhang, L.; Li, L.; Yang, W.; Li, J.; He, W. Nucleic acid spheres for treating capillarisation of liver sinusoidal endothelial cells in liver fibrosis. Nat. Commun. 2025, 16, 4517. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, J.P.; Schanz, O.; Garbers, C.; Zaremba, A.; Hegenbarth, S.; Kurts, C.; Beyer, M.; Schultze, J.L.; Kastenmuller, W.; Rose-John, S.; et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 2014, 8, 1318–1327. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.; Wang, X.; Zhao, D.; Liu, D.; Liu, J.; Chen, P.J.; Yang, D.; He, F.; Tang, L. Liver sinusoidal endothelial cell lectin inhibits CTL-dependent virus clearance in mouse models of viral hepatitis. J. Immunol. 2013, 190, 4185–4195. [Google Scholar] [CrossRef]
- Kurokawa, M.; Goya, T.; Kohjima, M.; Tanaka, M.; Iwabuchi, S.; Shichino, S.; Ueha, S.; Hioki, T.; Aoyagi, T.; Takahashi, M.; et al. Microcirculatory disturbance in acute liver injury is triggered by IFNgamma-CD40 axis. J. Inflamm. 2024, 21, 23. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Li, Y.; Li, X. Liver sinusoidal endothelial cell: An important yet often overlooked player in the liver fibrosis. Clin. Mol. Hepatol. 2024, 30, 303–325. [Google Scholar] [CrossRef]
- Sato-Espinoza, K.; Chotiprasidhi, P.; Huaman, M.R.; Diaz-Ferrer, J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J. Hepatol. 2024, 16, 452–464. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L. Epidemiology of NAFLD—Focus on diabetes. Diabetes Res. Clin. Pract. 2024, 210, 111648. [Google Scholar] [CrossRef]
- Sinha, R.A. Targeting nuclear receptors for NASH/MASH: From bench to bedside. Liver Res. 2024, 8, 34–45. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Alqahtani, S.A.; Alswat, K.; Yilmaz, Y.; Keklikkiran, C.; Funuyet-Salas, J.; Romero-Gomez, M.; Fan, J.G.; Zheng, M.H.; El-Kassas, M.; et al. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J. Hepatol. 2024, 80, 419–430. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.M.; Kehar, M.; Jimenez-Rivera, C. Liver Biopsy Handling of Metabolic-Associated Fatty Liver Disease (MAFLD): The Children’s Hospital of Eastern Ontario grossing protocol. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241227766. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Larsen, A.K.; McCourt, P.; Smedsrod, B.; Sorensen, K.K. The Scavenger Function of Liver Sinusoidal Endothelial Cells in Health and Disease. Front. Physiol. 2021, 12, 757469. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Leite, A.R.; Godinho, T.; Liberal, R.; Correia-Chaves, J.; Lourenco, I.M.; von Hafe, M.; Vale, C.; Fragao-Marques, M.; Pimentel-Nunes, P.; et al. Association of metabolic syndrome components and NAFLD with quality of life: Insights from a cross-sectional study. Prim. Care Diabetes 2024, 18, 196–201. [Google Scholar] [CrossRef]
- Susak, F.; Vrsaljko, N.; Vince, A.; Papic, N. TGF Beta as a Prognostic Biomarker of COVID-19 Severity in Patients with NAFLD-A Prospective Case-Control Study. Microorganisms 2023, 11, 1571. [Google Scholar] [CrossRef]
- Trampuz, S.R.; van Riet, S.; Nordling, A.; Ingelman-Sundberg, M. The Role of CTGF in Liver Fibrosis Induced in 3D Human Liver Spheroids. Cells 2023, 12, 302. [Google Scholar] [CrossRef]
- Garg, A.; Khan, S.; Luu, N.; Nicholas, D.J.; Day, V.; King, A.L.; Fear, J.; Lalor, P.F.; Newsome, P.N. TGFbeta(1) priming enhances CXCR3-mediated mesenchymal stromal cell engraftment to the liver and enhances anti-inflammatory efficacy. J. Cell. Mol. Med. 2023, 27, 864–878. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, Q.; Fu, Y.D.; Gao, S.Q.; Hu, Y.H.; Liu, W.; Chen, G.F.; Mu, Y.P.; Chen, J.M.; Liu, P. Amygdalin Ameliorates Liver Fibrosis through Inhibiting Activation of TGF-beta/Smad Signaling. Chin. J. Integr. Med. 2023, 29, 316–324. [Google Scholar] [CrossRef]
- Biquard, L.; Rautou, P.E. Autophagy and extracellular vesicles in the liver endothelium: Friends or foes? Hepatol. Int. 2024, 18, 1–3. [Google Scholar] [CrossRef]
- Kent, G.M.; Atkins, M.H.; Lung, B.; Nikitina, A.; Fernandes, I.M.; Kwan, J.J.; Andrews, T.S.; MacParland, S.A.; Keller, G.M.; Gage, B.K. Human liver sinusoidal endothelial cells support the development of functional human pluripotent stem cell-derived Kupffer cells. Cell Rep. 2024, 43, 114629. [Google Scholar] [CrossRef]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernandez-Gea, V. The Endothelium as a Driver of Liver Fibrosis and Regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef]
- Persad, R.; Huynh, H.Q.; Hao, L.; Ha, J.R.; Sergi, C.; Srivastava, R.; Persad, S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 251–260. [Google Scholar] [CrossRef]
- Sergi, C. EPAS 1, congenital heart disease, and high altitude: Disclosures by genetics, bioinformatics, and experimental embryology. Biosci. Rep. 2019, 39, BSR20182197. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuchner, J.; Rupp, M.; Salvador, C.; Meister, B.; Kiechl-Kohlendorfer, U.; Muller, T.; Geiger, K.; Sergi, C.; Obexer, P.; Ausserlechner, M.J. Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma. Oncotarget 2016, 7, 77591–77606. [Google Scholar] [CrossRef] [PubMed]
- Sako, S.; Takeshita, Y.; Takayama, H.; Goto, H.; Nakano, Y.; Ando, H.; Tsujiguchi, H.; Yamashita, T.; Arai, K.; Kaneko, S.; et al. Trajectories of Liver Fibrosis and Gene Expression Profiles in Nonalcoholic Fatty Liver Disease Associated with Diabetes. Diabetes 2023, 72, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, X.; Slevin, E.; Harrison, K.; Li, T.; Zhang, Y.; Klaunig, J.E.; Wu, C.; Shetty, A.K.; Dong, X.C.; et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 2022, 36, e22125. [Google Scholar] [CrossRef]
- Kumar, S.; Duan, Q.; Wu, R.; Harris, E.N.; Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113869. [Google Scholar] [CrossRef]
- Salazar, E.; Salazar, A.M.; Taylor, P.; Urdanibia, I.; Perez, K.; Rodriguez-Acosta, A.; Sanchez, E.E.; Guerrero, B. Contribution of endothelial cell and macrophage activation in the alterations induced by the venom of Micrurus tener tener in C57BL/6 mice. Mol. Immunol. 2019, 116, 45–55. [Google Scholar] [CrossRef]
- Benedicto, A.; Herrero, A.; Romayor, I.; Marquez, J.; Smedsrod, B.; Olaso, E.; Arteta, B. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci. Rep. 2019, 9, 13111. [Google Scholar] [CrossRef]
- Qiao, J.; Qi, K.; Chu, P.; Mi, H.; Yang, N.; Yao, H.; Xia, Y.; Li, Z.; Xu, K.; Zeng, L. Infusion of endothelial progenitor cells ameliorates liver injury in mice after haematopoietic stem cell transplantation. Liver Int. 2015, 35, 2611–2620. [Google Scholar] [CrossRef]
- Li, P.; Xie, W.; Wei, H.; Yang, F.; Chen, Y.; Li, Y. Transcriptome Analyses of Liver Sinusoidal Endothelial Cells Reveal a Consistent List of Candidate Genes Associated with Endothelial Dysfunction and the Fibrosis Progression. Curr. Issues Mol. Biol. 2024, 46, 7997–8014. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Tang, X.; Islam, S.; Tapia, A.; Chen, Z.B.; Ibrahim, S.H. Endotheliopathy in the metabolic syndrome: Mechanisms and clinical implications. Pharmacol. Ther. 2023, 244, 108372. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, F.; Fischer, J.; Innes, H.; Buch, S.; Moller, C.; Matz-Soja, M.; von Schonfels, W.; Kramer, B.; Langhans, B.; Kluners, A.; et al. High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis. JHEP Rep. 2023, 5, 100684. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Tacke, F.; Schwabe, R.F.; Henderson, N.C. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 2022, 4, 100524. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Li, J.; Xue, X.; Qin, L.; Li, Y.; Dou, Y.; Mu, X.; Li, X. Si-Wu-Tang improves liver fibrosis by restoring liver sinusoidal endothelial cell functionality and reducing communication with hepatic stellate cells. Chin. Med. 2024, 19, 179. [Google Scholar] [CrossRef]
- Yazdi, M.; Pohmerer, J.; Kafshgari, M.H.; Seidl, J.; Grau, M.; Hohn, M.; Vetter, V.; Hoch, C.C.; Wollenberg, B.; Multhoff, G.; et al. In Vivo Endothelial Cell Gene Silencing by siRNA-LNPs Tuned with Lipoamino Bundle Chemical and Ligand Targeting. Small 2024, 20, e2400643. [Google Scholar] [CrossRef]
- Bhandari, S.; Kyrrestad, I.; Simon-Santamaria, J.; Li, R.; Szafranska, K.J.; Dumitriu, G.; Romano, J.S.; Smedsrod, B.; Sorensen, K.K. Mouse liver sinusoidal endothelial cell responses to the glucocorticoid receptor agonist dexamethasone. Front. Pharmacol. 2024, 15, 1377136. [Google Scholar] [CrossRef]
- Yap, K.K.; Schroder, J.; Gerrand, Y.W.; Dobric, A.; Kong, A.M.; Fox, A.M.; Knowles, B.; Banting, S.W.; Elefanty, A.G.; Stanley, E.G.; et al. Liver specification of human iPSC-derived endothelial cells transplanted into mouse liver. JHEP Rep. 2024, 6, 101023. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Ruan, B.; Liu, J.J.; Duan, J.L.; Yue, Z.S.; Song, P.; Xu, H.; Ding, J.; Xu, C.; Dou, G.R.; et al. Notch-triggered maladaptation of liver sinusoidal endothelium aggravates nonalcoholic steatohepatitis through endothelial nitric oxide synthase. Hepatology 2022, 76, 742–758. [Google Scholar] [CrossRef]
- Casey, L.M.; Hughes, K.R.; Saunders, M.N.; Miller, S.D.; Pearson, R.M.; Shea, L.D. Mechanistic contributions of Kupffer cells and liver sinusoidal endothelial cells in nanoparticle-induced antigen-specific immune tolerance. Biomaterials 2022, 283, 121457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, J.; Liu, Y.; Wang, M.; Liu, Z.; Gao, Y.; Huang, L. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumor microenvironment for hepatocellular carcinoma. J. Nanobiotechnol. 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Tolksdorf, F.; Mikulec, J.; Geers, B.; Endig, J.; Sprezyna, P.; Heukamp, L.C.; Knolle, P.A.; Kolanus, W.; Diehl, L. The PDL1-inducible GTPase Arl4d controls T effector function by limiting IL-2 production. Sci. Rep. 2018, 8, 16123. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, V.; Fiorotto, R.; Cadamuro, M.; Fabris, L.; Strazzabosco, M. New insights on the role of vascular endothelial growth factor in biliary pathophysiology. JHEP Rep. 2021, 3, 100251. [Google Scholar] [CrossRef]
- Auvinen, K.; Lokka, E.; Mokkala, E.; Jappinen, N.; Tyystjarvi, S.; Saine, H.; Peurla, M.; Shetty, S.; Elima, K.; Rantakari, P.; et al. Fenestral diaphragms and PLVAP associations in liver sinusoidal endothelial cells are developmentally regulated. Sci. Rep. 2019, 9, 15698. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Duan, J.; Du, D.; Pu, Q.; Li, F. Gut microbiota depletion and FXR inhibition exacerbates zonal hepatotoxicity of sunitinib. Theranostics 2024, 14, 7219–7240. [Google Scholar] [CrossRef]
- Filali-Mouncef, Y.; Hunter, C.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The menage a trois of autophagy, lipid droplets and liver disease. Autophagy 2022, 18, 50–72. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Lekka, M.; Ahluwalia, B.S.; McCourt, P. Tuning of Liver Sieve: The Interplay between Actin and Myosin Regulatory Light Chain Regulates Fenestration Size and Number in Murine Liver Sinusoidal Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 9850. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Braet, F.; Kus, E.; Ginda-Makela, K.; Klejevskaja, B.; Campagna, R.; Chlopicki, S.; Szymonski, M. Actin-spectrin scaffold supports open fenestrae in liver sinusoidal endothelial cells. Traffic 2019, 20, 932–942. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Kus, E.; Braet, F.; Wisse, E.; Chlopicki, S.; Szymonski, M. Tracking Fenestrae Dynamics in Live Murine Liver Sinusoidal Endothelial Cells. Hepatology 2019, 69, 876–888. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Owczarczyk, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Atomic Force Microscopy Reveals the Dynamic Morphology of Fenestrations in Live Liver Sinusoidal Endothelial Cells. Sci. Rep. 2017, 7, 7994. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczny, B.; Owczarczyk, K.; Szafranska, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Morphology and force probing of primary murine liver sinusoidal endothelial cells. J. Mol. Recognit. 2017, 30, e2610. [Google Scholar] [CrossRef] [PubMed]
- Targosz-Korecka, M.; Jaglarz, M.; Malek-Zietek, K.E.; Gregorius, A.; Zakrzewska, A.; Sitek, B.; Rajfur, Z.; Chlopicki, S.; Szymonski, M. AFM-based detection of glycocalyx degradation and endothelial stiffening in the db/db mouse model of diabetes. Sci. Rep. 2017, 7, 15951. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczny, B.; Braet, F.; Wisse, E.; Lekka, M.; Szymonski, M. Biophysical nanocharacterization of liver sinusoidal endothelial cells through atomic force microscopy. Biophys. Rev. 2020, 12, 625–636. [Google Scholar] [CrossRef]
- Szafraniec, J.; Blazejczyk, A.; Kus, E.; Janik, M.; Zajac, G.; Wietrzyk, J.; Chlopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 9, 18867–18880. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Kus, E.; Chlopicki, S.; Szymonski, M. Quantification of fenestrations in liver sinusoidal endothelial cells by atomic force microscopy. Micron 2017, 101, 48–53. [Google Scholar] [CrossRef]
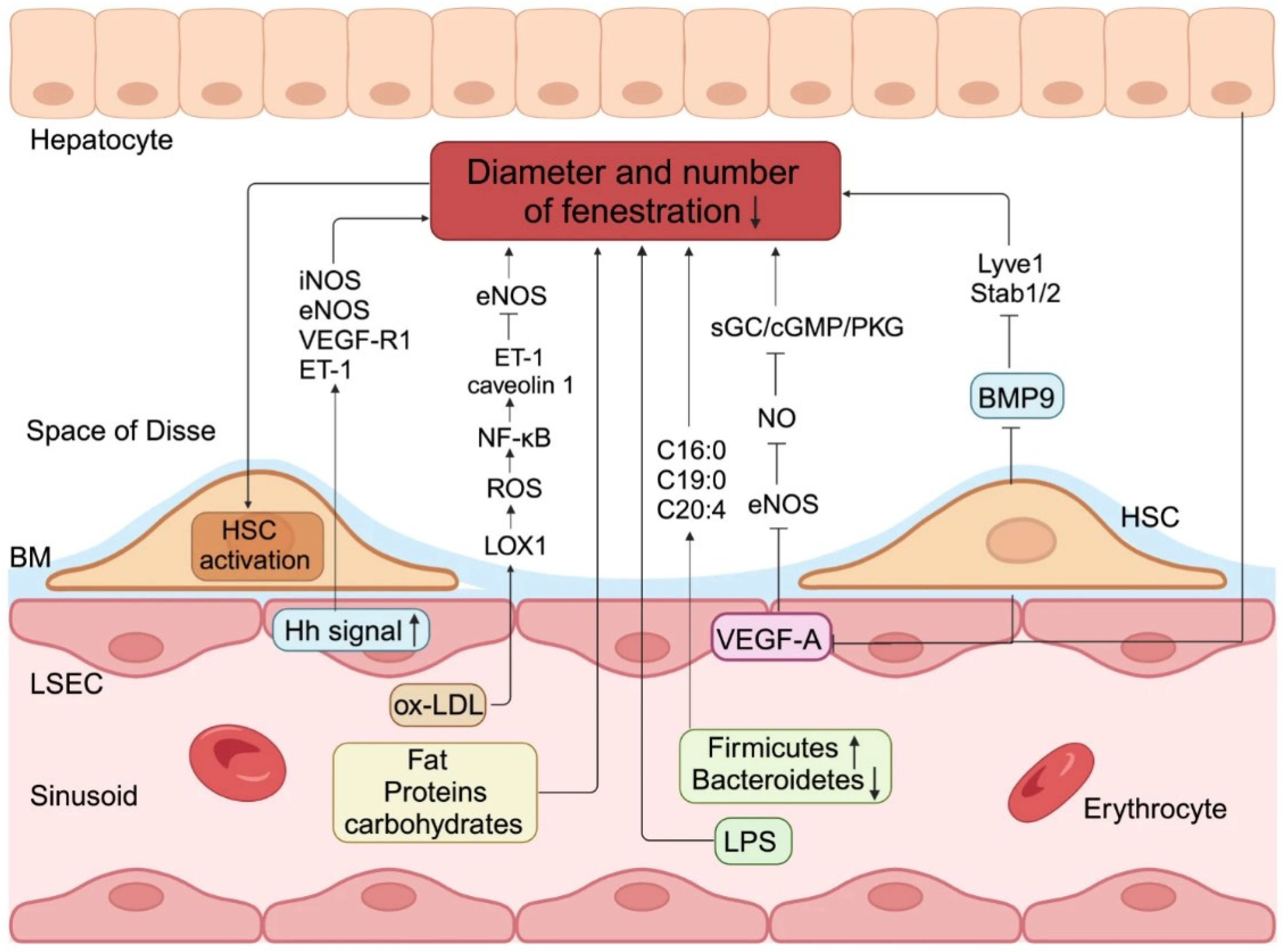
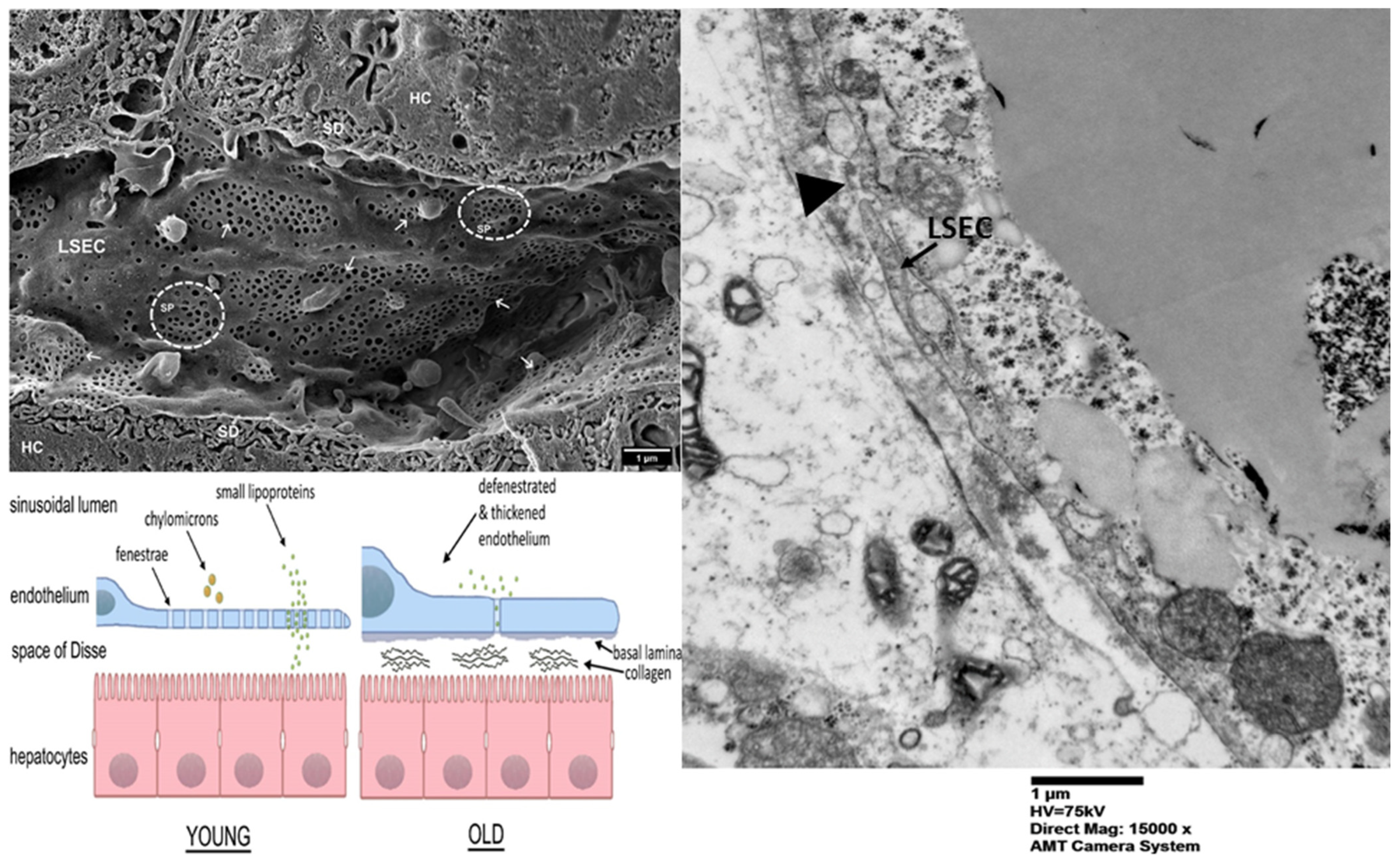
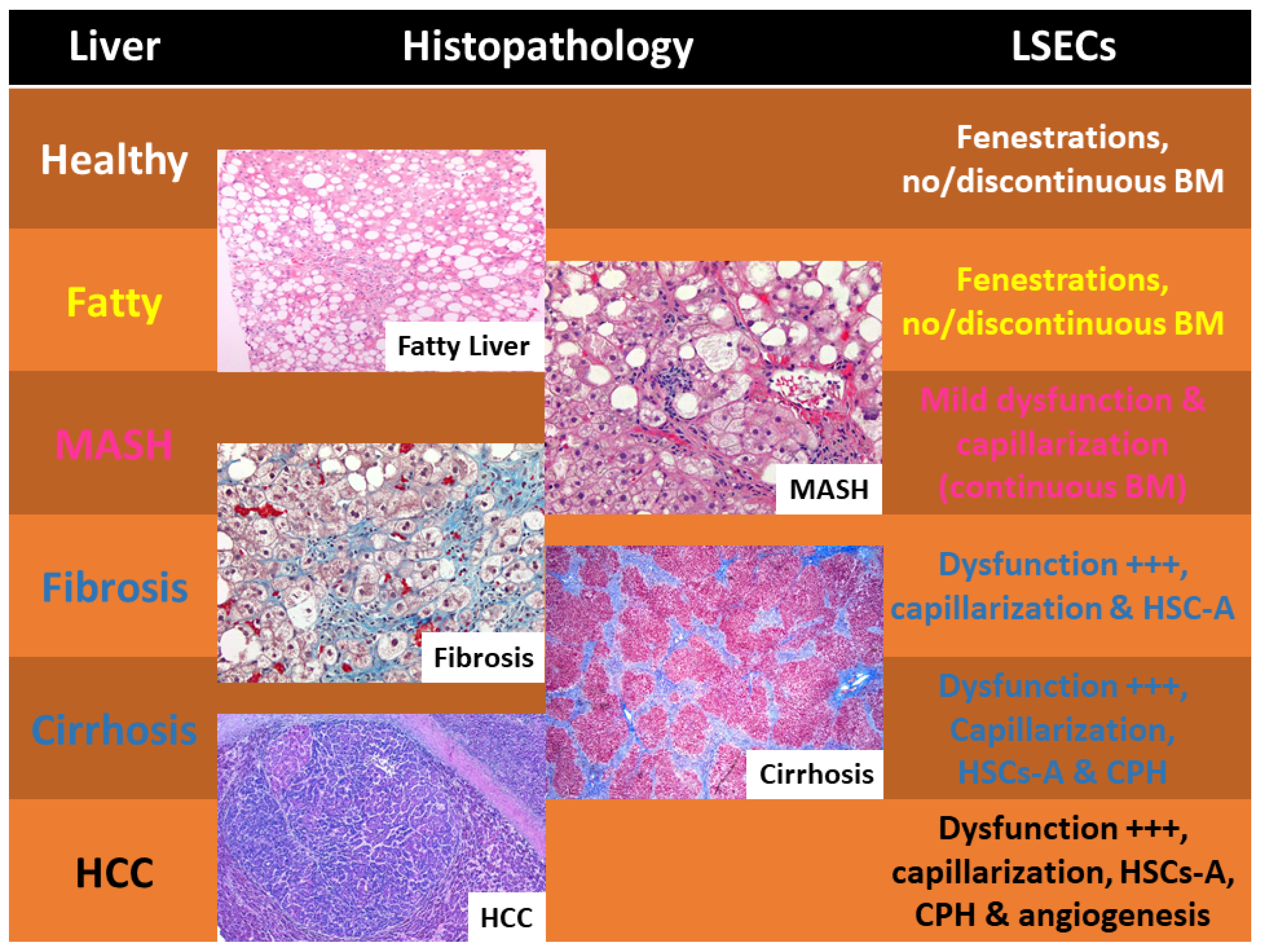
| Stage of Liver Disease | Therapeutic Interventions/Drugs |
|---|---|
| Healthy Liver | Healthy lifestyle, toxin-free nutrition, antioxidants, exercise |
| Fatty Liver | Lifestyle intervention, diet, daily exercise |
| MASH | Lifestyle intervention, diet, daily exercise, resmetirom, GLP-1 receptor agonists, and PPAR agonists |
| Fibrosis | ITDs [e.g., ASK1 or NF-κB ITDs (cenicriviroc, a dual CCR2/CCR5 inhibitor)], ECM Synthesis and Degradation drugs [BMS-986263, an HSP47 mRNA inhibitor, and agents that target galectin-3 (e.g., GR-MD-02)], liver cell-protecting drugs (e.g., emricasan, a pan-caspase inhibitor), REDs (e.g., liraglutide and semaglutide, GLP-1 analogs used for T2DM), others (pioglitazone, a PPARγ agonist, obeticholic acid, a FXR agonist, ARBs (e.g., losartan and candesartan), silymarin) |
| Cirrhosis | NSBBs (e.g., propranolol, nadolol, carvedilol), diuretics (e.g., spironolactone, furosemide), laxatives (lactulose or rifaximin), SBP antibiotics, MTDs (e.g., rifaximin), LTAIs, and statins |
| HCC | Anti-angiogenesis factors (sorafenib and ramucirumab) and immunomodulators [ICIs (e.g., atezolizumab (anti-PD-L1), bevacizumab (anti-VEGF), nivolumab (anti-PD-1), pembrolizumab (anti-PD-1), ipilimumab (anti-CTLA-4)), oncolytic viro-immunotherapy, adoptive T-cell transfer, and vaccines] |
| Therapeutic Approaches | Technology-Related Drugs | Directives |
|---|---|---|
| Vascular Modulation | Angiogenesis inhibitors, statins, FXR agonists | LB-based biomarker development (LBBD) |
| TGF-β Signaling | TGF-β inhibitors | RCTs on LSEC pathophysiology |
| Notch Pathway | Controlled release-associated nanoparticles, Notch inhibitors | RCTs on LSEC pathophysiology |
| Shear Stress | SSMs (Pirfenidone, Nintedanib, Galectin-3 inhibitors) | RCTs |
| Immune Checkpoint | ICMs | LSEC-microRNA modulators |
| Cytokine Interplay | Cytokine inhibitors | LSEC-directed nanoparticles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulmajeed, R.J.; Sergi, C.M. Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 8006. https://doi.org/10.3390/ijms26168006
Abdulmajeed RJ, Sergi CM. Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(16):8006. https://doi.org/10.3390/ijms26168006
Chicago/Turabian StyleAbdulmajeed, Reem J., and Consolato M. Sergi. 2025. "Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review" International Journal of Molecular Sciences 26, no. 16: 8006. https://doi.org/10.3390/ijms26168006
APA StyleAbdulmajeed, R. J., & Sergi, C. M. (2025). Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review. International Journal of Molecular Sciences, 26(16), 8006. https://doi.org/10.3390/ijms26168006







