Synergistic Anti-Obesity Effects of Lactiplantibacillus plantarum Q180 and Phaeodactylum tricornutum (CKDB-322) in High-Fat-Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
2.1. Characterization of P. tricornutum Using Gas Chromatography–Flame Ionization Detection (GC-FID)
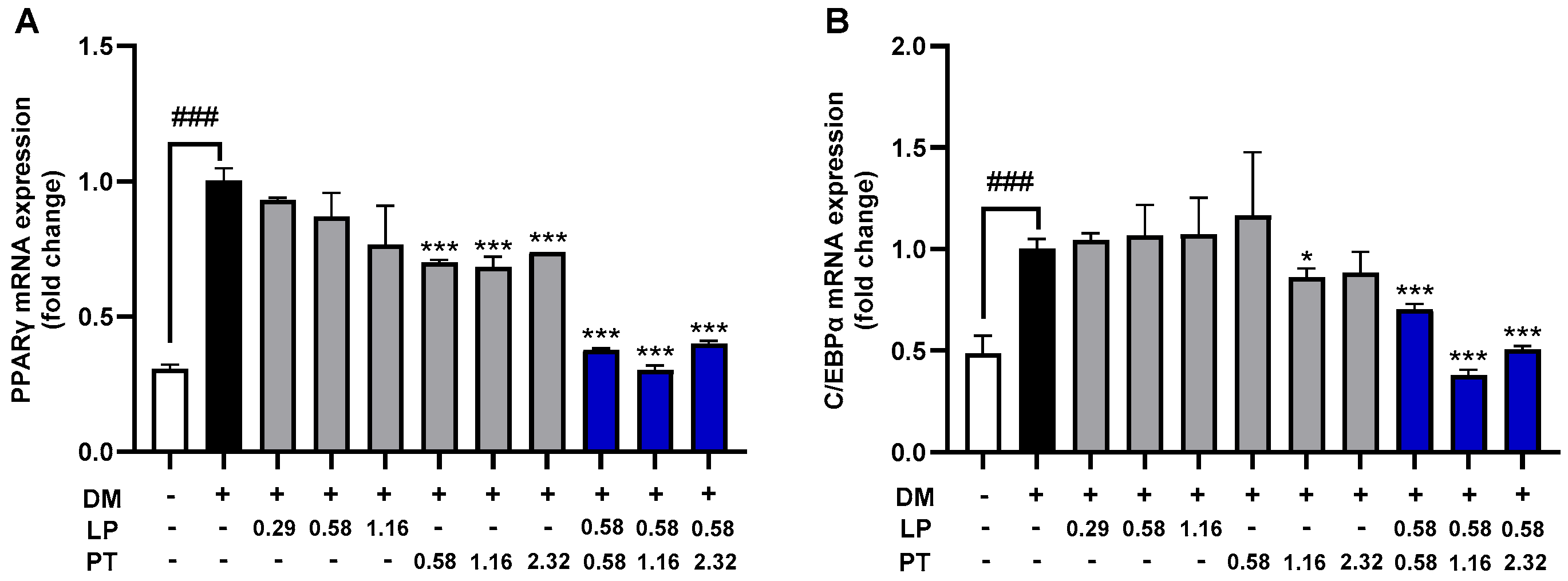
2.2. Effect of CKDB-322 on the mRNA Expression of Adipogenic Transcription Factors in 3T3-L1 Adipocytes
2.3. Effects of CKDB-322 on Lipolytic Activity In Vitro
2.4. Effect of CKDB-322 on Body Weight Gain, Food Intake, and Feed Efficiency Ratio (FER) in High-Fat-Diet (HFD)-Induced Obese Mice
2.5. Effects of CKDB-322 on Serum Biochemical Parameters in HFD-Induced Obese Mice
2.6. Effects of CKDB-322 on Body Fat Mass Accumulation in HFD-Induced Obese Mice
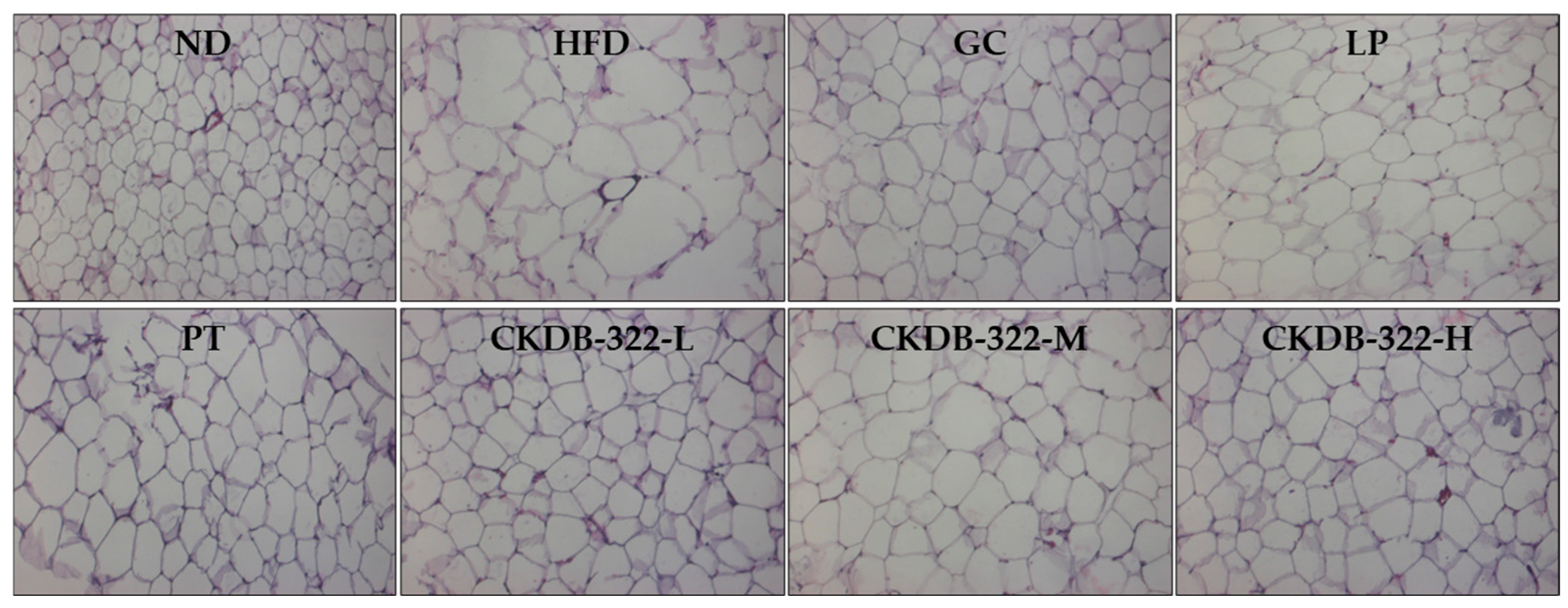
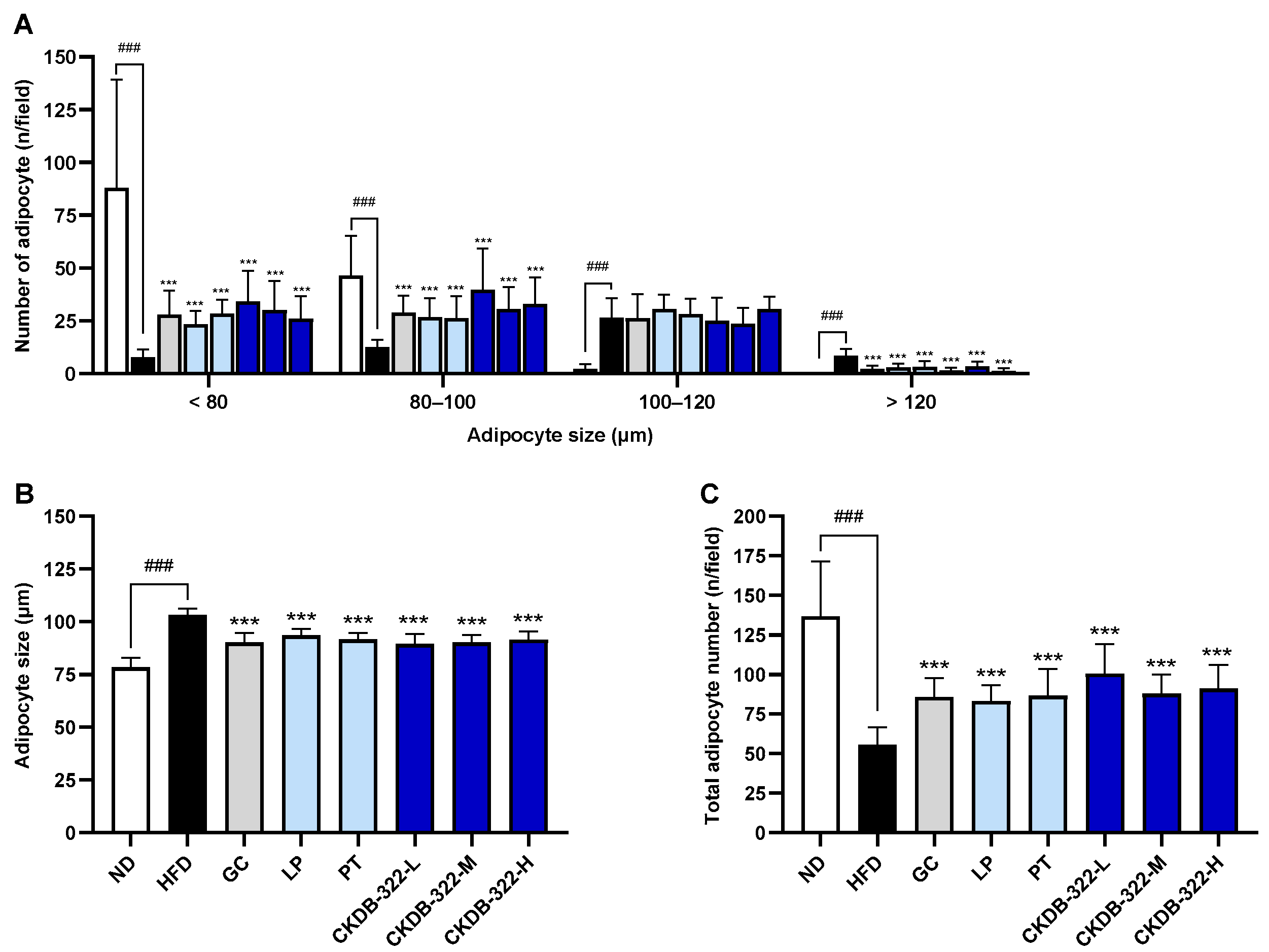
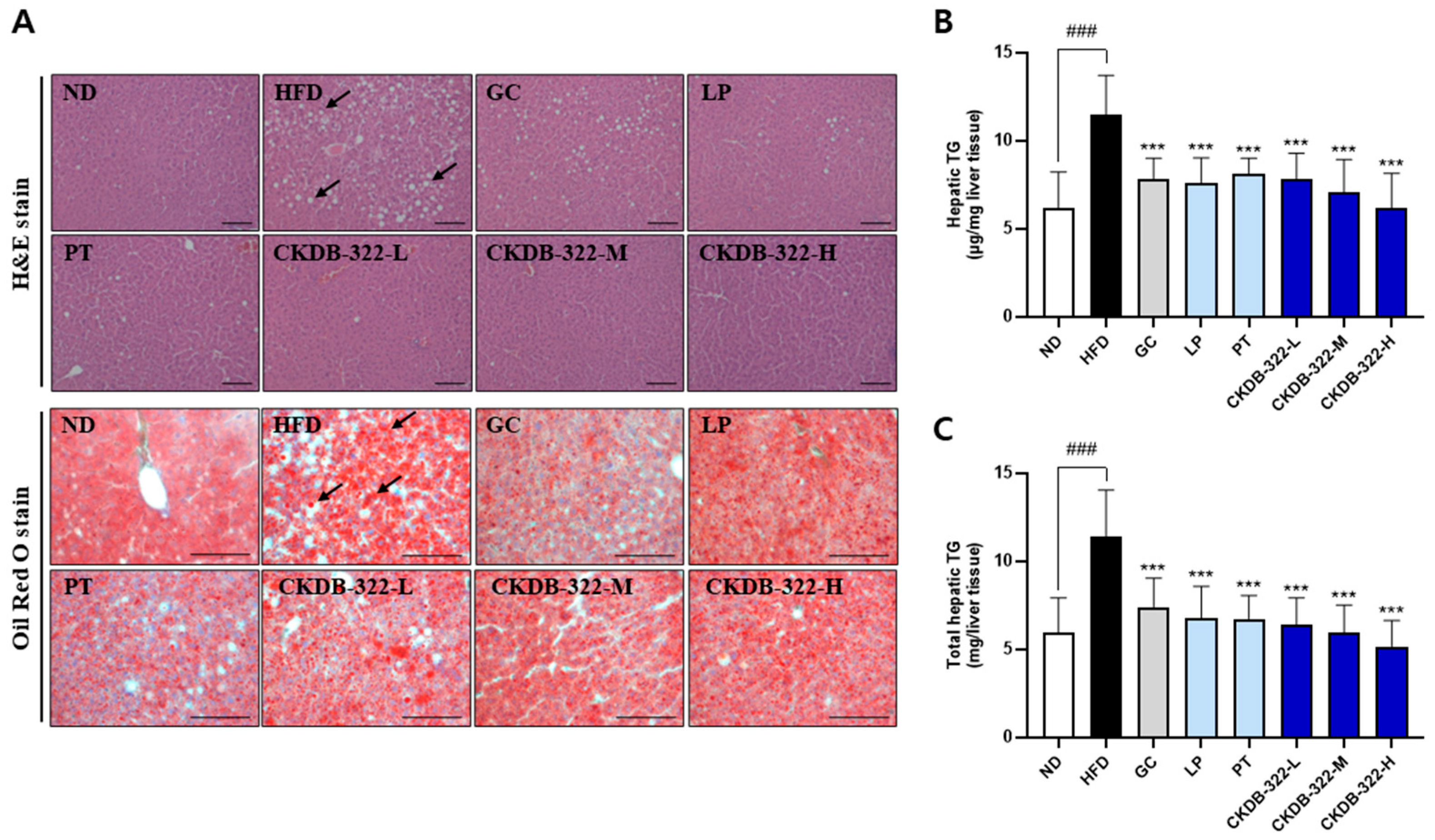
2.7. Effects of CKDB-322 on Histopathological Morphology of Epididymal Adipose Tissue in HFD-Induced Obese Mice
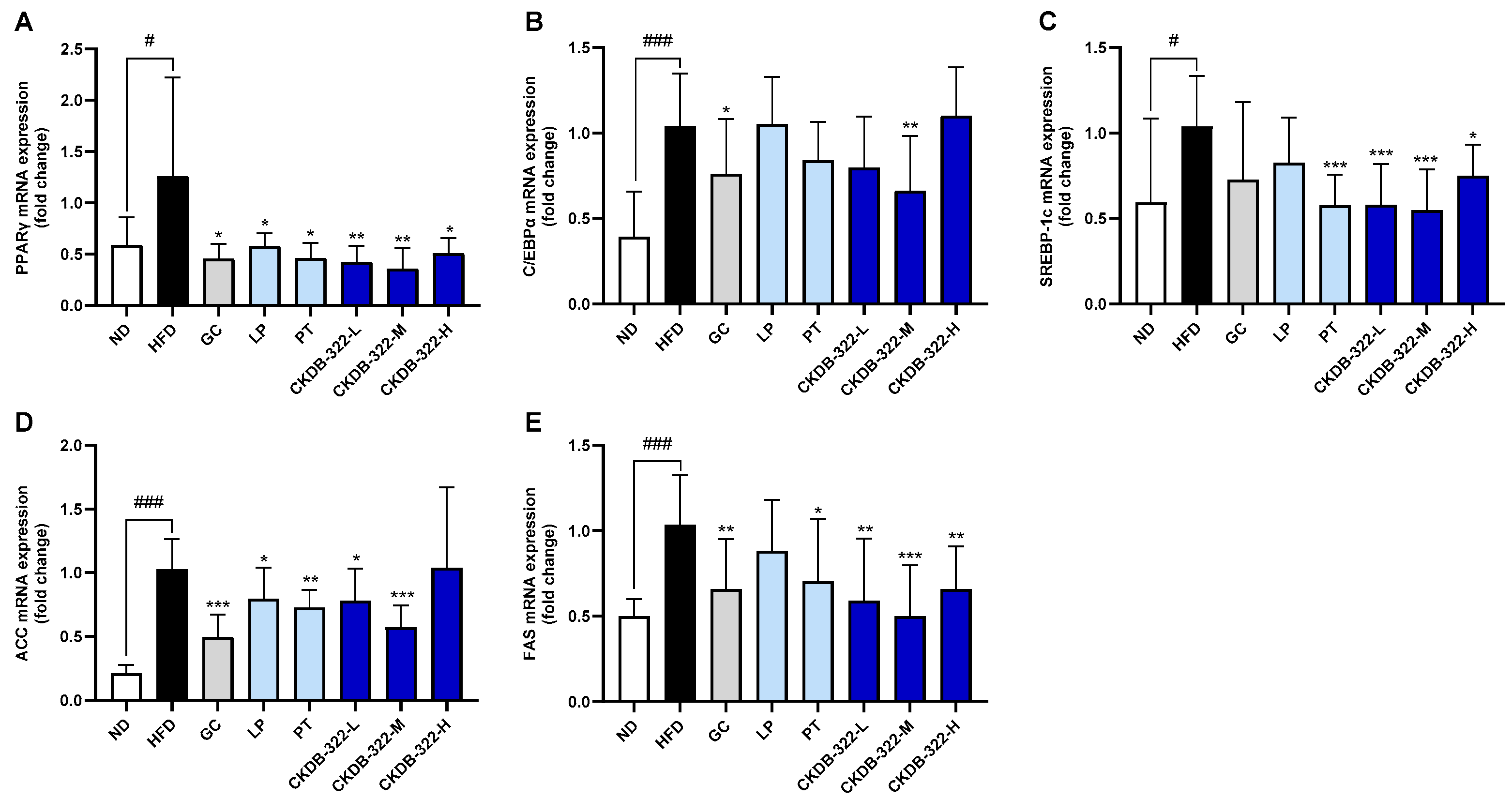
2.8. Effects of CKDB-322 on Gene Expression Related to Adipogenesis and Lipogenesis in Epididymal Adipose Tissue in HFD-Induced Obese Mice
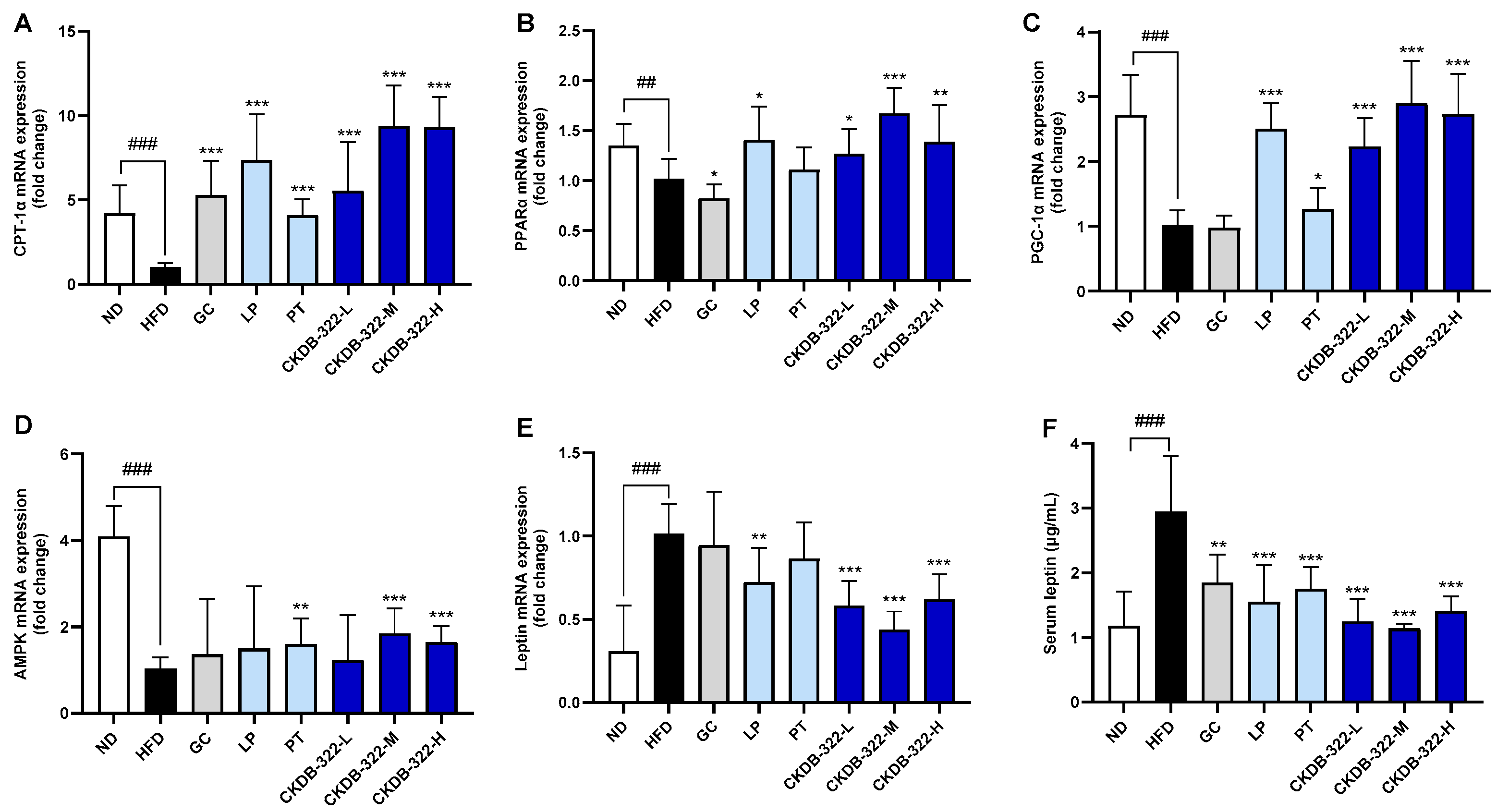
2.9. Effects of CKDB-322 on Markers Related to Fatty Acid Oxidation and Energy Metabolism in HFD-Induced Obese Mice
2.10. Effects of CKDB-322 on Hepatic Lipid Accumulation in HFD-Induced Obese Mice
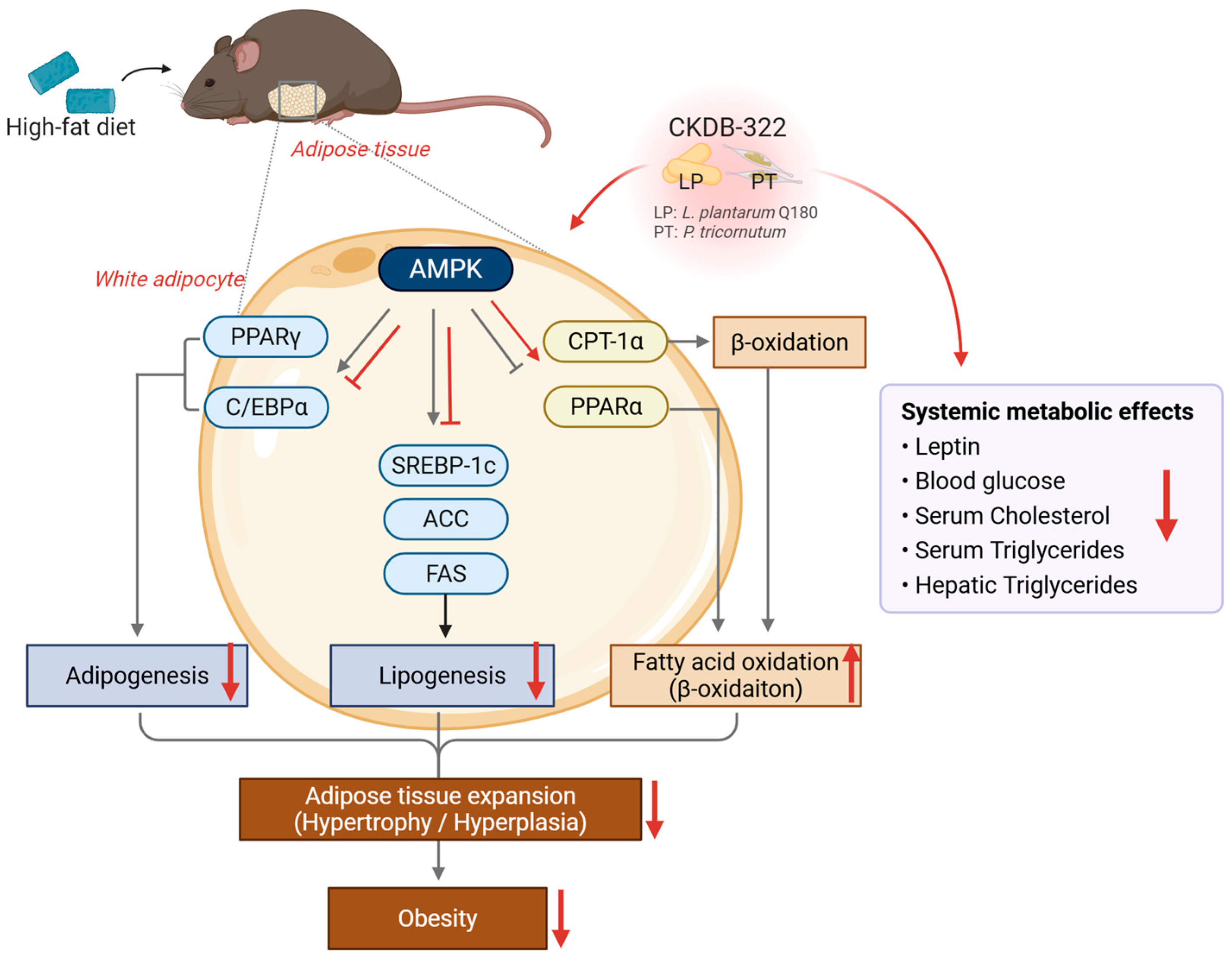
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Characterization of P. tricornutum Using GC-FID
4.2.1. Lipid Extraction from P. tricornutum
4.2.2. Derivatization of Fatty Acids into Methyl Esters
4.2.3. GC-FID Analysis of Fatty Acid Methyl Esters (FAMEs)
4.3. In Vitro Experiments
4.3.1. Cell Culture and Adipocyte Differentiation
4.3.2. RNA Extraction and RT-qPCR
4.3.3. In Vitro Lipolysis in 3T3-L1 Adipocytes
4.4. In Vivo Experiments
4.4.1. Animals and Experimental Design
4.4.2. Biochemical Analysis of Serum
4.4.3. Body Composition Analysis by Dual-Energy X-Ray Absorptiometry (DEXA)
4.4.4. Tissue Collection and Weight Measurement
4.4.5. Histological Analysis
4.4.6. Evaluation of Adipocyte Size and Number in Epididymal Adipose Tissue
4.4.7. RNA Extraction and RT-qPCR
4.4.8. Hepatic TG Content
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cottrell, E.C.; Ozanne, S.E. Early life programming of obesity and metabolic disease. Physiol. Behav. 2008, 94, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Vulchi, J.; Suryadevara, V.; Mohan, P.; Kamalanathan, S.; Sahoo, J.; Naik, D.; Sandhiya, S. Obesity and Metabolic Dysfunction-associated Fatty Liver Disease: Understanding the Intricate Link. J. Transl. Gastroenterol. 2023, 1, 74–86. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Flachs, P.; Brauner, P.; Sponarova, J.; Matejkova, O.; Prazak, T.; Ruzickova, J.; Bardova, K.; Kuda, O.; Kopecky, J. Role of energy charge and AMP-activated protein kinase in adipocytes in the control of body fat stores. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. 4), S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Marcondes-de-Castro, I.A.; Reis-Barbosa, P.H.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J. Gastroenterol. Hepatol. 2023, 38, 1868–1876. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, R. AMP-Activated Protein Kinase (AMPK): A Cellular Energy Sensor and the Guardian of Metabolism. Int. J. Biochem. Res. Rev. 2025, 34, 147–153. [Google Scholar] [CrossRef]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A review on probiotics and dietary bioactives: Insights on metabolic well-being, gut microbiota, and inflammatory responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Hols, P.; Bernard, E.; Rolain, T.; Zhou, M.; Siezen, R.J.; Bron, P.A. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 2010, 34, 199–230. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Jang, A.R.; Jung, D.H.; Lee, T.S.; Kim, J.K.; Lee, Y.B.; Lee, J.Y.; Kim, S.Y.; Yoo, Y.C.; Ahn, J.H.; Hong, E.H.; et al. Lactobacillus plantarum NCHBL-004 modulates high-fat diet-induced weight gain and enhances GLP-1 production for blood glucose regulation. Nutrition 2024, 128, 112565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus plantarum S9 alleviates lipid profile, insulin resistance, and inflammation in high-fat diet-induced metabolic syndrome rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, C.C.; Hsiao, Y.; Kao, C.H.; Chen, C.C.; Yang, H.J.; Tsai, R.Y. The Role of Lactobacillus plantarum in Reducing Obesity and Inflammation: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 7608. [Google Scholar] [CrossRef]
- He, G.; Long, H.; He, J.; Zhu, C. The Immunomodulatory Effects and Applications of Probiotic Lactiplantibacillus plantarum in Vaccine Development. Probiotics Antimicrob. Proteins 2024, 16, 2229–2250. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.I.; Chu, J.; Kim, B.K.; Choi, I.S.; Kim, J.Y. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.; Lim, S.D. The Inhibitory Effect of L. plantarum Q180 on Adipocyte Differentiation in 3T3-L1 and Reduction of Adipocyte Size in Mice Fed High-fat Diet. Korean J. Food Sci. Anim. Resour. 2018, 38, 99–109. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Peng, K.-T.; Xiong, B.-H.; Cheng, G.; Zhong, Y.-H.; Yu, L. Phosphomolybdic acid boosts polyunsaturated fatty acid and neutral lipid production in Phaeodactylum tricornutum. Front. Mar. Sci. 2025, 12, 2025. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-inflammatory effects of Phaeodactylum tricornutum extracts on human blood mononuclear cells and murine macrophages. J. Appl. Phycol. 2018, 30, 2837–2846. [Google Scholar] [CrossRef]
- Smeriglio, A.; Lionti, J.; Ingegneri, M.; Burlando, B.; Cornara, L.; Grillo, F.; Mastracci, L.; Trombetta, D. Xanthophyll-Rich Extract of Phaeodactylum tricornutum Bohlin as New Photoprotective Cosmeceutical Agent: Safety and Efficacy Assessment on In Vitro Reconstructed Human Epidermis Model. Molecules 2023, 28, 4190. [Google Scholar] [CrossRef]
- Maury, J.; Delbrut, A.; Villard, V.; Pradelles, R. A Standardized Extract of Microalgae Phaeodactylum tricornutum (Mi136) Inhibit D-Gal Induced Cognitive Dysfunction in Mice. Mar. Drugs 2024, 22, 99. [Google Scholar] [CrossRef]
- Yoo, C.; Maury, J.; Gonzalez, D.E.; Ko, J.; Xing, D.; Jenkins, V.; Dickerson, B.; Leonard, M.; Estes, L.; Johnson, S.; et al. Effects of Supplementation with a Microalgae Extract from Phaeodactylum tricornutum Containing Fucoxanthin on Cognition and Markers of Health in Older Individuals with Perceptions of Cognitive Decline. Nutrients 2024, 16, 2999. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Bäuerl, C.; Cortés-Macías, E.; Calvo-Lerma, J.; Collado, M.C.; Barba, F.J. The impact of liquid-pressurized extracts of Spirulina, Chlorella and Phaedactylum tricornutum on in vitro antioxidant, antiinflammatory and bacterial growth effects and gut microbiota modulation. Food Chem. 2023, 401, 134083. [Google Scholar] [CrossRef]
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 2021, 11, 971. [Google Scholar] [CrossRef]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; Abo-Shanab, W.A.; Ende, S.S.W.; Alquraishi, M.; El-Shenody, R.A. Optimizing Phaeodactylum tricornutum cultivation: Integrated strategies for enhancing biomass, lipid, and fucoxanthin production. Biotechnol. Biofuels Bioprod. 2025, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Weng, Y.; Ma, W.; Chang, C.; Gao, Y.; Huang, X.; Zhang, F. Improving Lipid Content in the Diatom Phaeodactylum tricornutum by the Knockdown of the Enoyl-CoA Hydratase Using CRISPR Interference. Curr. Issues Mol. Biol. 2024, 46, 10923–10933. [Google Scholar] [CrossRef] [PubMed]
- Pintaric, M.; Langerholc, T. Probiotic Mechanisms Affecting Glucose Homeostasis: A Scoping Review. Life 2022, 12, 1187. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Abdullah; Hafeez, M.A.; Guan, R.; Feng, F. Lactobacillus plantarum ZJUFB2 Prevents High Fat Diet-Induced Insulin Resistance in Association With Modulation of the Gut Microbiota. Front. Nutr. 2021, 8, 754222. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, W.; Jiang, S.; Wang, Y.; Chen, L.; Yang, G.; Liu, T. Heterotrophic modification of Phaeodactylum tricornutum Bohlin. Algal Res. 2023, 72, 103137. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-derived bioactive ingredients in functional foods for aging: Nutritional and therapeutic perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, B.; Lee, C.; Joung, H.; Kim, B.K.; Choi, I.S.; Hyun, C.K. Comprehensive amelioration of high-fat diet-induced metabolic dysfunctions through activation of the PGC-1alpha pathway by probiotics treatment in mice. PLoS ONE 2020, 15, e0228932. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S. The role of leptin and hypothalamic neuropeptides in energy homeostasis: Update on leptin in obesity. Growth Horm. IGF Res. 2001, 11 (Suppl. A), S85–S89. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shin, S.C.; Kim, G.J.; Eom, J.I.; Han, C.H.; Pan, C.H.; Lee, J.K. The beneficial effects of ethanol extract of the microalga Phaeodactylum tricornutum in alcoholic liver disease. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Hayamizu, K.; Ishii, Y.; Kaneko, I.; Suzuki, Y.; Goto, S.; Yoshino, G.; Shimasaki, H. Effects of garcinia cambogia (Hydroxycitric Acid) on visceral fat accumulation: A double-blind, randomized, placebo-controlled trial. Curr. Ther. Res. Clin. Exp. 2003, 64, 551–567. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). General Component Testing Method—2.1.5.4 Fatty Acid Determination (Method II). In Korean Food Standards Code; MFDS: Cheongju, Republic of Korea, 2024; Available online: https://www.foodsafetykorea.go.kr (accessed on 25 January 2024).

| Peak No. | Name | Contents (mg/100 g) | |
|---|---|---|---|
| 1 | Butyric acid | C4:0 | 0.00 |
| 2 | Caproic acid | C6:0 | 0.00 |
| 3 | Caprylic acid | C8:0 | 0.00 |
| 4 | Capric acid | C10:0 | 3.40 ± 0.17 |
| 5 | Undecanoic acid | C11:0 | 0.00 |
| 6 | Lauric acid | C12:0 | 29.23 ± 50.63 |
| 7 | Tridecanoic acid | C13:0 | 0.00 |
| 8 | Myristic acid | C14:0 | 936.07 ± 26.95 |
| 9 | Myristoleic acid | C14:1 | 51.70 ± 20.74 |
| 10 | Pentadecylic acid | C15:0 | 31.30 ± 0.82 |
| 11 | Cis-Pentadecenoic acid | C15:1 | 0.00 |
| 12 | Palmitic acid | C16:0 | 1290.00 ± 41.62 |
| 13 | Palmitoleic acid | C16:1n-7 | 2725.07 ± 76.21 |
| 14 | Heptadecanoic acid | C17:0 | 12.47 ± 0.40 |
| 15 | Cis-Heptadecenoic acid | C17:1 | 20.23 ± 0.60 |
| 16 | Stearic acid | C18:0 | 17.93 ± 0.47 |
| 17 | Trans-Oleic acid | C18:1-trans | 12.70 ± 0.72 |
| 18 | Oleic acid | C18:1n-9 | 1219.23 ± 49.16 |
| 19 | Trans-Linoleic acid | C18:2-trans | 82.47 ± 1.25 |
| 20 | Linoleic acid | C18:2n-6 | 572.73 ± 17.67 |
| 21 | Arachidic acid | C20:0 | 4.53 ± 0.06 |
| 22 | Gamma-linolenic acid (GLA) | C18:3n-6 | 73.77 ± 1.96 |
| 23 | Cis-11-eicosenoic acid | C20:1n-9 | 20.50 ± 0.56 |
| 24 | Alpha-linolenic acid (ALA) | C18:3n-3 | 28.67 ± 0.50 |
| 25 | Heneicosanoic acid | C21:0 | 0.00 |
| 26 | Eicosadienoic acid | C20:2n-6 | 71.57 ± 1.92 |
| 27 | Behenic acid | C22:0 | 36.20 ± 1.21 |
| 28 | Cis-11,14,17-Eicosatrienoic acid | C20:3n-3 | 20.80 ± 6.17 |
| 29 | Cis-13-docosenoic acid | C22:1n-9 | 72.33 ± 8.30 |
| 30 | Nonadecanoic acid | C19:0 | 0.00 |
| 31 | Tricosanoic acid | C23:0 | 0.00 |
| 32 | Arachidonic acid (AA) | C20:4n-6 | 372.53 ± 49.79 |
| 33 | Docosadienoic acid | C22:2n-6 | 35.67 ± 0.80 |
| 34 | Lignoceric acid | C24:0 | 221.83 ± 8.27 |
| 35 | Eicosapentaenoic acid (EPA) | C20:5n-3 | 3194.23 ± 81.27 |
| 36 | Nervonic acid | C24:1n-9 | 54.27 ± 2.59 |
| 37 | Docosahexaenoic acid (DHA) | C22:6n-3 | 300.37 ± 5.77 |
| Total | 11,511.67 ± 288.35 | ||
| Parameters | ND | HFD-Induced Obese Mice | ||||||
|---|---|---|---|---|---|---|---|---|
| HFD | GC | LP | PT | CKDB-322-L | CKDB-322-M | CKDB-322-H | ||
| Initial body weight (g) | 22.2 ± 1.2 | 22.6 ± 0.6 | 22.4 ± 1.3 | 22.4 ± 0.8 | 22.8 ± 1.2 | 22.5 ± 1.1 | 22.0 ± 0.9 | 22.3 ± 0.8 |
| Final body weight (g) | 29.3 ± 1.4 | 39.0 ± 3.0 ### | 34.5 ± 2.3 ** | 34.2 ± 1.8 *** | 36.0 ± 1.7 * | 33.5 ± 1.6 *** | 33.9 ± 2.1 *** | 33.0 ± 2.2 *** |
| Weight gain 1 (g) | 7.1 ± 1.9 | 16.4 ± 3.2 ### | 12.1 ± 2.7 ** | 11.8 ± 1.7 *** | 13.2 ± 2.2 * | 11.0 ± 1.4 *** | 11.9 ± 1.9 *** | 10.7 ± 2.2 *** |
| Food intake (g/day) | 2.8 ± 0.2 | 2.2 ± 0.0 ### | 2.2 ± 0.1 | 2.1 ± 0.0 *** | 2.1 ± 0.0 *** | 2.1 ± 0.1 *** | 2.1 ± 0.1 *** | 2.1 ± 0.1 *** |
| FER 2 | 0.047 ± 0.0 | 0.133 ± 0.0 ### | 0.100 ± 0.0 ** | 0.100 ± 0.0 ** | 0.112 ± 0.0 | 0.094 ± 0.0 *** | 0.102 ± 0.0 ** | 0.092 ± 0.0 *** |
| Parameters | ND | HFD-Induced Obese Mice | ||||||
|---|---|---|---|---|---|---|---|---|
| HFD | GC | LP | PT | CKDB-322-L | CKDB-322-M | CKDB-322-H | ||
| Glucose (mg/dL) | 168.5 ± 36.7 | 221.9 ± 23.9 ## | 168.1 ± 36.8 ** | 198.8 ± 30.1 | 193.3 ± 31.2 * | 177.0 ± 36.6 ** | 172.3 ± 17.9 *** | 256.8 ± 45.8 |
| TG (mg/dL) | 47.1 ± 8.9 | 61.1 ± 6.4 ### | 67.3 ± 16.1 | 61.9 ± 23.3 | 62.6 ± 5.7 | 49.8 ± 5.4 *** | 42.6 ± 9.2 *** | 44.5 ± 1.1 *** |
| Total cholesterol (mg/dL) | 106.2 ± 12.4 | 149.0 ± 9.0 ### | 120.9 ± 25.5 ** | 119.2 ± 12.1 *** | 142.5 ± 16.4 | 119.1 ± 21.3 *** | 117.3 ± 12.5 *** | 129.1 ± 15.8 ** |
| LDL-cholesterol (mg/dL) | 10.8 ± 2.7 | 18.0 ± 3.8 ### | 15.5 ± 3.6 | 14.7 ± 4.0 | 18.3 ± 3.8 | 13.5 ± 3.3 * | 14.4 ± 3.8 * | 16.4 ± 3.2 |
| HDL-cholesterol (mg/dL) | 97.0 ± 7.7 | 134.4 ± 10.0 ### | 106.9 ± 15.4 *** | 104.0 ± 14.2 *** | 125.0 ± 18.4 | 116.3 ± 10.9 ** | 115.3 ± 10.4 *** | 123.4 ± 17.5 |
| AST (U/L) | 110.9 ± 21.5 | 121.5 ± 32.8 | 118.1 ± 24.4 | 111.9 ± 28.3 | 114.2 ± 24.5 | 118.6 ± 32.6 | 104.1 ± 19.5 | 113.0 ± 27.5 |
| ALT (U/L) | 61.0 ± 22.0 | 86.3 ± 33.0 | 76.0 ± 37.4 | 65.6 ± 24.9 | 76.1 ± 19.7 | 64.3 ± 29.0 | 70.0 ± 20.5 | 82.1 ± 59.9 |
| Parameters | ND | HFD-Induced Obese Mice | ||||||
|---|---|---|---|---|---|---|---|---|
| HFD | GC | LP | PT | CKDB-322-L | CKDB-322-M | CKDB-322-H | ||
| Fat in tissue 1 (%) | 25.2 ± 3.6 | 40.5 ± 2.0 ### | 33.9 ± 5.1 ** | 34.9 ± 2.7 *** | 37.1 ± 4.2 * | 32.8 ± 4.6 *** | 35.2 ± 3.2 *** | 34.0 ± 4.0 *** |
| White adipose tissue (g) | ||||||||
| Total weight | 1.53 ± 0.44 | 4.75 ± 0.91 ### | 3.15 ± 0.88 *** | 3.28 ± 0.73 *** | 4.00 ± 0.76 | 2.95 ± 0.67 *** | 3.29 ± 0.50 *** | 3.24 ± 0.65 *** |
| Epididymal fat | 0.78 ± 0.23 | 2.44 ± 0.41 ### | 1.63 ± 0.48 *** | 1.67 ± 0.46 *** | 2.04 ± 0.44 | 1.50 ± 0.39 *** | 1.71 ± 0.29 *** | 1.61 ± 0.33 *** |
| Retroperitoneal fat | 0.32 ± 0.10 | 0.94 ± 0.12 ### | 0.69 ± 0.18 ** | 0.75 ± 0.15 ** | 0.91 ± 0.17 | 0.62 ± 0.10 *** | 0.71 ± 0.13 *** | 0.71 ± 0.14 *** |
| Mesenteric fat | 0.25 ± 0.07 | 0.77 ± 0.33 ### | 0.42 ± 0.16 ** | 0.41 ± 0.11 ** | 0.52 ± 0.12 * | 0.41 ± 0.12 ** | 0.42 ± 0.10 ** | 0.47 ± 0.16 * |
| Inguinal fat | 0.18 ± 0.08 | 0.59 ± 0.12 ### | 0.41 ± 0.12 ** | 0.45 ± 0.09 * | 0.54 ± 0.12 | 0.42 ± 0.14 ** | 0.45 ± 0.07 ** | 0.45 ± 0.12 * |
| Liver (g) | 0.95 ± 0.04 | 1.06 ± 0.12 # | 0.90 ± 0.06 ** | 0.88 ± 0.10 ** | 0.86 ± 0.06 *** | 0.80 ± 0.07 *** | 0.81 ± 0.05 *** | 0.84 ± 0.05 *** |
| Gene | Primer Sequence | |
|---|---|---|
| PPARγ | F: | 5′-GCCCACCAACTTCGGAATC-3′ |
| R: | 5′-TGCGAGTGGTCTTCCATCAC-3′ | |
| C/EBPα | F: | 5′-GAGCTGAGTGAGGCTCTCATTCT-3′ |
| R: | 5′-TGGGAGGCAGACGAAAAAAC-3′ | |
| SREBP-1c | F: | 5′-CCAGAGGGTGAGCCTGACAA-3′ |
| R: | 5′-AGCCTCTGCAATTTCCAGATCT-3′ | |
| ACC | F: | 5′-CGAGTCCTCTCCTCAGCTCC-3′ |
| R: | 5′-ATCGGGAGTGCTGGTTTAGC-3′ | |
| FAS | F: | 5′-CAAGTGTCCACCAACAAGCG-3′ |
| R: | 5′-GGAGCGCAGGATAGACTCAC-3′ | |
| CPT-1α | F: | 5′-GACTCCGCTCGCTCATTCC-3′ |
| R: | 5′-ACGCCACTCACGATGTTCTT-3′ | |
| PPARα | F: | 5′-CCGAACATTGGTGTTCGCAG-3′ |
| R: | 5′-AGATACGCCCAAATGCACCA-3′ | |
| PGC-1α | F: | 5′-TCTCAGTAAGGGGCTGGTTG-3′ |
| R: | 5′-TTCCGATTGGTCGCTACACC-3′ | |
| AMPK | F: | 5′-AGCCCTTCCTTCTCTTGCTC-3′ |
| R: | 5′-AGGATGCCTGAAAAGCTTGA-3′ | |
| Leptin | F: | 5′-GAGACCCCTGTGTCGGTTC-3′ |
| R: | 5′-CTGCGTGTGTGAAATGTCATTG-3′ | |
| β-actin | F: | 5′-CATTGCTGACAGGATGCAGAAGG-3′ |
| R: | 5′-TGCTGGAAGGTGGACAGTGAGG-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, H.-J.; Eom, J.-I.; Park, S.-J.; Shin, C.H.; Kim, S.-M.; Pan, C.-H.; Lee, J.K. Synergistic Anti-Obesity Effects of Lactiplantibacillus plantarum Q180 and Phaeodactylum tricornutum (CKDB-322) in High-Fat-Diet-Induced Obese Mice. Int. J. Mol. Sci. 2025, 26, 7991. https://doi.org/10.3390/ijms26167991
Noh H-J, Eom J-I, Park S-J, Shin CH, Kim S-M, Pan C-H, Lee JK. Synergistic Anti-Obesity Effects of Lactiplantibacillus plantarum Q180 and Phaeodactylum tricornutum (CKDB-322) in High-Fat-Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2025; 26(16):7991. https://doi.org/10.3390/ijms26167991
Chicago/Turabian StyleNoh, Hye-Ji, Jae-In Eom, Soo-Je Park, Chang Hun Shin, Se-Min Kim, Cheol-Ho Pan, and Jae Kwon Lee. 2025. "Synergistic Anti-Obesity Effects of Lactiplantibacillus plantarum Q180 and Phaeodactylum tricornutum (CKDB-322) in High-Fat-Diet-Induced Obese Mice" International Journal of Molecular Sciences 26, no. 16: 7991. https://doi.org/10.3390/ijms26167991
APA StyleNoh, H.-J., Eom, J.-I., Park, S.-J., Shin, C. H., Kim, S.-M., Pan, C.-H., & Lee, J. K. (2025). Synergistic Anti-Obesity Effects of Lactiplantibacillus plantarum Q180 and Phaeodactylum tricornutum (CKDB-322) in High-Fat-Diet-Induced Obese Mice. International Journal of Molecular Sciences, 26(16), 7991. https://doi.org/10.3390/ijms26167991






