Altered β-Adrenergic System, Cardiac Dysfunction, and Lethal Arrhythmia in a Rat Model of Metabolic Syndrome
Abstract
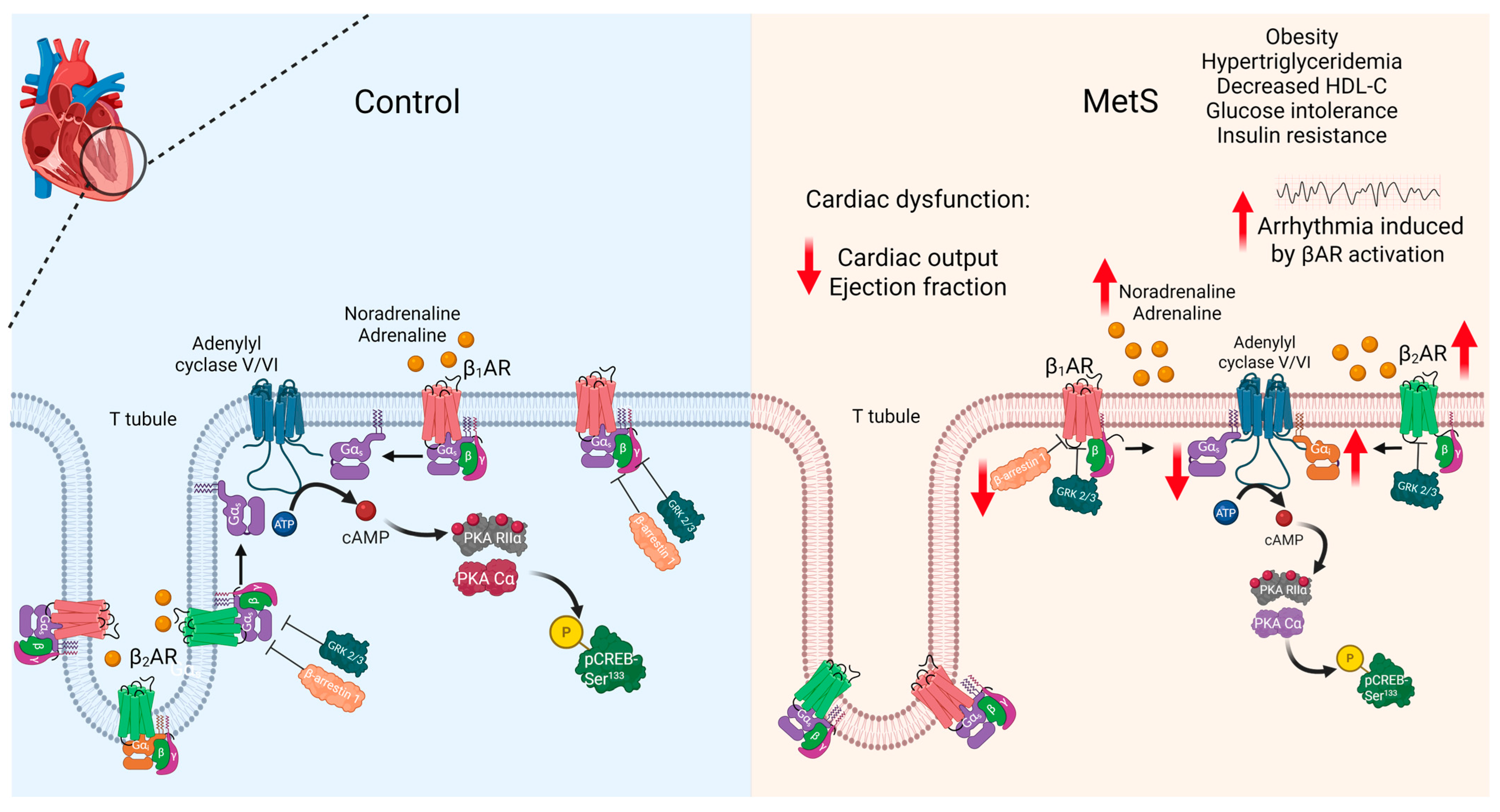
1. Introduction
2. Results
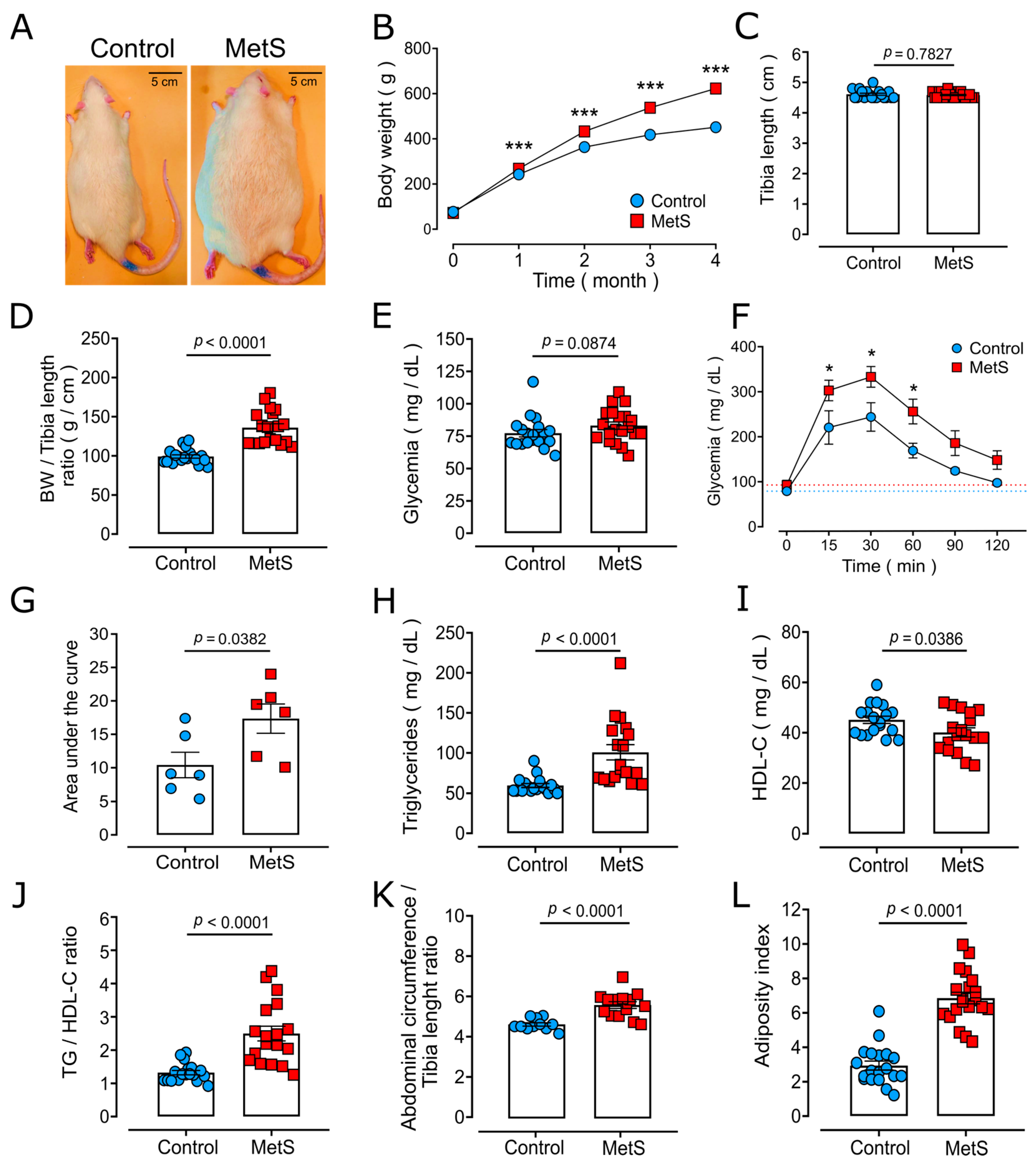
2.1. Characterization of the MetS Model
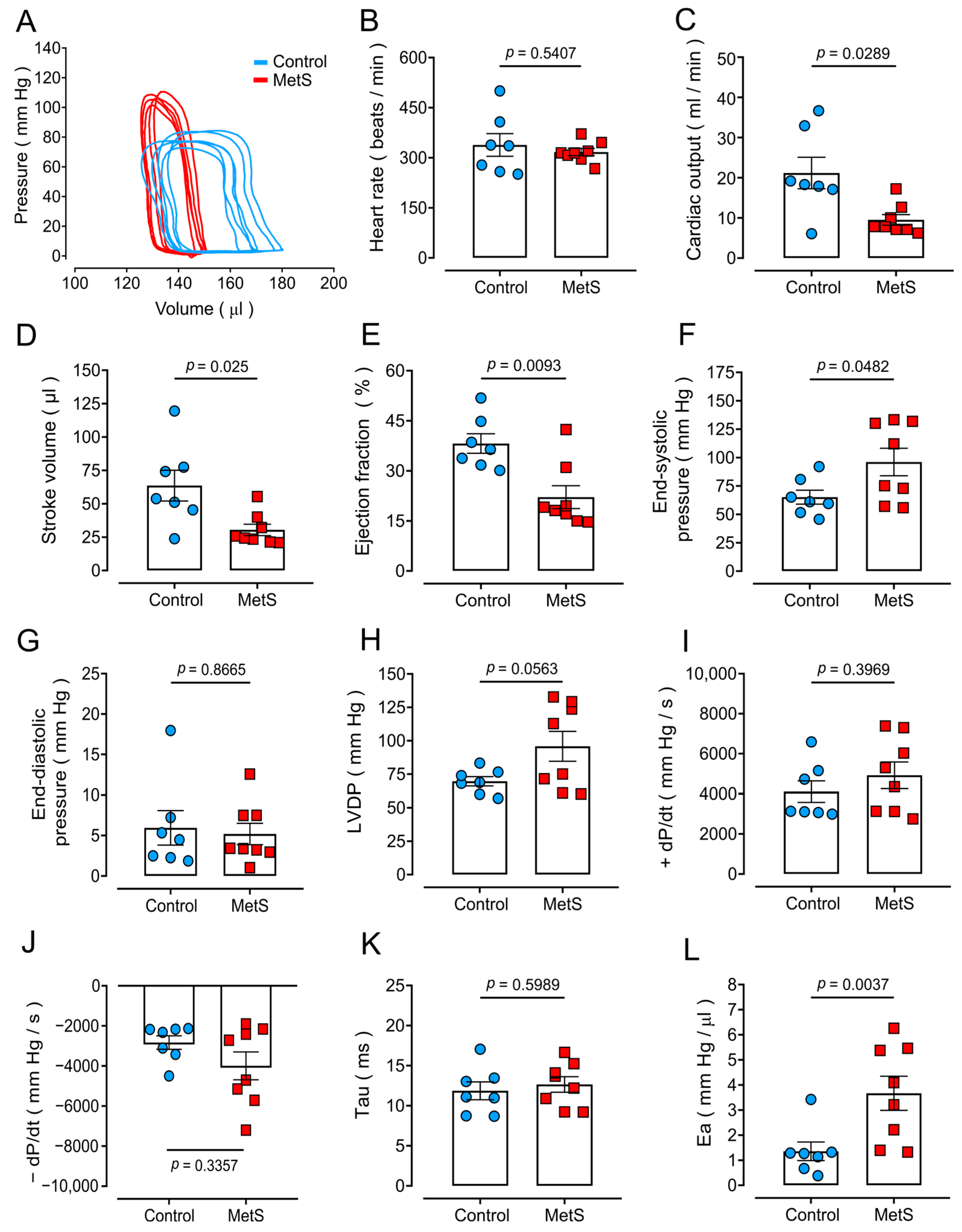
2.2. Altered Cardiac Function in MetS Rats
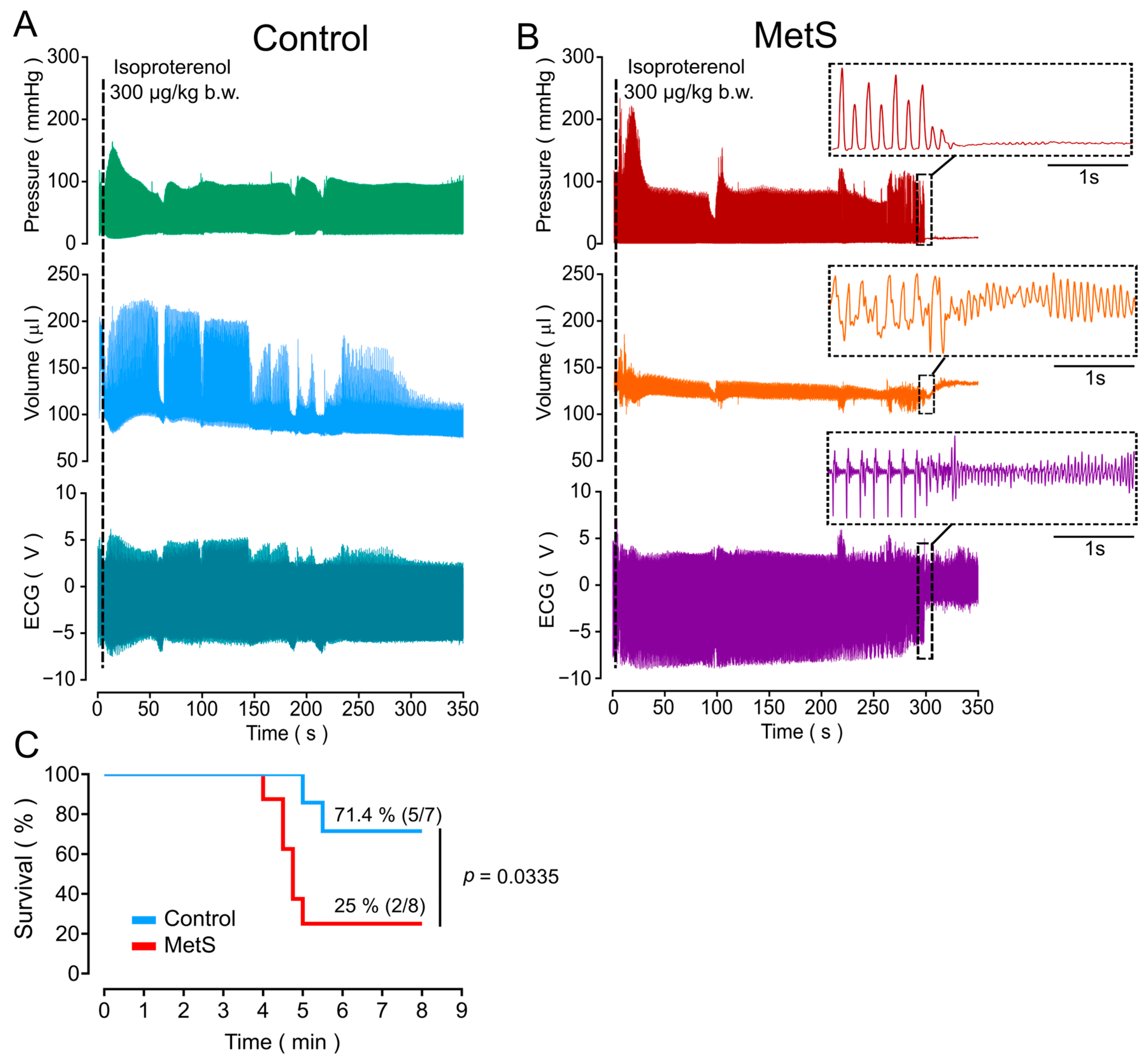
2.3. Higher Susceptibility of MetS Rats to Lethal Arrhythmia Induced by β-Adrenergic Stimulation
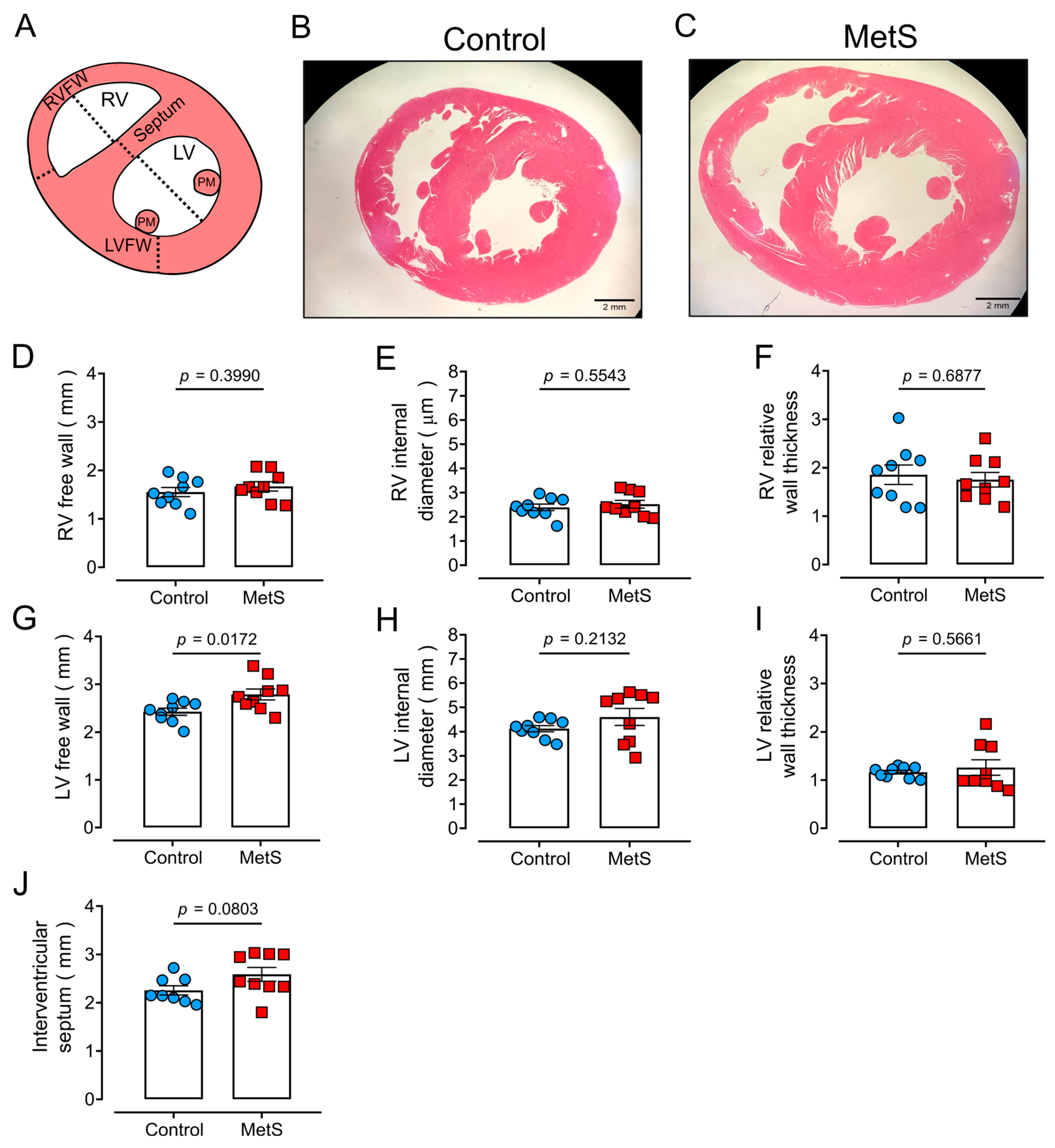
2.4. Ultrastructural Cardiac Remodeling in MetS Hearts
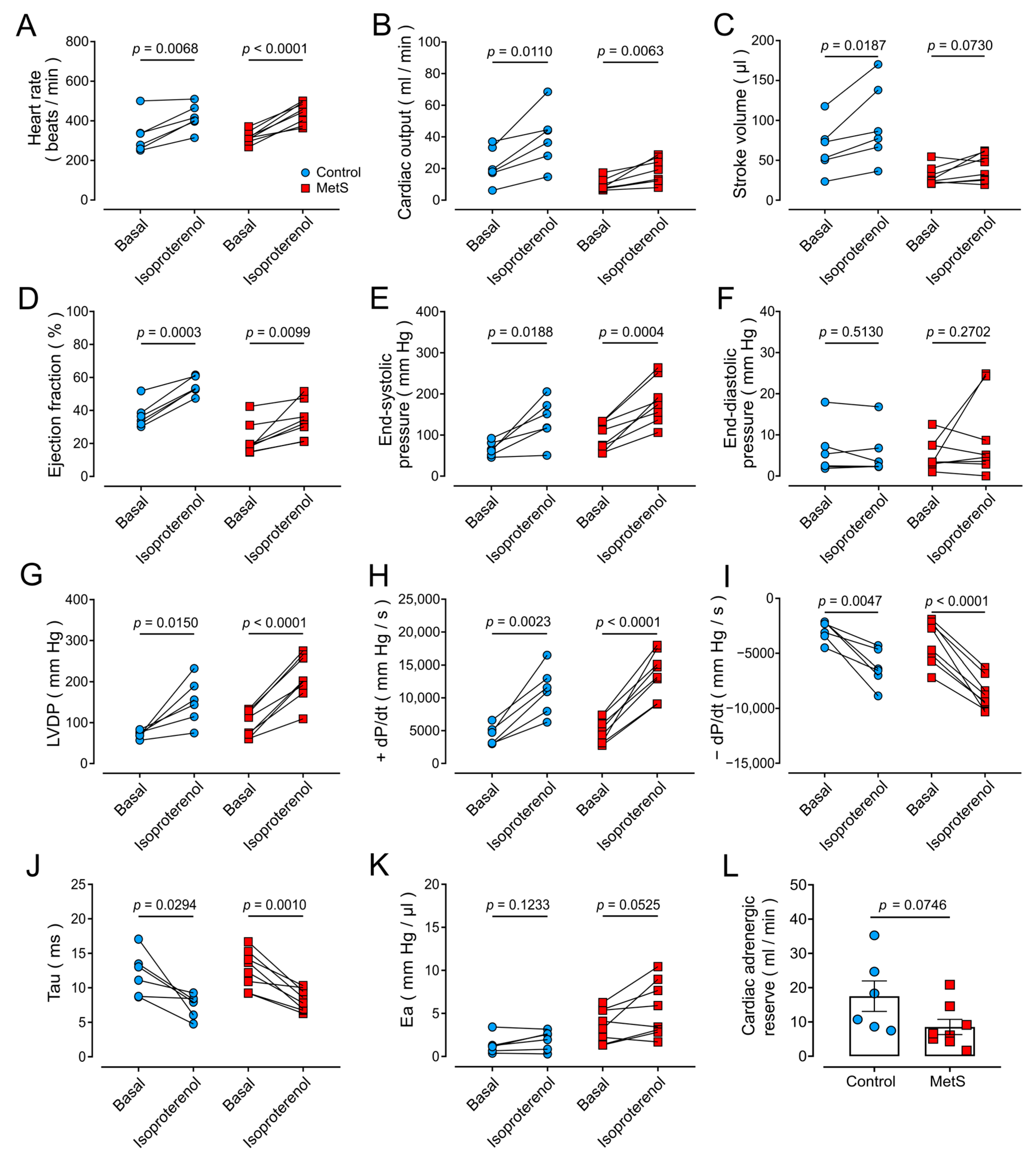
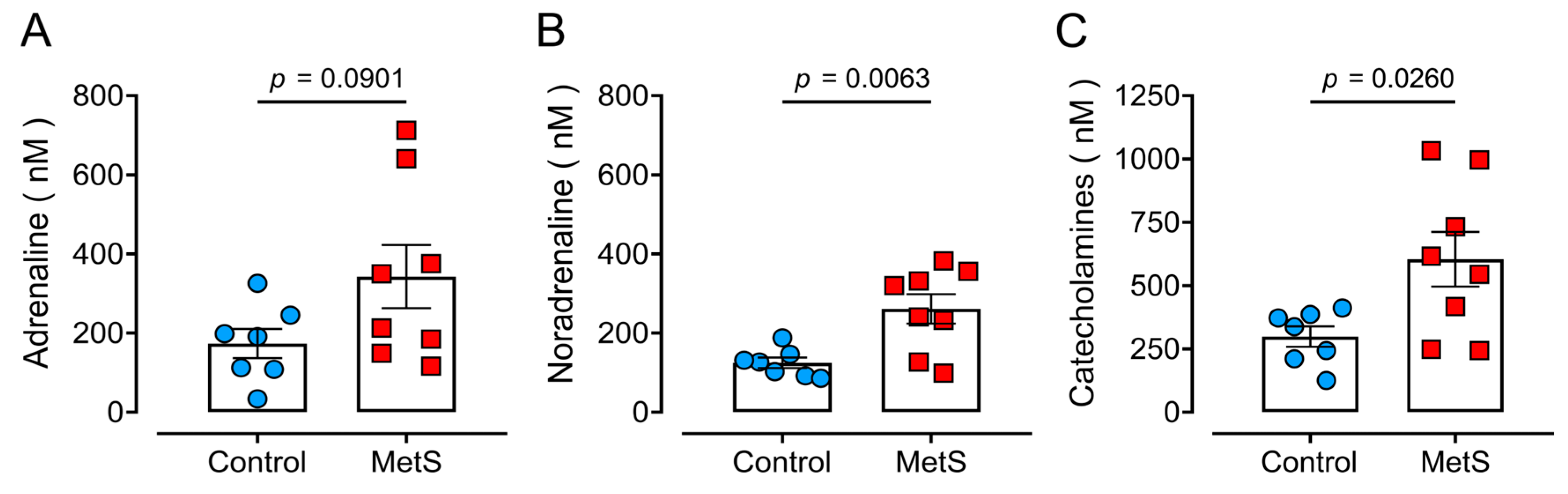
2.5. Hyperadrenergic Basal State in MetS Rats
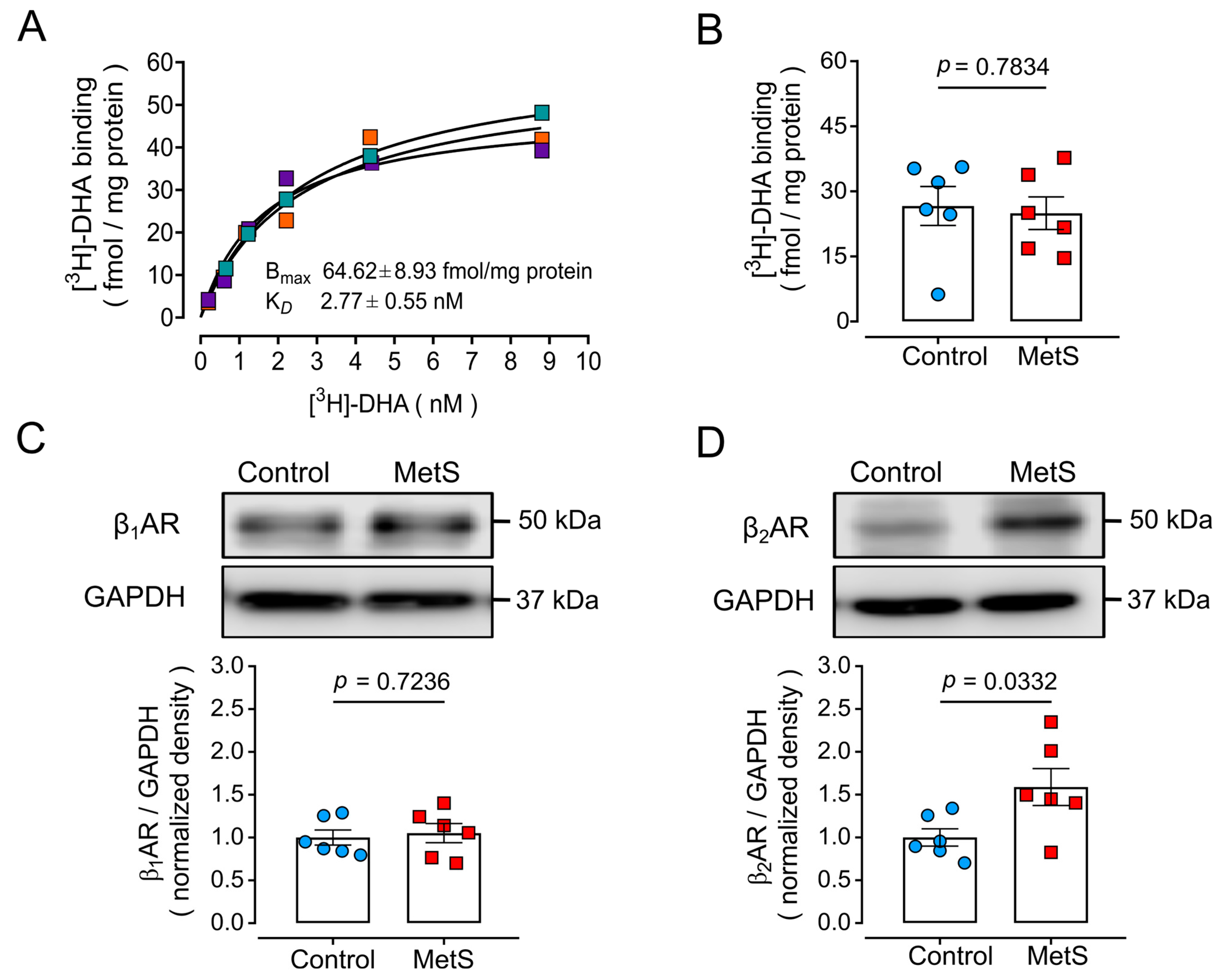
2.6. Altered βAR Expression in MetS Hearts
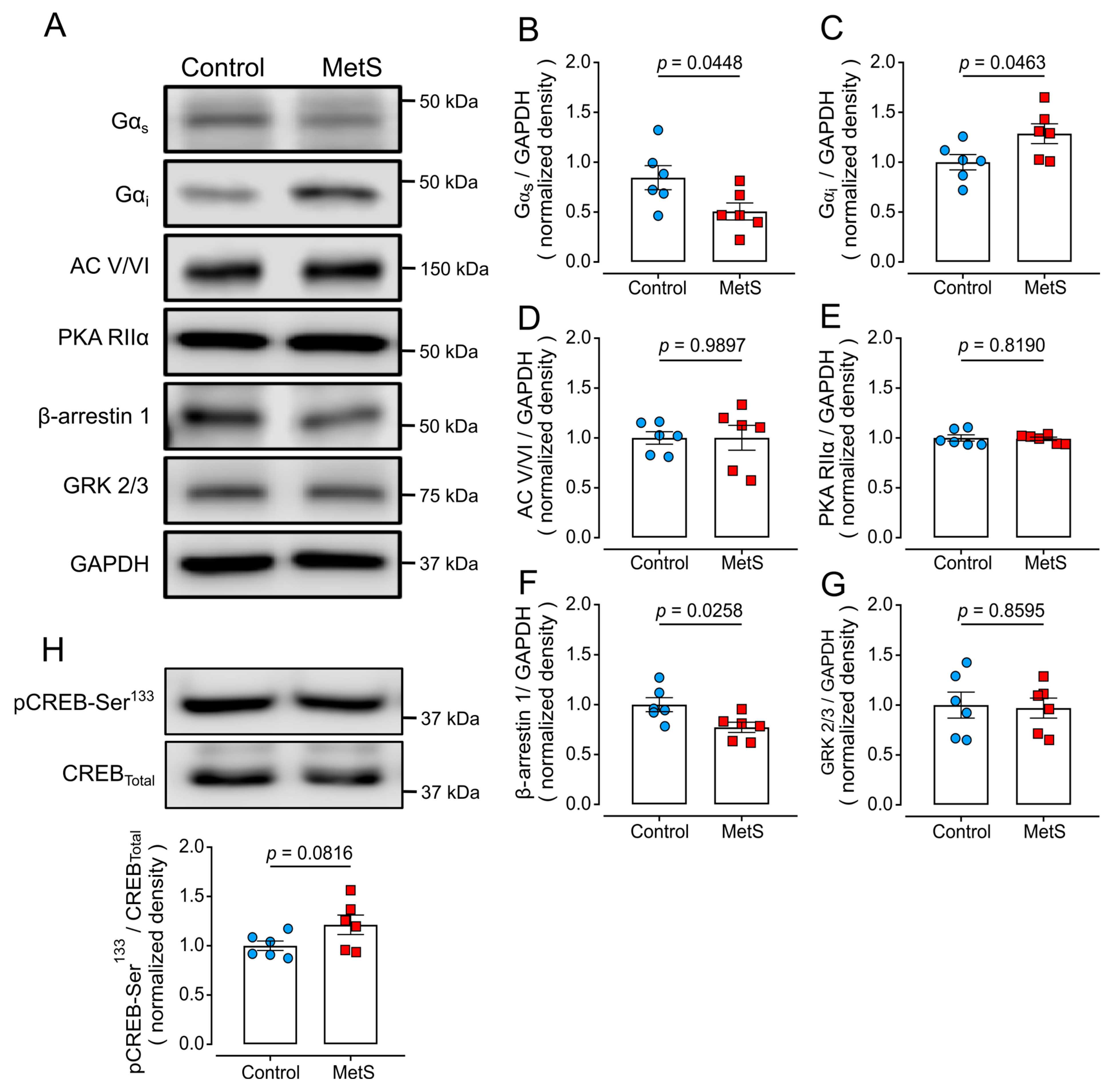
2.7. Altered Expression of Proteins of the β-Adrenergic Signaling Pathway in LV Extracts of MetS Rats
3. Discussion
Limitations of This Study
4. Materials and Methods
4.1. Sucrose-Induced MetS in Rats
4.2. Biochemical Characterization of the MetS Model
4.3. Determination of Obesity
4.4. Glucose Tolerance Test (GTT)
4.5. Determination of Serum Catecholamine Levels
4.6. Evaluation of Heart Hypertrophy and Fibrosis
4.7. Evaluation of Cardiac Function
4.8. Preparation of LV Homogenates
4.9. Preparation of Membranes for Radioligand Binding Assays
4.10. [3H]-Dihydroalprenolol ([3H]-DHA) Binding Assay
4.11. SDS-PAGE and Western Blotting
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| βAR | β-adrenergic receptor |
| BW | Body weight |
| CO | Cardiac output |
| CVD | Cardiovascular disease |
| Ea | Arterial elastance |
| EF | Ejection fraction |
| GPCR | G protein-coupled receptor |
| GRK | GPCR kinase |
| HDL-C | Cholesterol associated to high-density lipoproteins |
| HW | Heart weight |
| LV | Left ventricle |
| LVW | LV weight |
| LVFW | LV free wall |
| MetS | Metabolic syndrome |
| PKA | Protein kinase A |
| RV | Right ventricle |
| RVW | RV weight |
| RVFW | RV free wall |
| TG | Triglyceride |
| SV | Stroke volume |
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between Prediabetes and Risk of All Cause Mortality and Cardiovascular Disease: Updated Meta-Analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef]
- Grassi, G.; Dell’Oro, R.; Quarti-Trevano, F.; Scopelliti, F.; Seravalle, G.; Paleari, F.; Gamba, P.L.; Mancia, G. Neuroadrenergic and Reflex Abnormalities in Patients with Metabolic Syndrome. Diabetologia 2005, 48, 1359–1365. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Wu, S.; Zhang, G.Q.; Peng, M.; Wu, Y.; Zheng, X.; Ruan, C.; Zhang, W. Metabolic Syndrome Is Associated with and Predicted by Resting Heart Rate: A Cross-Sectional and Longitudinal Study. Heart 2015, 101, 44–49. [Google Scholar] [CrossRef]
- Lee, Z.S.; Critchley, J.A.; Tomlinson, B.; Young, R.P.; Thomas, G.N.; Cockram, C.S.; Chan, T.Y.; Chan, J.C. Urinary Epinephrine and Norepinephrine Interrelations with Obesity, Insulin, and the Metabolic Syndrome in Hong Kong Chinese. Metabolism 2001, 50, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Vanoli, J.; Molisano, C.; Merati, V.; Grassi, G. Heart Rate Thresholds for Cardiovascular Risk and Sympathetic Activation in the Metabolic Syndrome. Acta Diabetol. 2022, 59, 1429–1435. [Google Scholar] [CrossRef]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic Syndrome: A Sympathetic Disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to Pharmacology 2019/20: G Protein-coupled Receptors. Br. J. Pharmacol. 2019, 176, S46–S48. [Google Scholar] [CrossRef]
- Vago, T.; Bevilacqua, M.; Dagani, R.; Meroni, R.; Frigeni, G.; Santoli, C.; Norbiato, G. Comparison of Rat and Human Left Ventricle Beta-Adrenergic Receptors: Subtype Heterogeneity Delineated by Direct Radioligand Binding. Biochem. Biophys. Res. Commun. 1984, 121, 346–354. [Google Scholar] [CrossRef]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional β3-Adrenoceptor in the Human Heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac Excitation-Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Devic, E.; Xiang, Y.; Gould, D.; Kobilka, B. β-Adrenergic Receptor Subtype-Specific Signaling in Cardiac Myocytes from β1 and β2 Adrenoceptor Knockout Mice. Mol. Pharmacol. 2001, 60, 577–583. [Google Scholar] [CrossRef]
- Okatan, E.N.; Tuncay, E.; Hafez, G.; Turan, B. Profiling of Cardiac β-Adrenoceptor Subtypes in the Cardiac Left Ventricle of Rats with Metabolic Syndrome: Comparison with Streptozotocin-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 2015, 93, 517–525. [Google Scholar] [CrossRef]
- Fu, Q.; Hu, Y.; Wang, Q.; Liu, Y.; Li, N.; Xu, B.; Kim, S.; Chiamvimonvat, N.; Xiang, Y.K. High-Fat Diet Induces Protein Kinase A and G-Protein Receptor Kinase Phosphorylation of β2-Adrenergic Receptor and Impairs Cardiac Adrenergic Reserve in Animal Hearts. J. Physiol. 2017, 595, 1973–1986. [Google Scholar] [CrossRef] [PubMed]
- Landa-Galvan, H.V.; Rios-Castro, E.; Romero-Garcia, T.; Rueda, A.; Olivares-Reyes, J.A. Metabolic Syndrome Diminishes Insulin-Induced Akt Activation and Causes a Redistribution of Akt-Interacting Proteins in Cardiomyocytes. PLoS ONE 2020, 15, e0228115. [Google Scholar] [CrossRef]
- Romero-García, T.; Landa-Galvan, H.V.; Pavón, N.; Mercado-Morales, M.; Valdivia, H.H.; Rueda, A. Autonomous Activation of CaMKII Exacerbates Diastolic Calcium Leak during β-Adrenergic Stimulation in Cardiomyocytes of Metabolic Syndrome Rats. Cell Calcium 2020, 91, 102267. [Google Scholar] [CrossRef]
- Erickson, J.R. Mechanisms of CaMKII Activation in the Heart. Front. Pharmacol. 2014, 5, 59. [Google Scholar] [CrossRef]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Aizpurúa, M.; Maciel, P.M.; Reaven, G.M. Identification of Cardiometabolic Risk: Visceral Adiposity Index versus Triglyceride/HDL Cholesterol Ratio. Am. J. Med. 2014, 127, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.C.; Spurgeon, H.A.; Rakusan, K.; Weisfeldt, M.L.; Lakatta, E.G. Use of Tibial Length to Quantify Cardiac Hypertrophy: Application in the Aging Rat. Am. J. Physiol. 1982, 243, H941–H947. [Google Scholar] [CrossRef]
- Berg, B.N.; Harmison, C.R. Growth, Disease, and Aging in the Rat. J. Gerontol. 1957, 12, 370–377. [Google Scholar] [CrossRef]
- Biton, Y.; Goldenberg, I.; Kutyifa, V.; Baman, J.R.; Solomon, S.; Moss, A.J.; Szepietowska, B.; McNitt, S.; Polonsky, B.; Zareba, W.; et al. Relative Wall Thickness and the Risk for Ventricular Tachyarrhythmias in Patients with Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 67, 303–312. [Google Scholar] [CrossRef]
- Xiao, R.P.; Tomhave, E.D.; Wang, D.J.; Ji, X.; Boluyt, M.O.; Cheng, H.; Lakatta, E.G.; Koch, W.J. Age-Associated Reductions in Cardiac β1- and β2-Adrenergic Responses without Changes in Inhibitory G Proteins or Receptor Kinases. J. Clin. Investig. 1998, 101, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Göttle, M.; Geduhn, J.; König, B.; Gille, A.; Höcherl, K.; Seifert, R. Characterization of Mouse Heart Adenylyl Cyclase. J. Pharmacol. Exp. Ther. 2009, 329, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.; van Veen, T.A.B.; Vos, M.A.; Heck, A.J.R. Diversity of cAMP-Dependent Protein Kinase Isoforms and Their Anchoring Proteins in Mouse Ventricular Tissue. J. Proteome Res. 2007, 6, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Zoccarato, A.; Lissandron, V.; Terrin, A.; Li, X.; Houslay, M.D.; Baillie, G.S.; Zaccolo, M. Protein Kinase A Type I and Type II Define Distinct Intracellular Signaling Compartments. Circ. Res. 2008, 103, 836–844. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Devesa, A.; Fuster, V.; Vazirani, R.; García-Lunar, I.; Oliva, B.; España, S.; Moreno-Arciniegas, A.; Sanz, J.; Perez-Herreras, C.; Bueno, H.; et al. Cardiac Insulin Resistance in Subjects with Metabolic Syndrome Traits and Early Subclinical Atherosclerosis. Diabetes Care 2023, 46, 2050–2057. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Nammi, S. Chronic Treatment of (R)-α-lipoic Acid Reduces Blood Glucose and Lipid Levels in High-fat Diet and Low-dose Streptozotocin-induced Metabolic Syndrome and Type 2 Diabetes in Sprague-Dawley Rats. Pharmacol. Res. Perspect. 2017, 5, e00306. [Google Scholar] [CrossRef]
- Biessels, G.-J.; Cristino, N.A.; Rutten, G.-J.; Hamers, F.P.T.; Erkelens, D.W.; Gispen, W.H. Neurophysiological Changes in the Central and Peripheral Nervous System of Streptozotocin-Diabetic Rats. Brain 1999, 122, 757–768. [Google Scholar] [CrossRef]
- Gaitán-González, P.; Sánchez-Hernández, R.; Arias-Montaño, J.-A.; Rueda, A. Tale of Two Kinases: Protein Kinase A and Ca 2+/Calmodulin-Dependent Protein Kinase II in Pre-Diabetic Cardiomyopathy. World J. Diabetes 2021, 12, 1704–1718. [Google Scholar] [CrossRef]
- Fernández-Miranda, G.; Romero-Garcia, T.; Barrera-Lechuga, T.P.; Mercado-Morales, M.; Rueda, A. Impaired Activity of Ryanodine Receptors Contributes to Calcium Mishandling in Cardiomyocytes of Metabolic Syndrome Rats. Front. Physiol. 2019, 10, 520. [Google Scholar] [CrossRef]
- Burger, P.M.; Koudstaal, S.; Dorresteijn, J.A.N.; Savarese, G.; Van Der Meer, M.G.; De Borst, G.J.; Mosterd, A.; Visseren, F.L.J. Metabolic Syndrome and Risk of Incident Heart Failure in Non-Diabetic Patients with Established Cardiovascular Disease. Int. J. Cardiol. 2023, 379, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Perrone-Filardi, P.; Paolillo, S.; Costanzo, P.; Savarese, G.; Trimarco, B.; Bonow, R.O. The Role of Metabolic Syndrome in Heart Failure. Eur. Heart J. 2015, 36, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Matthia, E.L.; Setteducato, M.L.; Elzeneini, M.; Vernace, N.; Salerno, M.; Kramer, C.M.; Keeley, E.C. Circulating Biomarkers in Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e027618. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Schoenauer, R.; Ehler, E.; Agarkova, I.; Bennett, P.M. Cardiomyocyte Growth and Sarcomerogenesis at the Intercalated Disc. Cell. Mol. Life Sci. 2014, 71, 165–181. [Google Scholar] [CrossRef]
- Federico, M.; Portiansky, E.L.; Sommese, L.; Alvarado, F.J.; Blanco, P.G.; Zanuzzi, C.N.; Dedman, J.; Kaetzel, M.; Wehrens, X.H.T.; Mattiazzi, A.; et al. Calcium-calmodulin-dependent Protein Kinase Mediates the Intracellular Signalling Pathways of Cardiac Apoptosis in Mice with Impaired Glucose Tolerance. J. Physiol. 2017, 595, 4089–4108. [Google Scholar] [CrossRef]
- Carvajal, K.; Baños, G.; Moreno-Sánchez, R. Impairment of Glucose Metabolism and Energy Transfer in the Hypertriglyceridemic Rat Heart. Mol. Cell. Biochem. 2003, 249, 157–165. [Google Scholar] [CrossRef]
- Federico, M.; De La Fuente, S.; Palomeque, J.; Sheu, S. The Role of Mitochondria in Metabolic Disease: A Special Emphasis on Heart Dysfunction. J. Physiol. 2021, 599, 3477–3493. [Google Scholar] [CrossRef]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The Role of Mitochondrial Dynamics in Cardiovascular Diseases. Br. J. Pharmacol. 2021, 178, 2060–2076. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Radziuk, J.; Harper, M.-E.; Suuronen, E.J.; Ascah, K.J.; Beanlands, R.S.; DaSilva, J.N. Sympathetic Nervous Dysregulation in the Absence of Systolic Left Ventricular Dysfunction in a Rat Model of Insulin Resistance with Hyperglycemia. Cardiovasc. Diabetol. 2011, 10, 75. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, Y.-T.; Kalthof, B.; Iwase, M.; Sato, N.; Uechi, M.; Vatner, S.; Vatner, D. Identification and Functional Role of β-Adrenergic Receptor Subtypes in Primate and Rodent: In Vivo versus Isolated Myocytes. J. Mol. Cell. Cardiol. 1996, 28, 1307–1317. [Google Scholar] [CrossRef]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. β2-Adrenergic Receptor Redistribution in Heart Failure Changes cAMP Compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Fu, Q.; Xu, B.; Zhang, Y.; Kim, S.; Tan, R.; Barbagallo, F.; West, T.; Anderson, E.; et al. Inhibiting Insulin-Mediated β2-Adrenergic Receptor Activation Prevents Diabetes-Associated Cardiac Dysfunction. Circulation 2017, 135, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, Q.; Xiang, Y.K. Insulin and β Adrenergic Receptor Signaling: Crosstalk in Heart. Trends Endocrinol. Metab. 2017, 28, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bathgate-Siryk, A.; Dabul, S.; Pandya, K.; Walklett, K.; Rengo, G.; Cannavo, A.; De Lucia, C.; Liccardo, D.; Gao, E.; Leosco, D.; et al. Negative Impact of β-Arrestin-1 on Post-Myocardial Infarction Heart Failure via Cardiac and Adrenal-Dependent Neurohormonal Mechanisms. Hypertension 2014, 63, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Post, S.R.; Hilal-Dandan, R.; Urasawa, K.; Brunton, L.L.; Insel, P.A. Quantification of Signalling Components and Amplification in the β-Adrenergic-Receptor-Adenylate Cyclase Pathway in Isolated Adult Rat Ventricular Myocytes. Biochem. J. 1995, 311, 75–80. [Google Scholar] [CrossRef]
- Chelu, M.G.; Wehrens, X.H.T. Sarcoplasmic Reticulum Calcium Leak and Cardiac Arrhythmias. Biochem. Soc. Trans. 2007, 35, 952–956. [Google Scholar] [CrossRef]
- Federico, M.; Valverde, C.A.; Mattiazzi, A.; Palomeque, J. Unbalance Between Sarcoplasmic Reticulum Ca2 + Uptake and Release: A First Step Toward Ca2 + Triggered Arrhythmias and Cardiac Damage. Front. Physiol. 2020, 10, 1630. [Google Scholar] [CrossRef]
- Fernández-Sada, E.; Torres-Quintanilla, A.; Silva-Platas, C.; García, N.; Willis, B.C.; Rodríguez-Rodríguez, C.; De La Peña, E.; Bernal-Ramírez, J.; Treviño-Saldaña, N.; Oropeza-Almazán, Y.; et al. Proinflammatory Cytokines Are Soluble Mediators Linked with Ventricular Arrhythmias and Contractile Dysfunction in a Rat Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2017, 2017, 7682569. [Google Scholar] [CrossRef]
- Johnson, D.M.; Antoons, G. Arrhythmogenic Mechanisms in Heart Failure: Linking β-Adrenergic Stimulation, Stretch, and Calcium. Front. Physiol. 2018, 9, 1453. [Google Scholar] [CrossRef]
- Büttner, P.; Augstein, A.; Abdellatif, M.; Lourenço, A.; Leite-Moreira, A.; Falcão-Pires, I.; Werner, S.; Thiele, H.; Sedej, S.; Schauer, A.; et al. Lean ZSF1 Rats in Basic Research on Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2025, 12, 1474–1478. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Myagmar, B.-E.; Flynn, J.M.; Cowley, P.M.; Swigart, P.M.; Montgomery, M.D.; Thai, K.; Nair, D.; Gupta, R.; Deng, D.X.; Hosoda, C.; et al. Adrenergic Receptors in Individual Ventricular Myocytes: The β1 and α1B Are in All Cells, the α1A Is in a Subpopulation, and the β2 and β3 Are Mostly Absent. Circ. Res. 2017, 120, 1103–1115. [Google Scholar] [CrossRef]
- Park, M.; Reddy, G.R.; Wallukat, G.; Xiang, Y.K.; Steinberg, S.F. Β1-Adrenergic Receptor O-Glycosylation Regulates N-Terminal Cleavage and Signaling Responses in Cardiomyocytes. Sci. Rep. 2017, 7, 7890. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Steinberg, S.F. Carvedilol Prevents Redox Inactivation of Cardiomyocyte Β1-Adrenergic Receptors. JACC Basic. Transl. Sci. 2018, 3, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.F. Redox and Proteolytic Regulation of Cardiomyocyte Β1-Adrenergic Receptors—A Novel Paradigm for the Regulation of Catecholamine Responsiveness in the Heart. Front. Immunol. 2023, 14, 1306467. [Google Scholar] [CrossRef]
- Ramos-Jiménez, J.; Soria-Jasso, L.-E.; López-Colombo, A.; Reyes-Esparza, J.-A.; Camacho, J.; Arias-Montaño, J.-A. Histamine Augments β2-Adrenoceptor-Induced Cyclic AMP Accumulation in Human Prostate Cancer Cells DU-145 Independently of Known Histamine Receptors. Biochem. Pharmacol. 2007, 73, 814–823. [Google Scholar] [CrossRef] [PubMed]
| Control | MetS | p | |
|---|---|---|---|
| HW (g) | 2.22 ± 0.07 | 2.48 ± 0.06 | 0.0083 |
| RVW (g) | 0.52 ± 0.03 | 0.56 ± 0.02 | 0.3165 |
| LVW (g) | 1.16 ± 0.06 | 1.27 ± 0.03 | 0.0813 |
| HW/BW | 0.48 ± 0.01 | 0.40 ± 0.01 | 0.0009 |
| LVW/HW | 0.52 ± 0.01 | 0.52 ± 0.01 | 0.8772 |
| RVW/HW | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.5518 |
| HW/TL (g/cm) | 0.48 ± 0.02 | 0.54 ± 0.01 | 0.0089 |
| LVW/TL (g/cm) | 0.25 ± 0.01 | 0.28 ± 0.01 | 0.0870 |
| RVW/TL (g/cm) | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.3237 |
| Control | MetS | p Value | |
|---|---|---|---|
| HR | 31.00 ± 7.54% | 38.96 ± 4.99% | 0.3770 |
| CO | 92.90 ± 18.19% | 92.65 ± 26.34% | 0.9944 |
| SV | 46.09 ± 8.74% | 39.60 ± 17.98% | 0.7756 |
| EF | 50.64 ± 7.28% | 65.79 ± 21.05% | 0.5608 |
| ESP | 107.90 ± 34.35% | 101.60 ± 22.80% | 0.8752 |
| EDP | −1.84 ± 11.75% | 97.91 ± 88.68% | 0.3571 |
| LVDP | 113.30 ± 32.77% | 126.60 ± 19.37% | 0.7182 |
| +dP/dt | 165.50 ± 25.51% | 202.70 ± 39.50% | 0.4885 |
| −dP/dt | 127.00 ± 31.78% | 157.10 ± 35.55% | 0.5545 |
| τ | −34.54 ± 8.83% | −33.30 ± 4.61% | 0.8955 |
| Ea | 46.60 ± 24.60% | 61.36 ± 25.57% | 0.6229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, R.; Cruz-Villarreal, D.E.; Silva-Palacios, A.; Zúñiga-Muñoz, A.-M.; Soria-Castro, E.; Sánchez-Garibay, C.; Zazueta, C.; Olivares-Reyes, J.A.; Arias-Montaño, J.-A.; Rueda, A. Altered β-Adrenergic System, Cardiac Dysfunction, and Lethal Arrhythmia in a Rat Model of Metabolic Syndrome. Int. J. Mol. Sci. 2025, 26, 7989. https://doi.org/10.3390/ijms26167989
Sánchez-Hernández R, Cruz-Villarreal DE, Silva-Palacios A, Zúñiga-Muñoz A-M, Soria-Castro E, Sánchez-Garibay C, Zazueta C, Olivares-Reyes JA, Arias-Montaño J-A, Rueda A. Altered β-Adrenergic System, Cardiac Dysfunction, and Lethal Arrhythmia in a Rat Model of Metabolic Syndrome. International Journal of Molecular Sciences. 2025; 26(16):7989. https://doi.org/10.3390/ijms26167989
Chicago/Turabian StyleSánchez-Hernández, Rommel, Daphne E. Cruz-Villarreal, Alejandro Silva-Palacios, Alejandra-María Zúñiga-Muñoz, Elizabeth Soria-Castro, Carlos Sánchez-Garibay, Cecilia Zazueta, J. Alberto Olivares-Reyes, José-Antonio Arias-Montaño, and Angélica Rueda. 2025. "Altered β-Adrenergic System, Cardiac Dysfunction, and Lethal Arrhythmia in a Rat Model of Metabolic Syndrome" International Journal of Molecular Sciences 26, no. 16: 7989. https://doi.org/10.3390/ijms26167989
APA StyleSánchez-Hernández, R., Cruz-Villarreal, D. E., Silva-Palacios, A., Zúñiga-Muñoz, A.-M., Soria-Castro, E., Sánchez-Garibay, C., Zazueta, C., Olivares-Reyes, J. A., Arias-Montaño, J.-A., & Rueda, A. (2025). Altered β-Adrenergic System, Cardiac Dysfunction, and Lethal Arrhythmia in a Rat Model of Metabolic Syndrome. International Journal of Molecular Sciences, 26(16), 7989. https://doi.org/10.3390/ijms26167989









