Assessment of the Significance of LP-PLA2, DPYSL2, and 8-OHdG in the Oncological Diagnosis of Patients with Brain Tumors and Vitamin D Deficiency
Abstract
1. Introduction
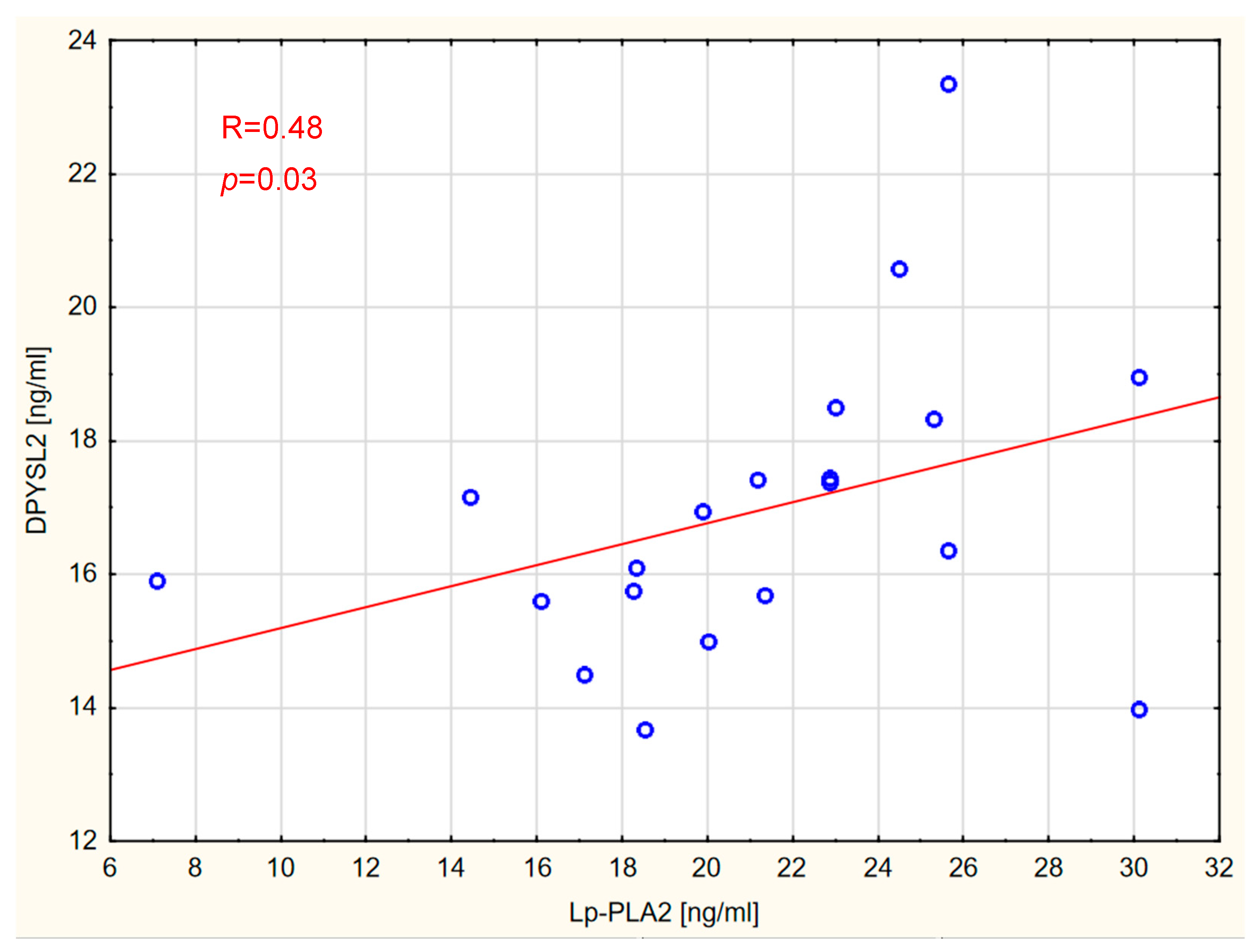
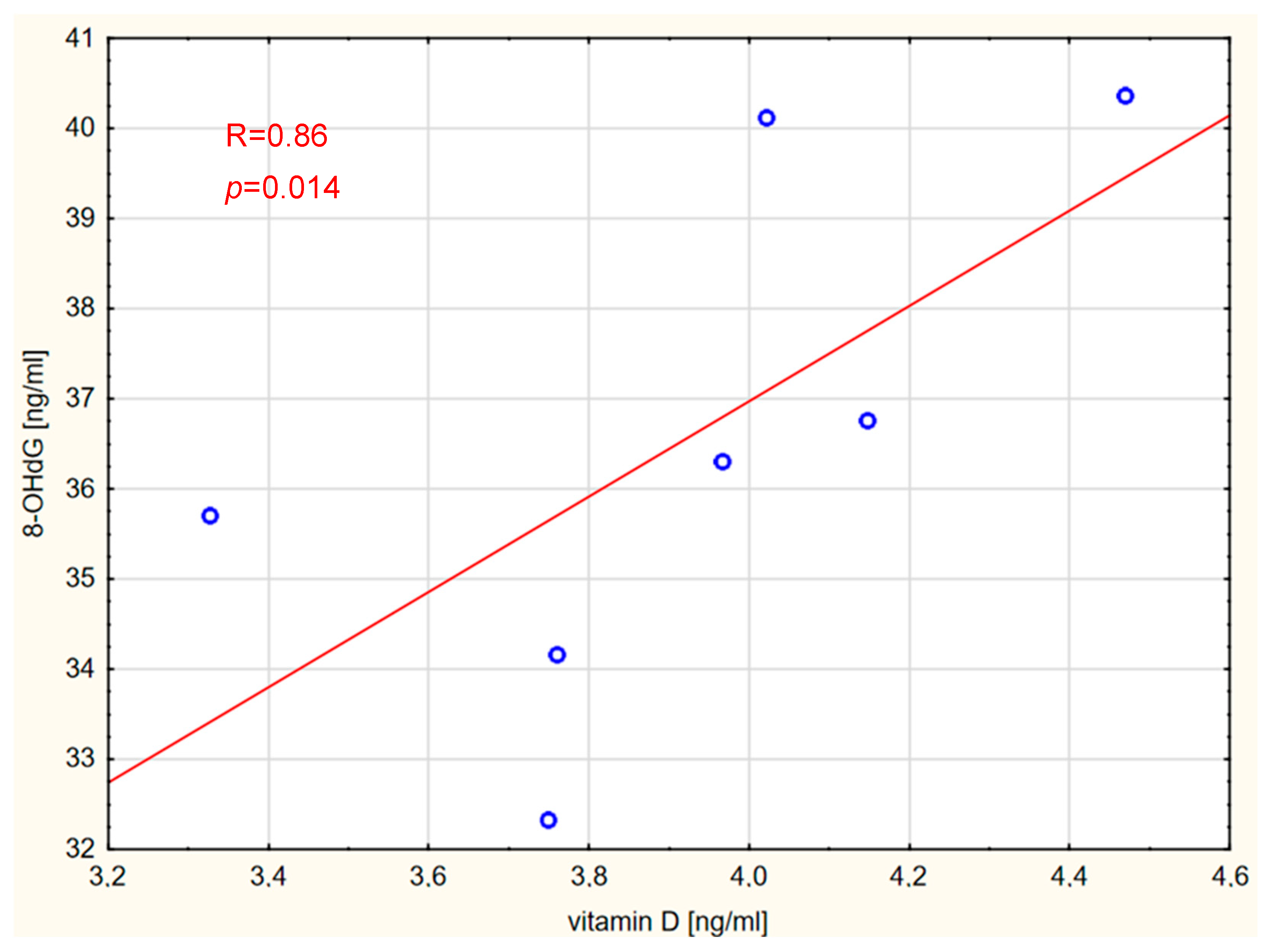
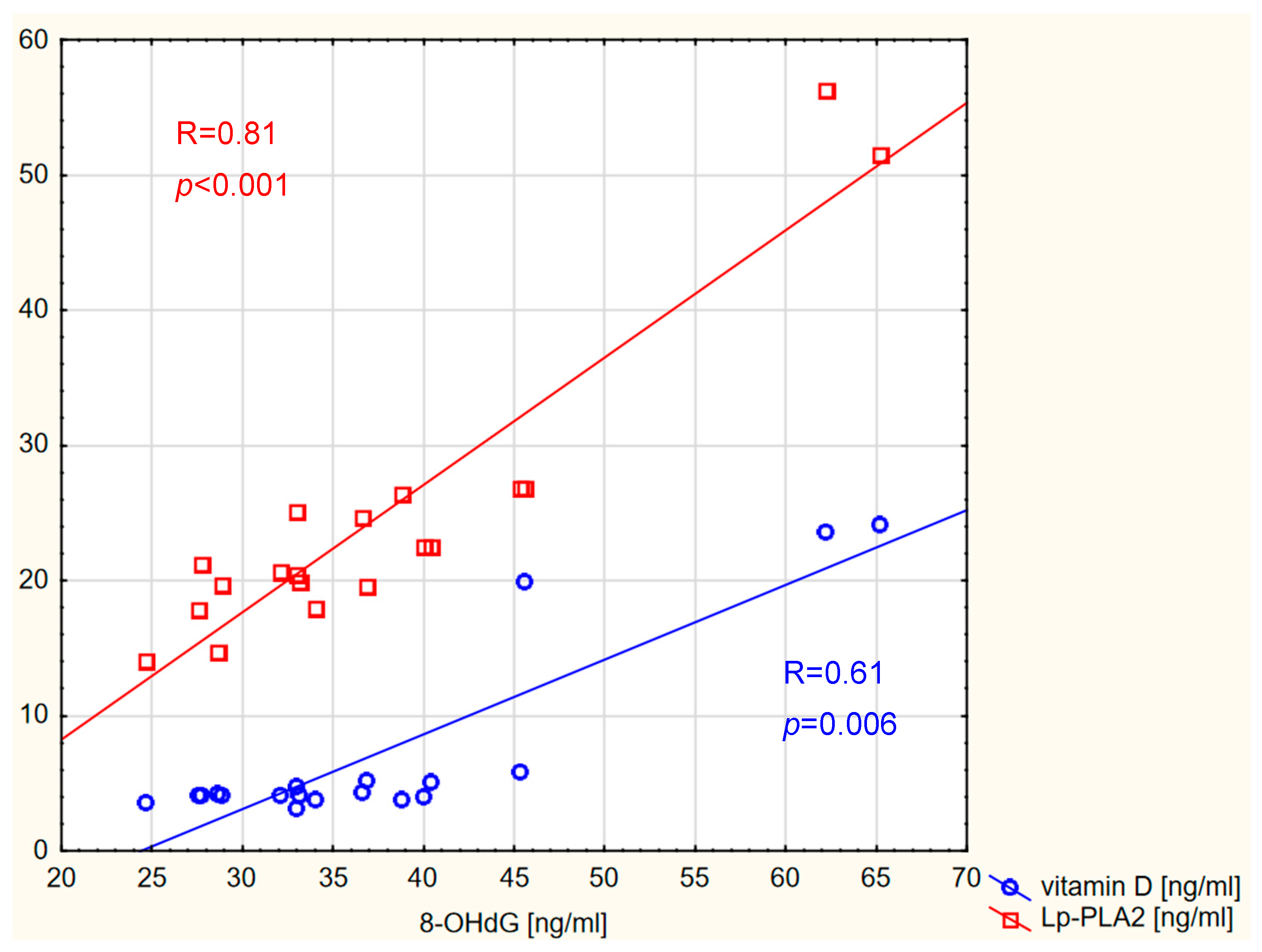
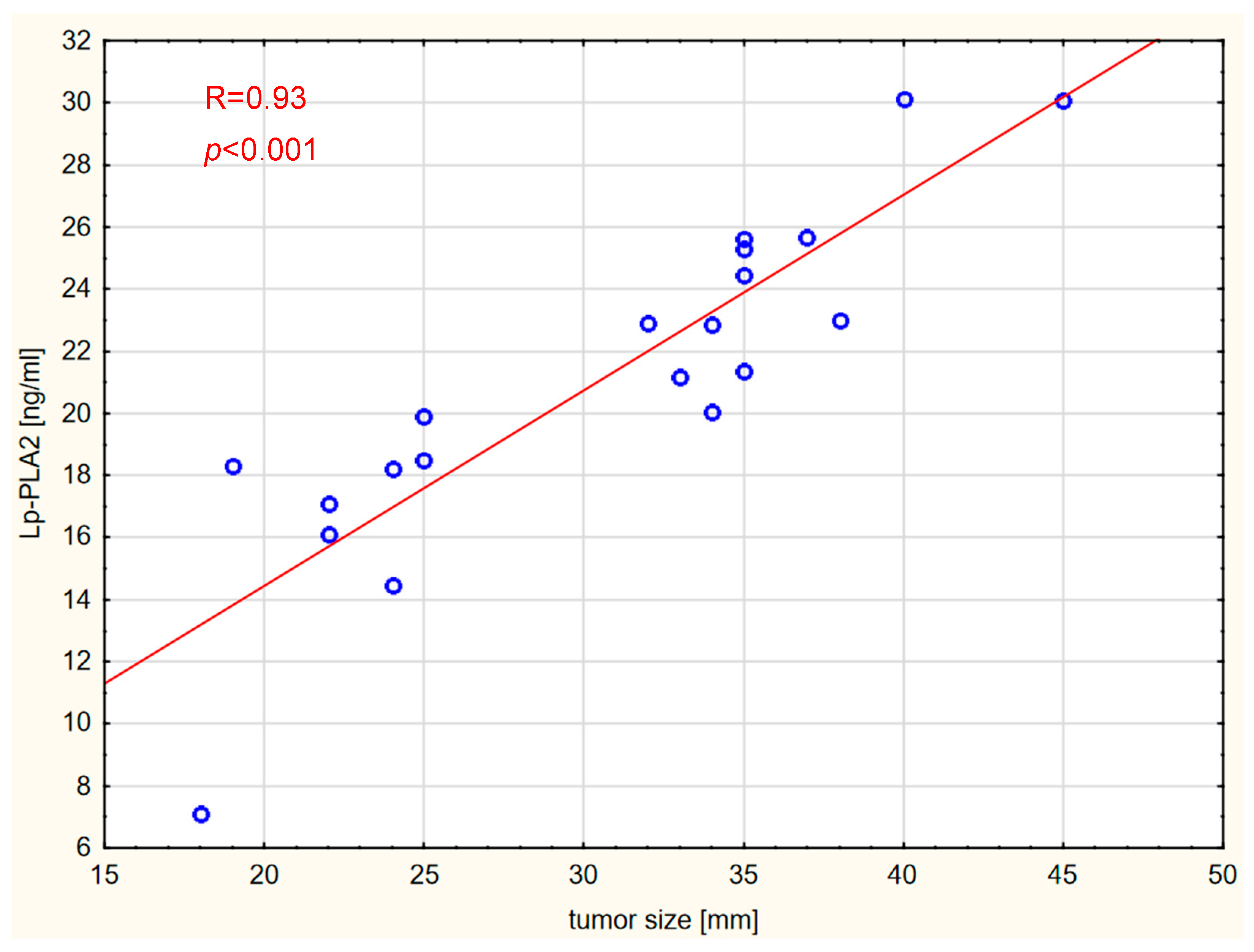
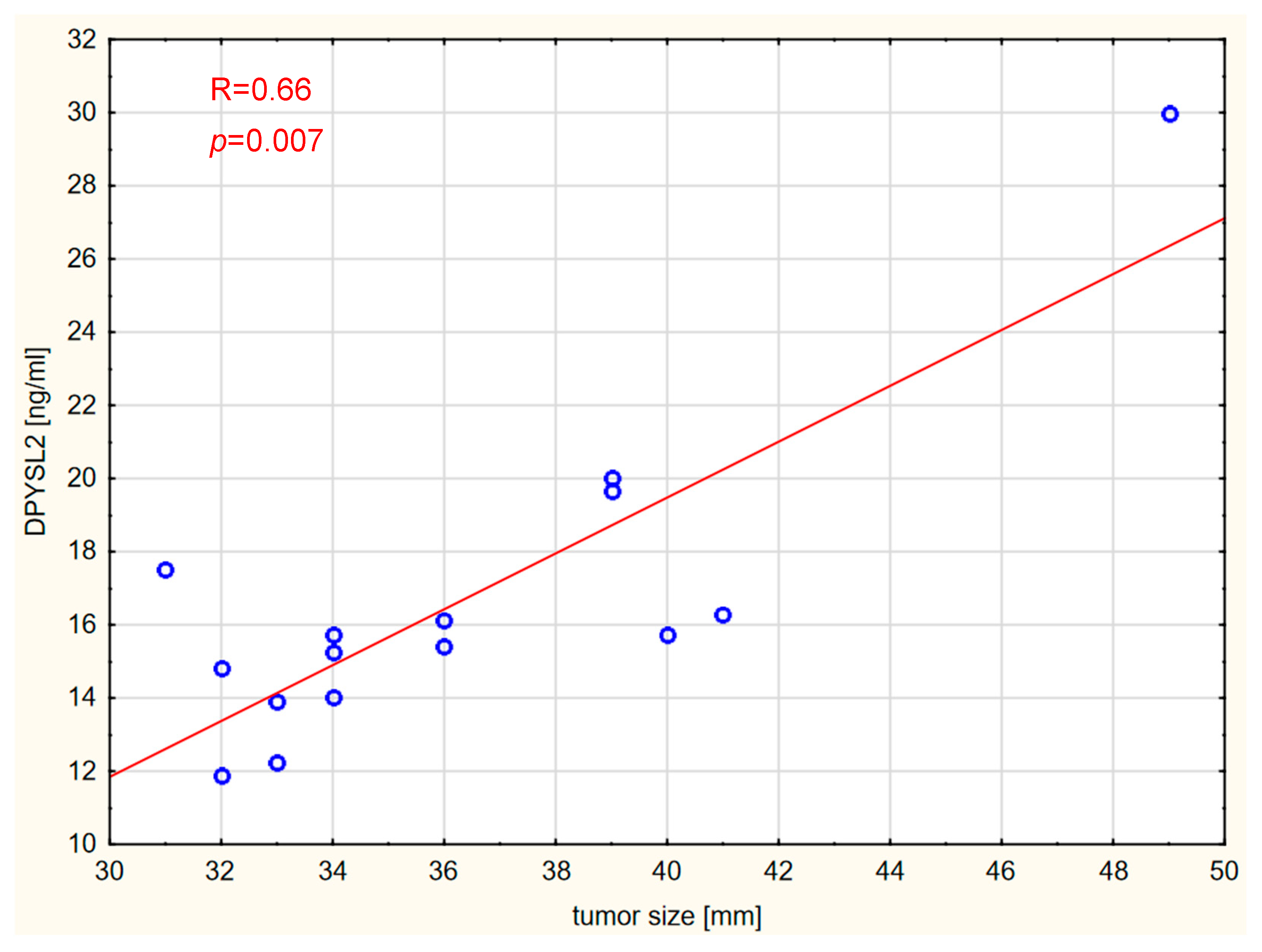
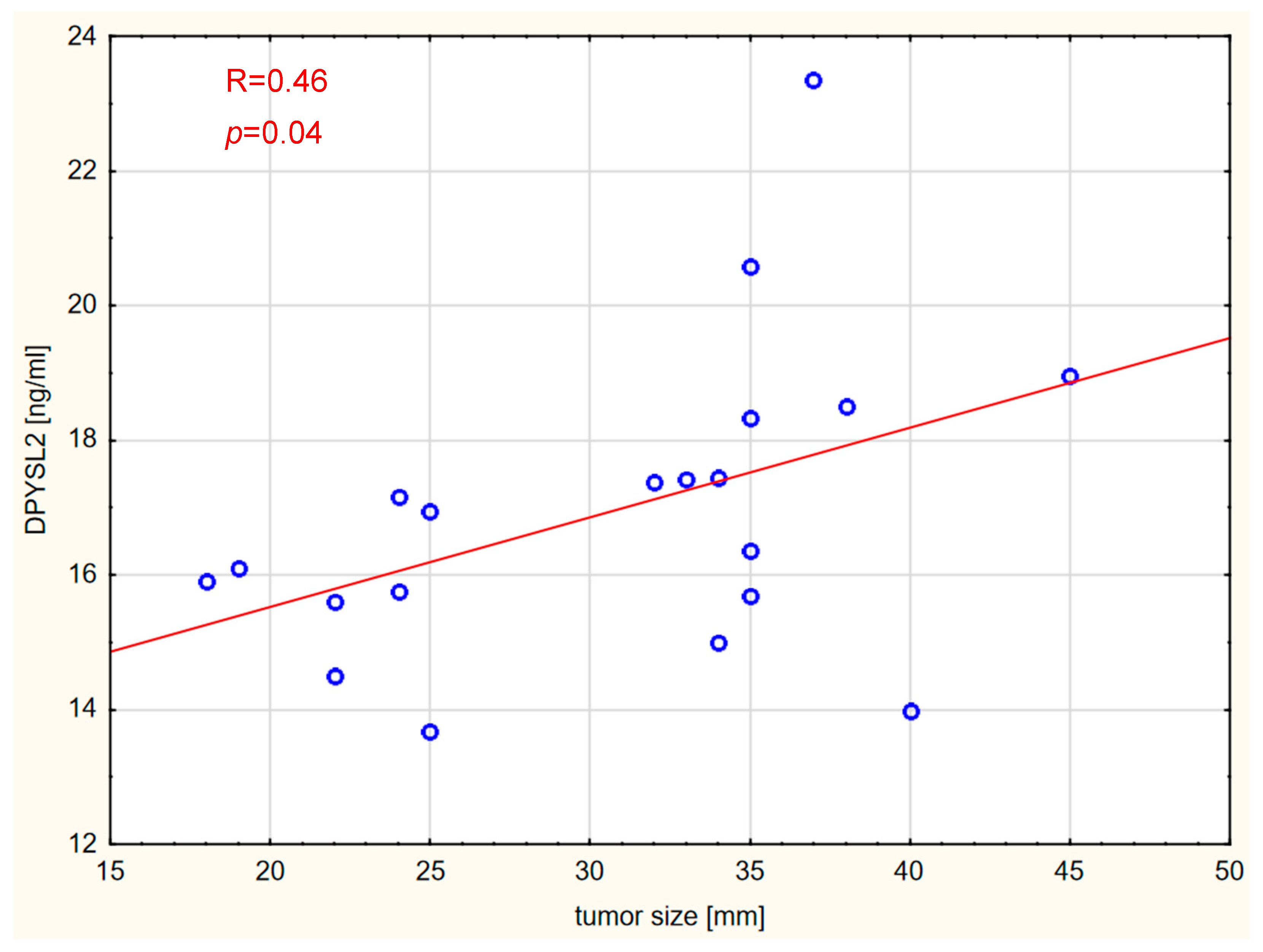
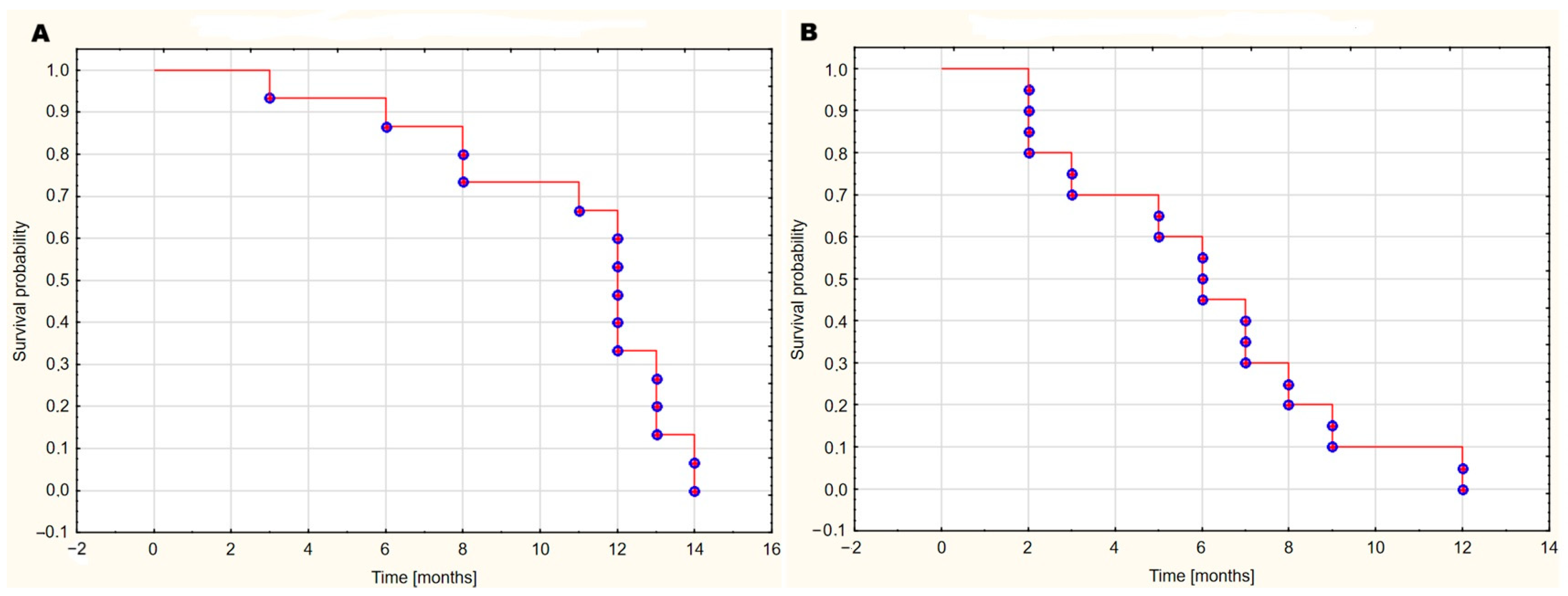
2. Results
3. Discussion
4. Materials and Methods
4.1. Bioethics
4.2. Study Cohort
4.3. Measurements
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AA | arachidonic acid |
| CNS | central nervous system |
| CRP | C-reactive protein |
| DPYSL2 | dihydropyrimidinase-like 2 |
| GBM | glioblastoma multiforme WHO 4 |
| IL-6 | interleukin-6 |
| IL-11 | interleukin-11 |
| IL-13 | interleukin-13 |
| JAK1/STAT3 | Janus kinase 1/signal transducer and activator of transcription 3 |
| Lp-PLA2 | lipoprotein-associated phospholipase A2 |
| LUAD | lung adenocarcinoma |
| NSCLC | non-small cell lung cancer |
| PGE2 | prostaglandin E2 |
| PGD2 | prostaglandin D2 |
| PGI | prostaglandin I |
| PLA2 | phospholipase A2 |
| PLA2G7 | group VIIA phospholipase A2 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor-alpha |
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro. Oncol. 2024, 26 (Suppl. S6), vi1–vi85. [Google Scholar] [CrossRef] [PubMed]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 8428–8442. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Wölfl, S. Vitamin D as a Novel Regulator of Tumor Metabolism: Insights on Potential Mechanisms and Implications for Anti-Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2184. [Google Scholar] [CrossRef]
- Lo, C.S.C.; Kiang, K.M.Y.; Leung, G.K.K. Anti-tumor effects of vitamin D in glioblastoma: Mechanism and therapeutic implications. Lab. Investig. 2022, 102, 118–125. [Google Scholar] [CrossRef]
- Elmaci, I.; Ozpinar, A.; Ozpinar, A.; Perez, J.L.; Altinoz, M.A. From epidemiology and neurometabolism to treatment: Vitamin D in pathogenesis of glioblastoma Multiforme (GBM) and a proposal for Vitamin D + all-trans retinoic acid + Temozolomide combination in treatment of GBM. Metab. Brain Dis. 2019, 34, 687–704. [Google Scholar] [CrossRef]
- Tellis, C.C.; Tselepis, A.D. Pathophysiological role and clinical significance of lipoprotein-associated phospholipase A2 (Lp-PLA2) bound to LDL and HDL. Curr. Pharm. Des. 2014, 20, 6256–6269. [Google Scholar] [CrossRef] [PubMed]
- Kupert, E.; Anderson, M.; Liu, Y.; Succop, P.; Levin, L.; Wang, J.; Wikenheiser-brokamp, K.; Chen, P.; Pinney, S.M.; Macdonald, T.; et al. Plasma secretory phospholipase A2-IIa as a potential biomarker for lung cancer in patients with solitary pulmonary nodules. BMC Cancer 2011, 11, 513. [Google Scholar] [CrossRef]
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021, 497, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S. Phospholipase A2 as targets for anti-cancer drugs. Biochem. Pharmacol. 2007, 74, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chiorean, E.G.; Chiorean, M.V.; Rex, D.K.; Robb, B.W.; Hahn, N.M.; Liu, Z.; Loehrer, P.J.; Harrison, M.L.; Xu, Y. Elevated phospholipase A2 activities in plasma samples from multiple cancers. PLoS ONE 2013, 8, e57081. [Google Scholar] [CrossRef]
- Se, Y.B.; Kim, S.H.; Kim, J.Y.; Kim, J.E.; Dho, Y.-S.; Kim, J.W.; Kim, Y.H.; Woo, H.G.; Kim, S.-H.; Kang, S.-H.; et al. Underexpression of HOXA11 is associated with treatment resistance and poor prognosis in glioblastoma. Cancer Res. Treat. 2017, 49, 387–398. [Google Scholar] [CrossRef]
- Grant, N.J.; Coates, P.J.; Woods, Y.L.; Bray, S.E.; Morrice, N.A.; Hastie, C.J.; Lamont, D.J.; Carey, F.A.; Sutherland, C. Phosphorylation of a splice variant of collapsin response mediator protein 2 in the nucleus of tumour cells links cyclin dependent kinase-5 to oncogenesis. BMC Cancer 2015, 15, 885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sethi, R.; Mangala, L.S.; Taylor, C.; Goldsmith, J.; Wang, M.; Masuda, K.; Karaminejadranjbar, M.; Mannion, D.; Miranda, F.; et al. Tuning microtubule dynamics to enhance cancer therapy by modulating FER-mediated CRMP2 phosphorylation. Nat. Commun. 2018, 9, 476, Erratum in Nat. Commun. 2022, 13, 3352. [Google Scholar] [CrossRef]
- Abu Rmaileh, A.; Solaimuthu, B.; Khatib, A.; Lavi, S.; Tanna, M.; Hayashi, A.; Ben Yosef, M.; Lichtenstein, M.; Pillar, N.; Shaul, Y.D. DPYSL2 interacts with JAK1 to mediate breast cancer cell migration. J. Cell Biol. 2022, 221, e202106078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: A meta-analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, D.; Tellis, C.; Tselepis, A.D. Oxidized phospholipids and lipoprotein- associated phospholipase A2 (Lp-PLA2) in atherosclerotic cardiovascular disease: An update. Biofactors 2022, 48, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hyppönen, E. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, I.T.; Mello, A.P.; Damasceno, N.R. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A2 (Lp-PLA2): A review. Lipids Health Dis. 2011, 10, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talmud, P.J.; Holmes, M.V. Deciphering the Causal Role of sPLA2s and Lp-PLA2 in Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2281–2289. [Google Scholar] [CrossRef]
- Borin, T.F.; Angara, K.; Rashid, M.H.; Achyut, B.R.; Arbab, A.S. Arachidonic Acid Metabolite as a Novel Therapeutic Target in Breast Cancer Metastasis. Int. J. Mol. Sci. 2017, 18, 2661. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S.; Leelahavanichkul, K. Arachidonic acid metabolism and its implication on head and neck cancer. In Head and Neck Cancer; Agulnik, D.M., Ed.; InTech: London, UK, 2012. [Google Scholar]
- Rosenson, R.S.; Stafforini, D.M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 2012, 53, 1767–1782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehtinen, L.; Vainio, P.; Wikman, H.; Huhtala, H.; Mueller, V.; Kallioniemi, A.; Pantel, K.; Kronqvist, P.; Kallioniemi, O.; Carpèn, O.; et al. PLA2G7 associates with hormone receptor negativity in clinical breast cancer samples and regulates epithelial-mesenchymal transition in cultured breast cancer cells. J. Pathol. Clin. Res. 2017, 3, 123–138. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Wang, H.; Yin, Z.; Huang, K.; Yu, M. High PLA2 level is correlated with glioblastoma progression via regulating DNA replication. J. Cell. Mol. Med. 2022, 26, 1466–1472. [Google Scholar] [CrossRef]
- Xu, C.; Reichert, E.C.; Nakano, T.; Lohse, M.; Gardner, A.A.; Revelo, M.P.; Topham, M.K.; Stafforini, D.M. Deficiency of phospholipase A2 group 7 decreases intestinal polyposis and colon tumorigenesis in Apc(Min/+) mice. Cancer Res. 2013, 73, 2806–2816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morigny, P.; Kaltenecker, D.; Zuber, J.; Machado, J.; Mehr, L.; Tsokanos, F.F.; Kuzi, H.; Hermann, C.D.; Voelkl, M.; Monogarov, G.; et al. Association of circulating PLA2G7 levels with cancer cachexia and assessment of darapladib as a therapy. J. Cachexia Sarcopenia Muscle 2021, 12, 1333–1351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Y.; Badmann, S.; Kraus, F.; Topalov, N.E.; Mayr, D.; Kolben, T.; Hester, A.; Beyer, S.; Mahner, S.; Jeschke, U.; et al. PLA2G7/PAF-AH as Potential Negative Regulator of the Wnt Signaling Pathway Mediates Protective Effects in BRCA1 Mutant Breast Cancer. Int. J. Mol. Sci. 2023, 24, 882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Liu, W.; Meng, F.; Jiang, Q.; Tang, W.; Liu, Z.; Lin, X.; Xue, R.; Zhang, S.; Dong, L. Inhibiting PLA2G7 reverses the immunosuppressive function of intratumoral macrophages and augments immunotherapy response in hepatocellular carcinoma. J. Immunother. Cancer 2024, 12, e008094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.J.; Nai, A.T.; He, G.C.; Xiao, F.; Li, Z.M.; Tang, S.Y.; Liu, Y.P.; Ai, X.H. DPYSL2 as potential diagnostic and prognostic biomarker linked to immune infiltration in lung adenocarcinoma. World J. Surg. Oncol. 2021, 19, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zu, L.; He, J.; Zhou, N.; Tang, Q.; Liang, M.; Xu, S. Identification of multiple organ metastasis-associated hub mRNA/miRNA signatures in non-small cell lung cancer. Cell Death Dis. 2023, 14, 798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.C.; Chen, H.C.; Chen, S.J.; Liu, H.P.; Hsieh, Y.Y.; Yu, C.J.; Tang, R.; Hsieh, L.L.; Yu, J.S.; Chang, Y.S. Identification of collapsin response mediator protein-2 as a potential marker of colorectal carcinoma by comparative analysis of cancer cell secretomes. Proteomics 2008, 8, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Jovčevska, I.; Šamec, N.; Mlakar, J.; Šribar, J.; Križaj, I.; Skoblar Vidmar, M.; Komel, R. Anti-vimentin, anti-TUFM, anti-NAP1L1 and anti-DPYSL2 nanobodies display cytotoxic effect and reduce glioblastoma cell migration. Ther. Adv. Med. Oncol. 2020, 12, 1–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol. Lett. 2020, 19, 2585–2594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-associated phospholipase A2: The story continues. Med. Res. Rev. 2020, 40, 79–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, J.; Zhao, Y. Lp-PLA2 silencing ameliorates inflammation and autophagy in nonalcoholic steatohepatitis through inhibiting the JAK2/STAT3 pathway. PeerJ 2023, 11, e15639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, D.; Yang, W.; Tang, T.; He, A.; Xu, X.; Jing, T.; Xia, D. PLA2G7 promotes immune evasion of bladder cancer through the JAK-STAT-PDL1 axis. Cell Death Dis. 2025, 16, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Shao, Z.; Lv, X.; Pu, F.; Gao, F.; Liu, L.; Shi, D. Anticancer effect of (S)-crizotinib on osteosarcoma cells by targeting MTH1 and activating reactive oxygen species. Anti-Cancer Drugs 2018, 29, 341–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuzgen, S.; Hanimoglu, H.; Tanriverdi, T.; Kacira, T.; Sanus, G.Z.; Atukeren, P.; Dashti, R.; Gumustas, K.; Canbaz, B.; Kaynar, M.Y. Relationship between DNA damage and total antioxidant capacity in patients with glioblastoma multiforme. Clin. Oncol. (R. Coll. Radiol.) 2007, 19, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Nair-Shalliker, V.; Armstrong, B.K.; Fenech, M. Does vitamin D protect against DNA damage? Mutat. Res. 2012, 733, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Bostick, R.M.; Long, Q.; Flanders, W.D.; McCullough, M.L.; Sidelnikov, E.; Daniel, C.R.; Rutherford, R.E.; Shaukat, A. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: A randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2010, 19, 280–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Group | ||||
|---|---|---|---|---|
| Experimental Group | Control Group | |||
| GBM WHO IV | Brain Metastases | Meningioma | Spinal Disk Disease | |
| n | 15 | 20 | 8 | 19 |
| Females | 4 | 9 | 5 | 12 |
| Males | 11 | 11 | 3 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromadzki, B.; Wiciński, M.; Siedlecki, Z.; Rzepiński, Ł.; Fajkiel-Madajczyk, A. Assessment of the Significance of LP-PLA2, DPYSL2, and 8-OHdG in the Oncological Diagnosis of Patients with Brain Tumors and Vitamin D Deficiency. Int. J. Mol. Sci. 2025, 26, 7990. https://doi.org/10.3390/ijms26167990
Gromadzki B, Wiciński M, Siedlecki Z, Rzepiński Ł, Fajkiel-Madajczyk A. Assessment of the Significance of LP-PLA2, DPYSL2, and 8-OHdG in the Oncological Diagnosis of Patients with Brain Tumors and Vitamin D Deficiency. International Journal of Molecular Sciences. 2025; 26(16):7990. https://doi.org/10.3390/ijms26167990
Chicago/Turabian StyleGromadzki, Bartłomiej, Michał Wiciński, Zygmunt Siedlecki, Łukasz Rzepiński, and Anna Fajkiel-Madajczyk. 2025. "Assessment of the Significance of LP-PLA2, DPYSL2, and 8-OHdG in the Oncological Diagnosis of Patients with Brain Tumors and Vitamin D Deficiency" International Journal of Molecular Sciences 26, no. 16: 7990. https://doi.org/10.3390/ijms26167990
APA StyleGromadzki, B., Wiciński, M., Siedlecki, Z., Rzepiński, Ł., & Fajkiel-Madajczyk, A. (2025). Assessment of the Significance of LP-PLA2, DPYSL2, and 8-OHdG in the Oncological Diagnosis of Patients with Brain Tumors and Vitamin D Deficiency. International Journal of Molecular Sciences, 26(16), 7990. https://doi.org/10.3390/ijms26167990




