1. Introduction
Wheat (
Triticum aestivum L.), one of the first domesticated food crops, has been a dietary staple for >8000 years, supporting major civilizations worldwide. Wheat plays a significant role in global food security and nutrition, providing ~20% of the daily intake of protein and calories. This essential grain meets the nutritional needs of billions. However, its production is increasingly challenged by biotic and abiotic stressors, such as climate change, diseases, and pests, which can negatively impact the quality and yield of wheat [
1].
Climate change–induced drought and salinity stress pose significant threats to global food security by adversely affecting plant growth, development, and productivity, leading to reduced crop yields. Drought tolerance has emerged as a cost-effective strategy to enhance plant resilience to abiotic stressors. Concurrently, salt stress, exacerbated by natural and human-induced factors, severely impacts food crop production, particularly wheat, by causing ion toxicity and osmotic stress. Recent advancements in biotechnology, such as the identification and manipulation of stress-responsive genes, offer promising avenues for developing wheat varieties with enhanced tolerance to these stressors [
2,
3].
Ubiquitination is a crucial post-translational modification that regulates cellular functions, including protein degradation, DNA repair, and stress responses. It is orchestrated by a cascade of enzymatic activities involving E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases. E3 ligases are the most diverse and play a crucial role in recognizing specific substrates and facilitating the transfer of ubiquitin molecules from E2 enzymes to the substrates, thereby marking them for degradation by the 26S proteasome [
4,
5]. The ubiquitin–proteasome system is essential for maintaining cellular homeostasis and regulating cell cycle progression, signal transduction, stress responses, etc. [
6,
7]. Among the diverse classes of E3 ligases, the U-box-type is characterized by a conserved U-box domain, which is essential for ubiquitin ligase activity. U-box-type E3 ligases are involved in protein quality control, stress responses, etc. [
8,
9]. The U-box domain interacts with E2, facilitating the transfer of ubiquitin to target proteins. This interaction is crucial for the specificity and efficiency of ubiquitination, making U-box E3 ligases a critical component of the ubiquitin–proteasome system [
10].
Plants have evolved intricate mechanisms to cope with abiotic stresses such as drought, salinity, and osmotic stress. Ubiquitination, mediated by E3 ligases, helps modulate the levels and activities of key stress-responsive proteins. This regulation is essential for plants to adapt and survive under adverse environmental conditions [
11,
12]. In addition to ubiquitination, plants deploy a complex antioxidant defense system to mitigate oxidative stress caused by abiotic stressors [
11]. Key antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), and peroxidases (PODs) such as ascorbate peroxidase (APX) and glutathione peroxidase. These enzymes play a crucial role in scavenging reactive oxygen species (ROS) and maintaining cellular redox homeostasis [
13]. The balance between ROS production and scavenging by antioxidant enzymes is critical for plant survival under stress, highlighting the importance of both ubiquitination and antioxidant defense mechanisms in plant stress responses.
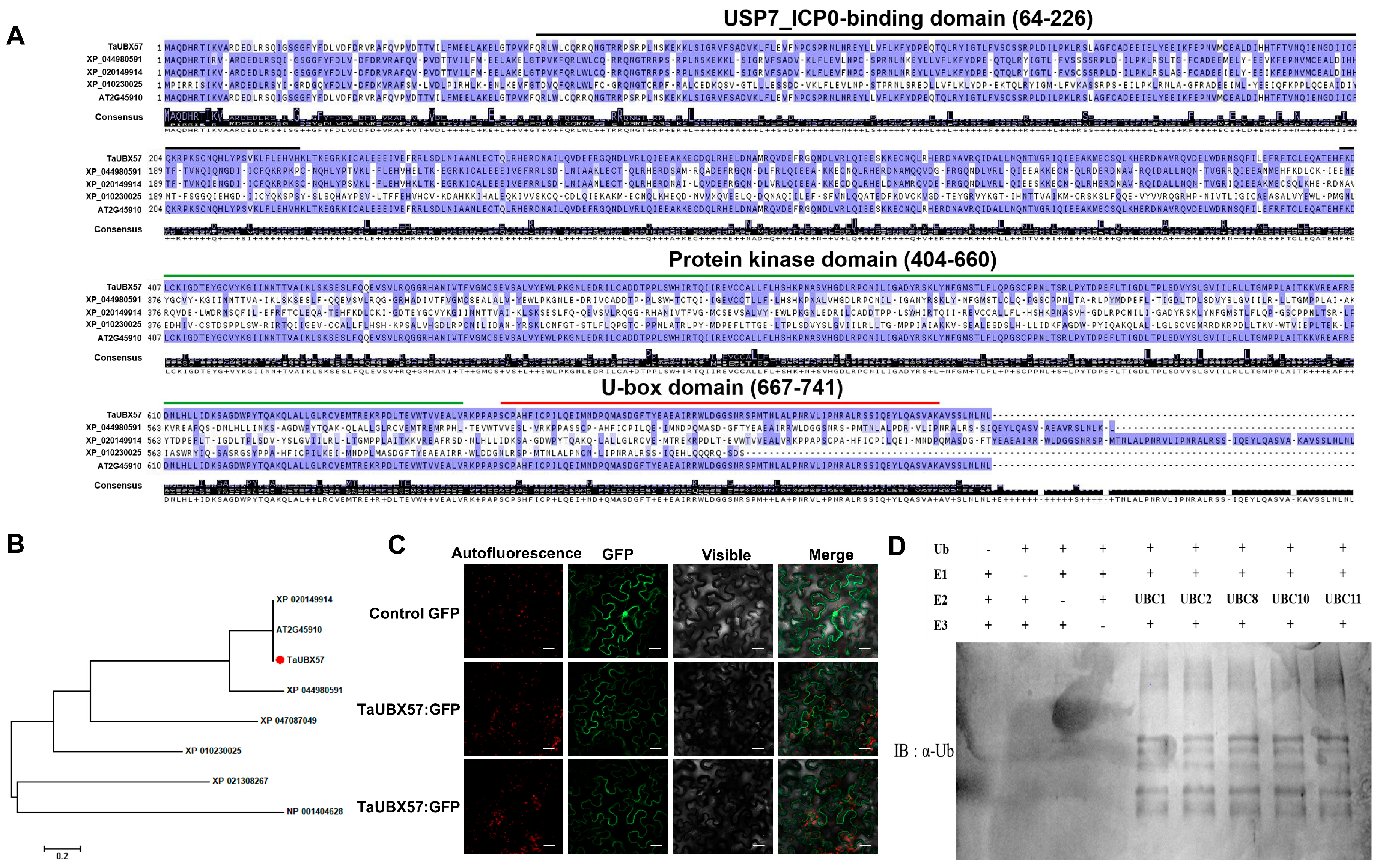
In this study, functional domain analysis using InterProScan revealed that TaUBX57 contains a conserved U-box domain, indicating its potential role in ubiquitination. The domain structure suggests that TaUBX57 functions like other U-box E3 ligases, participating in protein quality control and stress responses. Phylogenetic analysis revealed that TaUBX57 shares a close evolutionary relationship with other U-box E3 ligases across plant species, suggesting conserved function. The evolutionary conservation of U-box E3 ligases across plant species underscores their fundamental role in stress responses and cellular homeostasis. Despite the known importance of E3 ligases in plant stress responses, the functions of TaUBX57 remain largely unexplored. This study aimed to elucidate the role of TaUBX57 in abiotic stress tolerance by investigating its function through overexpression in transgenic Arabidopsis plants. Specifically, we aimed to analyze the domain structure and evolutionary relationships of TaUBX57 to gain insights into its functional aspects. By determining the subcellular localization of TaUBX57, we aimed to understand its interaction with cellular components and its role in cellular processes. We evaluated the impact of TaUBX57 overexpression on seed germination and root growth under osmotic, salt, and hormonal stress conditions to determine its role in stress tolerance. Additionally, we evaluated the physiological and biochemical responses of transgenic plants to drought and salt stress, focusing on parameters such as survival rates, growth performance, oxidative stress markers, and malondialdehyde (MDA) levels. Finally, we investigated the expression levels of antioxidant enzyme genes in transgenic plants under stress conditions, providing insights into how TaUBX57 confers stress tolerance and regulates cellular responses to stress.
3. Discussion
Overall, we analyzed TaUBX57 and confirmed its identity as a U-box-type E3 ubiquitin ligase (
Figure 1). Domain structure analysis and phylogenetic analysis indicated conserved function across species (
Figure 1A). Analysis of subcellular localization and the results of the ubiquitination assay suggested its role in stress response pathways (
Figure 1C). These findings lay the groundwork for further investigations into the specific substrates and pathways regulated by TaUBX57 in wheat and related species (
Figure 1D).
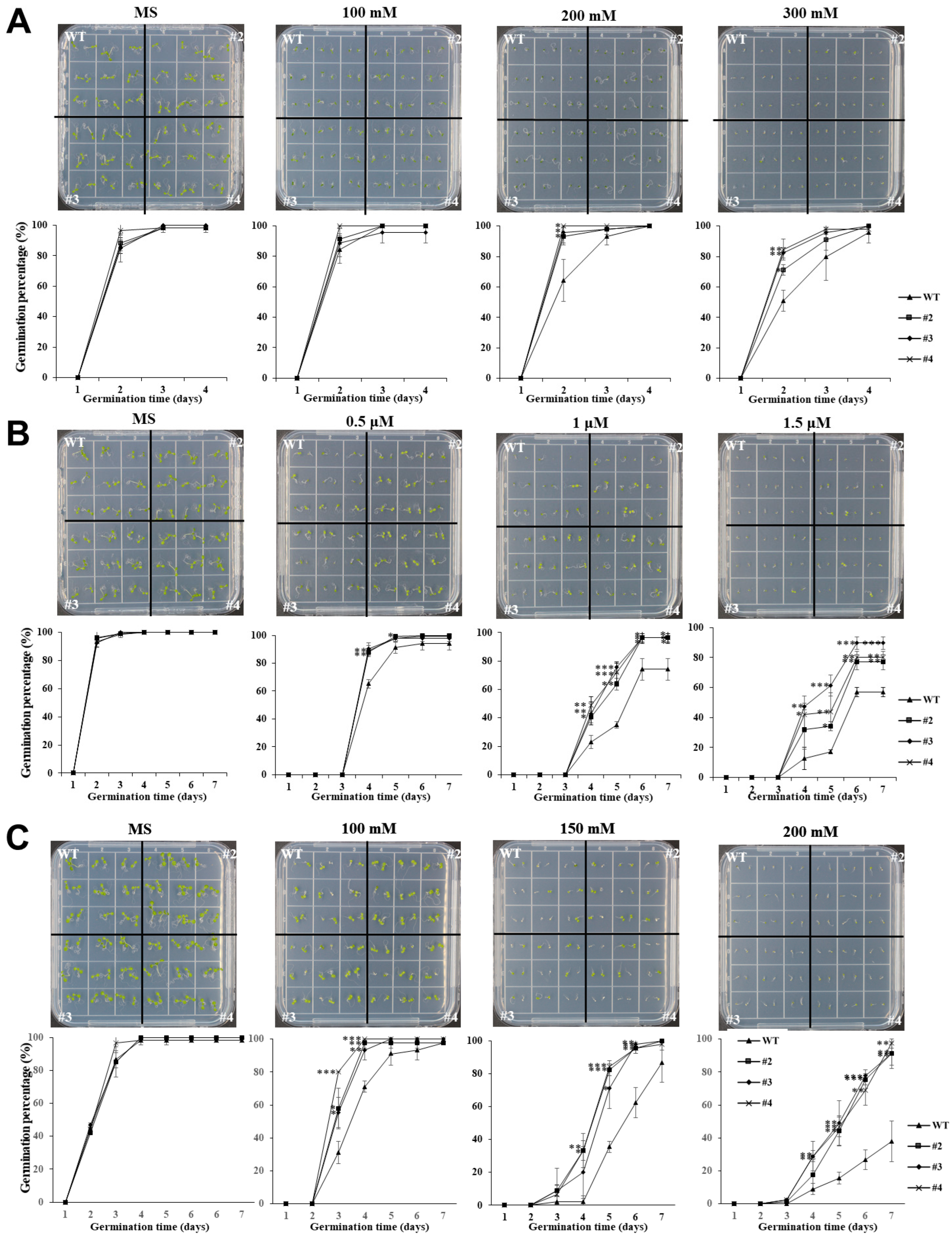
The germination assay of transgenic
Arabidopsis overexpressing
TaUBX57 revealed enhanced stress tolerance under various conditions. The
TaUBX57-overexpressing lines (#2–4) exhibited significantly higher germination rates than WT plants across all treatments, including osmotic stress induced by mannitol, ABA, and salt stress (
Figure 2). Specifically, these transgenic lines showed improved germination at higher mannitol concentrations (0, 100, 200, and 300 mM), increased tolerance under ABA (0, 0.5, 1.0, and 1.5 µM), and better performance under NaCl-induced salt stress (0, 100, 150, and 200 mM). Correspondingly, the accompanying bar graphs quantifying average root length indicate that the
TaUBX57-overexpressing lines had longer roots than WT plants across all treatments (
Figure 3). Under higher concentrations of mannitol, the
TaUBX57-overexpressing lines exhibited longer roots, suggesting enhanced tolerance to osmotic stress (
Figure 3A). Under ABA treatment conditions, the
TaUBX57-overexpressing lines showed significantly longer roots than WT plants, indicating that
TaUBX57 can modulate ABA-related growth inhibition (
Figure 3B). Under salt stress conditions, the
TaUBX57-overexpressing lines displayed longer roots than WT plants, particularly at higher NaCl concentrations, demonstrating that
TaUBX57 enhanced tolerance to salt stress (
Figure 3C). These results suggest that
TaUBX57 overexpression may enhance stress tolerance by supporting cellular water balance, modulating ABA signaling pathways, and managing ion homeostasis, thereby facilitating germination and enhancing stress tolerance at the seedling stage.
U-box E3 ligases played significant roles in managing plant responses to abiotic stressors, including drought, salt stress, and ABA. For instance, in rice, the U-box E3 ubiquitin ligase
OsPUB67 enhanced drought tolerance by promoting ROS scavenging and facilitating stomatal closure, a mechanism crucial for managing water loss and oxidative stress under drought conditions [
14]. Similarly, in apple, MdPUB29 positively regulated salt stress tolerance, likely by maintaining cellular ion balance and osmotic regulation.
MdPUB29 expression was significantly reduced under salt stress, highlighting its role in salt tolerance [
15]. Conversely, in soybean,
GmPUB21 negatively regulated the stress response to drought and salinity. The overexpression of GmPUB21 increased sensitivity to these stressors, characterized by higher ROS accumulation and altered stomatal behavior. However, silencing
GmPUB21 enhanced stress tolerance, suggesting that it modulated the stress response through ABA signaling [
16]. This dual role of GmPUB21 highlights the complex regulatory mechanisms that U-box E3 ligases can exert in different plants and stress conditions.
Poplar
PalPUB79 positively regulated ABA signaling and enhanced drought tolerance.
PalPUB79 expression was associated with increased ABA sensitivity, which helped in regulating the osmotic stress response under drought conditions [
17]. Moreover, a comprehensive study on sorghum identified multiple U-box genes involved in salt stress responses, indicating their significant role in enhancing salinity tolerance and potentially contributing to improved crop resilience [
18]. In Arabidopsis, U-box E3 ligases, such as AtPUB18, AtPUB19, and AtPUB44, negatively regulated seed germination under salt stress and ABA signaling, further demonstrating the diverse regulatory roles of these ligases [
19]. Recent research highlighted the role of U-box E3 ligases in modulating ABA signaling, with studies demonstrating their involvement in fine-tuning the response to ABA [
20].
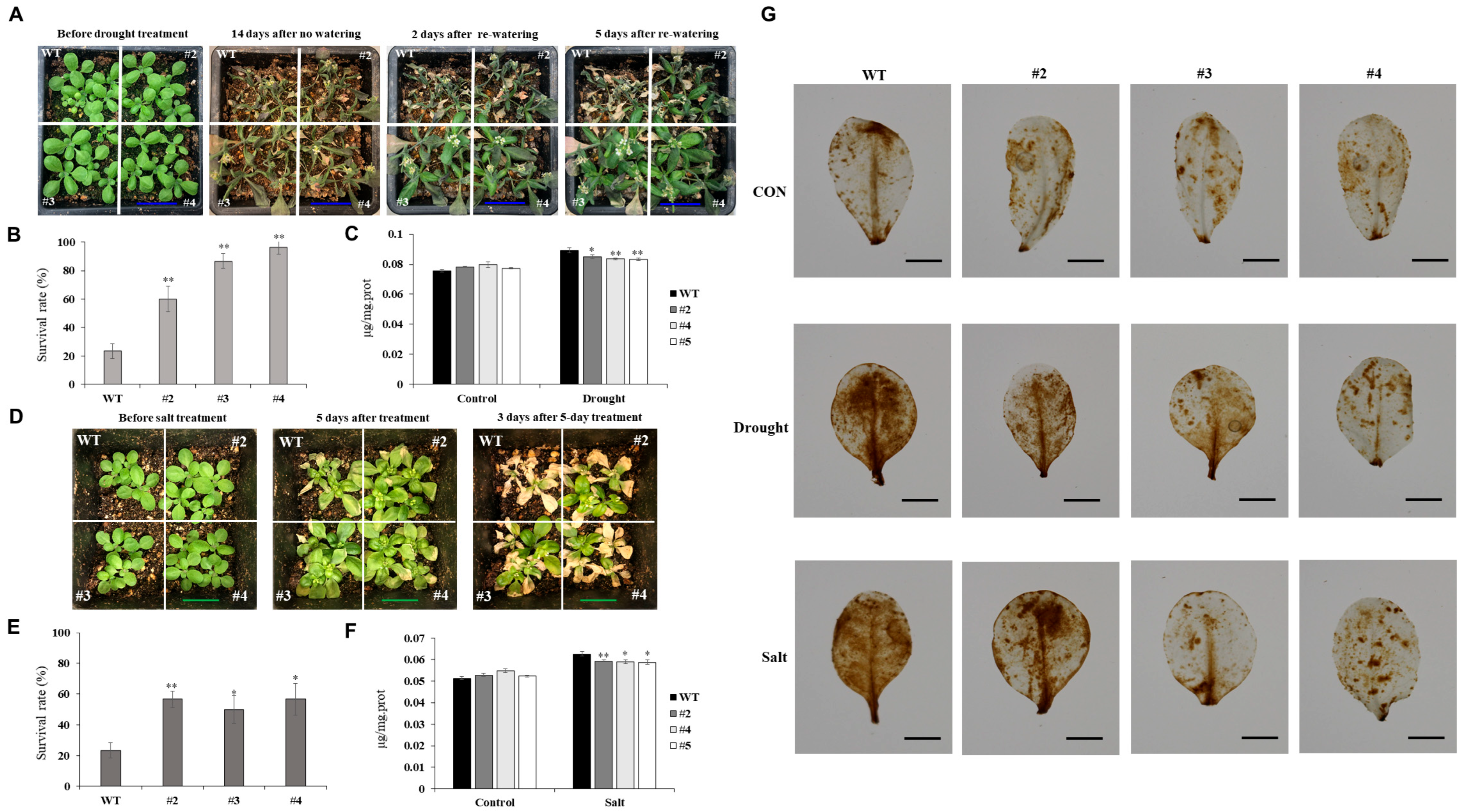
Although ROS are byproducts of normal cellular metabolism, they can accumulate to harmful levels under abiotic stressors, like drought and salinity, leading to the oxidative damage of proteins, lipids, and nucleic acids. Plants counteract this by activating a complex antioxidant defense system, including enzymatic (SOD, CAT, and POD) and nonenzymatic (ascorbate and GSH) components [
13,
21,
22]. These systems work together to scavenge excessive ROS, maintaining cellular redox balance and protecting plants from oxidative stress. Drought stress can exacerbate ROS production due to water deficit, whereas salt stress leads to osmotic stress and ionic toxicity, both resulting in increased ROS levels. The role of antioxidants is crucial in ROS detoxification, preventing cellular damage and ensuring plant survival under these conditions [
13,
21]. In our study, transgenic
Arabidopsis lines overexpressing
TaUBX57 demonstrated significantly enhanced antioxidant enzyme activities under both drought and salt stresses. The measured enzymes, including TAC, POD, APX, SOD, CAT, and GSH, were upregulated in these lines compared with WT plants, suggesting an enhanced capacity to detoxify ROS and protect cellular structures from oxidative damage (
Figure 5). Consequently, MDA content was lower in TaUBX57-overexpressing plants than in controls under both drought and salt stress conditions (
Figure 4C, F). Consistently, ROS damage, assessed through DAB staining, was lower in TaUBX57-overexpressing plants than in controls, further supporting high antioxidant defense in transgenic lines (
Figure 4G). This reduction in MDA and ROS damage highlights the role of TaUBX57 in mitigating oxidative stress, thereby contributing to the overall stress tolerance in these plants.
This study highlights the crucial role of U-box E3 ligases, specifically TaUBX57, in enhancing stress tolerance in plants. The findings underscore the potential of these proteins to enhance or mitigate stress responses, depending on the specific pathway or mechanism involved. These insights are invaluable for developing crops with improved tolerance to environmental stresses, especially as climate change increases the frequency and severity of abiotic stresses such as drought and salinity. Understanding the role of TaUBX57 in stress responses not only enriches our knowledge of the mechanisms of stress tolerance in wheat but also offers potential genetic targets for developing stress-tolerant crops. The ability to engineer crops that can better withstand environmental challenges is critical for ensuring agricultural sustainability and food security in a changing climate. This study provides a foundation for biotechnological applications aimed at enhancing crop resilience through genetic engineering and breeding programs.
Although the role of TaUBX57 in enhancing stress tolerance through increased antioxidant activity has been established, the specific protein substrates targeted for degradation by TaUBX57 remain unidentified. Identifying these substrates is crucial for understanding how TaUBX57 confers stress tolerance. Studies could employ proteomics approaches, such as yeast two-hybrid assays, to screen for interacting partners of TaUBX57. The findings of this study will be pivotal in elucidating the functional landscape of TaUBX57 in plant stress responses and could lead to the development of more resilient crop varieties.
4. Materials and Methods
4.1. Plant Materials and Stress Treatments
Colored wheat (K4191) of the F4:8 generation from the cross between “Woori-mil” (National Agrobiodiversity Center, RDA, Republic of Korea; accession no. IT172221) and “D-7” (an inbred line developed by Korea University; Fleming4/3/PIO2580//T831032/Hamlet) varieties was utilized as plant material [
23]. Wheat seeds were soaked in water until rooting and germination and transferred to half-strength Hoagland’s solution. The hydroponic culture system was maintained using Hoagland’s solution adjusted to pH 5.5–6.5, which was freshly prepared and renewed on a daily basis to maintain nutrient consistency and minimize microbial growth. The seedlings were grown for 10 d at 22 °C with a 16 h light/8 h dark photoperiod. To induce drought stress, seedlings were transferred to Hoagland’s solution containing 20% PEG-6000. For abscisic acid (ABA) treatment, seedlings were transferred to 100 μM ABA. For salt stress, 200 mM NaCl was used. The seedlings were exposed for 36 h before sampling.
4.2. Gene Cloning and Sequence Analysis
The open reading frame sequence of
TaUBX57 was obtained from Ensembl Plants (
https://plants.ensembl.org/Triticum_aestivum/Info/Index; accessed on 2 February 2023). Homologous genes in other species were identified using the NCBI database (
www.ncbi.nlm.nih.gov; accessed on 2 February 2023) and BLASTn tool. Significant sequences were downloaded, and domain prediction was performed using InterProScan (
https://www.ebi.ac.uk/interpro/search/sequence; accessed on 2 February 2023). Multiple sequence alignments were performed using ClustalW [
24], and a phylogenetic tree was constructed using the neighbor-joining method in MEGA7 software (version 7.0; MEGA Software, Pennsylvania State University, University Park, PA, USA).
4.3. In Vitro Ubiquitination Assay and Western Blotting
The full-length coding sequence of
TaUBX57 was cloned into pDEST-HisMBP (Plasmid #11085, Addgene, Cambridge, MA, USA) using LR Clonase (Thermo Fisher Scientific, Waltham, MA, USA). MBP-tagged TaUBX57 was expressed in
E. coli BL21 (DE3) pLysS and purified using amylose resin (New England BioLabs, Ipswich, MA, USA). The in vitro ubiquitination assay was performed as described by Choo and Zhang [
25] and Zeng [
26], with modifications. The assay was performed in a total reaction volume of 50 μL, containing 1 μg of purified MBP-TaUBX57, 10 μg of ubiquitin (Sigma, St. Louis, MO, USA), 40 ng of E1 (Boston Biochem, Cambridge, MA, USA), and 100 ng of various E2 enzymes. The reactions were incubated at 30 °C for 3 h, stopped with SDS sample buffer, and heated at 100 °C for 5 min. Ubiquitinated substrates were separated using 12% SDS-PAGE and identified via Western blotting using an anti-ubiquitin antibody (Sigma, MO, USA). The signals were detected using an ECL kit (Merck, Rahway, NJ, USA) and iBright CL1000 Imaging System (Thermo Scientific).
4.4. Subcellular Localization
For subcellular localization, full-length
TaUBX57 was cloned into the pMDC43 vector containing GFP using the Gateway method [
27]. The constructs pMDC43 and pMDC43 (control) were introduced into
Agrobacterium strain GV3101 and infiltrated into the abaxial surface of
Nicotiana benthamiana leaves. Fluorescence was detected 72 h post-infiltration using a confocal laser-scanning microscope (Zeiss LSM800, Oberkochen, Germany). Green fluorescence from GFP was visualized using excitation at 488 nm, with signal detection in the 510–530 nm emission range. Red autofluorescence from chloroplasts was observed by applying a 633 nm excitation laser and collecting emitted light above 650 nm.
4.5. RNA Extraction and Gene Expression
Total RNA was extracted from wheat seedlings using TRIzol reagent (Invitrogen, Waltham, MA, USA) and treated with DNase I to remove residual DNA. First-strand cDNA was synthesized from 1 μg of total RNA using a Power cDNA Synthesis Kit (iNtRON Biotechnology, Gyeonggi-do, Republic of Korea). RT-qPCR was performed using 2× SYBR Green Premix Ex Taq II (Takara, Kusatsu, Shiga, Japan) on a Bio-Rad Opus 96 System with the following parameters: 95 °C for 5 min followed by 45 cycles of 95 °C for 10 s and 65 °C for 30 s. Actin (AB18991) was used as an internal reference. Experiments were conducted with three biological replicates, and gene expression variations were evaluated using the 2
−ΔΔCt method [
28]. Specific primers are listed in
Table S1.
4.6. Arabidopsis Transformation and Stress Treatments
Arabidopsis thaliana ecotype Landsberg
erecta (Ler-0) was used as the wild type (WT). The full-length coding region of
TaUBX57 was cloned into pMDC32 and transformed into
Agrobacterium GV3101 for the transformation of
Arabidopsis using the floral dip method [
29]. Transgenic seeds were selected on 1/2 MS medium with 50 μg/mL hygromycin. Three independent homozygous T
3 transgenic lines (#2–4) were selected based on phenotypic consistency and elevated TaUBX57 transcript levels, as verified by RT-qPCR analysis (
Figure S2). For stress treatment, seedlings were transferred to soil and grown at 22 °C under long-day conditions (16 h light/8 h dark). Drought stress was induced by withholding water for 14 d, and salt stress was applied using 300 mM NaCl. Plant phenotypes were monitored during stress and survival and assessed after 5 d of rehydration.
4.7. Arabidopsis Seed Germination and Root Growth Assay
Sterilized seeds were sown on 1/2 MS medium supplemented with 0, 100, 200, and 300 mM mannitol; 0, 0.5, 1.0, and 1.5 μM ABA; or 0, 100, 150, and 200 mM NaCl. Germination rates were assessed daily over 7 d, noting radicle emergence and cotyledon greening. For root growth assays, 4-day-old seedlings were transferred to 1/2 MS medium with various concentrations of mannitol, ABA, and NaCl, and root lengths were measured after 7 d.
4.8. Antioxidant Activity Assays
Crude enzyme extracts were prepared from 100 mg of Arabidopsis tissue using a protein extraction solution containing 50 mM potassium phosphate buffer (pH 7.5). The activities of APX, CAT, POD, SOD, and total antioxidant capacity (TAC) were measured using commercial kits: APX (MBS2548460, MyBiosource, San Diego, CA, USA), CAT (MBS8243260, MyBiosource, CA, USA), POD (KTB1150, Abbkine, Wuhan, China), SOD (WST-1 method, MBS2540402, MyBiosource, CA, USA), and TAC (MAK187, Sigma, MO, USA). Protocols and calculations followed the manufacturers’ instructions.
4.9. MDA Content
MDA content was measured using a kit (MBS2540407, MyBiosource, San Diego, CA, USA). Approximately 0.1 g of Arabidopsis tissue was ground and extracted, followed by analysis according to the assay protocol.
4.10. 3,3′-Diaminobenzidine (DAB) Assay
ROS damage was visualized using DAB staining [
30]. Rosette leaves which had been subjected to drought and salt stress treatments for 5 d were immersed in 1% DAB solution until brown spots appeared, before being treated with destaining solution (acetic acid, glycerol, and 96% ethanol in a 1:1:3 ratio) and boiled. After destaining was complete, the leaves were photographed in water.
4.11. Statistical Analysis
Statistical significance was determined using SPSS software (version 25.0) using t tests. Significant differences are indicated by asterisks above columns in the figures (* p < 0.05, ** p < 0.01).