The Infant Oral Microbiome: Developmental Dynamics, Modulating Factors, and Implications for Oral and Systemic Health
Abstract
1. Introduction
2. Definition and Composition of the Human Oral Microbiome
2.1. Oral Microbial Colonization During the Lactation Stage
2.2. Influence of Breastfeeding on the Establishment of the Oral Microbiome
3. Development of the Oral Microbiome During Childhood
Environmental Factors and Maternal Transmission in Infant Oral Health
4. Changes in the Oral Microbiome During the Adolescent Stage
5. Interaction Between the Oral Microbiome, Oral Diseases, and Systemic Conditions
6. Influence of Food on the Formation of the Infant Oral Microbiome
7. Future Perspectives and a Comprehensive Approach to Children’s Oral Health
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casaburi, G.; Duar, R.M.; Brown, H.; Mitchell, R.D.; Kazi, S.; Chew, S.; Cagney, O.; Flannery, R.L.; Sylvester, K.G.; Frese, S.A.; et al. Author Correction: Metagenomic Insights of the Infant Microbiome Community Structure and Function across Multiple Sites in the United States. Sci. Rep. 2021, 11, 11050. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Perdijk, O.; Azzoni, R.; Marsland, B.J. The Microbiome: An Integral Player in Immune Homeostasis and Inflammation in the Respiratory Tract. Physiol. Rev. 2024, 104, 835–879. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The Human Microbiome in Evolution. BMC Biol. 2017, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Rowan-Nash, A.D.; Korry, B.J.; Mylonakis, E.; Belenky, P. Cross-Domain and Viral Interactions in the Microbiome. Microbiol. Mol. Biol. Rev. 2019, 83, 1–63. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- HOMD: Human Oral Microbiome Database. Available online: https://www.homd.org/ (accessed on 17 July 2025).
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The Oral Microbiome: Diversity, Biogeography and Human Health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The Role of Oral Microbiome in Respiratory Health and Diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kapoor, P.; Bhargava, D.; Bagga, D.K. Exploring the Oral Microbiome: An Updated Multidisciplinary Oral Healthcare Perspective. Discoveries 2023, 11, e165. [Google Scholar] [CrossRef] [PubMed]
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 17 July 2025).
- WHO. Highlights Oral Health Neglect Affecting Nearly Half of the World’s Population. Available online: https://www.who.int/news/item/18-11-2022-who-highlights-oral-health-neglect-affecting-nearly-half-of-the-world-s-population (accessed on 17 July 2025).
- Chimenos-Küstner, E.; Giovannoni, M.L.; Schemel-Suárez, M. Dysbiosis as a Determinant Factor of Systemic and Oral Pathology: Importance of Microbiome. Med. Clín. 2017, 149, 305–309. [Google Scholar] [CrossRef]
- Choo, S.W.; Mohammed, W.K.; Mutha, N.V.R.; Rostami, N.; Ahmed, H.; Krasnogor, N.; Tan, G.Y.A.; Jakubovics, N.S. Transcriptomic Responses to Coaggregation between Streptococcus gordonii and Streptococcus oralis. Appl. Environ. Microbiol. 2021, 87, e01558-21. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The Role of the Microbiome for Human Health: From Basic Science to Clinical Applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Yama, K.; Morishima, S.; Tsutsumi, K.; Jo, R.; Aita, Y.; Inokuchi, T.; Okuda, T.; Watai, D.; Ohara, K.; Maruyama, M.; et al. Oral Microbiota Development in the First 60 Months: A Longitudinal Study. J. Dent. Res. 2024, 103, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, M.; Li, S.; Lei, Y.; Xiang, X.; Guo, Z.; Wang, Q. Shaping Oral and Intestinal Microbiota and the Immune System during the First 1000 Days of Life. Front. Pediatr. 2025, 13, 1471743. [Google Scholar] [CrossRef]
- Damaceno, Q.S.; Gallotti, B.; Reis, I.M.M.; Totte, Y.C.P.; Assis, G.B.; Figueiredo, H.C.; Silva, T.F.; Azevedo, V.; Nicoli, J.R.; Martins, F.S. Isolation and Identification of Potential Probiotic Bacteria from Human Milk. Probiotics Antimicrob. Proteins 2023, 15, 491–501. [Google Scholar] [CrossRef]
- Kennedy, B.; Peura, S.; Hammar, U.; Vicenzi, S.; Hedman, A.; Almqvist, C.; Andolf, E.; Pershagen, G.; Dicksved, J.; Bertilsson, S.; et al. Oral Microbiota Development in Early Childhood. Sci. Rep. 2019, 9, 19025. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Q.; Liu, X.; Zheng, S.; Ma, L.; Chen, F.; Xu, T.; Xu, B. Exploring the Oral Microflora of Preschool Children. J. Microbiol. 2017, 55, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qiu, W.; Wang, J.; Zhao, A.; Zhou, C.; Sun, T.; Xiong, Z.; Cao, P.; Shen, W.; Chen, J.; et al. Effects of Vaginal Microbiota Transfer on the Neurodevelopment and Microbiome of Cesarean-Born Infants: A Blinded Randomized Controlled Trial. Cell Host Microbe 2023, 31, 1232–1247.e5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fiscella, K.A.; Gill, S.R. Oral Microbiome: Possible Harbinger for Children’s Health. Int. J. Oral Sci. 2020, 12, 12. [Google Scholar] [CrossRef]
- Bowland, G.B.; Weyrich, L.S. The oral-microbiome-brain axis and neuropsychiatric disorders: An anthropological perspective. Front. Psychiatry 2022, 13, 810008. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Selevsek, N.; Wolski, W.; Grossmann, J.; Bao, K.; Wahlander, A.; Trachsel, C.; Schlapbach, R.; Öztürk, V.Ö.; Afacan, B.; et al. Targeted Proteomics Guided by Label-Free Quantitative Proteome Analysis in Saliva Reveal Transition Signatures from Health to Periodontal Disease. Mol. Cell. Proteomics 2018, 17, 1392–1409. [Google Scholar] [CrossRef]
- Xu, X.; Shan, B.; Zhang, Q.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Oral Microbiome Characteristics in Children with and without Early Childhood Caries. J. Clin. Pediatr. Dent. 2023, 47, 58–67. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108. [Google Scholar] [CrossRef]
- Di Stefano, M.; Santonocito, S.; Polizzi, A.; Mauceri, R.; Troiano, G.; Lo Giudice, A.; Romano, A.; Mascitti, M.; Isola, G. A Reciprocal Link between Oral, Gut Microbiota during Periodontitis: The Potential Role of Probiotics in Reducing Dysbiosis-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 1084. [Google Scholar] [CrossRef]
- Shaiber, A.; Willis, A.D.; Delmont, T.O.; Roux, S.; Chen, L.-X.; Schmid, A.C.; Yousef, M.; Watson, A.R.; Lolans, K.; Esen, Ö.C.; et al. Functional and Genetic Markers of Niche Partitioning among Enigmatic Members of the Human Oral Microbiome. Genome Biol. 2020, 21, 292. [Google Scholar] [CrossRef]
- Bhandary, R.; Venugopalan, G.; Ramesh, A.; Tartaglia, G.M.; Singhal, I.; Khijmatgar, S. Microbial Symphony: Navigating the Intricacies of the Human Oral Microbiome and Its Impact on Health. Microorganisms 2024, 12, 571. [Google Scholar] [CrossRef]
- Xiao, Y.; Louwies, T.; Mars, R.A.T.; Kashyap, P.C. The Human Microbiome—A Physiologic Perspective. Compr. Physiol. 2024, 14, 5491–5519. [Google Scholar] [CrossRef]
- Villar, C.C.; Dongari-Bagtzoglou, A. Fungal diseases: Oral dysbiosis in susceptible hosts. Periodontology 2000 2021, 87, 166–180. [Google Scholar] [CrossRef]
- Xia, Q.; Pierson, S. HPV Infection and Oral Microbiota: Interactions and Future Implications. Int. J. Mol. Sci. 2025, 26, 1424. [Google Scholar] [CrossRef]
- D’Agostino, S.; Ferrara, E.; Valentini, G.; Stoica, S.A.; Dolci, M. Exploring Oral Microbiome in Healthy Infants and Children: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 11403. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-X.; Sun, M.-L.; Shi, P.-L.; Liu, P.; Chen, Y.-Y.; Peng, X. Research progress in the relationship between Veillonella and oral diseases. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2020, 38, 576–582. [Google Scholar] [CrossRef]
- Harris, J.W.; Brown, J.H. The Bacterial Content of the Uterus at Cesarean Section. Am. J. Obstet. Gynecol. 1927, 13, 133–143. [Google Scholar] [CrossRef]
- Stroup, P.E. Amniotic Fluid Infection and The Intact Fetal Membrane. Obstet. Gynecol. Surv. 1962, 19, 736–739. [Google Scholar] [CrossRef]
- Bearfield, C.; Davenport, E.S.; Sivapathasundaram, V.; Allaker, R.P. Possible Association between Amniotic Fluid Micro-Organism Infection and Microflora in the Mouth. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 527–533. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the Upper Genital Tract by Vaginal Bacterial Species in Nonpregnant Women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Sulyanto, R.M.; Thompson, Z.A.; Beall, C.J.; Leys, E.J.; Griffen, A.L. The Predominant Oral Microbiota Is Acquired Early in an Organized Pattern. Sci. Rep. 2019, 9, 10550. [Google Scholar] [CrossRef]
- Liu, P.; Wen, W.; Yu, K.F.; Tong, R.W.M.; Gao, X.; Lo, E.C.M.; Wong, M.C.M. Can Oral Microbiome Predict Low Birth Weight Infant Delivery? J. Dent. 2024, 146, 105018. [Google Scholar] [CrossRef]
- Lin, T.-H.; Lin, C.-H.; Pan, T.-M. The Implication of Probiotics in the Prevention of Dental Caries. Appl. Microbiol. Biotechnol. 2018, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Kapila, Y. Oral Microbiome Shifts during Pregnancy and Adverse Pregnancy Outcomes: Hormonal and Immunologic Changes at Play. Periodontol. 2000 2021, 87, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, H.; Metwally, A.; Tam, N.; Chen, Y.; Gong, D.; Wang, Q.; Begg, D.; Korf, I.; Ghosh, S.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Hirnle, L.; Szczepaniak, M.; Michalak, M.; Seremak-Mrozikiewicz, A.; Chlabicz, S. How much do pregnant women know about the importance of oral health in pregnancy? Questionnaire-based survey. BMC Pregnancy Childbirth 2023, 23, 348. [Google Scholar] [CrossRef]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of Lactation Stage, Gestational Age and Mode of Delivery on Breast Milk Microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Cravioto, A.; Tello, A.; Villafan, H.; Ruiz, J.; del Vedovo, S.; Neeser, J.-R. Inhibition of Localized Adhesion of Enteropathogenic Escherichia Coli to HEp-2 Cells by Immunoglobulin and Oligosaccharide Fractions of Human Colostrum and Breast Milk. J. Infect. Dis. 1991, 163, 1247–1255. [Google Scholar] [CrossRef]
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human Milk Microbiota Profiles in Relation to Birthing Method, Gestation and Infant Gender. Microbiome 2016, 4, 1. [Google Scholar] [CrossRef]
- Tunzi, C.R.; Harper, P.A.; Bar-Oz, B.; Valore, E.V.; Semple, J.L.; Watson-MacDonell, J.; Ganz, T.; Ito, S. β-Defensin Expression in Human Mammary Gland Epithelia. Pediatr. Res. 2000, 48, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Consales, A.; Cerasani, J.; Sorrentino, G.; Morniroli, D.; Colombo, L.; Mosca, F.; Giannì, M.L. The Hidden Universe of Human Milk Microbiome: Origin, Composition, Determinants, Role, and Future Perspectives. Eur. J. Pediatr. 2022, 181, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Reddel, S.; Pascucci, G.R.; Foligno, S.; Del Chierico, F.; Vernocchi, P.; Marzullo, A.; Pattumelli, M.G.; Palma, P.; Salvatori, G.; Putignani, L. A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants. Microorganisms 2022, 10, 468. [Google Scholar] [CrossRef]
- Al-Shehri, S.S.; Sweeney, E.L.; Cowley, D.M.; Liley, H.G.; Ranasinghe, P.D.; Charles, B.G.; Shaw, P.N.; Vagenas, D.; Duley, J.A.; Knox, C.L. Deep Sequencing of the 16S Ribosomal RNA of the Neonatal Oral Microbiome: A Comparison of Breast-Fed and Formula-Fed Infants. Sci. Rep. 2016, 6, 38309. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.A.; Adams, G.G.; Blum, J.; Byrne, S.J.; Carpenter, L.; Gussy, M.G.; Calache, H.; Catmull, D.V.; Reynolds, E.C.; Dashper, S.G. Breastmilk Influences Development and Composition of the Oral Microbiome. J. Oral Microbiol. 2022, 14, 2096287. [Google Scholar] [CrossRef]
- de Franchis, R.; Bozza, L.; Canale, P.; Chiacchio, M.; Cortese, P.; D’Avino, A.; De Giovanni, M.; Iacovo, M.D.; D’Onofrio, A.; Federico, A.; et al. The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial. Nutrients 2022, 14, 2486. [Google Scholar] [CrossRef]
- Oba, P.M.; Holscher, H.D.; Mathai, R.A.; Kim, J.; Swanson, K.S. Diet Influences the Oral Microbiota of Infants during the First Six Months of Life. Nutrients 2020, 12, 3400. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Z.; Lin, J.; Shan, C.; Abasijiang, A.; Zhao, J. Characterization of the Oral and Gut Microbiome in Children with Obesity Aged 3 to 5 Years. Front. Cell. Infect. Microbiol. 2023, 13, 1102650. [Google Scholar] [CrossRef]
- Dashper, S.G.; Mitchell, H.L.; Lê Cao, K.-A.; Carpenter, L.; Gussy, M.G.; Calache, H.; Gladman, S.L.; Bulach, D.M.; Hoffmann, B.; Catmull, D.V.; et al. Temporal Development of the Oral Microbiome and Prediction of Early Childhood Caries. Sci. Rep. 2019, 9, 19732. [Google Scholar] [CrossRef]
- Jo, R.; Yama, K.; Aita, Y.; Tsutsumi, K.; Ishihara, C.; Maruyama, M.; Takeda, K.; Nishinaga, E.; Shibasaki, K.; Morishima, S. Comparison of Oral Microbiome Profiles in 18-Month-Old Infants and Their Parents. Sci. Rep. 2021, 11, 861. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Zhao, L.; Sun, X.; Feng, Q. Microbiome Succession with Increasing Age in Three Oral Sites. Aging 2020, 12, 7874–7907. [Google Scholar] [CrossRef] [PubMed]
- Alcaina Lorente, A.; Saura López, V.; Pérez Pardo, A.; Guzmán Pina, S.; Cortés Lillo, O. Adolescent lifestyle and its influence on oral health. Pediatr. Aten. Primaria 2020, 22, 251–261. [Google Scholar]
- Willis, J.R.; González-Torres, P.; Pittis, A.A.; Bejarano, L.A.; Cozzuto, L.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Valentín, A.; Ksiezopolska, E.; Company, C.; et al. Citizen Science Charts Two Major “Stomatotypes” in the Oral Microbiome of Adolescents and Reveals Links with Habits and Drinking Water Composition. Microbiome 2018, 6, 218. [Google Scholar] [CrossRef]
- Sundström, K.; Mishra, P.P.; Pyysalo, M.J.; Lehtimäki, T.; Karhunen, P.J.; Pessi, T. Similarity of Salivary Microbiome in Parents and Adult Children. PeerJ 2020, 8, e8799. [Google Scholar] [CrossRef]
- Eriksson, L.; Lif Holgerson, P.; Johansson, I. Saliva and Tooth Biofilm Bacterial Microbiota in Adolescents in a Low Caries Community. Sci. Rep. 2017, 7, 5861. [Google Scholar] [CrossRef]
- Stahringer, S.S.; Clemente, J.C.; Corley, R.P.; Hewitt, J.; Knights, D.; Walters, W.A.; Knight, R.; Krauter, K.S. Nurture Trumps Nature in a Longitudinal Survey of Salivary Bacterial Communities in Twins from Early Adolescence to Early Adulthood. Genome Res. 2012, 22, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Bartha, V.; Exner, L.; Schweikert, D.; Woelber, J.P.; Vach, K.; Meyer, A.-L.; Basrai, M.; Bischoff, S.C.; Meller, C.; Wolff, D. Effect of the Mediterranean Diet on Gingivitis: A Randomized Controlled Trial. J. Clin. Periodontol. 2022, 49, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.M.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. Analysis of the Effects of Dietary Pattern on the Oral Microbiome of Elite Endurance Athletes. Nutrients 2019, 11, 614. [Google Scholar] [CrossRef]
- Pang, L.; Zhi, Q.; Jian, W.; Liu, Z.; Lin, H. The Oral Microbiome Impacts the Link between Sugar Consumption and Caries: A Preliminary Study. Nutrients 2022, 14, 3693. [Google Scholar] [CrossRef]
- Negrini, T.d.C.; Ren, Z.; Miao, Y.; Kim, D.; Simon-Soro, Á.; Liu, Y.; Koo, H.; Arthur, R.A. Dietary Sugars Modulate Bacterial-Fungal Interactions in Saliva and Inter-Kingdom Biofilm Formation on Apatitic Surface. Front. Cell. Infect. Microbiol. 2022, 12, 993640. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, T.L.; Cabudol, M.; Liu, J.X.; Garza, J.R.; Gansky, S.A.; Ramos-Gomez, F. A Qualitative Study of the Multi-Level Influences on Oral Hygiene Practices for Young Children in an Early Head Start Program. BMC Oral Health 2019, 19, 166. [Google Scholar] [CrossRef]
- Chankanka, O.; Levy, S.M.; Warren, J.J.; Chalmers, J.M. A Literature Review of Aesthetic Perceptions of Dental Fluorosis and Relationships with Psychosocial Aspects/Oral Health-Related Quality of Life. Community Dent. Oral Epidemiol. 2010, 38, 97–109. [Google Scholar] [CrossRef]
- Kouremenou-Dona, E.; Dona, A.; Papoutsis, J.; Spiliopoulou, C. Copper and Zinc Concentrations in Serum of Healthy Greek Adults. Sci. Total Environ. 2006, 359, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Avila, M.; Smith, D.; Meneses, F.; Sanin, L.H.; Hu, H. The Influence of Bone and Blood Lead on Plasma Lead Levels in Environmentally Exposed Adults. Environ. Health Perspect. 1998, 106, 473. [Google Scholar] [CrossRef]
- Xu, H.; Tian, B.; Shi, W.; Tian, J.; Wang, W.; Qin, M. Maturation of the Oral Microbiota during Primary Teeth Eruption: A Longitudinal, Preliminary Study. J. Oral Microbiol. 2022, 14, 2051352. [Google Scholar] [CrossRef]
- Mason, M.R.; Chambers, S.; Dabdoub, S.M.; Thikkurissy, S.; Kumar, P.S. Characterizing Oral Microbial Communities across Dentition States and Colonization Niches. Microbiome 2018, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Ramadugu, K.; Bhaumik, D.; Luo, T.; Gicquelais, R.E.; Lee, K.H.; Stafford, E.B.; Marrs, C.F.; Neiswanger, K.; McNeil, D.W.; Marazita, M.L.; et al. Maternal Oral Health Influences Infant Salivary Microbiome. J. Dent. Res. 2021, 100, 58–65. [Google Scholar] [CrossRef]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral Microbiome Development during Childhood: An Ecological Succession Influenced by Postnatal Factors and Associated with Tooth Decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Kahharova, D.; Brandt, B.W.; Buijs, M.J.; Peters, M.; Jackson, R.; Eckert, G.; Katz, B.; Keels, M.A.; Levy, S.M.; Fontana, M.; et al. Maturation of the Oral Microbiome in Caries-Free Toddlers: A Longitudinal Study. J. Dent. Res. 2020, 99, 159–167. [Google Scholar] [CrossRef]
- Lif Holgerson, P.; Esberg, A.; Sjödin, A.; West, C.E.; Johansson, I. A Longitudinal Study of the Development of the Saliva Microbiome in Infants 2 Days to 5 Years Compared to the Microbiome in Adolescents. Sci. Rep. 2020, 10, 9629. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Eriksen, C.; Boustedt, K.; Sonne, S.B.; Dahlgren, J.; Kristiansen, K.; Twetman, S.; Brix, S.; Roswall, J. Early Life Factors and Oral Microbial Signatures Define the Risk of Caries in a Swedish Cohort of Preschool Children. Sci. Rep. 2024, 14, 8463. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, Y.; Chen, L.; Huang, W.; Yang, D. Metagenomic Analysis of Oral Microbiome in Young Children Aged 6–8 Years Living in a Rural Isolated Chinese Province. Oral Dis. 2018, 24, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Li, F.; Feng, X.; Wong, M.C.M.; Lu, H. Plaque Biofilm Microbial Diversity in Infants Aged 12 Months and Their Mothers with or without Dental Caries: A Pilot Study. BMC Oral Health 2018, 18, 228. [Google Scholar] [CrossRef]
- Xu, H.; Tian, J.; Hao, W.; Zhang, Q.; Zhou, Q.; Shi, W.; Qin, M.; He, X.; Chen, F. Oral Microbiome Shifts From Caries-Free to Caries-Affected Status in 3-Year-Old Chinese Children: A Longitudinal Study. Front. Microbiol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Li, Y.; Saraithong, P.; Zhang, L.; Dills, A.; Paster, B.J.; Xiao, J.; Wu, T.T.; Jones, Z. Dynamics of Oral Microbiome Acquisition in Healthy Infants: A Pilot Study. Front. Oral Health 2023, 4, 1152601. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.K.L.; Seow, W.K.; Purdie, D.M.; Bird, P.S.; Walsh, L.J.; Tudehope, D.I. A Longitudinal Study of Streptococcus Mutans Colonization in Infants after Tooth Eruption. J. Dent. Res. 2003, 82, 504–508. [Google Scholar] [CrossRef]
- Jamieson, L.M.; Elani, H.W.; Mejia, G.C.; Ju, X.; Kawachi, I.; Harper, S.; Thomson, W.M.; Kaufman, J.S. Inequalities in Indigenous Oral Health: Findings from Australia, New Zealand, and Canada. J. Dent. Res. 2016, 95, 1375–1380. [Google Scholar] [CrossRef]
- Manton, D.J. Child Dental Caries—A Global Problem of Inequality. eClinicalMedicine 2018, 1, 3–4. [Google Scholar] [CrossRef]
- Singla, D. Distribution of Streptococcus mutans and Streptococcus sobrinus in Dental Plaque of Indian Pre-School Children Using PCR and SB-20M Agar Medium. J. Clin. Diagn. Res. 2016, 10, ZC60. [Google Scholar] [CrossRef] [PubMed]
- Saraithong, P.; Pattanaporn, K.; Chen, Z.; Khongkhunthian, S.; Laohapensang, P.; Chhun, N.; Pattanaporn, W.; Gaw, H.Y.; Li, Y. Streptococcus mutans and Streptococcus sobrinus Colonization and Caries Experience in 3- and 5-Year-Old Thai Children. Clin. Oral Investig. 2015, 19, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Hayashi, F.; Wakita, A.; Nagatani, Y.; Okada, M. Five-Year Longitudinal Study of Dental Caries Risk Associated with Streptococcus mutans and Streptococcus sobrinus in Individuals with Intellectual Disabilities. J. Oral Sci. 2017, 59, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Meriç, E.; Bolgül, B.; Duran, N.; Ay, E. Evaluation of Oral Streptococci in Saliva of Children with Severe Early Childhood Caries and Caries-Free. Eur. J. Paediatr. Dent. 2020, 21, 13–17. [Google Scholar] [CrossRef]
- Fregatto, L.F.; Costa, I.B.; De Bortoli Teixeira, D.; Duarte, J.C.M.; Mascarin, A.M.N.; da Silveira Junior, S.B.; Serva, B.E.B.M.; da Silva, R.G.; Junior, F.A.; Cola, P.C. Oral Hygiene and Oral Microbiota in Children and Young People with Neurological Impairment and Oropharyngeal Dysphagia. Sci. Rep. 2021, 11, 18090. [Google Scholar] [CrossRef]
- dos Santos, M.T.B.R.; Masiero, D.; Simionato, M.R.L. Risk Factors for Dental Caries in Children with Cerebral Palsy. Spec. Care Dentist. 2002, 22, 103–107. [Google Scholar] [CrossRef]
- Duarte, J.C.M.; Costa, I.B.; Teixeira, D.d.B.; Fregatto, L.F.; Mendes, C.G.; Mascarin, A.M.N.; da Silveira Junior, S.B.; Serva, B.E.B.M.; Comar, L.P.; da Silva, R.G.; et al. Biochemical and Microbiological Aspects of the Oral Cavity of Children and Young People with Neurological Impairment and Oropharyngeal Dysphagia. Life 2023, 13, 1342. [Google Scholar] [CrossRef]
- Hidas, A.; Cohen, J.; Beeri, M.; Shapira, J.; Steinberg, D.; Moskovitz, M. Salivary Bacteria and Oral Health Status in Children with Disabilities Fed through Gastrostomy. Int. J. Paediatr. Dent. 2010, 20, 179–185. [Google Scholar] [CrossRef]
- Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Mestnik, M.J.; Mayer, M.P.A.; Feres, M. Microbiological Profile of Untreated Subjects with Localized Aggressive Periodontitis. J. Clin. Periodontol. 2009, 36, 739–749. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Kinney, J.S.; Herr, A.E.; Braun, T.; Sugai, J.V.; Shelburne, C.A.; Rayburn, L.A.; Tran, H.M.; Singh, A.K.; Giannobile, W.V. Identification of Pathogen and Host-Response Markers Correlated with Periodontal Disease. J. Periodontol. 2009, 80, 436–446. [Google Scholar] [CrossRef]
- Wara-aswapati, N.; Pitiphat, W.; Chanchaimongkon, L.; Taweechaisupapong, S.; Boch, J.; Ishikawa, I. Red Bacterial Complex Is Associated with the Severity of Chronic Periodontitis in a Thai Population. Oral Dis. 2009, 15, 354–359. [Google Scholar] [CrossRef]
- Kebschull, M.; Papapanou, P.N. Periodontal Microbial Complexes Associated with Specific Cell and Tissue Responses. J. Clin. Periodontol. 2011, 38, 17–27. [Google Scholar] [CrossRef]
- Duijster, D.; de Jong-Lenters, M.; Verrips, E.; van Loveren, C. Establishing Oral Health Promoting Behaviours in Children—Parents’ Views on Barriers, Facilitators and Professional Support: A Qualitative Study. BMC Oral Health 2015, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kothadia, R.J.; Haider, S.S.; Mazumder, A.; Akhter, F.; Siddika, N.; Apu, E.H. Toothbrushing frequency among children and adolescents in 72 countries: Results from the Global School-based Health Survey. Dent. Med. Probl. 2024, 61, 495–506. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Blanco-Míguez, A.; Manghi, P.; Asnicar, F.; Dubois, L.; Golzato, D.; Armanini, F.; Cumbo, F.; Huang, K.D.; Manara, S.; et al. The Person-to-Person Transmission Landscape of the Gut and Oral Microbiomes. Nature 2023, 614, 125–135. [Google Scholar] [CrossRef]
- Arishi, R.A.; Lai, C.T.; Geddes, D.T.; Stinson, L.F. Impact of Breastfeeding and Other Early-Life Factors on the Development of the Oral Microbiome. Front. Microbiol. 2023, 14, 1236601. [Google Scholar] [CrossRef] [PubMed]
- Klaus, K.; Eichenauer, J.; Sprenger, R.; Ruf, S. Oral Microbiota Carriage in Patients with Multibracket Appliance in Relation to the Quality of Oral Hygiene. Head Face Med. 2016, 12, 28. [Google Scholar] [CrossRef]
- Koopman, J.E.; van der Kaaij, N.C.W.; Buijs, M.J.; Elyassi, Y.; van der Veen, M.H.; Crielaard, W.; ten Cate, J.M.; Zaura, E. The Effect of Fixed Orthodontic Appliances and Fluoride Mouthwash on the Oral Microbiome of Adolescents—A Randomized Controlled Clinical Trial. PLoS ONE 2015, 10, e0137318. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Nagarajan, R.; Kirakodu, S.; Gonzalez, O.A. Oral Microbiome and Gingival Gene Expression of Inflammatory Biomolecules with Aging and Periodontitis. Front. Oral Health 2021, 2, 725115. [Google Scholar] [CrossRef]
- Eshriqui, I.; Viljakainen, H.T.; Ferreira, S.R.G.; Raju, S.C.; Weiderpass, E.; Figueiredo, R.A.O. Breastfeeding May Have a Long-Term Effect on Oral Microbiota: Results from the Fin-HIT Cohort. Int. Breastfeed. J. 2020, 15, 42. [Google Scholar] [CrossRef]
- Willis, J.R.; Saus, E.; Iraola-Guzmán, S.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Blanco, A.; Puig-Sola, A.; et al. Citizen-Science Reveals Changes in the Oral Microbiome in Spain through Age and Lifestyle Factors. Npj Biofilms Microbiomes 2022, 8, 38. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Carlos, P.P.S.; Faria, G.A.; Pacheco, L.C.R.; da Costa, V.S.; Mendes, I.R.R.; de Oliveira, A.B.; Colombo, A.P.V. The Healthy Oral Microbiome: A Changing Ecosystem throughout the Human Lifespan. J. Dent. Res. 2025, 104, 235–242. [Google Scholar] [CrossRef]
- Ferrer, A. Metal poisoning. An. Sist. Sanit. Navar. 2003, 26, 141–153. [Google Scholar]
- Luo, J.; Hein, C.; Mücklich, F.; Solioz, M. Killing of Bacteria by Copper, Cadmium, and Silver Surfaces Reveals Relevant Physicochemical Parameters. Biointerphases 2017, 12, 020301. [Google Scholar] [CrossRef]
- Davis, E.; Bakulski, K.M.; Goodrich, J.M.; Peterson, K.E.; Marazita, M.L.; Foxman, B. Low Levels of Salivary Metals, Oral Microbiome Composition and Dental Decay. Sci. Rep. 2020, 10, 14640. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska-Wylęgała, B.E.; Wylęgała, A.A.; Zalejska-Fiolka, J.; Czuba, Z.; Toborek, M. Proinflammatory Cytokines and Antioxidant Enzymes as Salivary Biomarkers of Dentofacial Infections in Children. Dent. Med. Probl. 2024, 61. [Google Scholar] [CrossRef]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L.; Miranda, A.; Reinhart, B.; Meyers, D.; Woltkamp, D.; et al. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.M.; Relman, D.A. The Application of Ecological Theory towards an Understanding of the Human Microbiome. Science 2012, 336, 1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.; Zhou, X.; Cao, S.; Wu, T.; Cao, Y.; Xu, X. Human buccal mucosa microbiota succession across age. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2014, 32, 177–181. [Google Scholar]
- Kazarina, A.; Kuzmicka, J.; Bortkevica, S.; Zayakin, P.; Kimsis, J.; Igumnova, V.; Sadovska, D.; Freimane, L.; Kivrane, A.; Namina, A.; et al. Oral Microbiome Variations Related to Ageing: Possible Implications beyond Oral Health. Arch. Microbiol. 2023, 205, 116. [Google Scholar] [CrossRef]
- FDI’s Definition of Oral Health | FDI World Dental Federation. Available online: https://www.fdiworlddental.org/fdis-definition-oral-health (accessed on 18 July 2025).
- Rosan, B.; Lamont, R.J. Dental Plaque Formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Valledor-Alvarez, J.E.; Aguila-Rodríguez, C.A. Relationship between Systemic diseases and Oral diseases in the elderly. Rev. Arch. Méd. Camagüey 2022, 26. [Google Scholar]
- Zhou, Y.; Chen, D.R.; Zhi, Q.H.; Tao, Y.; Wang, X.; Feng, X.P.; Tai, B.J.; Hu, D.Y.; Wang, B.; Wang, C.X.; et al. The Prevalence and Associated Risk Indicators of Dental Fluorosis in China: Findings from the 4th National Oral Health Survey. Chin. J. Dent. Res. 2018, 21. [Google Scholar] [CrossRef]
- Verma, A.; Shetty, B.K.; Guddattu, V.; Chourasia, M.K.; Pundir, P. High Prevalence of Dental Fluorosis among Adolescents Is a Growing Concern: A School Based Cross-Sectional Study from Southern India. Environ. Health Prev. Med. 2017, 22, 17. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Hu, H.; Wei, X.; Wang, X.; Peng, Z.; Ma, R.; Zhao, Q.; Zhao, J.; Liu, J.; et al. Structural Changes in the Oral Microbiome of the Adolescent Patients with Moderate or Severe Dental Fluorosis. Sci. Rep. 2021, 11, 2897. [Google Scholar] [CrossRef]
- Guyonvarch, R.; Estivals, J.; Kérourédan, O. Minimally Invasive Direct Approach for Severe Dental Fluorosis: Balancing Aesthetics and Quality of Life. Clin. Case Rep. 2025, 13, e70254. [Google Scholar] [CrossRef]
- Al-Sarraj, F.; Albiheyri, R.; Qari, M.; Alotaibi, M.; Al-Zahrani, M.; Anwar, Y.; Alghamdi, M.A.; Nass, N.M.; Bouback, T.; Alotibi, I.; et al. Genetic Patterns of Oral Cavity Microbiome in Patients with Sickle Cell Disease. Int. J. Mol. Sci. 2024, 25, 8570. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Zheng, P.; Liu, E.; Bai, S.; Chen, S.; Pang, Y.; Xiao, X.; Yang, H.; Guo, J. Changes in Oral, Skin, and Gut Microbiota in Children with Atopic Dermatitis: A Case-Control Study. Front. Microbiol. 2024, 15, 1442126. [Google Scholar] [CrossRef]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Georges, F.M.; Do, N.T.; Seleem, D. Oral Dysbiosis and Systemic Diseases. Front. Dent. Med. 2022, 3, 995423. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- López, C.S.; Romero, F.P.; Sánchez, M.R. Action Mechanisms of the Oral Microbiota in the Development of Cancer. Scoping Review. Int. J. Odontostomatol. 2022, 16, 532–539. [Google Scholar] [CrossRef]
- Chandra Nayak, S.; Latha, P.B.; Kandanattu, B.; Pympallil, U.; Kumar, A.; Kumar Banga, H. The Oral Microbiome and Systemic Health: Bridging the Gap Between Dentistry and Medicine. Cureus 2025, 17, e78918. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S. From Focal Sepsis to Periodontal Medicine: A Century of Exploring the Role of the Oral Microbiome in Systemic Disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral Microbiota in Human Systematic Diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Kouhzad, M.; Taki, E.; Elahi, Z.; Khoshbayan, A.; Navidifar, T.; Darban-Sarokhalil, D. Oral Microbiota and Metabolites: Key Players in Oral Health and Disorder, and Microbiota-Based Therapies. Front. Microbiol. 2024, 15, 1431785. [Google Scholar] [CrossRef]
- Augimeri, G.; Caparello, G.; Caputo, I.; Reda, R.; Testarelli, L.; Bonofiglio, D. Mediterranean Diet: A Potential Player in the Link between Oral Microbiome and Oral Diseases. J. Oral Microbiol. 2024, 16, 2329474. [Google Scholar] [CrossRef]
- Lalli, M.K.; Salo, T.E.; Hakola, L.; Knip, M.; Virtanen, S.M.; Vatanen, T. Associations between Dietary Fibers and Gut Microbiome Composition in the EDIA Longitudinal Infant Cohort. Am. J. Clin. Nutr. 2025, 121, 83–99. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of Complementary Feeding Is Associated with Gut Microbiota Diversity and Composition and Short Chain Fatty Acid Concentrations over the First Year of Life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Qasem, W.; Azad, M.B.; Hossain, Z.; Azad, E.; Jorgensen, S.; Castillo San Juan, S.; Cai, C.; Khafipour, E.; Beta, T.; Roberts, L.J.; et al. Assessment of Complementary Feeding of Canadian Infants: Effects on Microbiome & Oxidative Stress, a Randomized Controlled Trial. BMC Pediatr. 2017, 17, 54. [Google Scholar] [CrossRef]
- Smilowitz, J.; Amicucci, M.; Nandita, E.; Galermo, A.; Tu, D.; Taft, D.; Meier, A.; Kurudimov, K.; Oakes, L.; Mills, D.; et al. The Introduction of Plant-Derived Glycans in Exclusively 6-Month Old Breastfed Infants Alters Fecal Glycan Profiles and Microbial Metabolism (IMiND Study). Curr. Dev. Nutr. 2020, 4, 1082. [Google Scholar] [CrossRef]
- Tungare, S.; Paranjpe, A.G. Diet and Nutrition to Prevent Dental Problems. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Banakar, M.; Fernandes, G.V.O.; Etemad-Moghadam, S.; Frankenberger, R.; Pourhajibagher, M.; Mehran, M.; Yazdi, M.H.; Haghgoo, R.; Alaeddini, M. The Strategic Role of Biotics in Dental Caries Prevention: A Scoping Review. Food Sci. Nutr. 2024, 12, 8651–8674. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Devine, D.A.; Vernon, J.J. Manipulating the Diseased Oral Microbiome: The Power of Probiotics and Prebiotics. J. Oral Microbiol. 2024, 16, 2307416. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.J.; Cao, K.-A.L.; Hughes, T.; Kumar, P.; Austin, C. How Does the Early Life Environment Influence the Oral Microbiome and Determine Oral Health Outcomes in Childhood? BioEssays News Rev. Mol. Cell. Dev. Biol. 2021, 43, e2000314. [Google Scholar] [CrossRef]
- Zou, J.; Du, Q.; Ge, L.; Wang, J.; Wang, X.; Li, Y.; Song, G.; Zhao, W.; Chen, X.; Jiang, B.; et al. Expert Consensus on Early Childhood Caries Management. Int. J. Oral Sci. 2022, 14, 35. [Google Scholar] [CrossRef]
- Tjandrawinata, R.R.; Amalia, N.; Tandi, Y.Y.P.; Athallah, A.F.; Afif Wibowo, C.; Aditya, M.R.; Muhammad, A.R.; Azizah, M.R.; Humardani, F.M.; Nojaid, A.; et al. The Forgotten Link: How the Oral Microbiome Shapes Childhood Growth and Development. Front. Oral Health 2025, 6, 1547099. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.; Kreth, J. Genetics of Sanguinis-Group Streptococci in Health and Disease. Microbiol. Spectr. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.S.; Al-Qadami, G.H.; Laheij, A.M.G.A.; Bossi, P.; Fregnani, E.R.; Wardill, H.R. From Pathogenesis to Intervention: The Importance of the Microbiome in Oral Mucositis. Int. J. Mol. Sci. 2023, 24, 8274. [Google Scholar] [CrossRef]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory Role of Oral Microbiota in Inflammatory Diseases and Allergic Conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef] [PubMed]
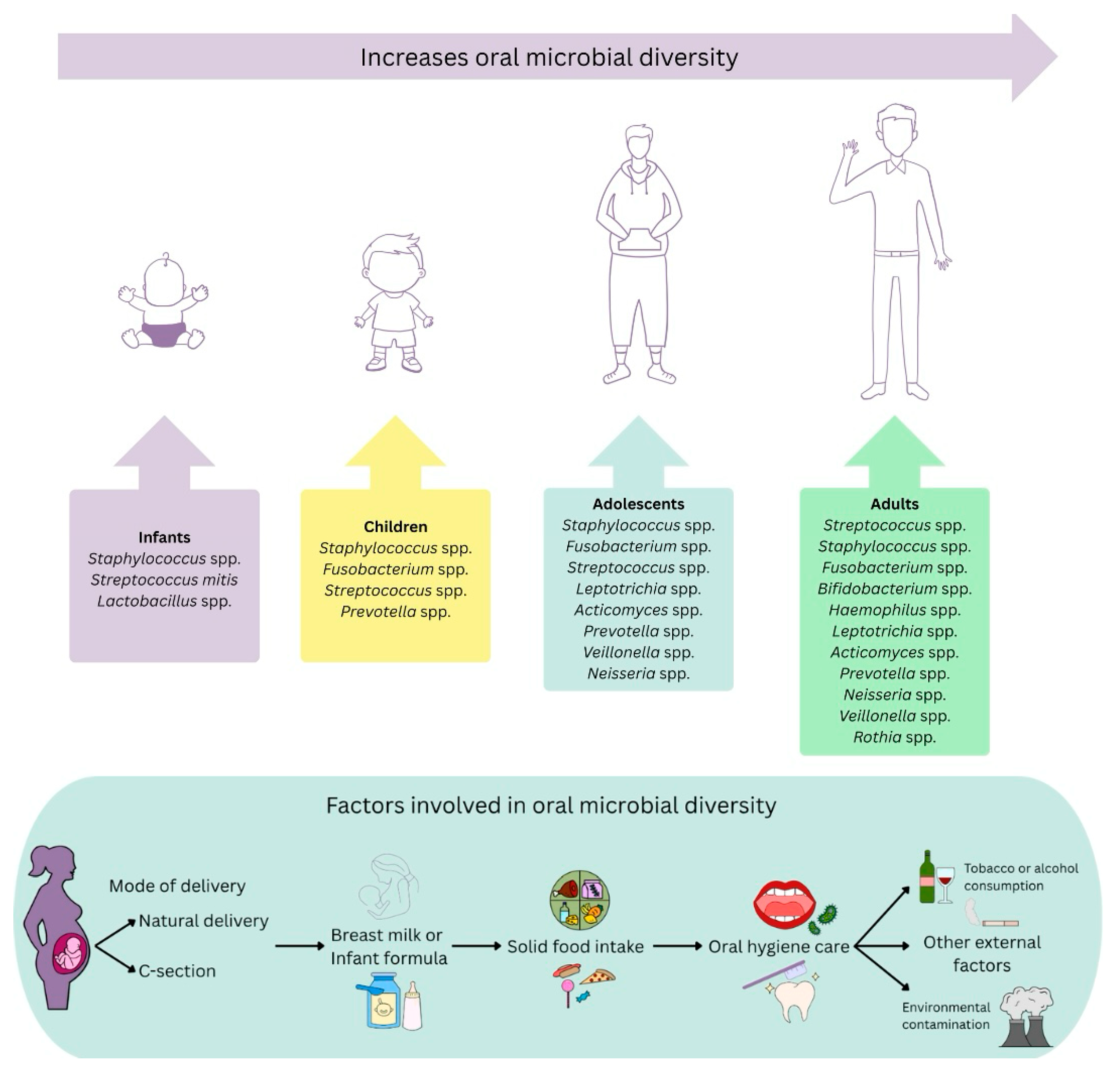
| Study Design | Characteristic Population | Nutrition or Other Variables | Microbial Diversity | Citation |
|---|---|---|---|---|
| Longitudinal observational study. 96 breast milk samples. | 39 healthy mothers | The participant’s diet was not evaluated | Predominant bacterial groups: Lactobacillus spp., Streptococcus spp., and Enterococcus spp. Increase in bacteria throughout lactation: ↑ Bifidobacterium spp. and Enterococcus spp. | [50] |
| Longitudinal observational study. Oral samples from infants. | 9 infants and their mothers | Breast milk, though diet was not evaluated | Streptococcus mitis was present in all infants and remained ubiquitous across ages. At 2 months, 6 species were found in ≥75% of infants. At 6 months, 7 more species were acquired. At 9 and 12 months: 4 additional species in ≥75% of infants. Most infant species were also prevalent in mothers except Streptococcus peroris, Leptotrichia Arg j44 uncultured, and Porphyromonas HF001. | [44] |
| Longitudinal observational study. Saliva samples from infants. | 39 infants aged 2–20 months, breastfed for 10 months | Breast milk vs. total absence of breastfeeding. Other dietary aspects were not addressed. | At 2 months, non-breastfed infants: higher oral bacterial diversity, ↑ Veillonella spp., and Bacteroidota. Breastfed infants: lower oral diversity, ↑ Streptococcus mitis. | [58] |
| Cross-sectional observational study. Saliva samples from healthy neonates | Healthy neonates divided into two groups according to the type of feeding | Breast milk vs. exclusive infant formula | Both groups dominated by the phylum Bacillota. Formula-fed infants: ↑ Bacteroidota Breastfed infants: ↑ Actinomycetota and Pseudomonadota. Streptococcus spp. showed an increasing trend between weeks 4 and 8 in both groups. | [57] |
| Randomized Controlled Clinical Trial | 394 healthy infants 4 to 6 months of age | Mediterranean Diet vs. industrial baby food | Greater abundance of beneficial taxa at 4 years. Increased microbial diversity at age 4 | [59] |
| Longitudinal observational study. Saliva samples from newborns. | Babies from 0 to 180 days old | Breast milk | Early colonizers: Streptococcus spp. and Staphylococcus spp. Late colonizers: Fusobacterium spp., Prevotella spp., Porphyromonas spp., Granulicatella spp., and Veillonella spp. | [56] |
| Longitudinal cohort study. Tongue and cheek swabs. | 12 infants, 0–6 months | Varied diet: breast milk, formula, and solid foods | Breast milk: lower oral bacterial diversity. ↑ Streptococcus spp. and ↓ Prevotella spp. Formula/solids: greater oral diversity. ↑ Prevotella spp. and Fusobacteriota. | [60] |
| Observational longitudinal study. Saliva samples from Swedish children at 6, 12, and 24 months of age. | 59 healthy children | Breast milk and antibiotic use | First two years of life: Significant increase in alpha diversity and decrease in beta diversity Breast milk and antibiotic use were associated with relative abundance of specific OTUs. ↓ from the family Streptococcaceae. | [22] |
| Observational cross-sectional study. CC cohort: children with dental caries. CH cohort: healthy teeth in the same children. HH Cohort: Healthy children without cavities. | 40 children from 3 to 6 years old | Participants’ diets were not assessed | Cohort CC: ↑ Lactobacillus spp., Veillonella spp. y Prevotella. Cohort CH: ↑ Actinomyces spp., Bifidobacterium spp. y Abiotrophia spp. Cohort HH: ↑ Neisseria spp., Leptotrichia spp., Porphyromonas spp., and Gemella spp. | [28] |
| Observational cross-sectional study. Oral and intestinal microbiota samples from children with and without obesity. | 30 children with obesity and 30 children with normal weight, aged 3 to 5 years | Participants’ diets were not assessed | Children with obesity: Lower bacterial diversity. ↑ Bacillota and ↓ Bacteroidota. Normal-weight children: Increased microbial diversity | [61] |
| Observational longitudinal study. Saliva samples from children and their parents | 27 healthy boys and 27 girls | Participants’ diets were not assessed | First week of life: ↑ Streptococcus spp., Rothia spp., and Gemella spp. Between 6 and 18 months: ↑ Neisseria spp., Haemophilus spp., and Fusobacterium spp. Between 36 and 60 months: no significant change compared to adults | [19] |
| Observational longitudinal study. Saliva samples from children and their mothers. Evaluation of caries in children. | 134 healthy children, from 2 months to 4 years of age | Participants’ diets were not assessed | From 1.9 months to 39 months of age: ↑ alpha diversity from 31 OTUs to 84 OTUs. In individuals with caries: greater abundance of Streptococcus mutans. | [62] |
| Cross-sectional observational study. Saliva samples from children and their parents. | 40 18-month-old children and their parents (mother and father) | Participants’ diets were not assessed | Microbial composition was significantly different between children and parents. Children’s microbiome was more similar to that of their mothers. In infants: ↑ Pseudomonadota and Fusobacteriota. In parents: ↑ Bacteroidota. Bacterial genera highly shared between infants and their parents: Granulicatella spp., Streptococcus spp., Veillonella spp., Neisseria spp., Haemophilus spp., Rothia spp. y Fusobacterium spp. | [63] |
| Cross-sectional observational study. Samples of gingival crevicular fluid, back of the tongue, and saliva | Healthy individuals of different age groups, from infancy to old age | No specific foods were evaluated | Decrease in alpha diversity and increase in beta diversity as age increases. | [64] |
| Observational cross-sectional study. Samples from different oral niches. | 20 healthy adults | Participants’ diets were not assessed | Significant differences in alpha diversity between the different sampled sites (p < 0.0001), but not between individuals (p = 0.876). | [9] |
| Observational cross-sectional study. Questionnaire to assess lifestyle habits | 39 schoolchildren aged 12 to 17 years old | Consumption of industrial pastries, sandwiches, and soft drinks | The oral microbiome was not analyzed directly. Significant associations were found between certain habits and oral health: frequency of tooth brushing (p = 0.005), consumption of pastries (p = 0.02), and consumption of soft drinks (p = 0.011). | [65] |
| Cross-sectional observational study. Saliva samples from adolescents | 1500 teenage girls | Analysis of general dietary habits, hygiene, and water composition | Two main patterns, “stomatotypes”, were identified: one dominated by Neisseria spp. and Haemophilus spp., and the other by Prevotella spp. and Veillonella spp. Water composition was associated with variations in bacterial genera | [66] |
| Cross-sectional observational study. Salivary microbiota profiles were analyzed | Two families: including parents and their adult children | No specific foods were evaluated | Predominant bacterial phyla in both families: Bacillota, Bacteroidota, Pseudomonadota, Fusobacteriota, and Actinomycetota. Individuals from the same family have bacterial similarity, with greater similarity between mothers and adult children. | [67] |
| Cross-sectional and longitudinal observational study. Biofilm and saliva samples. | 64 17-year-old adolescents with low caries prevalence and access to dental care from childhood | No specific foods were evaluated | In saliva: Bacillota was the predominant phylum (48%) and Actinomycetota (20%). In dental biofilm: Bacillota and Actinomycetota with similar abundance. In caries participants: ↑ Scardovia wiggsiae, Streptococcus mutans, Bifidobacterium longum, Leptotrichia spp. HOT498 and Selenomonas spp. in saliva. In dental biofilm, there was ↑ Corynebacterium matruchotii. | [68] |
| Longitudinal study. Saliva analysis at three points in time over 10 years | 107 individuals, including twins, from 12 to 24 years of age | No specific foods were evaluated | Twins shared more similar bacterial communities with each other. This similarity decreased with age and separation from the shared household. It is suggested that the environment has a greater impact than genetics. The most abundant genera: Veillonella spp., Actinomyces spp., and Streptococcus spp. | [69] |
| Randomized controlled clinical trial. Gingival parameters were evaluated. | 42 adults with gingivitis | Mediterranean Diet vs. Ultra-processed products | With a Mediterranean diet: significant reduction in the gingival index and bleeding on probing. Both groups: no change in plaque buildup | [70] |
| Longitudinal study of nutritional intervention. Analysis of saliva samples | 29 elite male athletes | High-carb vs. high-carb diet. Periodized vs. Periodized Carbs Low in carbohydrates and high in fat | Low-carb, high-fat diet: ↓ Neisseria spp. and ↑ Streptococcus spp. No significant changes in alpha diversity in the three groups. For athletes, the low-carb, high-fat diet can alter the composition of the oral microbiome | [71] |
| Cross-sectional observational study. Samples of supragingival plate | 40 adolescents with and without dental caries | Consumption of free sugars | Cavities and low sugar consumption: ↑ Lactobacillus spp., Streptococcus mutans, Actinomyces gerencseriae, Actinomyces dentails, Candida albicans, Scardovia wiggsiae, and Parascardovia acidifaciens. No cavities and high sugar consumption: more balanced microbiota, despite high sugar consumption. | [72] |
| In vitro experimental study using a human saliva model | Saliva samples from healthy adults were used to create biofilms of Streptococcus mutans and Candida albicans | Glucose, fructose, sucrose, starch, and starch-sucrose combinations | Sucrose and a combination of sucrose with starch: ↑ formation of bacterial-fungal aggregates in saliva. Dense biofilms were developed. Glucose and fructose: more dispersed biofilms with less acid production were developed. | [73] |
| Age Range of Children | Growth of Oral Microbial Diversity | Citation |
|---|---|---|
| Neonates 0–8 weeks of age | ↑ Streptococcus spp. and phylum Bacillota. | [53] |
| 0–2 months of age | ↑ Bifidobacterium spp. and Enterococcus spp. Streptococcus mitis remained ubiquitous across ages. | [42] |
| 4–6 months of age | Early colonizers Streptococcus spp. and Staphylococcus spp. | [74] |
| 0–6 months of age | Fusobacterium spp., Prevotella spp., Porphyromonas spp., Granulicatella spp., and Veillonella spp. | [52] |
| 6, 12, and 24 months of age. | ↑ Firmicutes and Proteobacteria | [22] |
| 1 year old | ↑ Pseudomonadota and Fusobacteriota. | [75] |
| 3–5 years old | Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus parainfluenzae. | [76] |
| 3–6 years old | ↑ Neisseria spp., Leptotrichia spp., Porphyromonas spp., and Gemella spp. | [28] |
| 11–15 years old | ↑ Streptococcus spp., Prevotella spp., Neisseria spp., and Rothia spp. | [77] |
| 12–24 years old | ↑ Veillonella spp., Actinomyces spp., and Streptococcus spp. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olate, P.; Martínez, A.; Sans-Serramitjana, E.; Cortés, M.; Díaz, R.; Hernández, G.; Paz, E.A.; Sepúlveda, N.; Quiñones, J. The Infant Oral Microbiome: Developmental Dynamics, Modulating Factors, and Implications for Oral and Systemic Health. Int. J. Mol. Sci. 2025, 26, 7983. https://doi.org/10.3390/ijms26167983
Olate P, Martínez A, Sans-Serramitjana E, Cortés M, Díaz R, Hernández G, Paz EA, Sepúlveda N, Quiñones J. The Infant Oral Microbiome: Developmental Dynamics, Modulating Factors, and Implications for Oral and Systemic Health. International Journal of Molecular Sciences. 2025; 26(16):7983. https://doi.org/10.3390/ijms26167983
Chicago/Turabian StyleOlate, Paula, Ailín Martínez, Eulàlia Sans-Serramitjana, Matías Cortés, Rommy Díaz, Genisley Hernández, Erwin A. Paz, Néstor Sepúlveda, and John Quiñones. 2025. "The Infant Oral Microbiome: Developmental Dynamics, Modulating Factors, and Implications for Oral and Systemic Health" International Journal of Molecular Sciences 26, no. 16: 7983. https://doi.org/10.3390/ijms26167983
APA StyleOlate, P., Martínez, A., Sans-Serramitjana, E., Cortés, M., Díaz, R., Hernández, G., Paz, E. A., Sepúlveda, N., & Quiñones, J. (2025). The Infant Oral Microbiome: Developmental Dynamics, Modulating Factors, and Implications for Oral and Systemic Health. International Journal of Molecular Sciences, 26(16), 7983. https://doi.org/10.3390/ijms26167983










