Abstract
Gestational diabetes mellitus (GDM) affects approximately 14% of pregnancies globally and has been hypothesized to be influenced by periconceptional and early pregnancy folic acid (FA) supplementation, a practice recommended to prevent neural tube defects. To evaluate this association, we conducted a systematic review of studies published between 2015 and 2024 examining FA use and GDM risk. Twelve studies met the inclusion criteria, including ten cohort studies and two case-control studies. While findings were mixed, several prospective studies suggested that high daily FA intake (≥800 μg) or prolonged use (>3–6 months) may be associated with increased odds of GDM, especially when initiated preconceptionally. Conversely, standard-dose supplementation (≈400 μg) appeared neutral or potentially protective in some populations. Notably, high folate status combined with low vitamin B12 was linked to increased GDM risk, suggesting metabolic interaction. Overall, most studies were of moderate to high methodological quality. Although current evidence is inconclusive, these results support cautious use of high-dose FA supplementation and the importance of individualized prenatal nutrition, particularly considering B12 status. Further research is needed to clarify biological mechanisms.
1. Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance first recognized during pregnancy [1], affects approximately 14% of pregnancies worldwide [2]. Its rising prevalence parallels global increases in obesity and type 2 diabetes, posing significant risks for both mother and child. The most common complications include an increased likelihood of requiring a cesarean section [3], preeclampsia [4], and a higher risk of progressing to type 2 diabetes mellitus (T2DM), although GDM resolves in most cases after delivery [5]. For the offspring it has been reported an increased likelihood of macrosomia [6] hypoglycemia [3,7], a higher incidence of congenital malformations [8], respiratory distress [9], lower Apgar scores at one and five minutes, and low birth weight [10] as well as higher T2DM risk later in life [11]. Identifying modifiable risk factors for GDM remains a key public health objective. One area of emerging interest is the potential metabolic impact of folic acid (FA) supplementation, a universally recommended intervention to prevent neural tube defects. Current guidelines advise 400 μg/day of FA from preconception through early pregnancy, with higher doses (5 mg/day) for high-risk women [12,13,14]. Beyond its neuroprotective role, folate participates in one-carbon metabolism and DNA methylation, suggesting broader physiological effects as explained by Figure 1 [15]. Recent studies have raised concerns that excessive FA intake may influence glucose metabolism, particularly when vitamin B12 deficiency coexists, potentially leading to insulin resistance through mechanisms like the methylfolate trap or immune-mediated pathways [16,17,18]. However, epidemiological findings are conflicting: while some studies associate high-dose or prolonged FA use with increased GDM risk, others report no association or even a protective effect [19,20,21]. To address this controversy, we systematically reviewed recent human studies on FA supplementation and GDM. Our goal was to clarify the strength and direction of the association, explore potential biological mechanisms, and consider implications for prenatal nutritional recommendations.
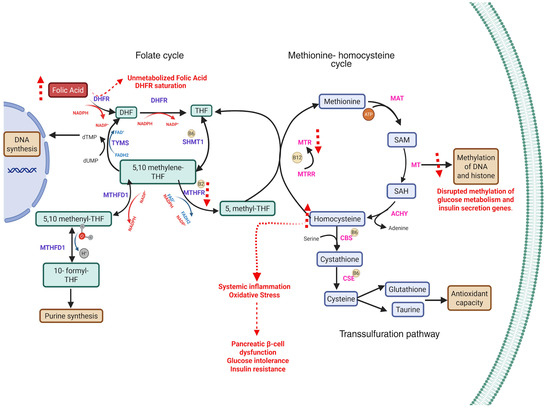
Figure 1.
Disruptions in one-carbon metabolism due to folate and vitamin B12 imbalance and their implications for gestational diabetes mellitus (GDM). The diagram outlines the folate cycle, methionine–homocysteine cycle, and transsulfuration pathway, highlighting the key enzymes and metabolites. Excess folic acid (FA) intake can lead to accumulation of unmetabolized folic acid (UMFA) and dihydrofolate (DHF), resulting in DHFR (dihydrofolate reductase) saturation and impaired formation of tetrahydrofolate (THF). This limits the availability of 5-methyltetrahydrofolate (5-methyl-THF), a methyl donor required by methionine synthase (MTR) for the remethylation of homocysteine (Hcy) to methionine in a vitamin B12-dependent reaction [22]. Elevated homocysteine may promote oxidative stress, systemic inflammation, pancreatic β-cell dysfunction, glucose intolerance, and insulin resistance [23,24]. While not depicted, hormonal changes during pregnancy (progesterone, leptin, adiponectin) may also contribute to insulin resistance. Red dashed arrows indicate disruptions specifically attributed to excessive folic acid exposure. Abbreviations: FA, folic acid; DHF, dihydrofolate; THF, tetrahydrofolate; SHMT1, serine hydroxymethyltransferase 1; 5,10-methylene-THF, 5,10-methylenetetrahydrofolate; 5,10-methenyl-THF, 5,10-methenyltetrahydrofolate; 10-formyl-THF, 10-formyltetrahydrofolate; MTHFD1, methylenetetrahydrofolate dehydrogenase 1; MTHFR, methylenetetrahydrofolate reductase; 5-methyl-THF, 5-methyltetrahydrofolate; MTRR, methionine synthase reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; MAT, methionine adenosyltransferase; MT, methyltransferase; TYMS, thymidylate synthase; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form).
2. Methods
This search protocol was designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [25] guidelines to ensure a comprehensive and transparent search of relevant literature.
2.1. Study Design
To identify relevant studies on the effect of folic acid consumption in GDM events, a systematic search was conducted in various electronic databases.
2.2. Eligibility Criteria
The search strategy was designed to capture relevant studies investigating the relationship between gestational diabetes mellitus and folic acid, as well as the effects of folic acid supplementation. The search was limited to studies published in the last 10 years and restricted to articles in English due to resource and time limitations. Randomized controlled trials, observational studies (cohorts, case-control) were included in the review. The primary outcomes were exposure to FA and GDM risk. We excluded studies that did not directly address the relationship between folic acid and GDM, as well as non-human studies, case reports, editorials, letters to the editor, and articles for which the full text was not available. To ensure high methodological quality and scientific rigor, the research team also agreed to exclude studies published in journals not listed in the Journal Citation Report (JCR). Although Web of Science provides access to JCR-indexed journals, we included PubMed and Scopus in the initial search strategy to enhance sensitivity and ensure broader coverage. This approach allowed us to capture potentially relevant studies that could later be screened for inclusion based on JCR indexing status.
2.3. Literature Search and Selection of Articles
The databases PubMed, Scopus, and Web of Science were used for this search. The search terms used included a combination of keywords and Medical Subject Headings (MeSH) related to gestational diabetes mellitus, folic acid, and supplementation. Boolean operators “AND” and “OR” were used to combine search terms appropriately. The search strategy was implemented using a combination of search terms, including variations of “gestational diabetes mellitus” AND “folic acid supplementation” AND “risk”, “gestational diabetes” AND “folic acid” AND “supplementation”, “GDM” AND “folic acid intake” AND “risk factor”, “diabetes in pregnancy” AND “folate supplementation” AND “association”, “pregnancy-induced diabetes” AND “folic acid” AND “increased risk”, “pregnancy-related diabetes” AND “folic acid use” AND “GDM incidence”, “gestational diabetes mellitus” AND “folate intake” AND “odds of GDM”, “hyperglycemia in pregnancy” AND “folic acid status” AND “glucose intolerance”, “gestational diabetes” AND “unmetabolized folic acid” AND “insulin resistance”, “GDM” AND “high-dose folic acid” AND “development of GDM”. Additionally, the reference lists of relevant articles found during the initial search were manually reviewed to identify additional studies that may have been missed.
All search results were recorded, and duplicates were removed. Identified studies underwent a selection process based on predefined inclusion and exclusion criteria to ensure the relevance and quality of studies included in the systematic review. Figure 2 shows the selection process.
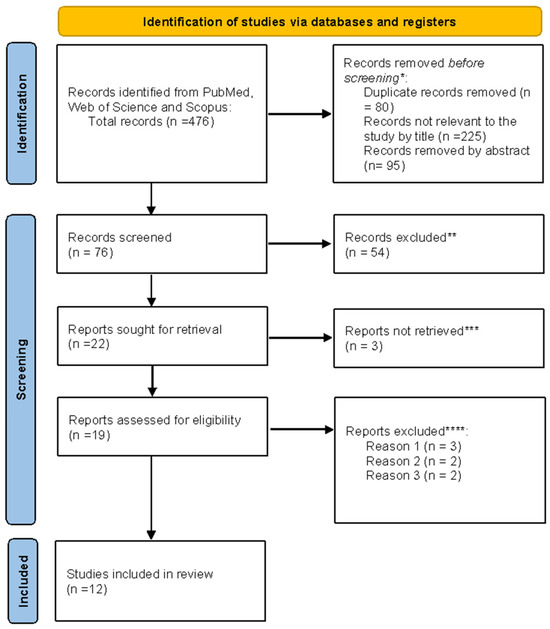
Figure 2.
PRISMA flowchart. * Studies not relevant to the present review ** No automation tools were used. All studies were assessed individually by a researcher. *** Unable to find full text. **** Reason 1: Study on animals. Reason 2: Wrong setting. Reason 3: The research question was not relevant.
2.4. Data Extraction
Twelve articles met the inclusion criteria and were chosen for further data extraction. Data extracted from each article included: title, study design, population characteristics (ethnicity, sample size), intervention details (folic acid dosage, duration, period), effect of FA, risk ratio, and odds ratio reported by the study, as well as key findings.
2.5. Risk of Bias Assessment Across Studies
Three authors independently evaluated the studies, and any differences among the authors were discussed by the investigation group until consensus was reached. The methodological quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS) [26], which assesses three domains: selection (four items), comparability (two items), and outcome (three items). Based on NOS criteria, studies receiving scores between 7 and 10 were categorized as high quality; low risk of bias, those scoring between 3 and 6 as moderate quality; moderate risk of bias, and those with lower scores as low quality; high risk of bias. In the present review, studies that obtained 6 or more stars were considered to be of high methodological quality and were reported as low risk of bias accordingly.
2.6. Synthesis of Results
Given substantial heterogeneity in study designs and reported measures, a meta-analysis was not performed. Instead, we carried out a narrative synthesis. We grouped the findings based on the nature of folic acid exposure (supplementation duration, dose, or timing relative to pregnancy) and whether the study suggested increased risk, decreased risk, or no effect on GDM. We also considered population differences that might explain discrepancies.
3. Results
As shown in Figure 2, a total of 476 records were identified through database searching (PubMed, Web of Science, and Scopus). After the removal of 80 duplicate records and exclusion of 225 titles and 95 abstracts that were not relevant to the research question, 76 records remained for screening. Following the title and abstract review, 54 articles were excluded. Of the 22 reports selected for full-text retrieval, 3 could not be obtained. Nineteen full-text articles were assessed for eligibility, of which five were excluded for predefined reasons (n = 3: animal studies; n = 2: inappropriate setting, 2 = research question not relevant). Ultimately, 12 studies met the inclusion criteria and were incorporated into the final synthesis. All stages of screening and selection were performed manually without automation tools.
3.1. Study Characteristics
As shown in Table 1, we included 12 studies, 9 were prospective cohort studies [20,27,28,29,30,31,32,33,34], 2 were case-control studies [35,36], and 1 was a retrospective cohort study [19]. Regarding population origin, 11 studies were conducted in Chinese populations, while 1 study involved Nordic populations from Norway and Sweden [19]. 9 out of 12 studies used the 75-g OGTT as suggested by the International Association of Diabetes and Pregnancy Study Groups [37]. The included studies displayed a wide variation in sample size, ranging from as few as 162 participants [36] to over 1.9 million pregnancies in population-based cohorts [19]. Most studies enrolled between 300 and 24,000 participants, with typical sample sizes around 2000 to 4000 in Chinese prospective cohorts [31,32,33,34]. Regarding methodological quality, six studies were assessed as having low risk of bias [19,28,29,30,35,36]. These studies generally featured rigorous designs and appropriate adjustment for confounders. In contrast, five studies were rated as having moderate risk of bias due to issues such as incomplete exposure characterization or limited statistical adjustment [20,27,29,32,33] none of the studies were judged to have high risk of bias, indicating an overall moderate to high methodological quality among the evidence included.

Table 1.
Studies evaluating the association between gestational diabetes mellitus and folic acid supplementation.
3.2. Folic Acid Supplementation and GDM
The association between FA-supplemented women and their risk for GDM was not consistent in the studies analyzed (Table 1). Some of them showed increased risk when FA was used in high doses or for long periods of time. For example, in the case of Li et al. [31], women who were supplemented with ≥ 800 μg/day for 3 or more months had a higher risk for GDM (aOR = 1.70, 95% CI: 1.30–3.36). Zhu et al. [36] also found that both not using FA (aOR = 7.25, 95% CI: 1.34–39.36) and using > 800 μg/day (aOR = 4.20, 95% CI: 1.03–17.22) were associated with increased risk compared to < 400 μg/day. Huang et al. [29] described something similar, but in a U-shape form, meaning that both no use and use for over 90 days increased the risk (OR = 3.45, 95% CI: 1.01–11.8). Early use in pregnancy during the first trimester was also associated with a higher risk, as reported by Zhu et al. [27] (OR 2.25 95% CI: 1.35–3.76). Lai et al. [28] reported that when folate was high and B12 was low, the risk was also higher (OR = 1.97, 95% CI: 1.05–3.68).
Not all studies showed a positive correlation. In contrast, some studies found protective effects or no relationship. Li et al. [35] reported that when the FA dose was given based on MTHFR/MTRR genotype, GDM incidence dropped to 0.27% in the genotype-tailored dossification group versus 3.24% in the control group; this difference reached statistical significance. Chen et al. [32] found that FA use was linked with a lower GDM risk (OR 0.82, 95% CI: 0.70–0.95). Guo et al. [20] and Zheng et al. [34] reported no association between FA and GDM risk. However, Zheng et al. [34] identified increased GDM risk with higher early pregnancy UMFA ≥P75 (aOR 1.36, 95% CI: 1.01–1.84) and ≥P90 (aOR 1.82, 95%, CI: 1.23–2.69), and Hcy ≥P75 (aOR 1.40, 95% CI: 1.04–1.88). In the Nordic cohort [19], self-reported use had minimal impact (OR 1.10, 95% CI: 1.06–1.14 in Norway, OR 0.89, 95% CI: 0.85–0.93 in Sweden), whereas prescribed 5 mg/day was linked with increased GDM risk (Norway OR 1.33, 95% CI: 1.15–1.53 and Sweden OR 1.56, 95% CI: 1.41–1.74). Cheng et al. [30] reported that FA supplementation ≥3 months before pregnancy increased GDM risk (ARR 1.72, 95% CI: 1.17–2.53); however, FA intake during pregnancy for ≥3 months had no association (ARR 0.92, 95% CI: 0.52–1.65). Li et al. [33] found that the effect varied according to timing and maternal characteristics; in obese women, both sufficient and deficient FA intake were associated with increased GDM risk (aORs 3.57. 95% CI: 2.02–6.34 and 10.82, 95% CI: 1.69–69.45, respectively), stratification by FA intake duration in obese women indicated a potential interaction when taken for less than 3 months, although this did not reach statistical significance (ROR = 1.63, 95% CI: 0.37–7.04).
4. Discussion
In this review, findings were mixed between FA supplementation and GDM risk. Evidence suggests that high-dose folic acid (≥800 μg/day) is associated with increased GDM risk, especially with prolonged use preconceptionally (≥3 months) [31,36]. In line with this, the Nordic cohort showed that 5mg/day doses were consistently linked to higher GDM risk [19]. The risk for GDM was further increased when mothers were obese (BMI ≥ 25 kg/m2) [27]. U-shaped association was observed with no FA supplementation or high-dose (≥800 μg/day) or long consumption duration (≥3 months) and elevated GDM risk [29,36].
Studies indicate that periconceptional folic acid supplementation is either neutral or mildly protective at the recommended guideline dose (400–800 μg/day) [38]; nonetheless, continuation in mid-pregnancy may increase GDM odds [30,31]. A protective effect was also reported where FA supplementation was individualized by MTHFR and MTRR single-nucleotide variants (SNVs) [35]; however, Zheng et al. found no association with folic acid-related genotypes [34].
A significant finding was that the higher risk for GDM was observed when a high level of folate in serum coexisted with low cobalamin (B12) status [28]. One more study reported a link between high levels of UMFA as well as Hcy in early pregnancy and GDM risk (aOR 1.82, 95%, CI: 1.23–2.69 and aOR 1.40, 95% CI: 1.04–1.88, respectively) [34]. In another study, RBC folate concentrations were measured in GDM and non-GDM pregnancies; notably, high folate concentrations were associated with GDM risk in the second trimester (OR = 2.17, 95% CI = 1.20–3.95) [39], which in turn provides empirical evidence for the anticipated biological mechanisms. Nevertheless, in a 2021 systematic review that included 12 studies about B12 and folate levels in pregnancy reported no association between serum folate and GDM risk, and a “conflicting” association between GDM risk and B12 deficiency [40].
4.1. Folic Acid and Potential Biological Mechanisms for GDM
Excessive FA intake has been found to disrupt one-carbon metabolism. It has been reported that high folate levels and B12 deficiency disrupted 1C metabolism; these findings are supported by increased total homocysteine and methylmalonic acid levels [41]. This is consistent with findings in the Folic Acid Clinical Trial, where high-dose folate acid intake increased folate serum level but no other biomarkers for one-carbon metabolism [42], which might in turn confirm some interdependency shown by other studies between FA and B12 in one-carbon metabolism disruption [28,43,44].
Evidence shows a correlation between FA intake and an increased risk for GDM. Numerous studies show that women supplementing with a ≥800 μg/day of FA, especially preconceptionally and in early pregnancy, have elevated odds of developing GDM [24,27,29,31]. Saravanan et al. [18] observed that higher folate concentrations in early pregnancy were associated with an increased risk of GDM, with each 12 nmol/L increase (1 SD) linked to an adjusted relative risk of 1.11 (95% CI: 1.036–1.182). Another study reported that individuals with GDM had significantly higher mean serum folate levels than non-GDM counterparts (37.6 ± 8 vs. 31.9 ± 11.2 nmol/L, p = 0.007) [45].
At least 60% of 193 countries mandate folic acid fortification [46]. In pregnant women who come from countries that mandate FA fortification and undergo folic acid supplementation, some studies show levels of unmetabolized folic acid (UMFA) were consistently higher [47,48,49]. The enzyme responsible for catalyzing the conversion from FA to dihydrofolate (DHF) and then to tetrahydrofolate (THF active form) is dihydrofolate reductase (DHFR). It has been reported that the function of DHFR in humans is slow and inadequate, as it becomes saturated when FA intake is higher than 331 μg, leaving UMFA levels increased [50]. Another study showed that even a 200 μg FA dose left measurable UMFA in serum after more than a few hours [51]. Therefore, a higher intake of FA could lead to an accumulation of UMFA as well as higher DHF levels; nevertheless, the UMFA role in GDM remains unclear.
High DHF levels from excessive folate supplementation may downregulate MTHFR, shifting folate metabolism from remethylating Hcy to nucleotide synthesis [52]. This shift might elevate Hcy levels. Elevated Hcy levels are consistently observed in pregnancies affected by GDM [53,54,55] and have been linked to oxidative stress, placental dysfunction [56,57], and impaired insulin secretion [58]. Interestingly, prolonged exposure to Hcy has been linked to reversible diminished pancreatic beta-cell function related to insulinotropic molecules [59]. It has also been proven that Hcy can reduce insulin secretion without beta-cell damage, even though it’s known for its cytotoxic effects [60]. Another potential mechanism by which Hcy levels may be relevant to maternal insulin resistance is through the modification of the immature form of the insulin receptor (pro-IR) via cysteine-homocysteinylation (C-Hcy) at the cysteine-825 residue. This modification has been shown to reduce mature insulin receptor levels across multiple tissues in mice [61]. High oxidative stress from Hcy effects may impair GLUT4 translocation [62], as well as endothelial damage [63]. High Hcy is linked to disrupted methylation patterns [64], particularly related to genes regulating glucose metabolism, insulin secretion, and sensitivity, such as MTNR1B, TCF7L2, CDKAL1, CDKN2A–CDKN2B, and HKDC1 [65], contributing to both maternal and adverse fetal outcomes. Paradoxically, while folate can lower homocysteine [66,67], this effect plateaus at high doses.
Regarding the previous evidence, FA excess increases Hcy because of MTHFR downregulation by DHFR saturation, which impairs Hcy remethylation. Notably, this remethylation process happens in a B12-dependent reaction by MTR [68]. Therefore, when there is a B12 deficiency, Hcy tends to be higher. MTR enzyme also uses 5-methyl-THF as a methyl donor in the reaction, so in the context of low B12, folate remains “trapped” in this methylated form; its accumulation in the nucleus has been linked to genomic instability, potentiating detrimental effects of FA high intake [69]. Recent literature shows that MTHFR C677T and A1298C contribute to GDM pathogenesis. The MTHFR 677 TT and 1298 AA genotypes are more prone to GDM dependent on one-carbon metabolism nutrients [70].
This might lead to functional folate deficiency despite high intake, homocysteine accumulation, and disrupted methylation patterns in GDM.
4.2. Methodological Considerations
This review followed PRISMA 2020 guidelines. We included only human studies indexed in major scientific databases. Study quality assessment was performed using the Newcastle–Ottawa Scale, with all studies rated as moderate to low bias risk, which enhances reliability in findings.
However, substantial heterogeneity among the included data, folic acid dose, timing, and GDM definitions limited comparability, so no metanalysis was performed. Almost all study designs were observational, which could result in potential residual confounding. Few cohorts adjusted for variables such as vitamin B12 status, dietary folate, or genetic variants affecting one-carbon metabolism, such as those found in MTHFR and MTRR. It is important to note that folic acid metabolism and response may vary depending on individual factors such as maternal body weight, nutritional status, age, ethnicity, and comorbidities. Most studies did not stratify results by these variables, which limits the applicability of the findings to broader populations. Most included studies were conducted in Chinese populations. Although these were derived from different provinces and hospitals, the concentration of data from a single country may limit generalizability to other ethnic and geographic populations.
5. Conclusions
This systematic review highlights a complex relationship between FA supplementation and the risk of GDM; while the standard FA dose (400 μg/day) remains essential for preventing neural tube defects, some studies suggest that no supplementation, high doses, or prolonged use—especially when started preconceptionally and in the presence of vitamin B12 deficiency—may increase GDM risk. Study heterogeneity limits definitive conclusions, but mechanistic evidence points to potential metabolic disturbances such as homocysteine accumulation, oxidative stress, and impaired insulin signaling. Given that FA fortification and supplementation are widespread practices globally, these findings suggest the need for individualized prenatal nutrition approaches. Therefore, we advocate that future research incorporates genetic, metabolic, and nutritional factors to better define optimal FA dosing and timing, aiming to balance fetal development benefits with minimized maternal metabolic risk.
Author Contributions
Conceptualization, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; methodology, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; software, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; validation, M.G.S.-P., A.E.G.-S., A.S.G.-C., R.C.-A., F.I.C.-M. and R.C.B.-R.; formal analysis, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; investigation, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; resources, M.G.S.-P., A.S.G.-C., A.E.G.-S., R.C.-A., F.I.C.-M. and R.C.B.-R.; data curation, M.G.S.-P., A.E.G.-S., A.S.G.-C., R.C.-A., F.I.C.-M. and R.C.B.-R.; writing—original draft preparation, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; writing—review and editing, M.G.S.-P., A.S.G.-C. and A.E.G.-S.; visualization, A.S.G.-C.; supervision, M.G.S.-P.; project administration, M.G.S.-P. and A.E.G.-S.; funding acquisition, M.G.S.-P., A.E.G.-S., A.S.G.-C., R.C.-A., F.I.C.-M. and R.C.B.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created the data supporting this review is from published studies that have been properly cited. This review was registered in the PROSPERO database: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251037606 accessed on 29 June 2025.
Acknowledgments
The authors gratefully acknowledge the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SEHICITI) for their institutional support and commitment to scientific advancement. We also extend our appreciation to the Universidad de Guadalajara (UDG) and the Doctorado en Investigación Multidisciplinaria en Salud (DIMS) for their continuous academic guidance and resources that enabled the development of this work. We appreciate the support of the Programa de Apoyo a la Mejora de las Condiciones de Producción de los miembros del SNII y del Sistema Nacional de Creadores de Arte (SNCA) (PROSNI program), from the Universidad de Guadalajara.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FA | Folic acid |
| GDM | Gestational diabetes mellitus |
| B12 | Vitamin B12 |
| UMFA | Unmetabolized folic acid |
| DHFR | Dihydrofolate reductase |
| THF | Tetrahydrofolate |
| MTHFR | Methylenetetrahydrofolate reductase |
| MTR | Methionine synthase |
| MTRR | Methionine synthase reductase |
| SAM | S-adenosylmethionine |
| SAH | S-adenosylhomocysteine |
| Hcy | Homocysteine |
| CBS | Cystathionine β-synthase |
| CSE | Cystathionine γ-lyase |
| MAT | Methionine adenosyltransferase |
| MT | Methyltransferase |
| TYMS | Thymidylate synthase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OGTT | Oral glucose tolerance test |
| aOR | Adjusted odds ratio |
| ARR | Adjusted risk ratio |
| OR | Odds ratio |
| CI | Confidence interval |
| BMI | Body mass index |
| NOS | Newcastle–Ottawa Scale |
| ROR | Relative odds ratio |
| PBG | Plasma blood glucose |
| MV | Multivitamin |
| wk. | Week |
| D | Day |
| 5-methyl-THF | 5-methyltetrahydrofolate |
| IR | Insulin receptor |
| C-Hcy | Cysteine-homocysteinylation |
| ROS | Reactive oxygen species |
| T2DM | Type 2 diabetes mellitus |
| SNP | Single nucleotide polymorphism |
References
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and Management of Gestational Diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Spradley, F.T. Metabolic Abnormalities and Obesity’s Impact on the Risk for Developing Preeclampsia Spradley FT. Metabolic Abnormalities and Obesity’s Impact on the Risk for Devel-Oping Preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, 5–12. [Google Scholar] [CrossRef]
- You, H.; Hu, J.; Liu, Y.; Luo, B.; Lei, A. Risk of Type 2 Diabetes Mellitus after Gestational Diabetes Mellitus: A Systematic Review & Meta-Analysis. Indian J. Med. Res. 2021, 154, 62–77. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66, 14–20. [Google Scholar] [CrossRef]
- Desoye, G.; Nolan, C.J. The Fetal Glucose Steal: An Underappreciated Phenomenon in Diabetic Pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Sun, Y.; Du, Y.; Santillan, M.K.; Santillan, D.A.; Snetselaar, L.G.; Bao, W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus with Congenital Anomalies of the Newborn. Diabetes Care 2020, 43, 2983–2990. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Zhang, D. Maternal Diabetes Mellitus and Risk of Neonatal Respiratory Distress Syndrome: A Meta-Analysis. Acta Diabetol. 2019, 56, 729–740. [Google Scholar] [CrossRef]
- Mistry, S.K.; Gupta, R.D.; Alam, S.; Kaur, K.; Shamim, A.A.; Puthussery, S. Gestational Diabetes Mellitus (GDM) and Adverse Pregnancy Outcome in South Asia: A Systematic Review. Endocrinol. Diabetes Metab. 2021, 4, e00285. [Google Scholar] [CrossRef]
- Song, Q.; Wang, L.; Liu, H.; Liang, Z.; Chen, Y.; Sun, D.; Li, W.; Leng, J.; Yang, X.; Cardoso, M.A.; et al. Maternal GDM Status, Genetically Determined Blood Glucose, and Offspring Obesity Risk: An Observational Study. Obesity 2020, 29, 204. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of Women’s Nutrition before and during Early Pregnancy on Maternal and Infant Outcomes: A Systematic Review. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and Safety of Periconceptional Oral Folate Supplementation for Preventing Birth Defects. Cochrane Database Syst. Rev. 2015, 2015, CD007950. [Google Scholar] [CrossRef]
- Balogun, O.O.; da Silva Lopes, K.; Ota, E.; Takemoto, Y.; Rumbold, A.; Takegata, M.; Mori, R. Vitamin Supplementation for Preventing Miscarriage. Cochrane Database Syst. Rev. 2016, 2016, CD004073. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Stover, P.J. Chapter 1 Folate-Mediated One-Carbon Metabolism. Vitam. Horm. 2008, 79, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, S.; Zhong, Z.; Guo, Y.; Xia, T.; Chen, Y.; Ding, L. The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2766. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Han, X.; Zhou, W.; Long, W.; Wang, H.; Yu, B.; Zhang, B. Association of Folate and Vitamin B12 Imbalance with Adverse Pregnancy Outcomes among 11,549 Pregnant Women: An Observational Cohort Study. Front. Nutr. 2022, 9, 947118. [Google Scholar] [CrossRef]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of Maternal Vitamin B12 and Folate Levels in Early Pregnancy with Gestational Diabetes: A Prospective UK Cohort Study (PRiDE Study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef]
- Pazzagli, L.; Chacon, S.S.; Karampelias, C.; Cohen, J.M.; Broms, G.; Kieler, H.; Odsbu, I.; Selmer, R.; Andersson, O.; Cesta, C.E. Association between Folic Acid Use during Pregnancy and Gestational Diabetes Mellitus: Two Population-Based Nordic Cohort Studies. PLoS ONE 2022, 17, e0272046. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, R.; Corsi, D.J.; White, R.R.; Smith, G.; Rodger, M.; Retnakaran, R.; Walker, M.; Wen, S.W. Folic Acid Supplementation in Early Pregnancy, Homocysteine Concentration, and Risk of Gestational Diabetes Mellitus. J. Obstet. Gynaecol. Can. 2022, 44, 196–199. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Zhang, J. Association between Maternal Folate Status and Gestational Diabetes Mellitus. Food Sci. Nutr. 2021, 9, 2042–2052. [Google Scholar] [CrossRef]
- Lionaki, E.; Ploumi, C.; Tavernarakis, N. One-Carbon Metabolism: Pulling the Strings behind Aging and Neurodegeneration. Cells 2022, 11, 214. [Google Scholar] [CrossRef]
- Yang, H.; Qin, D.; Xu, S.; He, C.; Sun, J.; Hua, J.; Peng, S. Folic Acid Promotes Proliferation and Differentiation of Porcine Pancreatic Stem Cells into Insulin-Secreting Cells through Canonical Wnt and ERK Signaling Pathway. J. Steroid Biochem. Mol. Biol. 2021, 205, 105772. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.M.; Arthurs, A.L.; Smith, M.D.; Roberts, C.T.; Jankovic-Karasoulos, T. High Folate, Perturbed One-Carbon Metabolism and Gestational Diabetes Mellitus. Nutrients 2022, 14, 3930. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Zhu, B.; Ge, X.; Huang, K.; Mao, L.; Yan, S.; Xu, Y.; Huang, S.; Hao, J.; Zhu, P.; Niu, Y.; et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence from a Prospective Cohort Study. Diabetes Care 2016, 39, e36–e37. [Google Scholar] [CrossRef]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.Y.; Shek, L.P.C.; Yap, F.K.P.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High Folate and Low Vitamin B12 Status during Pregnancy Is Associated with Gestational Diabetes Mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Li, L.; Chen, Y.; Yang, Y.; Yang, Y.; Hu, Y.; Zhao, Y.; Tang, H.; Xu, D.; et al. Duration of Periconceptional Folic Acid Supplementation and Risk of Gestational Diabetes Mellitus. Asia Pac. J. Clin. Nutr. 2019, 28, 321–329. [Google Scholar] [CrossRef]
- Cheng, G.; Sha, T.; Gao, X.; He, Q.; Wu, X.; Tian, Q.; Yang, F.; Tang, C.; Wu, X.; Xie, Q.; et al. The Associations between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus, and Adverse Birth Outcomes Based on a Birth Cohort. Int. J. Environ. Res. Public Health 2019, 16, 4511. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Huang, L.; Zhong, C.; Chen, R.; Zhou, X.; Chen, X.; Li, X.; Cui, W.; Xiong, T.; et al. High-Dose Folic Acid Supplement Use from Prepregnancy through Midpregnancy Is Associated with Increased Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2019, 42, E113–E115. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Li, Y.; Zhou, W.; Zhou, N.; Yang, H.; Chen, Q.; Li, Y.; Huang, Q.; Chen, Z. Association of Folic Acid Supplementation in Early Pregnancy with Risk of Gestational Diabetes Mellitus: A Longitudinal Study. Nutrients 2022, 14, 4061. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Du, Z.; Shen, Q.; Jiang, L.; Sui, L.; Zhang, N.; Wang, H.; Li, G. Joint Effect of Maternal Pre-Pregnancy Body Mass Index and Folic Acid Supplements on Gestational Diabetes Mellitus Risk: A Prospective Cohort Study. BMC Pregnancy Childbirth 2023, 23, 202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Zhang, P.; Chen, T.; Yan, X.; Li, L.; Shao, L.; Song, Z.; Han, W.; Wang, J.; et al. Gestational Diabetes Mellitus Is Associated with Distinct Folate-Related Metabolites in Early and Mid-Pregnancy: A Prospective Cohort Study. Diabetes Metab. Res. Rev. 2024, 40, e3814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, J.; Xu, M.; Xu, M.; Yang, Y.; Lu, W.; Yu, X.; Ma, J.; Pan, J. Individualized Supplementation of Folic Acid According to Polymorphisms of Methylenetetrahydrofolate Reductase (MTHFR), Methionine Synthase Reductase (MTRR) Reduced Pregnant Complications. Gynecol. Obstet. Investig. 2015, 79, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, Y.; Fu, Y.; Sun, W.; Chen, J.; Yu, N.; Zhao, M. Association of Folic Acid Supplementation, Dietary Folate Intake and Serum Folate Levels with Risk of Gestational Diabetes Mellitus: A Matched Case-Control Study. J. Nutr. Sci. Vitaminol. 2023, 69, 28–37. [Google Scholar] [CrossRef]
- Metzger, B.E. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Wilson, R.D.; O’Connor, D.L. Guideline No. 427: Folic Acid and Multivitamin Supplementation for Prevention of Folic Acid–Sensitive Congenital Anomalies. J. Obstet. Gynaecol. Can. 2022, 44, 707–719.e1. [Google Scholar] [CrossRef]
- Xie, K.; Xu, P.; Fu, Z.; Gu, X.; Li, H.; Cui, X.; You, L.; Zhu, L.; Ji, C.; Guo, X. Association of Maternal Folate Status in the Second Trimester of Pregnancy with the Risk of Gestational Diabetes Mellitus. Food Sci. Nutr. 2019, 7, 3759–3765. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Meng, D.; Yang, L.; Meng, X.; Liu, F. Vitamin B12 and Folate Levels During Pregnancy and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 670289. [Google Scholar] [CrossRef]
- Selhub, J.; Morris, M.S.; Jacques, P.F. In Vitamin B12 Deficiency, Higher Serum Folate Is Associated with Increased Total Homocysteine and Methylmalonic Acid Concentrations. Proc. Natl. Acad. Sci. USA 2007, 104, 19995–20000. [Google Scholar] [CrossRef]
- Murphy, M.S.Q.; Muldoon, K.A.; Sheyholislami, H.; Behan, N.; Lamers, Y.; Rybak, N.; White, R.R.; Harvey, A.L.J.; Gaudet, L.M.; Smith, G.N.; et al. Impact of High-Dose Folic Acid Supplementation in Pregnancy on Biomarkers of Folate Status and 1-Carbon Metabolism: An Ancillary Study of the Folic Acid Clinical Trial (FACT). Am. J. Clin. Nutr. 2021, 113, 1361–1371. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Yan, X.; Wang, Y.; Shi, C.; Wu, X.; Liu, H.; Zhang, L.; Zhang, X.; Liu, J.; et al. Joint Effects of Folate and Vitamin B12 Imbalance with Maternal Characteristics on Gestational Diabetes Mellitus. J. Diabetes 2019, 11, 744–751. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy with Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal Folate, One-Carbon Metabolism and Pregnancy Outcomes. Matern. Child. Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Halsey, J.; Sherliker, P.; Pan, H.; Chen, Z.; Bennett, D.A.; Clarke, R. Global Heterogeneity in Folic Acid Fortification Policies and Implications for Prevention of Neural Tube Defects and Stroke: A Systematic Review. EClinical Med. 2024, 67, 102366. [Google Scholar] [CrossRef] [PubMed]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.I. High Concentrations of Folate and Unmetabolized Folic Acid in a Cohort of Pregnant Canadian Women and Umbilical Cord Blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; O’Connor, D.; Koren, G. Circulating Unmetabolized Folic Acid: Relationship to Folate Status and Effect of Supplementation. Obstet. Gynecol. Int. 2012, 2012, 485179. [Google Scholar] [CrossRef]
- Sulistyoningrum, D.C.; Sullivan, T.R.; Skubisz, M.; Palmer, D.J.; Wood, S.; Ueland, P.M.; McCann, A.; Makrides, M.; Green, T.J.; Best, K.P. Maternal Serum Unmetabolized Folic Acid Concentration Following Multivitamin and Mineral Supplementation with or without Folic Acid after 12 Weeks Gestation: A Randomized Controlled Trial. Matern. Child. Nutr. 2024, 20, e13668. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The Extremely Slow and Variable Activity of Dihydrofolate Reductase in Human Liver and Its Implications for High Folic Acid Intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized Folic Acid in Serum: Acute Studies in Subjects Consuming Fortified Food and Supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef]
- Petrova, B.; Maynard, A.G.; Wang, P.; Kanarek, N. Regulatory Mechanisms of One-Carbon Metabolism Enzymes. J. Biol. Chem. 2023, 299, 105457–105458. [Google Scholar] [CrossRef]
- Deng, M.; Zhou, J.; Tang, Z.; Xiang, J.; Yi, J.; Peng, Y.; Di, L.; Zhai, X.; Yang, M.; Du, Y. The Correlation between Plasma Total Homocysteine Level and Gestational Diabetes Mellitus in a Chinese Han Population. Sci. Rep. 2020, 10, 18679. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, J.; Yang, M.; Shao, Y.; Liu, J.; Wu, Q.; Xu, Q.; Wang, H.; He, X.; Chen, Y.; et al. Serum Homocysteine Level and Gestational Diabetes Mellitus: A Meta-analysis. J. Diabetes Investig. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Lu, L.-P.; Yi, M.-H.; Shen, C.-Y.; Lu, G.-Q.; Jia, J.; Wu, H. Study on the Correlation between Homocysteine-Related Dietary Patterns and Gestational Diabetes Mellitus:A Reduced-Rank Regression Analysis Study. BMC Pregnancy Childbirth 2022, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Jankovic-Karasoulos, T.; Smith, M.D.; Leemaqz, S.; Mittinty, M.; Williamson, J.; McCullough, D.; Arthurs, A.L.; Dekker, G.A.; Roberts, C.T.; Affiliations, A.; et al. Folate Overload and the Placental Hormone Axis: A Hidden Risk for Gestational Diabetes Mellitus. medRxiv 2025. [Google Scholar] [CrossRef]
- Thakur, P.; Bhalerao, A.; Thakur, P.; Bhalerao, A. High Homocysteine Levels During Pregnancy and Its Association with Placenta-Mediated Complications: A Scoping Review. Cureus 2023, 15, e35244. [Google Scholar] [CrossRef]
- Patterson, S.; Flatt, P.R.; Brennan, L.; Newsholme, P.; McClenaghan, N.H. Detrimental Actions of Metabolic Syndrome Risk Factor, Homocysteine, on Pancreatic β-Cell Glucose Metabolism and Insulin Secretion. J. Endocrinol. 2006, 189, 301–310. [Google Scholar] [CrossRef]
- Patterson, S.; Scullion, S.M.J.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. Prolonged Exposure to Homocysteine Results in Diminished but Reversible Pancreatic β-Cell Responsiveness to Insulinotropic Agents. Diabetes Metab. Res. Rev. 2007, 23, 324–334. [Google Scholar] [CrossRef]
- Patterson, S.; Flatt, P.R.; McClenaghan, N.H. Homocysteine and Other Structurally-Diverse Amino Thiols Can Alter Pancreatic Beta Cell Function without Evoking Cellular Damage. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1109–1114. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.Y.; Liu, L.; Qiao, Y.N.; Geng, H.R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.Y. Homocysteine Inhibits Pro-Insulin Receptor Cleavage and Causes Insulin Resistance via Protein Cysteine-Homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef]
- Pessler, D.; Rudich, A.; Bashan, N. Oxidative Stress Impairs Nuclear Proteins Binding to the Insulin Responsive Element in the GLUT4 Promoter. Diabetologia 2001, 44, 2156–2164. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of Homocysteine-Mediated Endothelial Injury and Its Consequences for Atherosclerosis. Front. Cardiovasc. Med. 2023, 9, 1109445. [Google Scholar] [CrossRef]
- Linares-Pineda, T.; Peña-Montero, N.; Fragoso-Bargas, N.; Gutiérrez-Repiso, C.; Lima-Rubio, F.; Suarez-Arana, M.; Sánchez-Pozo, A.; Tinahones, F.J.; Molina-Vega, M.; Picón-César, M.J.; et al. Epigenetic Marks Associated with Gestational Diabetes Mellitus across Two Time Points during Pregnancy. Clin. Epigenetics 2023, 15, 110. [Google Scholar] [CrossRef]
- Kong, D.; Kowalik, O.; Garratt, E.; Godfrey, K.M.; Chan, S.Y.; Teo, A.K.K. Genetics and Epigenetics in Gestational Diabetes Contributing to Type 2 Diabetes. Trends Endocrinol. Metabol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149. [Google Scholar] [CrossRef] [PubMed]
- Almassinokiani, F.; Kashanian, M.; Akbari, P.; Mossayebi, E.; Sadeghian, E. Folic Acid Supplementation Reduces Plasma Homocysteine in Postmenopausal Women. J. Obstet. Gynaecol. 2016, 36, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Palmer, A.M.; Kamynina, E.; Field, M.S.; Stover, P.J. Folate Rescues Vitamin B12 Depletion-Induced Inhibition of Nuclear Thymidylate Biosynthesis and Genome Instability. Proc. Natl. Acad. Sci. USA 2017, 114, E4095–E4102. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, B.; Zhang, Y.; Zhou, Y. MTHFR Gene Polymorphisms in Diabetes Mellitus. Clin. Chim. Acta 2024, 561, 119825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).