Systematic Evaluation of Biodegradation of Azo Dyes by Microorganisms: Efficient Species, Physicochemical Factors, and Enzymatic Systems
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Azo Dyes
3.1.1. Azo Dyes and Their Structural Versatility
3.1.2. Toxicological Potential of Azo Dyes
4. Conventional Methods for Treating Azo Contaminants: Principles, Challenges, and Perspectives for Environmental Remediation
Application of Biological Treatments in the Remediation of Industrial Effluents
| Type of Microorganism | Species | Treated Azo Dyes | Mechanism Involved | Conditions (pH, Temperature, and Presence or Absence of O2) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Bacteria | Pseudomonas putida | Reactive Red 120 | Azoreductase | pH 7.4 | 92.6 | [143] |
| Reactive Black 5 | 35 °C | 92.6 | ||||
| Reactive Blue 13 | Anaerobic conditions | 88.0 | ||||
| Bacillus subtilis (DY1KVG) | Azo dye mixtures: Reactive red, Reactive brown, Reactive black | Azoreductase | pH 7–8.5 | 87.3 | [144] | |
| 50–70 °C | ||||||
| Aerobic conditions | ||||||
| Escherichia coli | Basic Orange 2 | Azoreductase | pH 4 | 89.8 | [145] | |
| 40 °C | ||||||
| Aerobic conditions | ||||||
| Pseudomonas geniculata Ka38 | Methyl orange | Azoreductase | pH 7 | 89.0 | [146] | |
| 30 °C | ||||||
| Mixed conditions (aerobic/anaerobic) | ||||||
| Filamentous fungi | Oudemansiella canarii (EF72) | Congo Red | Laccase | pH 5.5 | 80.0 | [88] |
| 30 °C | ||||||
| Presence of O2 | ||||||
| Trametes hirsuta D7 | Acid Blue 29 Reactive Blue 4 | Laccase | pH 4–5 | 86–90 | [88] | |
| 25 °C | ||||||
| Aerobic conditions | ||||||
| Nigrospora sp | Synazol Red HF-6BN | Ligninolytic enzymes | pH 5–7 | 85.0 | ||
| 25–30 °C | ||||||
| Aerobic conditions | ||||||
| Trametes versicolor | Direct Pink B | Manganese Peroxidase | pH 5.2 | 72.4 | [147] | |
| 29.6 | ||||||
| Aerobic conditions | ||||||
| Aspergillus terreus | Acid Blue 29 Disperse Red 1 Congo Red | Ligninolytic enzymes | pH 7 | 92.7 | [88,148] | |
| 30 °C | 90.5 | |||||
| Aerobic conditions | 96.7 | |||||
| Aspergillus niger | pH 7 | 93.4 | ||||
| 30 °C | 84.2 | |||||
| Aerobic conditions | 96.5 | |||||
| Aspergillus flavus | pH 7 | 92.4 | ||||
| 30 °C | 80.5 | |||||
| Aerobic conditions | 96.3 | |||||
| Aspergillus fumigatus | Direct Pink B | Manganese Peroxidase | pH 7 | 91.8 | [147] | |
| 35 °C | 95.5 | |||||
| Aerobic conditions | 97.0 | |||||
| Actinobacteria | Streptomyces albidoflavus 3MGH | Reactive Orange 122 | Reductive enzymes (such as azoreductase) and oxidative enzymes (such as laccase) | pH 6 | 94.4 | [149] |
| Direct Blue 15 | 36 °C | 86.3 | ||||
| Black Direct 38 | Aerobic conditions | 68.2 | ||||
| Arthrobacter bambusae DP-A9 | Methyl red Brilliant black | Azoreductase Peroxidase Laccase | pH 7 30 °C Aerobic conditions | 100 | [150] | |
| 100 | ||||||
| 100 | ||||||
| Dermacoccus nishinomyaensis DP-D10 | 100 | |||||
| 74.0 | ||||||
| 97.6 | ||||||
| Leifsonia shinshuensis DP-L11 | ||||||
| Streptomyces maritimus (A011) | Amido Black10B | Extracellular enzymes: peroxidases (lignin peroxidase, manganese peroxidase), Laccases | pH 7–9 | 85.4 | [151] | |
| 30–40 °C | ||||||
| Anoxic conditions | ||||||
| Yeasts | Geotrichum candidum | Congo red | Manganese peroxidase, Lignin peroxidase | pH 5–6 | 85.4 | [152] |
| 25–30 °C | ||||||
| Microaerophilic/anoxic conditions | ||||||
| Sterigmatomyces haophilus SSA1575 | Reactive Black 5 | NADH-dichlorophenol indophenol (NADH--DCIP). Reductase Lignin peroxidase (LiP). | pH 5 | 100 | [153] | |
| 30 °C | ||||||
| Aerobic conditions | ||||||
| Meyerozyma guilliermondii A4 | Acid Red B | Azoreductase. NADH-DCIP reductase. Lignin peroxidase. Manganese peroxidase. Laccase | pH 6 35 °C Aerobic conditions | >97 | [154,155] | |
| Acid Orange II | ||||||
| Acid Scarlet GR | ||||||
| Acid Red 3R | ||||||
| Reactive Brilliant | ||||||
| Red K-2G | ||||||
| Reactive Violet KN-4R | ||||||
| Reactive Yellow 3R | ||||||
| Meyerozyma caribbica | Acid Orange 7 | Manganese peroxidase | pH 5–7 | 93.8 | [155] | |
| 28 °C | ||||||
| Aerobic conditions | ||||||
| Microalgae | Lychaete pellucida | Reactive Blue 4 | Biosorption | pH 8.0 | 96–97 | [156] |
| Reactive Red 120 | 25 °C | 95–97 | ||||
| Brilliant Reactive Yellow 3G | The study reports photoautotrophic culture conditions with continuous light exposure and O2 supplementation at room temperature | 96–97 | ||||
| Reactive Green 12 | ||||||
| Chlorella vulgaris | Reactive Black 5 | Azoreductase | pH 5–8 | 80 | [157] | |
| Direct Blue 71 | 40 °C | 78 | ||||
| Scattered Red 1 | The study reports mixotrophic culture conditions, with controlled light exposure to enhance dye degradation | 84 |
5. Enzymatic Systems in the Biodegradation of Azo Dyes: Reductive and Oxidative Mechanisms in Bioremediation
6. Fungi Remediation of Synthetic Dyes: A Biotechnological Alternative for the Treatment of Textile Effluents
| Fungi | Enzyme | Azo Dye | Optimal Conditions (pH, T (°C), Absence or Presence of O2) | Metabolites/Degraded Products | Mechanism of Action | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Fusarium oxysporum | Laccase Azorreductasa | Reactive Black 5 Orange II | - | Aniline. Phenolic compounds | Oxidation of phenolic groups (laccases) and cleavage of the azo bond (azoreductases) | 89.8 91.3 | [181] |
| 30 °C | |||||||
| Aerobiosis | |||||||
| Aspergillus tamari | Laccase | Crystal Violet | pH 7 | N,N,N′,N′-Tetramethylpararosaniline. 2-(Methylamino)phenol. Benzophenone. 4-methyl amino phenol. 4-(Dimethylamino)benzaldehyde. | Oxidative catalysis of the azo bond | 33.8 | [182] |
| Congo Red | 27 °C | 74.0 | |||||
| Aerobiosis | |||||||
| Aspergillus flavus ASP1 | Laccase Lignin peroxidase Quinine reductase | Reactive Orange 16 | pH 3 | Aniline. 6-(acetylamino)naphthalene-2-sulfonic acid | (Lac/LiP)-mediated azo bond cleavage and detoxification by quinine reductase | 100 | [183] |
| 30 °C | |||||||
| Aerobic conditions | |||||||
| Trametes versicolor | Laccase | Remazol Red | pH 5 | - | Laccase oxidizes the dye by transferring electrons from the substrate (RR) to molecular oxygen, resulting in the formation of water. | 54.0 | [184] |
| 45 °C | |||||||
| - | |||||||
| Peroneutypa scoparia | Laccase | Acid Red 97 | pH 6 | - | Reduction of the azo bond | 87.5 | [185] |
| 40 °C | |||||||
| - | |||||||
| Irpex lacteus F17 | Manganese Peroxidase | Malachite green | - | - | Reduction of the azo bond and oxidation of aromatic rings | 96.0 | |
| - | |||||||
| - | |||||||
| Bjerkandera adusta | Reactive Blue 120 | pH 5 | - | Mn3+-mediated radical oxidation | 90 | ||
| 28 °C | |||||||
| Aerobic conditions | |||||||
| Paraconiothyrium variabile | Laccase | Acid Red 18 | - | - | Oxidative degradation of the azo group | 90 | |
| Direct Red 81 | - | 68.3 | |||||
| - | |||||||
| RH-2 Consortium * | Laccase Manganese Peroxidase | Congo red | pH 5 | - | Enzymatic oxidation of the azo bond | 97.1 | [186] |
| 28 °C | |||||||
| Aerobic conditions |
7. Photoautotrophic Microalgae and Microbial Consortia in the Biotransformation of Azo Dyes
| Algae Strain/Consortium | Azo Dye | Mechanisms Involved | Metabolites/Degraded Products | Optimal Conditions (Concentration (mg/L), pH, Temperature) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Chlorella vulgaris | Direct Green 6 | Reductive cleavage of azo bonds by azoreductase and decomposition by peroxidases and laccase | - | 200 mg/L | 78.4 | [194] |
| pH 8 | ||||||
| 25 °C | ||||||
| Direct Black 22 | Photodegradation (primary), enzymatic degradation (secondary), and adsorption | - | 30 mg/L | - | [194] | |
| pH 7.2 | ||||||
| 28 °C | ||||||
| Oedogonium subplagiostomum AP1 | Methyl orange | Biosorption | Phenols*. Aromatic amines*. Organic acids*. | 500 mg/L | 97.0 | [195] |
| pH 6.5 | ||||||
| 30 °C | ||||||
| Oscillatoria sp. y S. obliquus | Reactive Orange 122 | Enzymatic degradation, adsorption | Cyclic amines*. Phenolic compounds*. | 20 mg/L | 98.5 | [196] |
| pH 11 | ||||||
| 25 °C | ||||||
| Consortium (Chlorella, Pseudomonas putida, and Lactobacillus plantarum) | Reactive Blue 40 | Synergistic degradation: - Azo bond cleavage (azoreductases) - Oxidation of intermediates (laccases/peroxidases) - Adsorption in biomass | Aromatic amines* (typical product of azo bond cleavage). Phenols and modified alcohols*. | 1000 mg/L | 99.0 | [191] |
| 11 | ||||||
| 35 °C | ||||||
| Fucus vesiculosus | Methyl orange | Biosorption | N1,N1-dimethylbenzene-1,4-diamine. 4-aminobenzenesulfonate. | 57.6 | 76.8 | [197] |
| pH 9 | ||||||
| 25 °C | ||||||
| Chlamydomonas mexicana | Red HE8B | Combined enzymatic biodegradation mechanism (Laccases and peroxidases) | N-phenylhydroxylamine. Naphthalen-1-ol. Sodium 5-hydroxynaphthalene-2-sulfonate. | 5 mg/L | 62 | [188] |
| pH 7 | 39 | |||||
| 27 °C | ||||||
| Reactive Green 27 |
8. Yeast-Based Biocatalytic Systems for Azo Dye Degradation: Enzymes, Biosorptive Dynamics, and Biotechnological Potential in Textile Effluent Remediation
| Yeast Strain/Consortium | Dye | Mechanisms Involved | Metabolites/Degraded Products | Conditions (Time (h), pH, T (°C), Dye Concentration (mg/L)) | Removal/ Decolorization (%) | Ref. |
|---|---|---|---|---|---|---|
| Cyberlindnera fabianii | Acid Red 14 | Laccase (Lac), Tyrosinase (Tyr), Manganese Peroxidase (Mnp), Azoreductase (AzoR) | - | 12 h | 97 | [205] |
| pH 5 | ||||||
| 30 °C | ||||||
| 50 mg/L | ||||||
| Saccharomyces cerevisiae | Acid Orange 7 | Biosorption (Immobilization in Fe3O4) | - | 2.3 h | ||
| pH 6.5 | ||||||
| 35 °C | ||||||
| 50 mg/L | ||||||
| Violet crystal | The enzymes involved are not identified. | - | 24 h | 84.9 | [203,207] | |
| pH 7 | ||||||
| 30 °C | ||||||
| 1000 mg/L | ||||||
| Pichia kudriavzevii SDG12 | Reactive Black 5 | Azoreductase | Unspecified amines and aromatic compounds | 18 h | 100 | [208] |
| pH 7 | ||||||
| 32 °C | ||||||
| 100 mg/L | ||||||
| Candida tropicalis A1 | Acid Red B | Azoreductase (AZR), Laccase (Lac), Manganese peroxidase (MnP), Lignin peroxidases (LiP) | 4-amino-naphthalene-1-sulfonic acid, 4-hydrazinylnaphthalene-1-sulfonic acid, naphthalene-1,2,4-triol, 1-phenylethenol | 12 h | 966 | [209] |
| pH 7 | ||||||
| 30 °C | ||||||
| 70 mg/L | ||||||
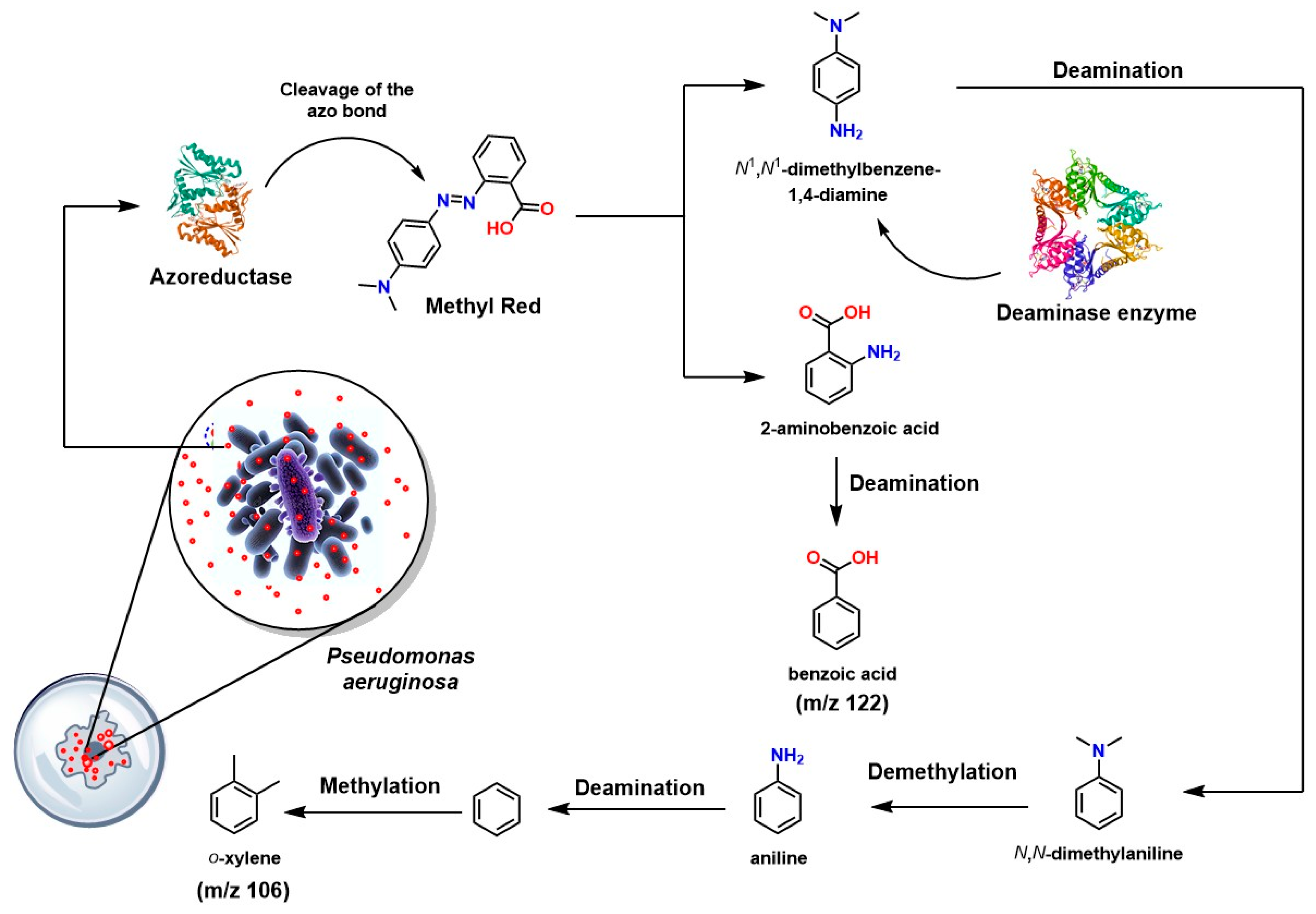
| Galactomyces geotrichum MTCC 1360 | Methyl Red | - | 2-Aminobenzoic acid N,N-Dimethyl-p-phenylenediamine | 1 h | 100 | [204] |
| pH 3 | ||||||
| 30 °C | ||||||
| 100 mg/L | ||||||
| Sterigmatomyces halophilus SSA-1575 | Reactive Black 55 | NADH-DCIP reductase Lignin peroxidase (LiP) Azoreductase | Catechol cis-9-octadecenoic acid Aniline 4-methylsulfonyl aniline Benzene 2-(4′-aminophenyl) sulfonyltholNaphthalene-1,2,4-triol2,7,8-triaminonaphthalenol | 12 h | 98.7 | [210] |
| pH 5 | ||||||
| 30 °C | ||||||
| 750 mg/L | ||||||
| Candida zeylanoides | Reactive Orange 16 (RO16) | Reductases (azoreductase, NADH-dichlorophenolindophenol reductase) | 4-(Methyl sulfonyl)aniline, α-Hydroxybenzene propanoic acid | 5 days | 100 | [206] |
| - | ||||||
| 28 °C | ||||||
| 150 mg/L | ||||||
| Mixed consortium (Pleurotus ostreatus and Candida zeylanoides) | Reactive Orange 16 (RO16) | Manganese peroxidase (MnP), Laccase | 4-(Ethenyl Sulfonyl) benzene, (Methylsulfonyl) benzene, 2-(Phenyl Sulfonyl) ethanol, 4-(Ethenyl Sulfonyl) aniline, α-Hydroxybenzenepropanoic acid | 11 days | 87.5 | |
| - | ||||||
| 28 °C | ||||||
| 150 mg/L | ||||||
| Y-BC-SH Consortium * | Reactive Black 5 | Lipase, Xylanase, Laccase, Azoreductase, LiP, MnP | 2,7,8-triaminenonaphthalen-1-ol, 2-chloro-4,6-diamino-1,3,5-triazine, aniline, 2-naphthol, lauric anhydride | 3 h | 100 | [185] |
| pH 8 | ||||||
| 18 °C | ||||||
| 200 mg/L | ||||||
| HYC Consortium ** | NADH-DCIP reductase, azoreductase, veratryl alcohol oxidase, aldehyde dehydrogenase | 1,3,5-Trimethylbenzene (TMB), benzoic acid (BA), 2,4-Di-tert-butyl phenol (DTBP) | 48 h | 96.1 | [201] | |
| pH 7 | ||||||
| 35 °C | ||||||
| 50 mg/L |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial Decolouration of Azo Dyes: A Review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo Dyes: Past, Present and the Future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261, Erratum in J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Alsantali, R.I.; Raja, Q.A.; Alzahrani, A.Y.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; El Guesmi, N.; Moussa, Z.; Ahmed, S.A. Miscellaneous Azo Dyes: A Comprehensive Review on Recent Advancements in Biological and Industrial Applications. Dye. Pigment. 2022, 199, 110050. [Google Scholar] [CrossRef]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal Decolouration and Degradation of Azo Dyes: A Review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Tiwari, A.; Joshi, M.; Salvi, N.; Gupta, D.; Gandhi, S.; Rajpoot, K.; Tekade, R.K. Chapter 21—Toxicity of Pharmaceutical Azo Dyes. In Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 569–603. ISBN 978-0-323-98367-9. [Google Scholar]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the Analysis of Azo Dyes Employed in Food Industry—A Review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Priya, P.S.; Murugan, R.; Arockiaraj, J. Hues of Risk: Investigating Genotoxicity and Environmental Impacts of Azo Textile Dyes. Environ. Sci. Pollut. Res. 2024, 31, 33190–33211. [Google Scholar] [CrossRef]
- Shetty, A.; Goyal, A. Total Organic Carbon Analysis in Water—A Review of Current Methods. Mater. Today Proc. 2022, 65, 3881–3886. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Bao, J.; Wang, X.; Yu, W.; Shao, L. Degradation of Azo Dyes with Different Functional Groups in Simulated Wastewater by Electrocoagulation. Water 2022, 14, 123. [Google Scholar] [CrossRef]
- Khare, U.K.; Bose, P.; Vankar, P.S. Impact of Ozonation on Subsequent Treatment of Azo Dye Solutions. J. Chem. Technol. Biotechnol. 2007, 82, 1012–1022. [Google Scholar] [CrossRef]
- Akter, T.; Protity, A.T.; Shaha, M.; Al Mamun, M.; Hashem, A. The Impact of Textile Dyes on the Environment. In Nanohybrid Materials for Treatment of Textiles Dyes; Ahmad, A., Jawaid, M., Mohamad Ibrahim, M.N., Yaqoob, A.A., Alshammari, M.B., Eds.; Springer Nature: Singapore, 2023; pp. 401–431. ISBN 978-981-99-3901-5. [Google Scholar]
- Ma, S.; Lee, S.; Kim, K.; Im, J.; Jeon, H. Purification of Organic Pollutants in Cationic Thiazine and Azo Dye Solutions Using Plasma-Based Advanced Oxidation Process via Submerged Multi-Hole Dielectric Barrier Discharge. Sep. Purif. Technol. 2021, 255, 117715. [Google Scholar] [CrossRef]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. Biodegradation and Detoxification of Textile Azo Dyes by Bacterial Consortium under Sequential Microaerophilic/Aerobic Processes. EXCLI J. 2015, 14, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Ajaz, M.; Shakeel, S.; Rehman, A. Microbial Use for Azo Dye Degradation—A Strategy for Dye Bioremediation. Int. Microbiol. 2020, 23, 149–159. [Google Scholar] [CrossRef]
- Singh, K.R.; Poluri, K.M. Facile Synthesis and Physicochemical Characterization of κ-Carrageenan-Silver-Bentonite Based Nanocatalytic Platform for Efficient Degradation of Anionic Azo Dyes. Environ. Res. 2023, 231, 116145. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Sheng, D.; Zhang, C.; Gong, J.; Fu, Z.; Wang, Y.; Li, W.; Xia, L.; Xu, W. Salt-Free Reactive Dyeing of Cotton Fibers in a Ternary Solvent System with Different Reactive Dye Chemistries. Cellulose 2023, 30, 463–479. [Google Scholar] [CrossRef]
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity Assessment and Microbial Degradation of Azo Dyes. Indian J. Exp. Biol. 2006, 44, 618. [Google Scholar]
- Rubalajyothi, P.; Rajendran, A.; Gangadhar, L.; Pandiyan, V. Chronic Neurological Effects and Photocatalytic Investigation of AZO Dyes. Neurosci. Inform. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Fouad, F.A.; Youssef, D.G.; Shahat, F.M.; Abd El-Ghany, M.N. Role of Microorganisms in Biodegradation of Pollutants. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 221–260. ISBN 978-3-031-09710-2. [Google Scholar]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile Industry Effluent Treatment Techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-Bed Adsorption of Reactive Azo Dye onto Granular Activated Carbon Prepared from Waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Ying, T.Y.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Magnetic Graphene Oxide-Biomass Activated Carbon Composite for Dye Removal. Korean J. Chem. Eng. 2020, 37, 2179–2191. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of Anionic Azo-Dyes from Aqueous Solutions onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Bazgir, A.; Khorshidi, A.; Kamani, H.; Ashrafi, S.D.; Naghipour, D. Modeling of Azo Dyes Adsorption on Magnetic NiFe2O4/RGO Nanocomposite Using Response Surface Methodology. J. Environ. Health Sci. Eng. 2019, 17, 931–947. [Google Scholar] [CrossRef]
- Mossavi, E.; Hosseini Sabzevari, M.; Ghaedi, M.; Ahmadi Azqhandi, M.H. Adsorption of the Azo Dyes from Wastewater Media by a Renewable Nanocomposite Based on the Graphene Sheets and Hydroxyapatite/ZnO Nanoparticles. J. Mol. Liq. 2022, 350, 118568. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Nikitha, M.R.; Karthikeyan, P.; Meenakshi, S. Perceptive Removal of Toxic Azo Dyes from Water Using Magnetic Fe3O4 Reinforced Graphene Oxide–Carboxymethyl Cellulose Recyclable Composite: Adsorption Investigation of Parametric Studies and Their Mechanisms. Surf. Interfaces 2020, 21, 100648. [Google Scholar] [CrossRef]
- Banerjee, P.; Barman, S.R.; Mukhopadhayay, A.; Das, P. Ultrasound Assisted Mixed Azo Dye Adsorption by Chitosan–Graphene Oxide Nanocomposite. Chem. Eng. Res. Des. 2017, 117, 43–56. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Atenas, G.M.; Mielczarski, E. Role of Iron Surface Oxidation Layers in Decomposition of Azo-Dye Water Pollutants in Weak Acidic Solutions. Appl. Catal. B Environ. 2005, 56, 289–303. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Priya, A.K.; Ghfar, A.A.; Sekar, K.; Santhamoorthy, M.; Arthi, M.; Soto-Moscoso, M. The Influence of Heterostructured TiO2/ZnO Nanomaterials for the Removal of Azo Dye Pollutant. Chemosphere 2022, 308, 136161. [Google Scholar] [CrossRef]
- Topkaya, E.; Konyar, M.; Yatmaz, H.C.; Öztürk, K. Pure ZnO and Composite ZnO/TiO2 Catalyst Plates: A Comparative Study for the Degradation of Azo Dye, Pesticide and Antibiotic in Aqueous Solutions. J. Colloid Interface Sci. 2014, 430, 6–11. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C. Degradation of Azo Dyes by Laccase: Biological Method to Reduce Pollution Load in Dye Wastewater. Clean Technol. Environ. Policy 2015, 17, 1443–1456. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Senthil Kumar, P. Sustainable Approach on the Biodegradation of Azo Dyes: A Short Review. Curr. Opin. Green Sustain. Chem. 2022, 33, 100578. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.L. Bio-Removal of Azo Dyes: A Review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Xing, L.; Zhang, X.; Li, X.; Zhang, D. Recent Advances in the Biodegradation of Azo Dyes. World J. Microbiol. Biotechnol. 2021, 37, 137. [Google Scholar] [CrossRef]
- Vitor, V.; Corso, C.R. Decolorization of Textile Dye by Candida Albicans Isolated from Industrial Effluents. J. Ind. Microbiol. Biotechnol. 2008, 35, 1353–1357. [Google Scholar] [CrossRef]
- Charumathi, D.; Das, N. Packed Bed Column Studies for the Removal of Synthetic Dyes from Textile Wastewater Using Immobilised Dead C. Tropicalis. Desalination 2012, 285, 22–30. [Google Scholar] [CrossRef]
- Aksu, Z.; Dönmez, G. A Comparative Study on the Biosorption Characteristics of Some Yeasts for Remazol Blue Reactive Dye. Chemosphere 2003, 50, 1075–1083. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Cervantes, F.J. Impact and Application of Electron Shuttles on the Redox (Bio) Transformation of Contaminants: A Review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef]
- Dos Santos, A.B.; Cervantes, F.J.; Van Lier, J.B. Review Paper on Current Technologies for Decolourisation of Textile Wastewaters: Perspectives for Anaerobic Biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef]
- Jafari, N.; Soudi, M.R.; Kasra-Kermanshahi, R. Biodegradation Perspectives of Azo Dyes by Yeasts. Microbiology 2014, 83, 484–497. [Google Scholar] [CrossRef]
- Song, L.; Shao, Y.; Ning, S.; Tan, L. Performance of a Newly Isolated Salt-Tolerant Yeast Strain Pichia Occidentalis G1 for Degrading and Detoxifying Azo Dyes. Bioresour. Technol. 2017, 233, 21–29. [Google Scholar] [CrossRef]
- Charumathi, D.; Das, N. Bioaccumulation of Synthetic Dyes by Candida tropicalis Growing in Sugarcane Bagasse Extract Medium. Adv. Biol. Res. 2010, 4, 233–240. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Campos, A.C.E.; Treuherz, A.; Murasaki, R.T.; Gonzalez, D.; Mújica, O.J. New Health Science Descriptors to classify and retrieve information on equity. Rev. Panam. Salud Publica 2020, 44, e98. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Scells, H.; Locke, D.; Zuccon, G. MeSH Term Suggestion for Systematic Review Literature Search. In Proceedings of the 25th Australasian Document Computing Symposium, Virtual, 9 December 2021; Association for Computing Machinery: New York, NY, USA, 2022. [Google Scholar]
- Riva, J.J.; Malik, K.M.P.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What Is Your Research Question? An Introduction to the PICOT Format for Clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167. [Google Scholar]
- Ballew, B.S. Elsevier’s Scopus® Database. J. Electron. Resour. Med. Libr. 2009, 6, 245–252. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W. A Tale of Two Databases: The Use of Web of Science and Scopus in Academic Papers. Scientometrics 2020, 123, 321–335. [Google Scholar] [CrossRef]
- Haque, M.M.; Haque, M.A.; Mosharaf, M.K.; Marcus, P.K. Decolorization, Degradation and Detoxification of Carcinogenic Sulfonated Azo Dye Methyl Orange by Newly Developed Biofilm Consortia. Saudi J. Biol. Sci. 2021, 28, 793–804. [Google Scholar] [CrossRef]
- Haque, M.M.; Haque, M.A.; Mosharaf, M.K.; Marcus, P.K. Novel Bacterial Biofilm Consortia That Degrade and Detoxify the Carcinogenic Diazo Dye Congo Red. Arch. Microbiol. 2021, 203, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Tipre, D.; Dave, S.R. Biotreatment of Azo Dye Containing Textile Industry Effluent by a Developed Bacterial Consortium Immobilised on Brick Pieces in an Indigenously Designed Packed Bed Biofilm Reactor. World J. Microbiol. Biotechnol. 2023, 39, 83. [Google Scholar] [CrossRef]
- John, A.; Yang, H.H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Saini, R.; Choudhary, K. Chapter 32—Toxic Potential of Azo Dyes: A Broader Understanding. In Hazardous Chemicals; Chawla, M., Singh, J., Kaushik, R.D., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 469–481. ISBN 978-0-323-95235-4. [Google Scholar]
- Goswami, D.; Mukherjee, J.; Mondal, C.; Bhunia, B. Bioremediation of Azo Dye: A Review on Strategies, Toxicity Assessment, Mechanisms, Bottlenecks and Prospects. Sci. Total Environ. 2024, 954, 176426. [Google Scholar] [CrossRef]
- Hihara, T.; Okada, Y.; Morita, Z. Relationship between Photochemical Properties and Colourfastness Due to Light-Related Effects on Monoazo Reactive Dyes Derived from H-Acid, γ-Acid, and Related Naphthalene Sulfonic Acids. Dye. Pigment. 2004, 60, 23–48. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; Lgaz, H.; El Bachiri, A.; El Harfi, A. Dyes: Classification, Pollution, and Environmental Effects. In Dye Biodegradation, Mechanisms and Techniques: Recent Advances; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 1–50. ISBN 978-981-16-5932-4. [Google Scholar]
- Phan, K.-H.; Le, L.-T.; Ninh, T.-T.N.; Tran, C.-S.; Nguyen, T.-T.; Nguyen, D.-T.-D.; Tra, V.-T.; Tran, T.-D.; Nguyen, T.-B.; Mai, T.-P.; et al. Decolorization and Degradation of Azo Dyes in Thermophilic Biological Wastewater Treatment Process: A Mini-Review. Case Stud. Chem. Environ. Eng. 2024, 10, 101018. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Balalaie, S.; Ramezanpour, S.; Bararjanian, M.; Gross, J.H. DABCO-Catalyzed Efficient Synthesis of Naphthopyran Derivatives via One-Pot Three-Component Condensation Reaction at Room Temperature. Synth. Commun. 2008, 38, 1078–1089. [Google Scholar] [CrossRef]
- Dhungana, B.; Peng, H.; Kutarna, S.; Umbuzeiro, G.; Shrestha, S.; Liu, J.; Jones, P.D.; Subedi, B.; Giesy, J.P.; Cobb, G.P. Abundances and Concentrations of Brominated Azo Dyes Detected in Indoor Dust. Environ. Pollut. 2019, 252, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W.; Rangnekar, D.W.; Sonawane, N.D. 2-Aminothiophenes by the Gewald Reaction. J. Heterocycl. Chem. 1999, 36, 333–345. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions; Springer: Berlin/Heidelberg, Germany, 2023; Volume 30, ISBN 0123456789. [Google Scholar]
- Zhang, M.-M.; Chen, W.-M.; Chen, B.-Y.; Chang, C.-T.; Hsueh, C.-C.; Ding, Y.; Lin, K.-L.; Xu, H. Comparative Study on Characteristics of Azo Dye Decolorization by Indigenous Decolorizers. Bioresour. Technol. 2010, 101, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.; Armagan, B.; Turan, M.; Çelik, M.S. Comparison of the Adsorption Characteristics of Azo-Reactive Dyes on Mezoporous Minerals. Dye. Pigment. 2004, 62, 49–60. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Terent’ev, A.O. Azo Dyes and the Microbial World: Synthesis, Breakdown, and Bioactivity. Microbiol. Res. 2025, 16, 100. [Google Scholar] [CrossRef]
- Mahmood, S.; Azeem, K.; Muhammad, A.; Tariq, M.; Crowley, D.E. Detoxification of Azo Dyes by Bacterial Oxidoreductase Enzymes. Crit. Rev. Biotechnol. 2016, 36, 639–651. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial Decolorization and Degradation of Azo Dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Jeong, S.W.; Yang, J.E.; Choi, Y.J. Microbial Treatment of Azo Dyes Using Biogenic Bimetallic Iron–Molybdenum Nanoparticles. Korean J. Chem. Eng. 2024, 41, 2059–2067. [Google Scholar] [CrossRef]
- Lu, X.; Liu, R. Treatment of Azo Dye-Containing Wastewater Using Integrated Processes. In Biodegradation of Azo Dyes; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–155. ISBN 978-3-642-11847-0. [Google Scholar]
- Mota, I.G.C.; Renata Alexandra Moreira Das, N.; Sara Sayonara Da Cruz, N.; Bruna Leal Lima, M.; Ana Heloneida De Araújo, M.; Passos, T.S. Artificial Dyes: Health Risks and the Need for Revision of International Regulations. Food Rev. Int. 2023, 39, 1578–1593. [Google Scholar] [CrossRef]
- Ozyurt, M.; Ataçag, H. Biodegradation of Azo Dyes: A Review. Fresenius Environ. Bull. 2003, 12, 1294–1302. [Google Scholar]
- Shyla, H.; Saha, P.; Rao, K.V.B. Biodegradation and Decolorization of Two Different Azo Dyes, Reactive Blue 221 and Direct Black 38, and Assessment of the Degraded Dye Metabolites. Desalin. Water Treat. 2018, 123, 338–347. [Google Scholar] [CrossRef]
- Mishra, D. Food Colors and Associated Oxidative Stress in Chemical Carcinogenesis. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Singapore, 2021; pp. 1–14. [Google Scholar]
- Libardi, N.; Schallemberger, J.B.; Hassemer, M.E.N.; da Costa, R.H.R.; Soccol, C.R.; de Souza Vandenberghe, L.P. A Combination of Biosorption and Enzymatic Degradation of Azo Dyes. In Microbial Remediation of Azo Dyes with Prokaryotes; CRC Press: Boca Raton, FL, USA, 2022; pp. 259–277. [Google Scholar]
- Kamani, H.; Hosseinzehi, M.; Ghayebzadeh, M.; Azari, A.; Ashrafi, S.D.; Abdipour, H. Degradation of Reactive Red 198 Dye from Aqueous Solutions by Combined Technology Advanced Sonofenton with Zero Valent Iron: Characteristics/ Effect of Parameters/Kinetic Studies. Heliyon 2024, 10, e23667. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Al Arni, S.; Ghareba, S.; Solisio, C.; Alves Palma, M.S.; Converti, A. Methods of Reactive Red 141 Dye Decolorization, Treatment, and Removal from Industrial Wastewaters: A Critical Review. Environ. Eng. Sci. 2020, 38, 577–591. [Google Scholar] [CrossRef]
- Deb, H.; Hasan, M.K.; Islam, M.Z.; Yang, S.; Zhang, Y.; Yao, J. Deep Analysis of Adsorption Isotherm for Rapid Sorption of Acid Blue 93 and Reactive Red 195 on Reactive Graphene. Environ. Sci. Pollut. Res. 2024, 31, 67410–67428. [Google Scholar] [CrossRef]
- Nho, S.W.; Cui, X.; Kweon, O.; Jin, J.; Chen, H.; Moon, M.S.; Kim, S.J.; Cerniglia, C.E. Phylogenetically Diverse Bacteria Isolated from Tattoo Inks, an Azo Dye-Rich Environment, Decolorize a Wide Range of Azo Dyes. Ann. Microbiol. 2021, 71, 35. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Vinayak, A.; Mudgal, G.; Kesari, K.K. Azo Dye Bioremediation: An Interdisciplinary Path to Sustainable Fashion. Environ. Technol. Innov. 2024, 36, 103832. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Uthman, T.O. A Review of History, Properties, Classification, Applications and Challenges of Natural and Synthetic Dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sharma, R.S.; Mishra, V. Deciphering the Ecological Impact of Azo Dye Pollution through Microbial Community Analysis in Water–Sediment Microcosms. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Dissanayake, M.; Liyanage, N.; Herath, C.; Rathnayake, S.; Fernando, E.Y. Mineralization of Persistent Azo Dye Pollutants by a Microaerophilic Tropical Lake Sediment Mixed Bacterial Consortium. Environ. Adv. 2021, 3, 100038. [Google Scholar] [CrossRef]
- Bardi, L.; Marzona, M. Factors Affecting the Complete Mineralization of Azo Dyes. In Biodegradation of Azo Dyes; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 195–210. ISBN 978-3-642-11847-0. [Google Scholar]
- Zafar, S.; Bukhari, D.A.; Rehman, A. Azo Dyes Degradation by Microorganisms—An Efficient and Sustainable Approach. Saudi J. Biol. Sci. 2022, 29, 103437. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and Prospects for a Hazard-Free Conversion by Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4740. [Google Scholar] [CrossRef]
- Kumari, U. Textile Dyes and Their Impact on the Natural Environment. In Dye Pollution from Textile Industry: Challenges and Opportunities for Sustainable Development; Singh, P., Ed.; Springer Nature: Singapore, 2024; pp. 17–30. ISBN 978-981-97-5341-3. [Google Scholar]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Banerjee, P.; Ulker, O.C. Combinative Ex Vivo Studies and in Silico Models ProTox-II for Investigating the Toxicity of Chemicals Used Mainly in Cosmetic Products. Toxicol. Mech. Methods 2022, 32, 542–548. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.; Karmakar, S.; Arora, L.S.; Mishra, V. Ecotoxic Potential of a Presumably Non-Toxic Azo Dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef]
- Luan, F.; Xu, X.; Liu, H.; Cordeiro, M.N.D.S. Review of Quantitative Structure-Activity/Property Relationship Studies of Dyes: Recent Advances and Perspectives. Color. Technol. 2013, 129, 173–186. [Google Scholar] [CrossRef]
- Toropova, A.P.; Toropov, A.A.; Roncaglioni, A.; Benfenati, E. Monte Carlo Technique to Study the Adsorption Affinity of Azo Dyes by Applying New Statistical Criteria of the Predictive Potential. SAR QSAR Environ. Res. 2022, 33, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Alsaggaf, A.; Assirey, E.; Naqvi, A.; Okasha, R.M.; Afifi, T.H.; Hagar, M. A New Family of Benzo[h]Chromene Based Azo Dye: Synthesis, In-Silico and DFT Studies with in Vitro Antimicrobial and Antiproliferative Assessment. Int. J. Mol. Sci. 2021, 22, 2807. [Google Scholar] [CrossRef]
- Kamenická, B. Chemical Degradation of Azo Dyes Using Different Reducing Agents: A Review. J. Water Process Eng. 2024, 61, 105350. [Google Scholar] [CrossRef]
- Saxena, A.; Gupta, S. Toxicological Impact of Azo DyesAzo Dyes and Their Microbial Degraded Byproducts on Flora and Fauna. In Innovations in Environmental Biotechnology; Arora, S., Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer Nature: Singapore, 2022; pp. 319–343. ISBN 978-981-16-4445-0. [Google Scholar]
- Gomaa, H.; Emran, M.Y.; El-Gammal, M.A. Biodegradation of Azo Dye Pollutants Using Microorganisms. In Handbook of Biodegradable Materials; Springer: Cham, Switzerland, 2022; pp. 1–29. [Google Scholar] [CrossRef]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over Review on Recently Developed Techniques, Mechanisms and Intermediate Involved in the Advanced Azo Dye Degradation for Industrial Applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Liu, H.; Huang, J.; Li, L. Sulfide-Mediated Azo Dye Degradation and Microbial Community Analysis in a Single-Chamber Air Cathode Microbial Fuel Cell. Bioelectrochemistry 2020, 131, 107349. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.; Ibrahim, A.H.; Al-Rawi, S.S.; Majeed, A.; Iqbal, M.A.; Kashif, M.; Abidin, Z.U.; Arbaz, M.; Ali, S.; Hussain, S.A.; et al. Visible Light Assisted Photooxidative Facile Degradation of Azo Dyes in Water Using a Green Method. RSC Adv. 2024, 14, 16138–16149. [Google Scholar] [CrossRef]
- Biswas, K.; Ahamed, Z.; Dutta, T.; Mallick, B.; Khuda-Bukhsh, A.R.; Biswas, J.K.; Mandal, S.K. Green Synthesis of Silver Nanoparticles from Waste Leaves of Tea (Camellia Sinensis) and Their Catalytic Potential for Degradation of Azo Dyes. J. Mol. Struct. 2024, 1318, 139448. [Google Scholar] [CrossRef]
- Dadvar, E.; Kalantary, R.R.; Ahmad Panahi, H.; Peyravi, M. Efficiency of Polymeric Membrane Graphene Oxide-TiO2 for Removal of Azo Dye. J. Chem. 2017, 2017, 6217987. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Kucharczyk, A. Adsorptive Removal of Direct Azo Dyes from Textile Wastewaters Using Weakly Basic Anion Exchange Resin. Int. J. Mol. Sci. 2023, 24, 4886. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Menazea, A.A.; Ali, H. Selective Adsorption of Cationic Azo Dyes onto Zeolite Nanorod-Based Membranes Prepared via Laser Ablation. J. Mater. Sci. Mater. Electron. 2021, 32, 19352–19367. [Google Scholar] [CrossRef]
- Mohajerani, M.; Mehrvar, M.; Ein-Mozaffari, F. An Overview of the Integration of Advanced Oxidation Technologies and Other Processes for Water and Wastewater Treatment. Int. J. Eng. 2009, 3, 120–146. [Google Scholar]
- Kawsar, M.; Sahadat Hossain, M.; Tabassum, S.; Bahadur, N.M.; Ahmed, S. Synthesis of Different Types of Nano-Hydroxyapatites for Efficient Photocatalytic Degradation of Textile Dye (Congo Red): A Crystallographic Characterization. RSC Adv. 2024, 14, 11570–11583. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of Azo Dyes Using Low Iron Concentration of Fenton and Fenton-like System. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef]
- Fan, J.; Fan, F.; Wang, W.; Zhang, H.; Wang, L.; Chang, J.; Liang, Q.; Wang, D.; Liu, Z.; Shao, L. Treatment of Acid Red 73 Wastewater by the O3/RSR-BCR Process. Chem. Eng. Process.-Process Intensif. 2021, 160, 108296. [Google Scholar] [CrossRef]
- Fu, L.; You, S.-J.; Zhang, G.; Yang, F.-L.; Fang, X. Degradation of Azo Dyes Using In-Situ Fenton Reaction Incorporated into H2O2-Producing Microbial Fuel Cell. Chem. Eng. J. 2010, 160, 164–169. [Google Scholar] [CrossRef]
- Ribeiro, J.A.S.; Alves, J.F.; Salgado, B.C.B.; Oliveira, A.C.; Araújo, R.S.; Rodríguez-Castellón, E. Heterogeneous Photo-Fenton Degradation of Azo Dyes over a Magnetite-Based Catalyst: Kinetic and Thermodynamic Studies. Catalysts 2024, 14, 591. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Sun, J.-Y.; Sun, R.-X.; Qiao, L.-P.; Guo, H.-Q.; Fan, M.-H. Degradation of Azo Dye Acid Black 1 Using Low Concentration Iron of Fenton Process Facilitated by Ultrasonic Irradiation. Ultrason. Sonochem. 2007, 14, 761–766. [Google Scholar] [CrossRef]
- Guivarch, E.; Trevin, S.; Lahitte, C.; Oturan, M.A. Degradation of Azo Dyes in Water by Electro-Fenton Process. Environ. Chem. Lett. 2003, 1, 38–44. [Google Scholar] [CrossRef]
- Tantak, N.P.; Chaudhari, S. Degradation of Azo Dyes by Sequential Fenton’s Oxidation and Aerobic Biological Treatment. J. Hazard. Mater. 2006, 136, 698–705. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Wang, G.-L.; Qiao, L.-P. Degradation of Azo Dye Amido Black 10B in Aqueous Solution by Fenton Oxidation Process. Dye. Pigment. 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Kumar, J.E.; Sahoo, M.K. A Review on Effect of Operational Parameters for the Degradation of Azo Dyes by Some Advanced Oxidation Processes. Sustain. Chem. Environ. 2025, 11, 100274. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Tan, F.; Cheng, X.; Ma, Q.; Wu, D.; Li, P.; Zhang, F.; Ma, J. Degradation of Congo Red by UV Photolysis of Nitrate: Kinetics and Degradation Mechanism. Sep. Purif. Technol. 2021, 262, 118276. [Google Scholar] [CrossRef]
- Chowdhury, A.P.; Anantharaju, K.S.; Keshavamurthy, K.; Rokhum, S.L. Recent Advances in Efficient Photocatalytic Degradation Approaches for Azo Dyes. J. Chem. 2023, 2023, 9780955. [Google Scholar] [CrossRef]
- Arora, C.; Kumar, P.; Soni, S.; Mittal, J.; Mittal, A.; Singh, B. Efficient Removal of Malachite Green Dye from Aqueous Solution Using Curcuma Caesia Based Activated Carbon. Desalin. Water Treat. 2020, 195, 341–352. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Advances in Application of Cotton-Based Adsorbents for Heavy Metals Trapping, Surface Modifications and Future Perspectives. Ecotoxicol. Environ. Saf. 2020, 201, 110825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, H.; Song, M.; Xu, Z.; Sun, Z.; Xu, Q.; Chen, Y.; Liao, X. Interface Engineering of Ti-MOFs: Adsorption of Anionic, Cationic and Neutral Dyes in Wastewater. J. Mol. Struct. 2023, 1283, 135268. [Google Scholar] [CrossRef]
- Joseph, J.; Radhakrishnan, R.C.; Johnson, J.K.; Joy, S.P.; Thomas, J. Ion-Exchange Mediated Removal of Cationic Dye-Stuffs from Water Using Ammonium Phosphomolybdate. Mater. Chem. Phys. 2020, 242, 122488. [Google Scholar] [CrossRef]
- Rathod, P.B.; Singh, M.P.; Taware, A.S.; Deshmukh, S.U.; Tagad, C.K.; Kulkarni, A.; Kanagare, A.B. Comprehensive Insights into Water Remediation: Chemical, Biotechnological, and Nanotechnological Perspectives. Environ. Pollut. Bioavailab. 2024, 36, 2329660. [Google Scholar] [CrossRef]
- Zainudin, N.F.; Sam, S.T.; Wong, Y.S.; Ismail, H.; Walli, S.; Inoue, K.; Kawamura, G.; Tan, W.K. Degradation of Diazo Congo Red Dye by Using Synthesized Poly-Ferric-Silicate-Sulphate through Co-Polymerization Process. Polymers 2023, 15, 237. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, X.; Ding, A.; Xu, J. Magnesium Silicate Polymer as a Coagulant for Reactive Dye Removal from Wastewater: Considering the Intrinsic PH in Magnesium Silicate Polymer and Coagulation Behavior. ACS Omega 2020, 5, 26094–26100. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (Go) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ikram, M.; Zahoor, M.; Naeem, M.; Islam, N.U.; Shah, A.B.; Shahzad, B. Bacterial Oxidoreductive Enzymes as Molecular Weapons for the Degradation and Metabolism of the Toxic Azo Dyes in Wastewater: A Review. Z. Phys. Chem. 2023, 237, 187–209. [Google Scholar] [CrossRef]
- Dias, A.A.; Lucas, M.S.; Sampaio, A.; Peres, J.A.; Bezerra, R.M.F. Decolorization of Azo Dyes by Yeasts. In Biodegradation of Azo Dyes; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 183–193. ISBN 978-3-642-11847-0. [Google Scholar]
- Yu, J.; Ogata, D.; Gai, Z.; Taguchi, S.; Tanaka, I.; Ooi, T.; Yao, M. Structures of AzrA and of AzrC Complexed with Substrate or Inhibitor: Insight into Substrate Specificity and Catalytic Mechanism. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Choudhury, A.R.; Jose, P.A.; Suganya, K.; Senthilkumar, M.; Prabhakaran, J.; Gopal, N.O.; Choi, J.; Kim, K.; Anandham, R.; et al. Long-Term Exposure to Azo Dyes from Textile Wastewater Causes the Abundance of Saccharibacteria Population. Appl. Sci. 2021, 11, 379. [Google Scholar] [CrossRef]
- Sehar, S.; Rasool, T.; Syed, H.M.; Mir, M.A.; Naz, I.; Rehman, A.; Shah, M.S.; Akhter, M.S.; Mahmood, Q.; Younis, A. Recent Advances in Biodecolorization and Biodegradation of Environmental Threatening Textile Finishing Dyes. 3 Biotech 2022, 12, 186. [Google Scholar] [CrossRef]
- Khudhair, S.H.; Al-Fayaad, D.B.M. Bacterial Biodegradation of Congo Red Dye Using Local Bacterial Isolates. Iraqi J. Sci. 2022, 63, 4674–4682. [Google Scholar] [CrossRef]
- Zhao, B.-H.; Zhang, B.-L.; Zhang, B.-Y.; Liu, R.-X.; Liu, X.-M. The Bio-Decolorization of Methyl Orange by S.Putrefaciens CN32 and Responding Mechanism to Salinity Stress Based on Transcriptomic Analysis. Water Res. 2025, 272, 122923. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, A.; Walaszczyk, A.; Paraszkiewicz, K. Omics-Based Approaches in Research on Textile Dye Microbial Decolorization. Molecules 2024, 29, 2771. [Google Scholar] [CrossRef]
- Kamal, I.M.; Abdeltawab, N.F.; Ragab, Y.M.; Farag, M.A.; Ramadan, M.A. Biodegradation, Decolorization, and Detoxification of Di-Azo Dye Direct Red 81 by Halotolerant, Alkali-Thermo-Tolerant Bacterial Mixed Cultures. Microorganisms 2022, 10, 994. [Google Scholar] [CrossRef]
- Masarbo, R.S.; Karegoudar, T.B. Decolourisation of Toxic Azo Dye Fast Red E by Three Bacterial Strains: Process Optimisation and Toxicity Assessment. Int. J. Environ. Anal. Chem. 2022, 102, 2686–2696. [Google Scholar] [CrossRef]
- Pundir, A.; Thakur, M.S.; Prakash, S.; Kumari, N.; Sharma, N.; Parameswari, E.; He, Z.; Nam, S.; Thakur, M.; Puri, S.; et al. Fungi as Versatile Biocatalytic Tool for Treatment of Textile Wastewater Effluents. Environ. Sci. Eur. 2024, 36, 185. [Google Scholar] [CrossRef]
- Nagraj; Chaurasia, P.K.; Bharati, S.L.; Sharma, N.; Kumar, J.; Sivalingam, A.M. Degradation of Dyes by Fungi: An Overview on Recent Updates. Microbe 2025, 6, 100232. [Google Scholar] [CrossRef]
- Takkar, S.; Tyagi, B.; Kumar, N.; Kumari, T.; Iqbal, K.; Varma, A.; Thakur, I.S.; Mishra, A. Biodegradation of Methyl Red Dye by a Novel Actinobacterium Zhihengliuella Sp. ISTPL4: Kinetic Studies, Isotherm and Biodegradation Pathway. Environ. Technol. Innov. 2022, 26, 102348. [Google Scholar] [CrossRef]
- Gomaa, H.; Emran, M.Y.; El-Gammal, M.A. Biodegradation of Azo Dye Pollutants Using Microorganisms. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 781–809. ISBN 978-3-031-09710-2. [Google Scholar] [CrossRef]
- Samuchiwal, S.; Abhishek, S.; Koushalya, S.; Shubha, S.; Malik, A. Unveiling the Azo-Reductase Mechanism in Pseudomonas Putida for Efficient Decolorization of Textile Reactive Dyes: An in-Silico Study. J. Biomol. Struct. Dyn. 2025, 43, 5164–5177. [Google Scholar] [CrossRef]
- Krithika, A.; Gayathri, K.V.; Kumar, D.T.; Doss, C.G.P. Mixed Azo Dyes Degradation by an Intracellular Azoreductase Enzyme from Alkaliphilic Bacillus Subtilis: A Molecular Docking Study. Arch. Microbiol. 2021, 203, 3033–3044. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Ullah, R.; Farraj, D.A.A.; Elshikh, M.S.; Zekker, I.; Gulfam, N. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia Coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Zhu, C.; Mahmood, Z.; Siddique, M.S.; Wang, H.; Anqi, H.; Sillanpää, M. Structure-Based Long-Term Biodegradation of the Azo Dye: Insights from the Bacterial Community Succession and Efficiency Comparison. Water 2021, 13, 3017. [Google Scholar] [CrossRef]
- Fayyaz, I.; Saddick, S.; Mahmood, R.T.; Asad, M.J.; Hussain, M.A.; Hu, J.; Awais, M.; Khan, M.I.; Saydaxmetova, S. Biodegradation of Azo and Disperse Dyes by Trametes versicolor: Process Optimization and MnP Enzyme Dynamics. Results Eng. 2025, 25, 103980. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.M.; Alshehrei, F.; Alsamhary, K.; Almansob, A. Decolorization of Acid Blue 29, Disperse Red 1 and Congo Red by Different Indigenous Fungal Strains. Chemosphere 2021, 271, 129532. [Google Scholar] [CrossRef] [PubMed]
- El Awady, M.E.; El-Shall, F.N.; Mohamed, G.E.; Abd-Elaziz, A.M.; Abdel-Monem, M.O.; Hassan, M.G. Exploring the Decolorization Efficiency and Biodegradation Mechanisms of Different Functional Textile Azo Dyes by Streptomyces Albidoflavus 3MGH. BMC Microbiol. 2024, 24, 210. [Google Scholar] [CrossRef]
- Kumaran, S.; Ngo, A.C.R.; Schultes, F.P.J.; Saravanan, V.S.; Tischler, D. In Vitro and in Silico Analysis of Brilliant Black Degradation by Actinobacteria and a Paraburkholderia sp. Genomics 2022, 114, 110266. [Google Scholar] [CrossRef]
- Kameche, K.; Amrani, S.; Mouzaoui, S.; Aït-Amar, H. Biodegradation of Diazo Dye Evans Blue by Four Strains of Streptomyces Isolated from Soils of Algeria. Biocatal. Agric. Biotechnol. 2022, 46, 102529. [Google Scholar] [CrossRef]
- Aounallah, F.; Hkiri, N.; Fouzai, K.; Elaoud, A.; Ayed, L.; Asses, N. Biodegradation Pathway of Congo Red Azo Dye by Geotrichum Candidum and Toxicity Assessment of Metabolites. Catal. Lett. 2024, 154, 6064–6079. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Sun, J.; Fareed, M.F.; Kenawy, E.R.; Ali, S.S. Ecofriendly Biodegradation of Reactive Black 5 by Newly Isolated Sterigmatomyces Halophilus SSA1575, Valued for Textile Azo Dye Wastewater Processing and Detoxification. Sci. Rep. 2020, 10, 12370. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cui, J.; Xu, B.; Jiang, Y.; Fu, C.; Tan, L. A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms. J. Fungi 2023, 9, 851. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Sun, J. Performance of Meyerozyma Caribbica as a Novel Manganese Peroxidase-Producing Yeast Inhabiting Wood-Feeding Termite Gut Symbionts for Azo Dye Decolorization and Detoxification. Sci. Total Environ. 2022, 806, 150665. [Google Scholar] [CrossRef]
- Khalaf, H.A.; El-Sheekh, M.M.; Makhlof, M.E.M. Lychaete Pellucida as a Novel Biosorbent for the Biodegradation of Hazardous Azo Dyes. Environ. Monit. Assess. 2023, 195, 929. [Google Scholar] [CrossRef]
- Ishchi, T.; Sibi, G. Azo Dye Degradation by Chlorella vulgaris: Optimization and Kinetics. Int. J. Biol. Chem 2020, 14, 1–7. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Degradation of Azo Dyes: Bacterial Potential for Bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Ayub, A.; Wani, A.K.; Chopra, C.; Sharma, D.K.; Amin, O.; Wani, A.W.; Singh, A.; Manzoor, S.; Singh, R. Advancing Dye Degradation: Integrating Microbial Metabolism, Photocatalysis, and Nanotechnology for Eco-Friendly Solutions. Bacteria 2025, 4, 15. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Danish, M.; Singh, R.S.; Rafatullah, M.; HPS, A.K. Exploiting Microbial Biomass in Treating Azo Dyes Contaminated Wastewater: Mechanism of Degradation and Factors Affecting Microbial Efficiency. J. Water Process Eng. 2021, 43, 102255. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes From Wastewater: A Review. Front. Environ. Sci. 2021, 9, 764958. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Syranidou, E.; Crotti, E.; Choukrallah, R.; Kalogerakis, N.; Borin, S. Root Bacteria Recruited by Phragmites Australis in Constructed Wetlands Have the Potential to Enhance Azo-Dye Phytodepuration. Microorganisms 2019, 7, 384. [Google Scholar] [CrossRef]
- Sandhya, S. Biodegradation of Azo Dyes Under Anaerobic Condition: Role of Azoreductase. In Biodegradation of Azo Dyes; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–57. ISBN 978-3-642-11847-0. [Google Scholar]
- Shweta, D.; Sanjeev, G. Biodegradation of Environmentally Hazardous Azo Dyes and Aromatic Amines Using Klebsiella Pneumoniae. J. Environ. Eng. 2018, 144, 4018035. [Google Scholar] [CrossRef]
- Bhatia, D.; Kanwar, R.S.; Singh, J.; Sharma, N.R.; Khandare, R.V. Degradation and Decolorization of Disperse Red 167 Dye with an In-Situ Isolated Azo-Reductase Enzyme Producing Bacterium Paenochrobactrum Glaciei. Int. J. Environ. Sci. Technol. 2023, 20, 2389–2404. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Musa, S.A.; Fugu, M.B.; Mohammed, A.I.; Adam, H.B.; Wakil, I.M. A Comprehensive Review on Anthranilic Acid-Derived Schiff Bases and Their Metal Chelates: Structures and Applications. Chem. Rev. Lett. 2023, 6, 350–389. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Rahim, A.; Hanafiah, M.M.; Oyekanmi, A.A.; Shah, A.B.; Mahnashi, M.H.; Al Ali, A.; Jalal, N.A.; et al. Biodegradation of Azo Dye Methyl Red by Pseudomonas Aeruginosa: Optimization of Process Conditions. Int. J. Environ. Res. Public Health 2022, 19, 9962. [Google Scholar] [CrossRef]
- Thangaraj, S.; Bankole, P.O.; Sadasivam, S.K. Microbial Degradation of Azo Dyes by Textile Effluent Adapted, Enterobacter Hormaechei under Microaerophilic Condition. Microbiol. Res. 2021, 250, 126805. [Google Scholar] [CrossRef]
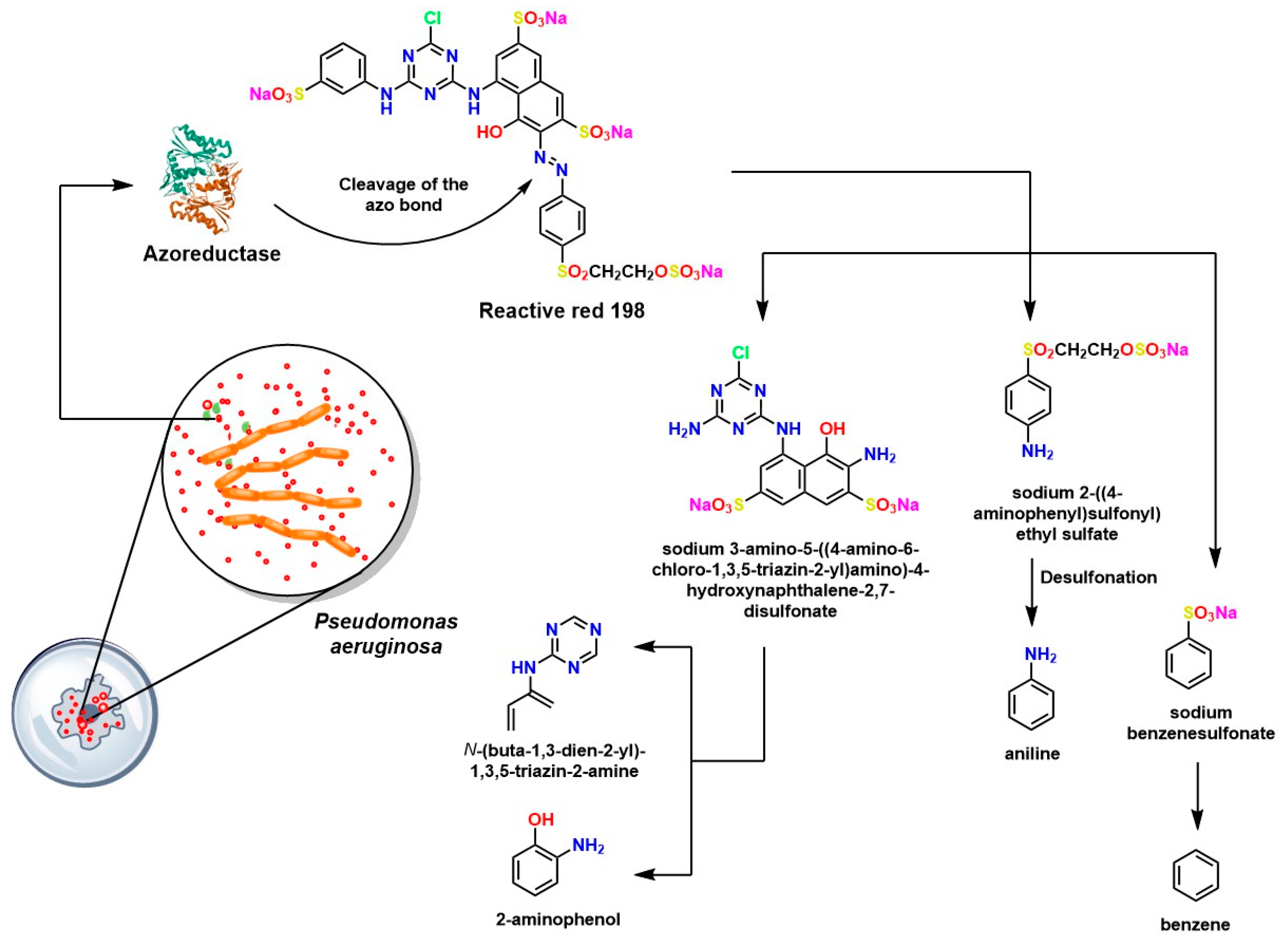
- Thangaraj, S.; Bankole, P.O.; Sadasivam, S.K.; Kumarvel, V. Biodegradation of Reactive Red 198 by Textile Effluent Adapted Microbial Strains. Arch. Microbiol. 2022, 204, 12. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Kumar Arora, N.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S.; et al. Microbial Fuel Cells for Azo Dye Degradation: A Perspective Review. J. Ind. Eng. Chem. 2025, 142, 45–67. [Google Scholar] [CrossRef]
- Santos, G.C.; Corso, C.R. Comparative Analysis of Azo Dye Biodegradation by Aspergillus Oryzae and Phanerochaete Chrysosporium. Water. Air. Soil Pollut. 2014, 225, 2026. [Google Scholar] [CrossRef]
- Rosu, C.M.; Avadanei, M.; Gherghel, D.; Mihasan, M.; Mihai, C.; Trifan, A.; Miron, A.; Vochita, G. Biodegradation and Detoxification Efficiency of Azo-Dye Reactive Orange 16 by Pichia kudriavzevii CR-Y103. Water. Air. Soil Pollut. 2018, 229, 15. [Google Scholar] [CrossRef]
- Arif, M. Catalytic Degradation of Azo Dyes by Bimetallic Nanoparticles Loaded in Smart Polymer Microgels. RSC Adv. 2023, 13, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Bouacem, K.; Rekik, H.; Jaouadi, N.Z.; Zenati, B.; Kourdali, S.; El Hattab, M.; Badis, A.; Annane, R.; Bejar, S.; Hacene, H.; et al. Purification and Characterization of Two Novel Peroxidases from the Dye-Decolorizing Fungus Bjerkandera Adusta Strain CX-9. Int. J. Biol. Macromol. 2018, 106, 636–646. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.P.; Macrae, A.; Ribeiro, B.D.; do Nascimento, R.P. Decolorization and Detoxification of Different Azo Dyes by Phanerochaete Chrysosporium ME-446 under Submerged Fermentation. Braz. J. Microbiol. 2021, 52, 727–738. [Google Scholar] [CrossRef]
- Thampraphaphon, B.; Phosri, C.; Pisutpaisal, N.; Thamvithayakorn, P.; Chotelersak, K.; Sarp, S.; Suwannasai, N. High Potential Decolourisation of Textile Dyes from Wastewater by Manganese Peroxidase Production of Newly Immobilised Trametes Hirsuta PW17-41 and FTIR Analysis. Microorganisms 2022, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.; Joshi, S.J. Biodecolorization and Biodegradation of Dyes: A Review. Open Biotechnol. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Bowman, K.; Breheny, M.; Chandra, T.S.; Keshavarz, T.; Kyazze, G. Degradation of Azo Dye (Acid Orange 7) in a Microbial Fuel Cell: Comparison Between Anodic Microbial-Mediated Reduction and Cathodic Laccase-Mediated Oxidation. Front. Energy Res. 2019, 7, 101. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, D.; Li, R.; Wang, T.; Zhu, Y. Textile Dye Biodecolorization by Manganese Peroxidase: A Review. Molecules 2021, 26, 4403. [Google Scholar] [CrossRef]
- Thoa, L.T.K.; Thao, T.T.P.; Nguyen-Thi, M.-L.; Chung, N.D.; Ooi, C.W.; Park, S.-M.; Lan, T.T.; Quang, H.T.; Khoo, K.S.; Show, P.L.; et al. Microbial Biodegradation of Recalcitrant Synthetic Dyes from Textile-Enriched Wastewater by Fusarium Oxysporum. Chemosphere 2023, 325, 138392. [Google Scholar] [CrossRef]
- Henagamage, A.P.; Peries, C.M. Degradation and Decolorization of Textile Azo Dyes by Effective Fungal-Bacterial Consortium. Mol. Biol. Rep. 2023, 50, 8901–8914. [Google Scholar] [CrossRef]
- Qin, W.; Guo, S.; Li, Q.; Tang, A.; Liu, H.; Liu, Y. Biotransformation of the Azo Dye Reactive Orange 16 by Aspergillus Flavus A5P1: Performance, Genetic Background, Pathway, and Mechanism. J. Hazard. Mater. 2024, 468, 133562. [Google Scholar] [CrossRef] [PubMed]
- Hürmüzlü, R.; Okur, M.; Saraçoğlu, N. Immobilization of Trametes versicolor Laccase on Chitosan/Halloysite as a Biocatalyst in the Remazol Red RR Dye. Int. J. Biol. Macromol. 2021, 192, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Al-Tohamy, R.; Xie, R.; El-Sheekh, M.M.; Sun, J. Construction of a New Lipase- and Xylanase-Producing Oleaginous Yeast Consortium Capable of Reactive Azo Dye Degradation and Detoxification. Bioresour. Technol. 2020, 313, 123631. [Google Scholar] [CrossRef]
- Thakor, R.; Mistry, H.; Tapodhan, K.; Bariya, H. Efficient Biodegradation of Congo Red Dye Using Fungal Consortium Incorporated with Penicillium Oxalicum and Aspergillus Tubingensis. Folia Microbiol. 2022, 67, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Khadir, A.; Muthu, S.S. Biological Approaches in Dye-Containing Wastewater; Springer: Singapore, 2022; Volume 2, ISBN 9789811905254. [Google Scholar]
- Mustafa, G.; Zahid, M.T.; Kurade, M.B.; Alvi, A.; Ullah, F.; Yadav, N.; Park, H.-K.; Khan, M.A.; Jeon, B.-H. Microalgal and Activated Sludge Processing for Biodegradation of Textile Dyes. Environ. Pollut. 2024, 349, 123902. [Google Scholar] [CrossRef]
- Deka, R.; Shreya, S.; Mourya, M.; Sirotiya, V.; Rai, A.; Khan, M.J.; Ahirwar, A.; Schoefs, B.; Bilal, M.; Saratale, G.D.; et al. A Techno-Economic Approach for Eliminating Dye Pollutants from Industrial Effluent Employing Microalgae through Microbial Fuel Cells: Barriers and Perspectives. Environ. Res. 2022, 212, 113454. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Yadav, S.; Bhattacharya, A.; Aseri, G.K.; Jain, N. Constructed Wetland–Microbial Fuel Cell (CW-MFC) Mediated Bio-Electrodegradation of Azo Dyes from Textile Wastewater. Lett. Appl. Microbiol. 2025, 78, ovaf010. [Google Scholar] [CrossRef]
- Ayed, L.; Ladhari, N.; El Mzoughi, R.; Chaieb, K. Decolorization and Phytotoxicity Reduction of Reactive Blue 40 Dye in Real Textile Wastewater by Active Consortium: Anaerobic/Aerobic Algal-Bacterial-Probiotic Bioreactor. J. Microbiol. Methods 2021, 181, 106129. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Gessesse, A. Adaptive Response of Thermophiles to Redox Stress and Their Role in the Process of Dye Degradation From Textile Industry Wastewater. Front. Physiol. 2022, 13, 908370. [Google Scholar] [CrossRef]
- Alaguprathana, M.; Poonkothai, M. Haematological, Biochemical, Enzymological and Histological Responses of Labeo Rohita Exposed to Methyl Orange Dye Solution Treated with Oedogonium Subplagiostomum AP1. Environ. Sci. Pollut. Res. 2021, 28, 17602–17612. [Google Scholar] [CrossRef]
- Moradi, Z.; Haghjou, M.M.; Zarei, M.; Sharifan, H. Harnessing Chlorella vulgaris for the Phycoremediation of Azo Dye: A Comprehensive Analysis of Metabolic Responses and Antioxidant System. Algal Res. 2024, 82, 103660. [Google Scholar] [CrossRef]
- Maruthanayagam, A.; Mani, P.; Kaliappan, K.; Chinnappan, S. In Vitro and In Silico Studies on the Removal of Methyl Orange from Aqueous Solution Using Oedogonium Subplagiostomum AP1. Water. Air. Soil Pollut. 2020, 231, 232. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shanshoury, A.R.; Abou-El-Souod, G.W.; Gharieb, D.Y.; El Shafay, S.M. Decolorization of Dyestuffs by Some Species of Green Algae and Cyanobacteria and Its Consortium. Int. J. Environ. Sci. Technol. 2021, 18, 3895–3906. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hamouda, R.A.; Saddiq, A.A.; Alkinani, M.H. Simultaneous Bioremediation of Cationic Copper Ions and Anionic Methyl Orange Azo Dye by Brown Marine Alga Fucus Vesiculosus. Sci. Rep. 2021, 11, 3555. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Cassoni, A.C.; Alves, S.; Pintado, M.E.; Castro, P.M.L.; Moreira, P. Screening for a More Sustainable Solution for Decolorization of Dyes and Textile Effluents Using Candida and Yarrowia spp. J. Environ. Manag. 2022, 307, 114421. [Google Scholar] [CrossRef]
- Li, M.; Zhou, P.; Chen, M.; Yu, H.; Ye, L. Spatiotemporal Regulation of Astaxanthin Synthesis in S. Cerevisiae. ACS Synth. Biol. 2022, 11, 2636–2649. [Google Scholar] [CrossRef]
- Rana, S.; Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Role of Candida in the Bioremediation of Pollutants: A Review. Lett. Appl. Microbiol. 2023, 76, ovad103. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Xie, R.; Schagerl, M.; Khalil, M.A.; Sun, J. Decolorization of Reactive Azo Dye Using Novel Halotolerant Yeast Consortium HYC and Proposed Degradation Pathway. Ecotoxicol. Environ. Saf. 2023, 263, 115258. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Yoshida, T.; Fernandez-lafuente, R. DyP-Type Peroxidases: Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 5556. [Google Scholar] [CrossRef] [PubMed]
- Azeez, R.A.; Al-Zuhairi, F.K.I. Biosorption of Dye by Immobilized Yeast Cells on the Surface of Magnetic Nanoparticles. Alexandria Eng. J. 2022, 61, 5213–5222. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Jamal, A.; Ilyas, M.; Zubair, M.; Khan, G.; Atieh, M.A. Bioremediation of Dyes: Current Status and Prospects. J. Water Process Eng. 2020, 38, 101680. [Google Scholar] [CrossRef]
- Danouche, M.; Ferioun, M.; Bahafid, W.; El Ghachtouli, N. Mycoremediation of Azo Dyes Using Cyberlindnera Fabianii Yeast Strain: Application of Designs of Experiments for Decolorization Optimization. Water Environ. Res. 2021, 93, 1402–1416. [Google Scholar] [CrossRef]
- Šlosarčíková, P.; Plachá, D.; Malachová, K.; Rybková, Z.; Novotný, Č. Biodegradation of Reactive Orange 16 Azo Dye by Simultaneous Action of Pleurotus Ostreatus and the Yeast Candida Zeylanoides. Folia Microbiol. 2020, 65, 629–638. [Google Scholar] [CrossRef]
- Zahmatkesh Anbarani, M.; Nourbakhsh, S.; Toolabi, A.; Bonyadi, Z. Biodegradation of Crystal Violet Dye by Saccharomyces cerevisiae in Aqueous Medium. Heliyon 2023, 9, e19460. [Google Scholar] [CrossRef]
- Gholizadeh-Balderlou, F.; Soudi, M.R.; Darvishi, F. Performance of Pichia kudriavzevii SDG12 in Decolorization and Biodegradation of Azo Dye under Optimized Conditions. J. Water Process Eng. 2025, 71, 107168. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Ning, S.; Shi, S.; Tan, L. Magnetically Stimulated Azo Dye Biodegradation by a Newly Isolated Osmo-Tolerant Candida tropicalis A1 and Transcriptomic Responses. Ecotoxicol. Environ. Saf. 2021, 209, 111791. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Kenawy, E.R.; Sun, J.; Ali, S.S. Performance of a Newly Isolated Salt-Tolerant Yeast Strain Sterigmatomyces Halophilus SSA-1575 for Azo Dye Decolorization and Detoxification. Front. Microbiol. 2020, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
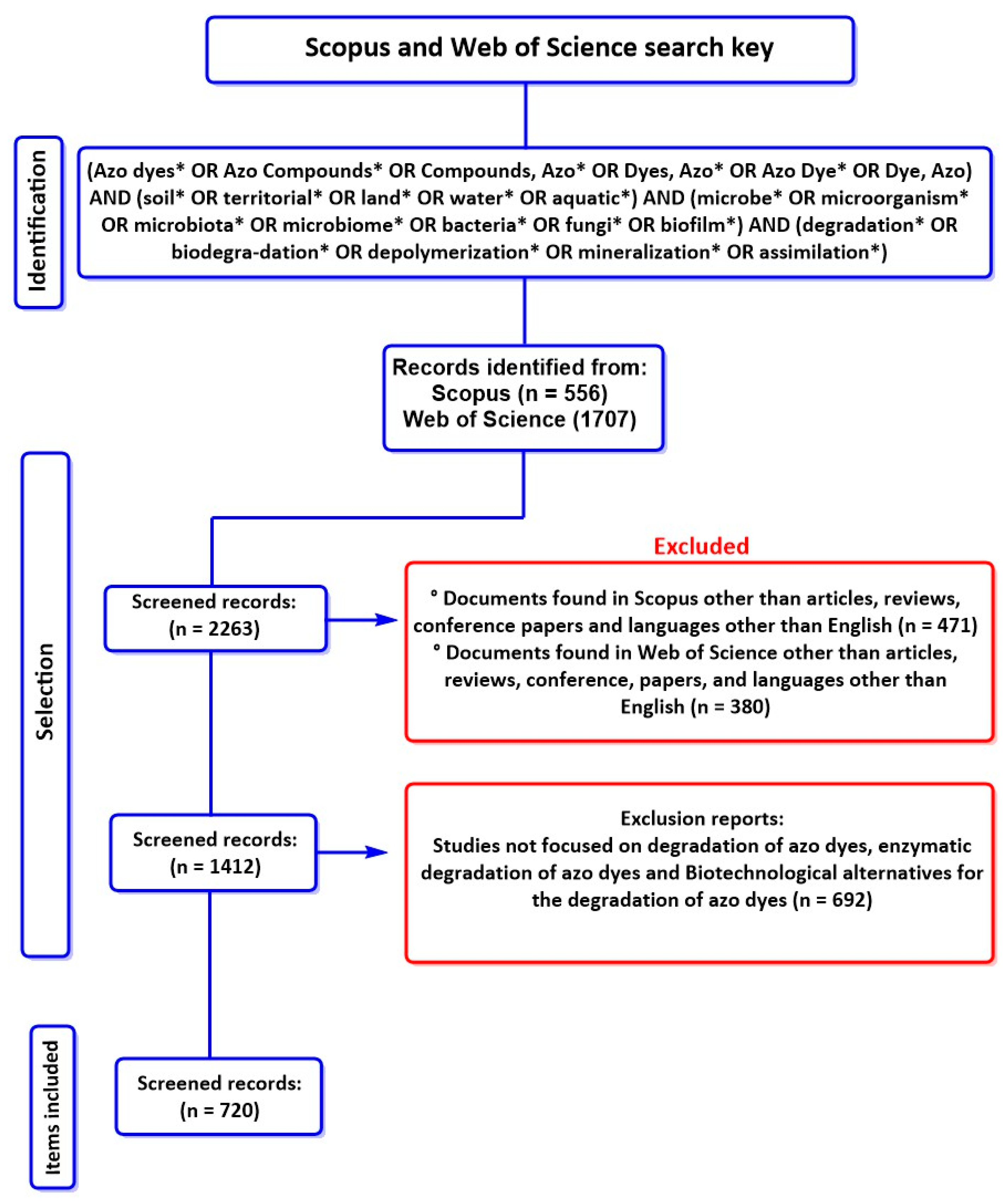



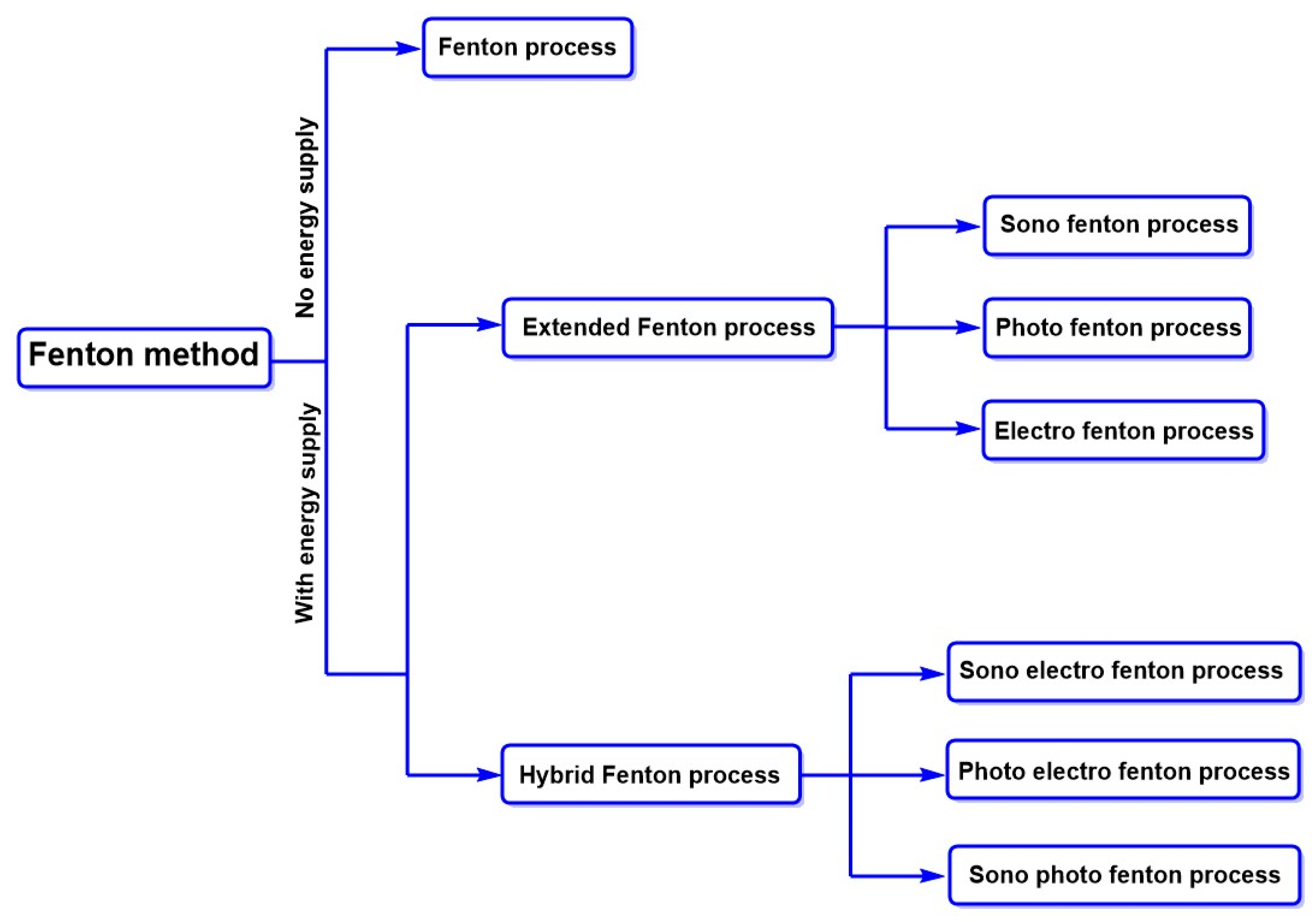

| STEP 1 | Study Idea | Degradation of Azo Dyes by Aquatic and Terrestrial Microorganisms | |||||
|---|---|---|---|---|---|---|---|
| STEP 2 | Study problem | P | Azo dyes | ||||
| I | Degradation/removal of azo dyes by microorganisms such as yeast, bacteria, fungi, microalgae, and consortia | ||||||
| C | Degradation/removal of azo dyes by conventional treatments | ||||||
| O | Bioremediation | ||||||
| STEP 3 | Research question | Can biodegradation based on yeast, bacteria, fungi, microalgae, and consortia offer a viable and environmentally friendly solution for detoxifying aquatic ecosystems severely affected by persistent azo dyes? | |||||
| STEP 4 | DeCS | Azo compounds | DeCS | ||||
| Accumulation in water and land | Soil*, Water* | ||||||
| Aquatic and terrestrial microorganisms | Microorganism* | ||||||
| Bioremediation | Bioremediation (Environmental Health) | ||||||
| STEP 5 | MeSH similarity in PUBMED | Azo dyes | Azo Compounds* | Compounds, Azo* | Dyes, Azo* | Azo Dye* | Dye, Azo |
| Soil*, Water* | Soil* | Water* | Aquatic* | ||||
| Microorganism* | Microbe* | Microbiota* | Bacteria* | Fungi* | Microbiome* | ||
| Bioremediation | Degradation* | Biodegradation* | Depolymarization* | Mineralization* | Assimilation* | ||
| STEP 6 | Search approach by variables | Azo dye | Azo dyes* OR Azo Compounds* OR Compounds, Azo* OR Dyes, Azo* OR Azo Dye* OR Dye, Azo | ||||
| Biodegradation, Environmental | Soil* OR territorial* OR land* OR water* OR aquatic* | ||||||
| Microorganism | Microbe* OR microorganism* OR microbiota* OR microbiome* OR bacteria* OR fungi* OR biofilm* | ||||||
| Bioremediation | Degradation* OR biodegradation* OR depolymerization* OR mineralization* OR assimilation* | ||||||
| STEP 7 | Advanced search key | (Azo dyes* OR Azo Compounds* OR Compounds, Azo* OR Dyes, Azo* OR Azo Dye* OR Dye, Azo) AND (soil* OR territorial* OR land* OR water* OR aquatic*) AND (microbe* OR microorganism* OR microbiota* OR microbiome* OR bacteria* OR fungi* OR biofilm*) AND (degradation* OR biodegradation* OR depolymerization* OR mineralization* OR assimilation*) | |||||
| Method/Technique | Removal Mechanism | Advantages | Disadvantages * | Ref. |
|---|---|---|---|---|
| Adsorption | Dye removal by adhesion to the surface of an adsorbent | Reuse of adsorbents, high efficiency, and short times for removing dye from wastewater | -Only soluble dyes. -High energy consumption. | [120,121,122] |
| Ion exchange | Use of resins that allow ionic exchange between the substances involved | The ion exchange process can effectively remove cationic dyes from contaminated water | The resulting sludge may contain concentrated metals, posing challenges for disposal | [123,124] |
| Coagulation and flocculation | In this process, coagulants are used to destabilize dissolved dyes, enabling their removal through sedimentation. | Coagulation–flocculation is a simple and widely used process for removing dyes from wastewater. | The initial pH and the dosage of the coagulant have a significant influence on the coagulation and flocculation process. | [125,126] |
| Membrane filtration | They use membranes with small pores that trap solutes larger than themselves, allowing the passage of a dye-free solution | -High separation efficiency, reliability, cost-effectiveness, and simplicity. -They have low operating costs compared to conventional technologies. | -Production of toxic byproducts. -Sludge production. | [127,128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrascal-Hernández, D.C.; Orozco-Beltrán, E.J.; Insuasty, D.; Márquez, E.; Grande-Tovar, C.D. Systematic Evaluation of Biodegradation of Azo Dyes by Microorganisms: Efficient Species, Physicochemical Factors, and Enzymatic Systems. Int. J. Mol. Sci. 2025, 26, 7973. https://doi.org/10.3390/ijms26167973
Carrascal-Hernández DC, Orozco-Beltrán EJ, Insuasty D, Márquez E, Grande-Tovar CD. Systematic Evaluation of Biodegradation of Azo Dyes by Microorganisms: Efficient Species, Physicochemical Factors, and Enzymatic Systems. International Journal of Molecular Sciences. 2025; 26(16):7973. https://doi.org/10.3390/ijms26167973
Chicago/Turabian StyleCarrascal-Hernández, Domingo Cesar, Erney José Orozco-Beltrán, Daniel Insuasty, Edgar Márquez, and Carlos David Grande-Tovar. 2025. "Systematic Evaluation of Biodegradation of Azo Dyes by Microorganisms: Efficient Species, Physicochemical Factors, and Enzymatic Systems" International Journal of Molecular Sciences 26, no. 16: 7973. https://doi.org/10.3390/ijms26167973
APA StyleCarrascal-Hernández, D. C., Orozco-Beltrán, E. J., Insuasty, D., Márquez, E., & Grande-Tovar, C. D. (2025). Systematic Evaluation of Biodegradation of Azo Dyes by Microorganisms: Efficient Species, Physicochemical Factors, and Enzymatic Systems. International Journal of Molecular Sciences, 26(16), 7973. https://doi.org/10.3390/ijms26167973







