Galactooligosaccharides Promote Gut Barrier Integrity and Exert Anti-Inflammatory Effects in DSS-Induced Colitis Through Microbiota Modulation
Abstract
1. Introduction
2. Results
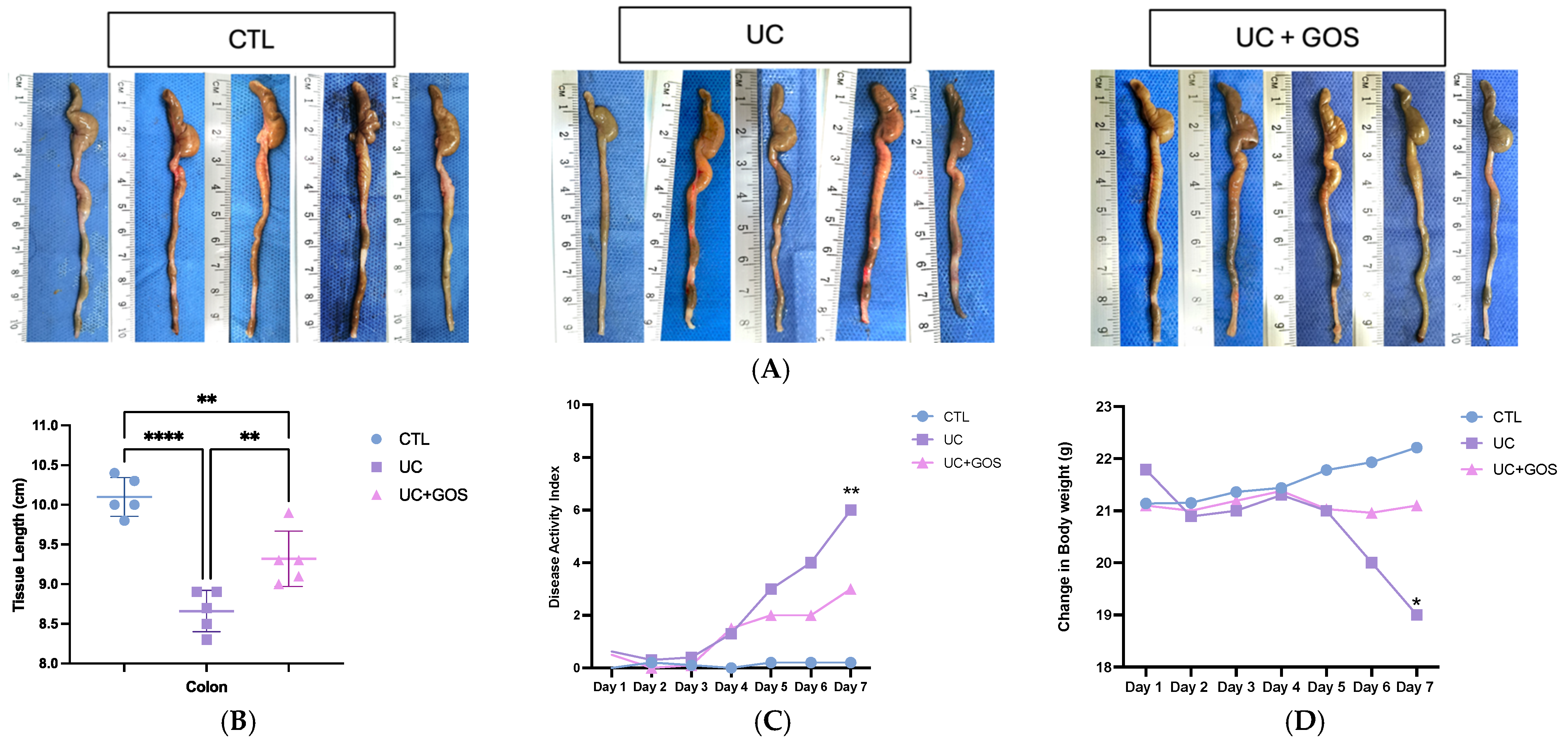
2.1. GOS from Lupinus albus Attenuates the Damage of DSS Ulcerative Colitis
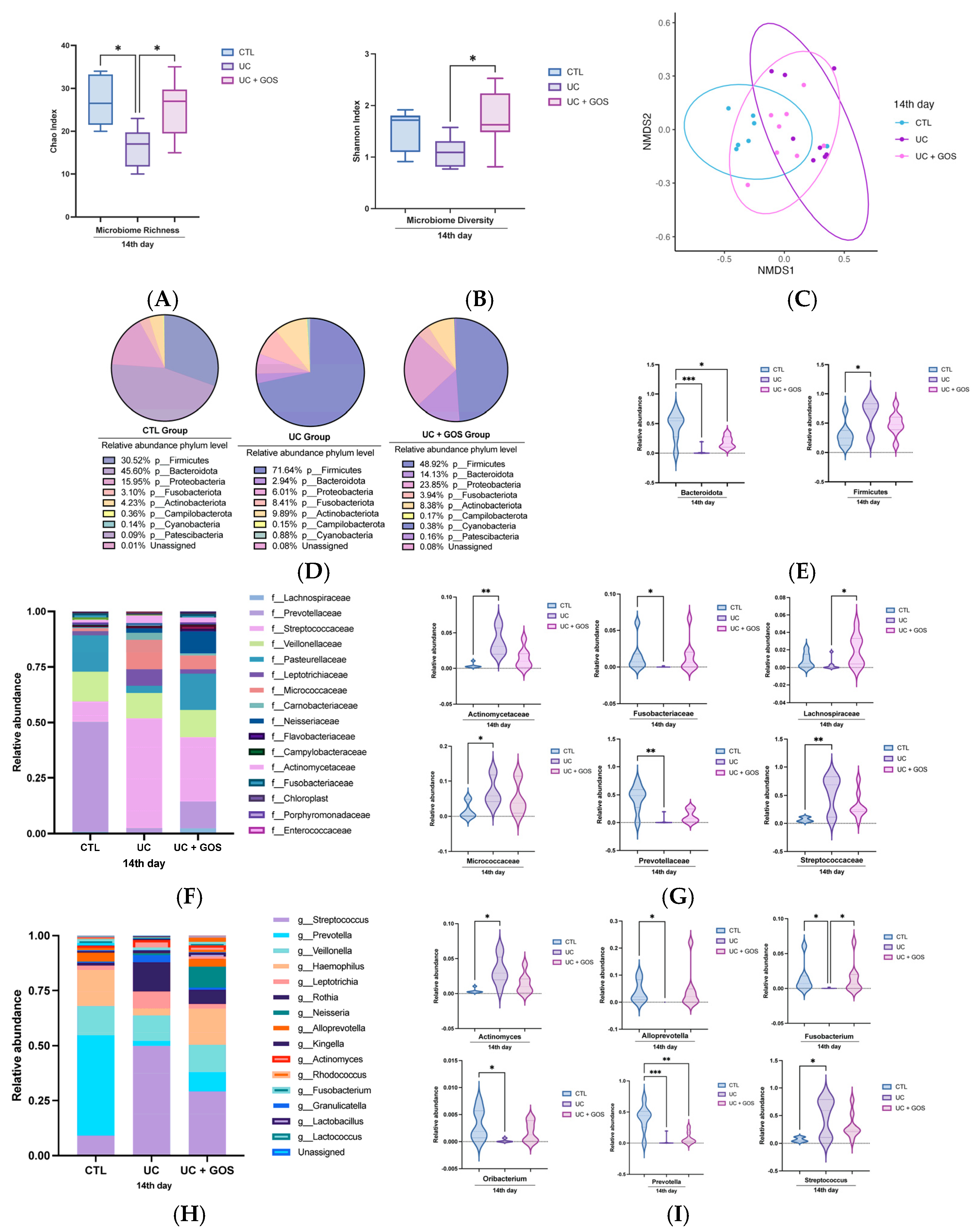
2.2. Modification of the Gut Microbiota by the DSS-Induced Ulcerative Colitis and GOS Administration
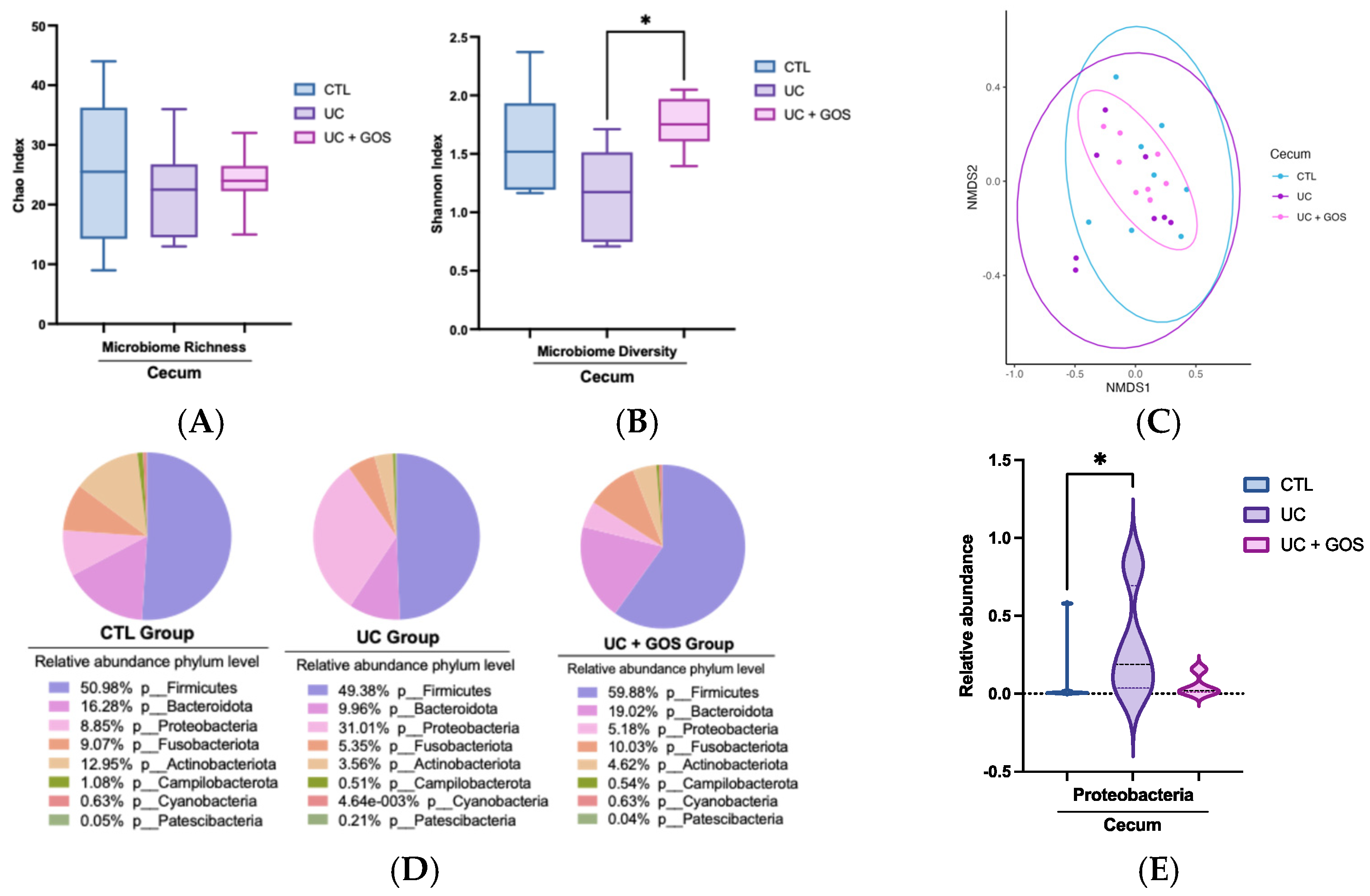
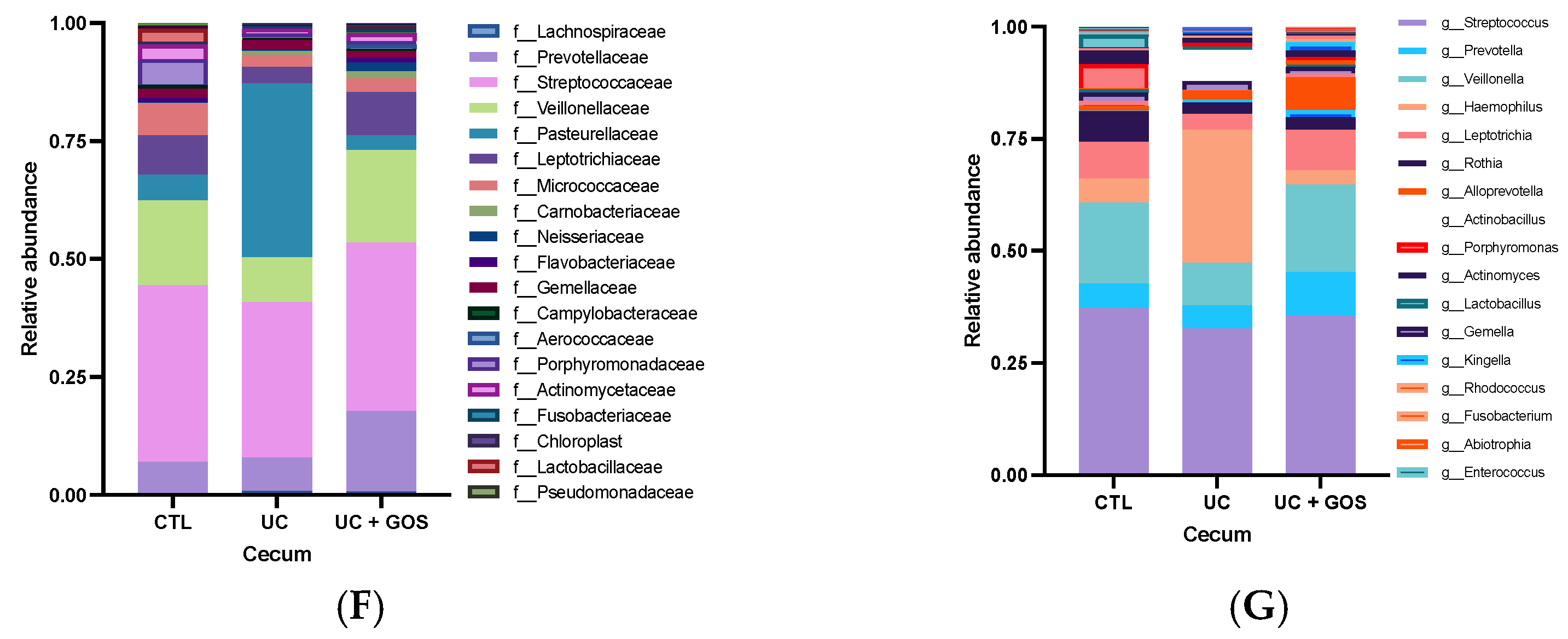
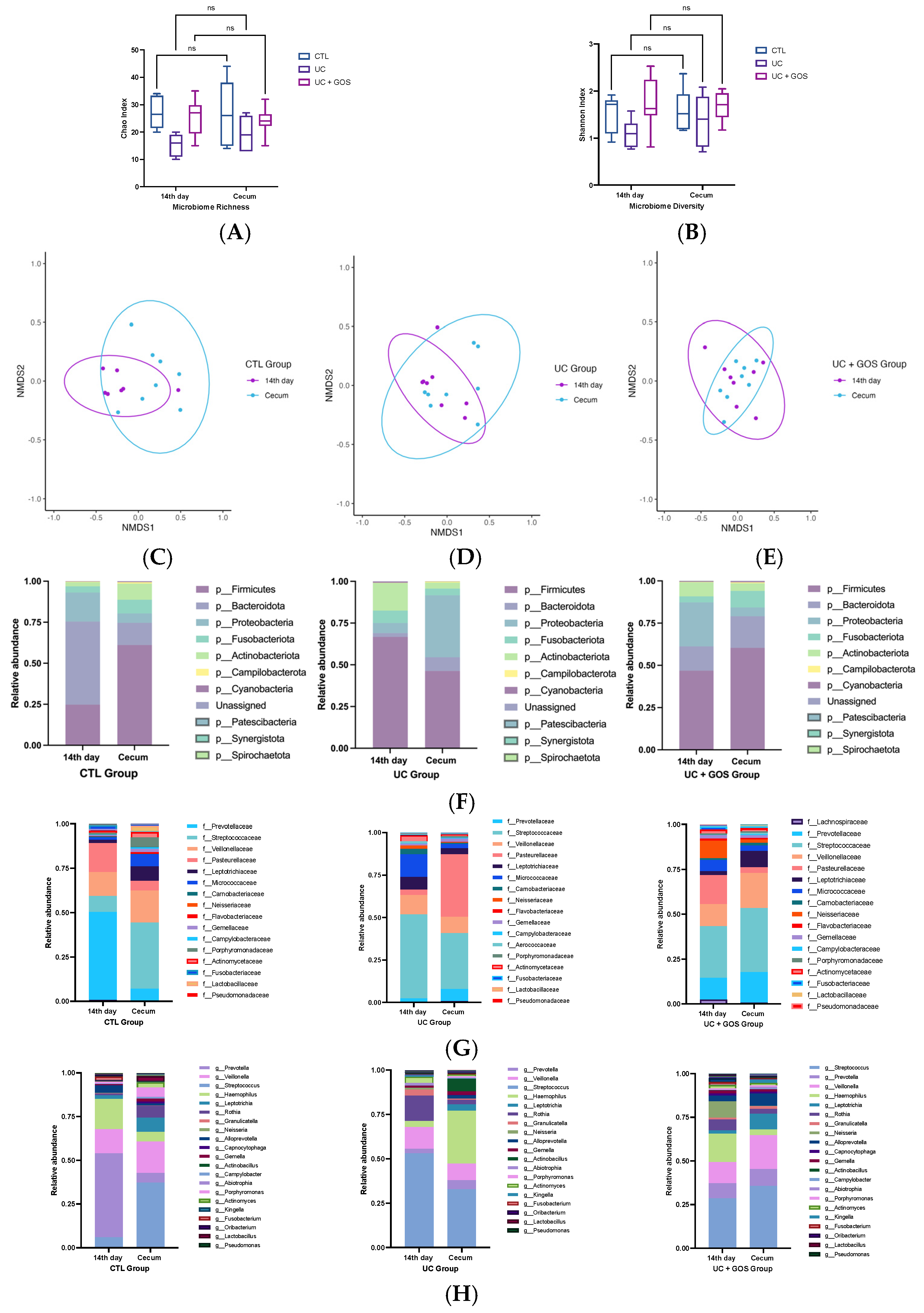
2.3. DSS-Induced Ulcerative Colitis Modifies Cecum Microbiota Diversity
2.4. Comparison of Gut Microbiota Composition Between 14-Day and Cecum Feces
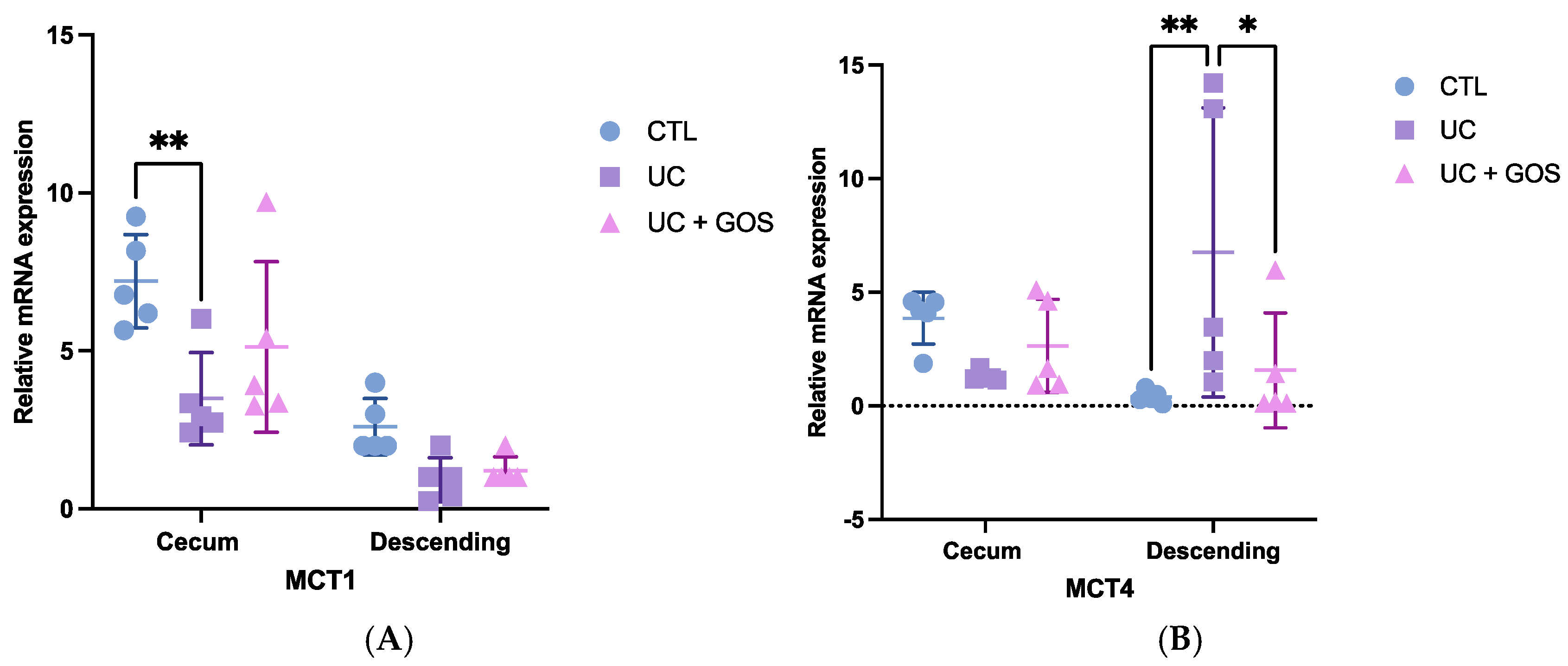
2.5. DSS-Induced Ulcerative Colitis Alters Monocarboxylate Transporter Expression
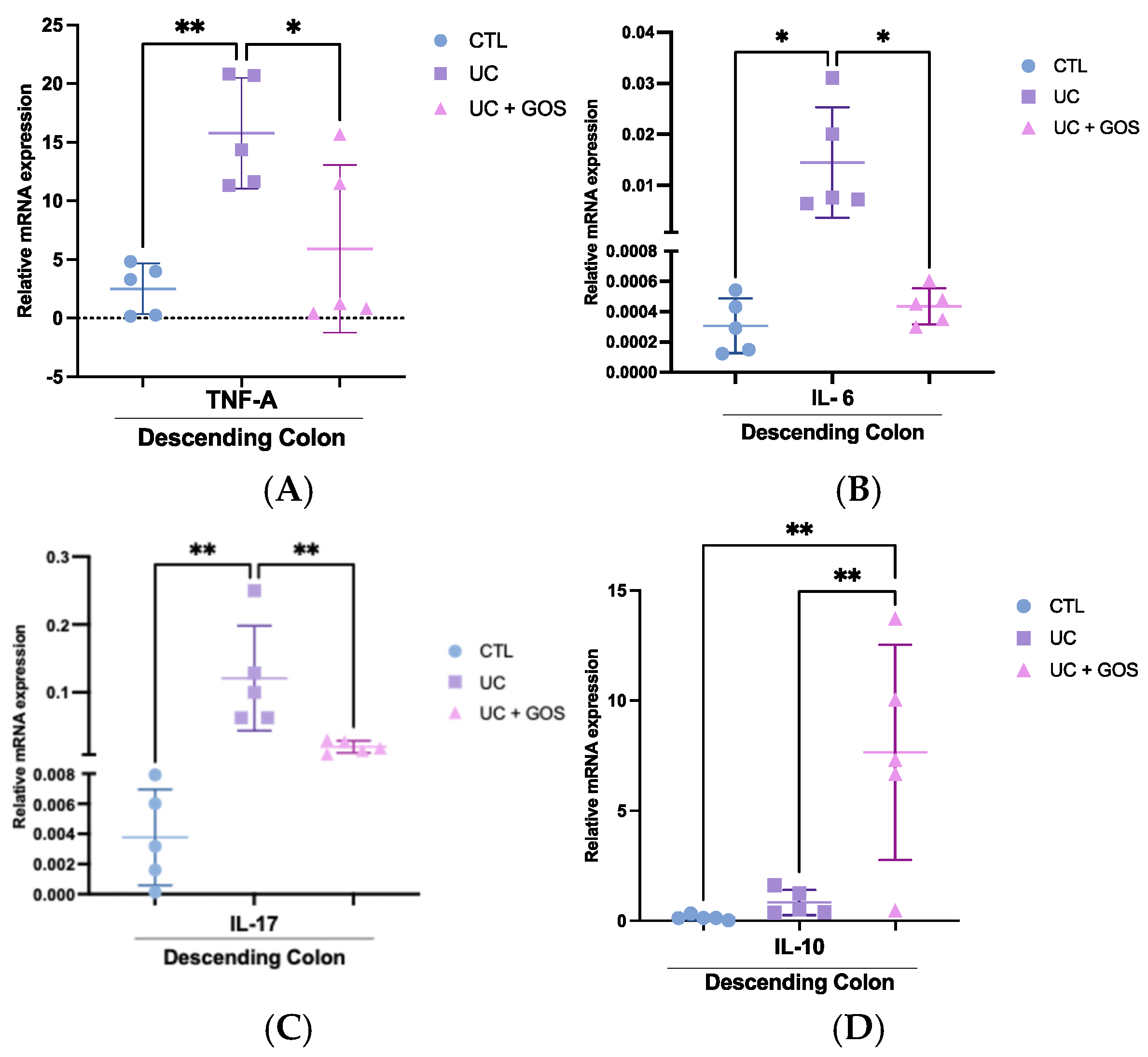
2.6. GOS Administration Reduces the Expression of Inflammatory Cytokines in Descending Colonic Tissue
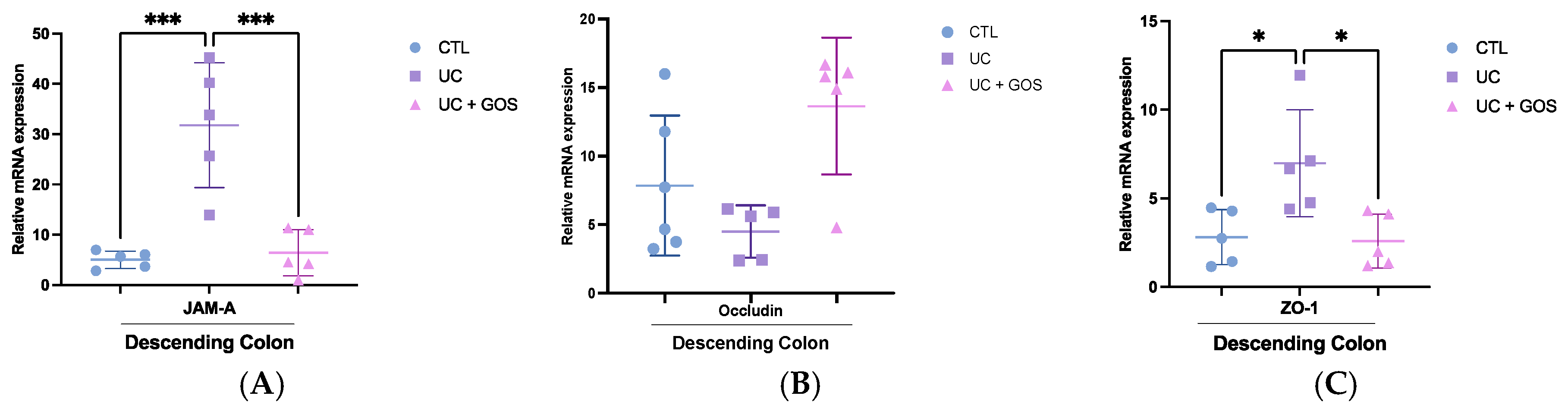
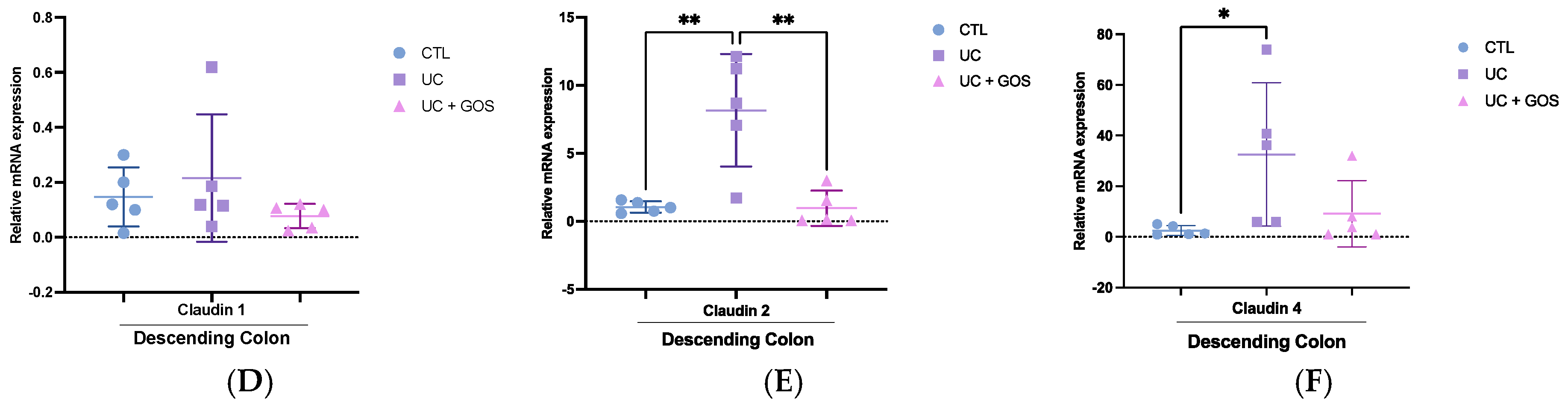
2.7. GOS Treatment Modulates Tight Junction Protein Expression in Descending Colonic Tissue
3. Discussion
4. Materials and Methods
4.1. Extraction of GOS from Lupinus albus
4.2. Mice
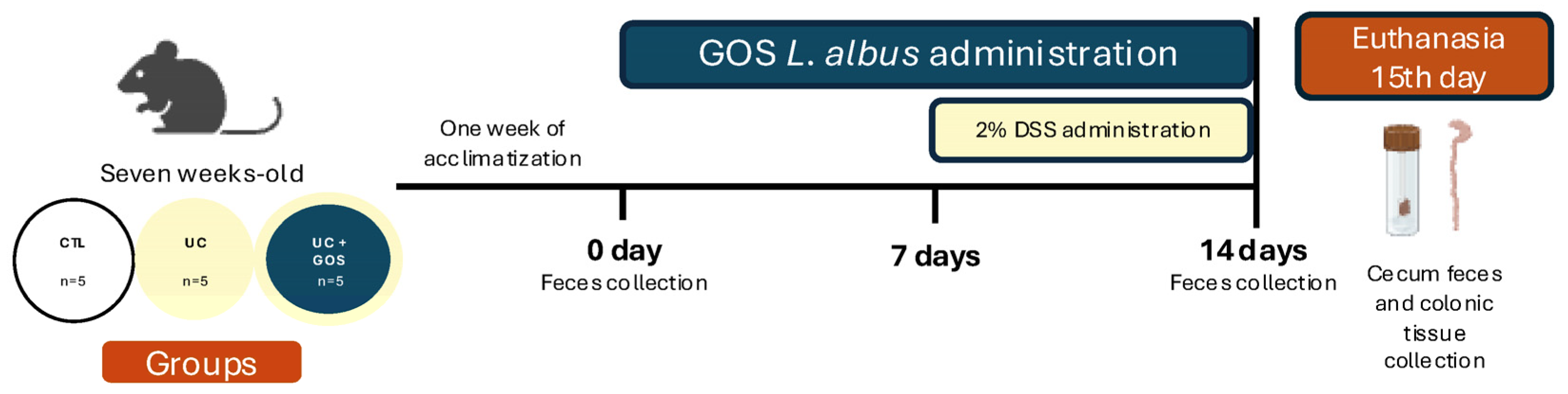
4.3. Experimental Design
4.4. Assessment of Ulcerative Colitis Severity
4.5. Mucus Layer Measurement
4.6. Feces Collection Protocol
4.7. Sample Preparation and DNA Sequencing
4.8. Bioinformatics Analysis and Statistics
4.9. Real-Time RT-PCR
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ASA | 5-Aminosalicylic Acid |
| °C | Degrees Celsius |
| ABCG2 | ATP-Binding Cassette Subfamily G Member 2 |
| α-SMA | Alpha-Smooth Muscle Actin |
| ANOVA | Analysis of Variance |
| AOAC | Association of Official Analytical Chemists |
| ASV | Amplicon Sequence Variant |
| ATP | Adenosine Triphosphate |
| BCFA | Branched Chain Fatty Acids |
| BLAST | Basic Local Alignment Search Tool |
| BW | Body Weight |
| CD | Crohn’s Disease |
| CTL | Control |
| DAI | Disease Activity Index |
| DNA | Desoxyribonucleic Acid |
| DSS | Dextran Sulfate Sodium |
| GOS | Galactooligosaccharides |
| IBD | Inflammatory Bowel Disease |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-17 | Interleukin 17 |
| IL-18 | Interleukin 18 |
| IL-22 | Interleukin 22 |
| INF-γ | Interferon Gamma |
| JAM-A | Junctional Adhesion Molecule A |
| LPS | Lipopolysaccharide |
| MCT1 | Monocarboxylate Transporter 1 |
| MCT4 | Monocarboxylate Transporter 4 |
| mM | Millimolar |
| NCBI | National Center for Biotechnology Information |
| NF-κB | Nuclear Factor Kappa B |
| NMDS | Non-Metric Multidimensional Scaling |
| p-JAM-A | Phosphorylated Junctional Adhesion Molecule A |
| PAS | Periodic Acid–Schiff |
| pH | Potential of Hydrogen |
| RFOs | Raffinose oligosaccharides |
| RNA | Ribonucleic Acid |
| rpm | Revolutions per minute |
| rRNA | Ribosomal Ribonucleic Acid |
| SCFA | Short-Chain Fatty Acids |
| SD | Standard Deviation |
| SOBS | Observed Species |
| TCA | Tricarboxylic Acid |
| TJ | Tight Junction |
| TLR2 | Toll-like Receptor 2 |
| TNF-α | Tumor Necrosis Factor Alpha |
| μm | Micrometers |
| UC | Ulcerative Colitis |
| UC + GOS | Ulcerative Colitis + Galactooligosaccharides |
| ZO-1 | Zonula Occludens 1 |
References
- Choi, Y.-S.; Kim, J.-K.; Kim, W.-J. Clinical characteristics and prognosis of patients with ulcerative colitis that shows rectal sparing at initial diagnosis. World J. Gastrointest. Endosc. 2021, 13, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primer 2020, 6, 74. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. Semin. Immunopathol. 2025, 47, 2. [Google Scholar] [CrossRef]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Čužić, S.; Antolić, M.; Ognjenović, A.; Stupin-Polančec, D.; Petrinić Grba, A.; Hrvačić, B.; Dominis Kramarić, M.; Musladin, S.; Požgaj, L.; Zlatar, I.; et al. Claudins: Beyond Tight Junctions in Human IBD and Murine Models. Front. Pharmacol. 2021, 12, 682614. [Google Scholar] [CrossRef]
- Nakai, D.; Miyake, M. Intestinal Membrane Function in Inflammatory Bowel Disease. Pharmaceutics 2024, 16, 29. [Google Scholar] [CrossRef]
- Fan, S.; Boerner, K.; Muraleedharan, C.K.; Nusrat, A.; Quiros, M.; Parkos, C.A. Epithelial JAM-A is fundamental for intestinal wound repair in vivo. JCI Insight 2022, 7, e158934. [Google Scholar] [CrossRef]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Świrkosz, G.; Szczygieł, A.; Logoń, K.; Wrześniewska, M.; Gomułka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Ulcerative Colitis—A Literature Review. Biomedicines 2023, 11, 3144. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Xue, J.; Lee, S.A.; Liu, L.; Riordan, S.M. Bacterial Species Associated with Human Inflammatory Bowel Disease and Their Pathogenic Mechanisms. Front. Microbiol. 2022, 13, 801892. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Lambert, D.W.; Wood, I.S.; Ellis, A.; Shirazi-Beechey, S.P. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br. J. Cancer 2002, 86, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Yong, C.; Jin, Y.; Rong, S.; Xue, K.; Cao, B.; Wei, H. Unraveling the microbial mysteries: Gut microbiota’s role in ulcerative colitis. Front. Nutr. 2025, 12, 1519974. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Yang, Z.; Zhao, K.; Li, S.; He, N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022, 13, 6875–6893. [Google Scholar] [CrossRef]
- Jadhav, A.; Jagtap, S.; Vyavahare, S.; Sharbidre, A.; Kunchiraman, B. Reviewing the potential of probiotics, prebiotics and synbiotics: Advancements in treatment of ulcerative colitis. Front. Cell. Infect. Microbiol. 2023, 13, 1268041. [Google Scholar] [CrossRef]
- Ignatova, I.; Arsov, A.; Petrova, P.; Petrov, K. Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation. Molecules 2025, 30, 803. [Google Scholar] [CrossRef]
- Mei, Z.; Yuan, J.; Li, D. Biological activity of galacto-oligosaccharides: A review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef]
- Roselli, M.; Maruszak, A.; Grimaldi, R.; Harthoorn, L.; Finamore, A. Galactooligosaccharide Treatment Alleviates DSS-Induced Colonic Inflammation in Caco-2 Cell Model. Front. Nutr. 2022, 9, 862974. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Tao, X.; Sun, Z.; Hao, W.; Wei, X. Galactooligosaccharides protects against DSS-induced murine colitis through regulating intestinal flora and inhibiting NF-κB pathway. Life Sci. 2020, 242, 117220. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Méndez, L.A.; Gurrola-Díaz, C.M.; Zepeda-Nuño, J.S.; Vega-Magaña, N.; Lopez-Roa, R.I.; Íñiguez-Gutiérrez, L.; García-López, P.M.; Fafutis-Morris, M.; Delgado-Rizo, V. In Vivo Healthy Benefits of Galacto-Oligosaccharides from Lupinus albus (LA-GOS) in Butyrate Production through Intestinal Microbiota. Biomolecules 2021, 11, 1658. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Casimiro, S.; Fernandes, J.; Hartmann, R.M.; Schemitt, E.; Picada, J.; Costa, L.; Marroni, N.; Raymundo, A.; Lima, A.; et al. Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients 2022, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Wilson, B.; Eyice, Ö.; Koumoutsos, I.; Lomer, M.C.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients 2021, 13, 3598. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Yin, J.; Zhang, B.; Wang, J.; Wang, S. Differential responses on gut microbiota and microbial metabolome of 2′-fucosyllactose and galactooligosaccharide against DSS-induced colitis. Food Res. Int. 2022, 162, 112072. [Google Scholar] [CrossRef]
- Do, K.-H.; Ko, S.-H.; Kim, K.B.; Seo, K.; Lee, W.-K. Comparative Study of Intestinal Microbiome in Patients with Ulcerative Colitis and Healthy Controls in Korea. Microorganisms 2023, 11, 2750. [Google Scholar] [CrossRef]
- Molinero, N.; Taladrid, D.; Zorraquín-Peña, I.; De Celis, M.; Belda, I.; Mira, A.; Bartolomé, B.; Moreno-Arribas, M.V. Ulcerative Colitis Seems to Imply Oral Microbiome Dysbiosis. Curr. Issues Mol. Biol. 2022, 44, 1513–1527. [Google Scholar] [CrossRef]
- Kojima, A.; Nomura, R.; Ooshima, T.; Nakano, K. Aggravation of Inflammatory Bowel Diseases by oral streptococci. Oral Dis. 2013, 20, 359–366. [Google Scholar] [CrossRef]
- Kojima, A.; Nakano, K.; Wada, K.; Takahashi, H.; Katayama, K.; Yoneda, M.; Higurashi, T.; Nomura, R.; Hokamura, K.; Muranaka, Y.; et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2012, 2, 332. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sasahira, M.; Go, T.T.; Yo, S.; Ninomiya, T.; Osawa, M.; Handa, O.; Umegami, E.; Inoue, R.; Shiotani, A. Characteristics of Mucosa-Associated Microbiota in Ulcerative Colitis Patients with 5-Aminosalicylic Acid Intolerance. Biomedicines 2024, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lkhagva, E.; Kim, S.; Zhai, C.; Islam, M.M.; Kim, H.J.; Hong, S.-T. The gut microbe pair of Oribacterium sp. GMB0313 and Ruminococcus sp. GMB0270 confers complete protection against SARS-CoV-2 infection by activating CD8+ T cell-mediated immunity. Gut Microbes 2024, 16, 2342497. [Google Scholar] [CrossRef]
- Park, H.; Yeo, S.; Kang, S.; Huh, C.S. Longitudinal Microbiome Analysis in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Microorganisms 2021, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.L.; Krishna, M.; Kellermayer, R. Weight loss is a sufficient and economical single outcome measure of murine dextran sulfate sodium colitis. FASEB Bioadv. 2019, 1, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, X.; Chen, X.; Wang, Z.; Xia, J.; Wang, B.; Wang, M.; Ding, Y. Identification of differentially expressed genes associated with ferroptosis in ulcerative colitis. PLoS ONE 2025, 20, e0327990. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548. [Google Scholar] [CrossRef]
- Olshan, K.L.; Zomorrodi, A.R.; Pujolassos, M.; Troisi, J.; Khan, N.; Fanelli, B.; Kenyon, V.; Fasano, A.; Leonard, M.M. Microbiota and Metabolomic Patterns in the Breast Milk of Subjects with Celiac Disease on a Gluten-Free Diet. Nutrients 2021, 13, 2243. [Google Scholar] [CrossRef]
- Fatahi-Bafghi, M. Characterization of the Rothia spp. and their role in human clinical infections. Infect. Genet. Evol. 2021, 93, 104877. [Google Scholar] [CrossRef]
- Nishihara, Y.; Ogino, H.; Tanaka, M.; Ihara, E.; Fukaura, K.; Nishioka, K.; Chinen, T.; Tanaka, Y.; Nakayama, J.; Kang, D.; et al. Mucosa-associated gut microbiota reflects clinical course of ulcerative colitis. Sci. Rep. 2021, 11, 13743. [Google Scholar] [CrossRef]
- Kövér, Z.; Johansen Nordskag, V.; Bán, Á.; Gajdács, M.; Urbán, E. The role of Actinomyces spp. and related organisms in cervicofacial infections: Pathomechanism, diagnosis and therapeutic aspects. Anaerobe 2023, 82, 102767. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhou, Y.; Wang, C.; Wu, B.; Wan, J. Actinomyces and Alimentary Tract Diseases: A Review of Its Biological Functions and Pathology. BioMed Res. Int. 2018, 2018, 3820215. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Lo Muzio, L.; Troiano, G. Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review. J. Clin. Med. 2020, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lv, Z.; Chen, F.; Zhang, Y.; Xu, Z.; Huo, J.; Liu, W.; Yu, S.; Tuersun, A.; Zhao, J.; et al. Dysbiosis of human tumor microbiome and aberrant residence of Actinomyces in tumor-associated fibroblasts in young-onset colorectal cancer. Front. Immunol. 2022, 13, 1008975. [Google Scholar] [CrossRef]
- Collins, K.H.; Schwartz, D.J.; Lenz, K.L.; Harris, C.A.; Guilak, F. Taxonomic changes in the gut microbiota are associated with cartilage damage independent of adiposity, high fat diet, and joint injury. Sci. Rep. 2021, 11, 14560. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef]
- Sohn, J.; Li, L.; Zhang, L.; Settem, R.P.; Honma, K.; Sharma, A.; Falkner, K.L.; Novak, J.M.; Sun, Y.; Kirkwood, K.L. Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells. Mol. Oral Microbiol. 2022, 37, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Fleming, B.A.; Mulvey, M.A. Commensal Strains of Neisseria Use DNA to Poison Their Pathogenic Rivals. Cell Host Microbe 2019, 26, 156–158. [Google Scholar] [CrossRef]
- Aho, E.L.; Ogle, J.M.; Finck, A.M. The Human Microbiome as a Focus of Antibiotic Discovery: Neisseria mucosa Displays Activity Against Neisseria gonorrhoeae. Front. Microbiol. 2020, 11, 577762. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Higashi, D.; Goytia, M.; Rendón, M.A.; Pilligua-Lucas, M.; Bronnimann, M.; McLean, J.A.; Duncan, J.; Trees, D.; Jerse, A.E.; et al. Commensal Neisseria Kill Neisseria gonorrhoeae through a DNA-Dependent Mechanism. Cell Host Microbe 2019, 26, 228–239.e8. [Google Scholar] [CrossRef]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol. Spectr. 2022, 10, e01651-22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D.; et al. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef]
- Overstreet, A.-M.C.; Ramer-Tait, A.E.; Suchodolski, J.S.; Hostetter, J.M.; Wang, C.; Jergens, A.E.; Phillips, G.J.; Wannemuehler, M.J. Temporal Dynamics of Chronic Inflammation on the Cecal Microbiota in IL-10-/- Mice. Front. Immunol. 2021, 11, 585431. [Google Scholar] [CrossRef]
- Peng, Y.; Wei, J.; Jia, X.; Luan, F.; Man, M.; Ma, X.; Luo, Y.; Li, Y.; Li, N.; Wang, Q.; et al. Changes in the microbiota in different intestinal segments of mice with sepsis. Front. Cell. Infect. Microbiol. 2023, 12, 954347. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Pochart, P.; Doré, J.; Béra-Maillet, C.; Bernalier, A.; Corthier, G. Comparative Study of Bacterial Groups within the Human Cecal and Fecal Microbiota. Appl. Environ. Microbiol. 2001, 67, 4939–4942. [Google Scholar] [CrossRef]
- Xu, K.; Tan, J.; Lin, D.; Jiang, H.; Chu, Y.; Zhou, L.; Zhang, J.; Lu, Y. Gut microbes of the cecum versus the colon drive more severe lethality and multi-organ damage. Int. Immunopharmacol. 2025, 147, 114029. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef]
- Park, S.J.; Smith, C.P.; Wilbur, R.R.; Cain, C.P.; Kallu, S.R.; Valasapalli, S.; Sahoo, A.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. An overview of MCT1 and MCT4 in GBM: Small molecule transporters with large implications. Am. J. Cancer Res. 2018, 8, 1967. [Google Scholar]
- Fisel, P.; Schaeffeler, E.; Schwab, M. Clinical and Functional Relevance of the Monocarboxylate Transporter Family in Disease Pathophysiology and Drug Therapy. Clin. Transl. Sci. 2018, 11, 352–364. [Google Scholar] [CrossRef]
- Iwanaga, T.; Takebe, K.; Kato, I.; Karaki, S.-I.; Kuwahara, A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed. Res. 2006, 27, 243–254. [Google Scholar] [CrossRef]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Babl, N.; Decking, S.-M.; Voll, F.; Althammer, M.; Sala-Hojman, A.; Ferretti, R.; Korf, C.; Schmidl, C.; Schmidleithner, L.; Nerb, B.; et al. MCT4 blockade increases the efficacy of immune checkpoint blockade. J. Immunother. Cancer 2023, 11, e007349. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, I.; Bang, H.; Kim, H.C.; Lee, W.Y.; Yun, S.H.; Lee, J.; Lee, S.J.; Park, Y.S.; Kim, K.-M.; et al. MCT4 Expression Is a Potential Therapeutic Target in Colorectal Cancer with Peritoneal Carcinomatosis. Mol. Cancer Ther. 2018, 17, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: Upregulation of claudin-1, claudin-3, claudin-4, and β-catenin. Int. J. Colorectal Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef]
- Vetrano, S.; Rescigno, M.; Rosaria Cera, M.; Correale, C.; Rumio, C.; Doni, A.; Fantini, M.; Sturm, A.; Borroni, E.; Repici, A.; et al. Unique Role of Junctional Adhesion Molecule-A in Maintaining Mucosal Homeostasis in Inflammatory Bowel Disease. Gastroenterology 2008, 135, 173–184. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.-D. Mechanism of Diapedesis: Importance of the Transcellular Route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Weight, C.M.; Luissint, A.-C.; Hilgarth, R.S.; Brazil, J.C.; Ettel, M.; Nusrat, A.; Parkos, C.A. Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation. Mol. Biol. Cell 2019, 30, 566–578. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte–Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Ibrahim, S.; Zhu, X.; Luo, X.; Feng, Y.; Wang, J. PIK3R3 regulates ZO-1 expression through the NF-κB pathway in inflammatory bowel disease. Int. Immunopharmacol. 2020, 85, 106610. [Google Scholar] [CrossRef]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Tan, Y.; Guan, Y.; Sun, Y.; Zheng, C. Correlation of Intestinal Mucosal Healing and Tight Junction Protein Expression in Ulcerative Colitis Patients. Am. J. Med. Sci. 2019, 357, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Tsai, K.-W.; Liao, J.-B.; Kuo, W.-T.; Chang, Y.-C.; Yang, Y.-F. High expression of tight junction protein 1 as a predictive biomarker for bladder cancer grade and staging. Sci. Rep. 2022, 12, 1496. [Google Scholar] [CrossRef]
- Gulewicz, P.; Ciesiołka, D.; Frias, J.; Vidal-Valverde, C.; Frejnagel, S.; Trojanowska, K.; Gulewicz, K. Simple Method of Isolation and Purification of α-Galactosides from Legumes. J. Agric. Food Chem. 2000, 48, 3120–3123. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.-K. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef]
- Hatanaka, H.; Tsukui, M.; Takada, S.; Kurashina, K.; Choi, Y.L.; Soda, M.; Yamashita, Y.; Haruta, H.; Hamada, T.; Ueno, T.; et al. Identification of transforming activity of free fatty acid receptor 2 by retroviral expression screening. Cancer Sci. 2010, 101, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; An, B.S.; Yang, H.; Kang, H.S.; Jung, E.M.; Jeung, E.B. Tissue-specific expression of occludin, zona occludens-1, and junction adhesion molecule a in the duodenum, ileum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J. Physiol. Pharmacol. 2013, 64, 11–18. [Google Scholar]
- Schutkowski, A.; Wege, N.; Stangl, G.I.; König, B. Tissue-Specific Expression of Monocarboxylate Transporters during Fasting in Mice. PLoS ONE 2014, 9, e112118. [Google Scholar] [CrossRef]
- Furukawa, C.; Ishizuka, N.; Hayashi, H.; Fujii, N.; Manabe, A.; Tabuchi, Y.; Matsunaga, T.; Endo, S.; Ikari, A. Up-regulation of claudin-2 expression by aldosterone in colonic epithelial cells of mice fed with NaCl-depleted diets. Sci. Rep. 2017, 7, 12223. [Google Scholar] [CrossRef]
- Ahmad, R.; Chaturvedi, R.; Olivares-Villagómez, D.; Habib, T.; Asim, M.; Shivesh, P.; Polk, D.B.; Wilson, K.T.; Washington, M.K.; Van Kaer, L.; et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014, 7, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Stark, M.A.; Zarbock, A.; Burcin, T.L.; Vaswani, D.; Foley, P.; Ley, K. Interleukin-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J. Immunol. 2009, 181, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-B.; Zhao, D.-Y.; Qi, Q.-Q.; Long, X.; Li, X.; Chen, F.-X.; Zuo, X.-L. BDNF modulates intestinal barrier integrity through regulating the expression of tight junction proteins. Neurogastroenterol. Motil. 2017, 29, e12967. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-H.; Kim, N.; Sohn, S.H.; Lee, S.M.; Nam, R.H.; Na, H.Y.; Lee, D.H.; Surh, Y.-J. Effects of 17beta;-Estradiol on Colonic Permeability and Inflammation in an Azoxymethane/Dextran Sulfate Sodium-Induced Colitis Mouse Model. Gut Liver 2018, 12, 682–693. [Google Scholar] [CrossRef]

| Score | Weight Loss | Stool Consistency | Blood in Feces |
|---|---|---|---|
| 0 | 0 | Normal | Negative |
| 1 | 1–5% | Soft but still formed | Trace amounts; blood steak |
| 2 | 5–10% | Soft and unformed | Moderate bleeding; blood clot |
| 3 | 10–20% | Loose | Visible, bloody stool |
| 4 | >20% | Diarrhea | Gross bleeding |
| Score | Presence of Inflammatory Cells | Extent of Leukocyte Infiltration | Severity of Fibrosis | Epithelial Damage | Mucosal Damage |
|---|---|---|---|---|---|
| 0 | Normal tissue | Rare (1–5 cells per field) | Normal | Normal | Normal tissue |
| 1 | Rare (1–5 per field) | Increased in lamina propria (5–10 cells) | Mild | Loss of the basal one-third of the crypt | 1–2 foci of ulcerations |
| 2 | Slight increase (5–10 per field) | Confluent in the submucosal part | Moderate | Loss of the basal two-thirds of the crypt | 2–4 foci of ulcerations |
| 3 | More obvious increase | Transmural infiltration | Severe | Entire crypt loss | Confluent or extensive ulceration |
| 4 | Significantly elevated (full infiltration) | - | Very Severe | Focal erosion | - |
| 5 | - | - | Confluent erosion | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godínez-Méndez, L.A.; Vega-Magaña, A.N.; Peña-Rodríguez, M.; Valencia-Hernández, G.A.; Muñoz-Sánchez, G.; Iñiguez-Gutiérrez, L.; López-Roa, R.; Ramos-Márquez, M.E.; Fafutis-Morris, M.; Delgado-Rizo, V. Galactooligosaccharides Promote Gut Barrier Integrity and Exert Anti-Inflammatory Effects in DSS-Induced Colitis Through Microbiota Modulation. Int. J. Mol. Sci. 2025, 26, 7968. https://doi.org/10.3390/ijms26167968
Godínez-Méndez LA, Vega-Magaña AN, Peña-Rodríguez M, Valencia-Hernández GA, Muñoz-Sánchez G, Iñiguez-Gutiérrez L, López-Roa R, Ramos-Márquez ME, Fafutis-Morris M, Delgado-Rizo V. Galactooligosaccharides Promote Gut Barrier Integrity and Exert Anti-Inflammatory Effects in DSS-Induced Colitis Through Microbiota Modulation. International Journal of Molecular Sciences. 2025; 26(16):7968. https://doi.org/10.3390/ijms26167968
Chicago/Turabian StyleGodínez-Méndez, Lucila A., Alejandra Natali Vega-Magaña, Marcela Peña-Rodríguez, Gisela Anay Valencia-Hernández, Germán Muñoz-Sánchez, Liliana Iñiguez-Gutiérrez, Rocío López-Roa, Martha Eloisa Ramos-Márquez, Mary Fafutis-Morris, and Vidal Delgado-Rizo. 2025. "Galactooligosaccharides Promote Gut Barrier Integrity and Exert Anti-Inflammatory Effects in DSS-Induced Colitis Through Microbiota Modulation" International Journal of Molecular Sciences 26, no. 16: 7968. https://doi.org/10.3390/ijms26167968
APA StyleGodínez-Méndez, L. A., Vega-Magaña, A. N., Peña-Rodríguez, M., Valencia-Hernández, G. A., Muñoz-Sánchez, G., Iñiguez-Gutiérrez, L., López-Roa, R., Ramos-Márquez, M. E., Fafutis-Morris, M., & Delgado-Rizo, V. (2025). Galactooligosaccharides Promote Gut Barrier Integrity and Exert Anti-Inflammatory Effects in DSS-Induced Colitis Through Microbiota Modulation. International Journal of Molecular Sciences, 26(16), 7968. https://doi.org/10.3390/ijms26167968







