From Gut to Lungs: The Hidden Respiratory Impacts of IBD: A Systematic Review of the Literature
Abstract
1. Introduction
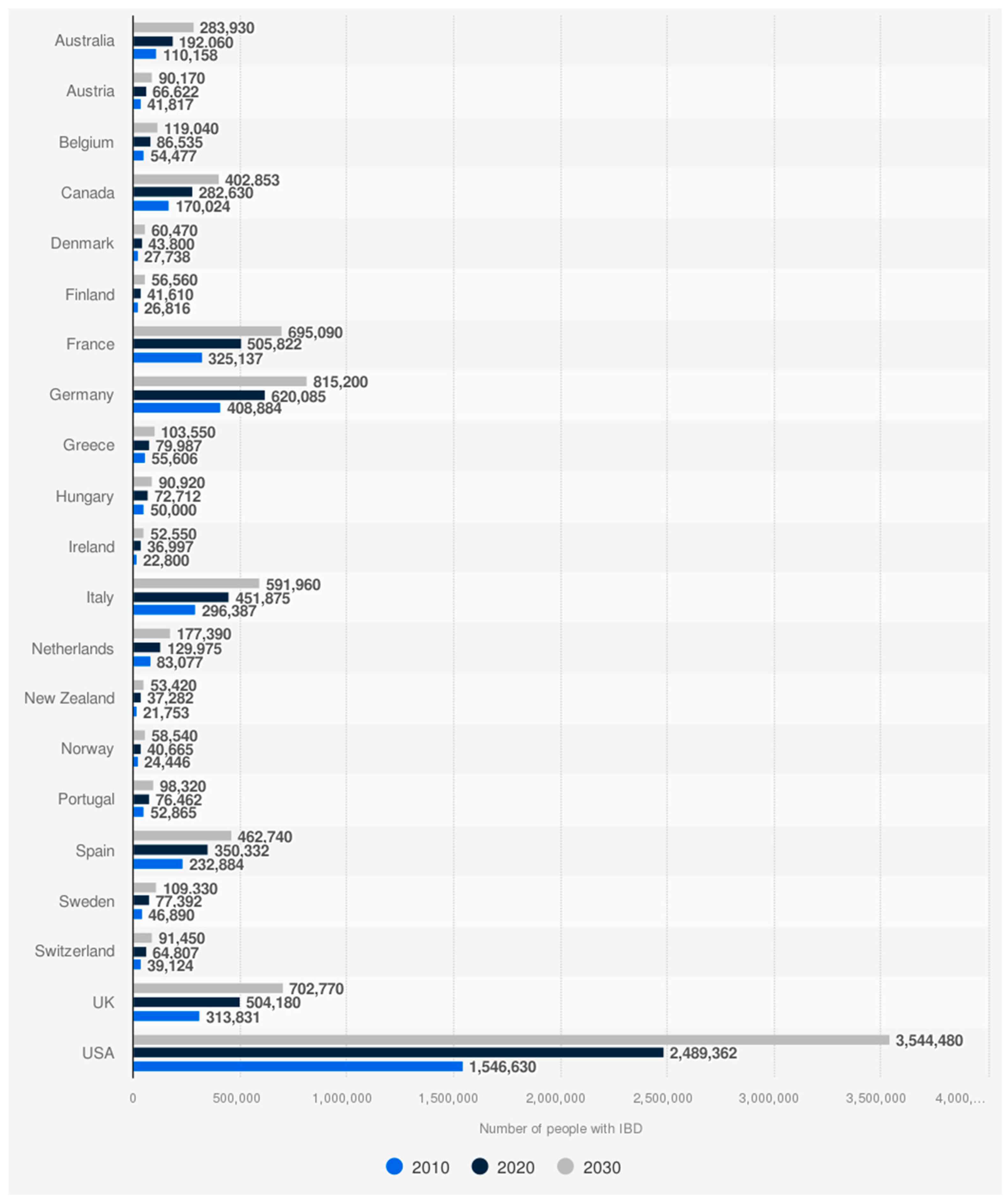
1.1. Epidemiology
1.2. Pulmonary Involvement
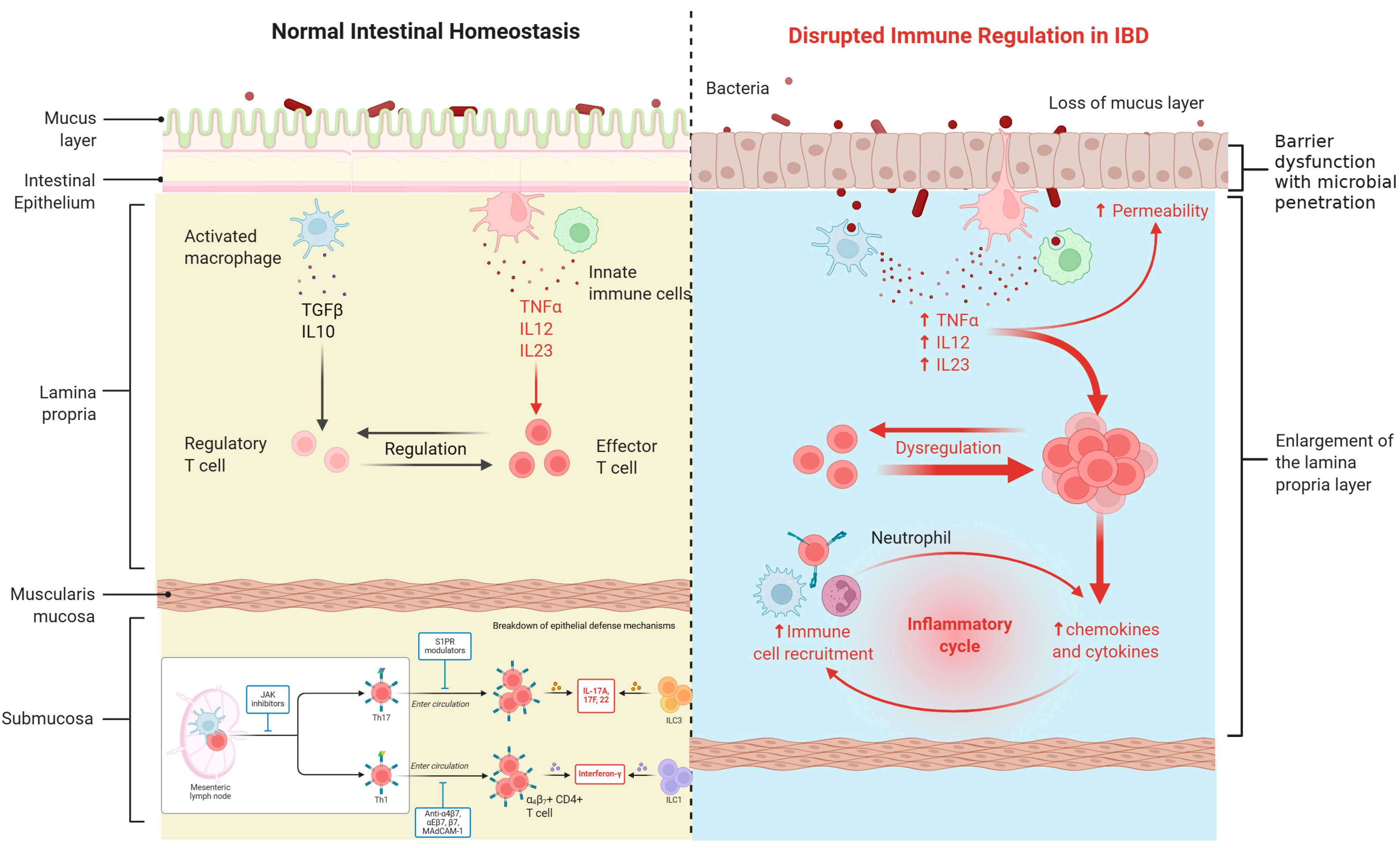
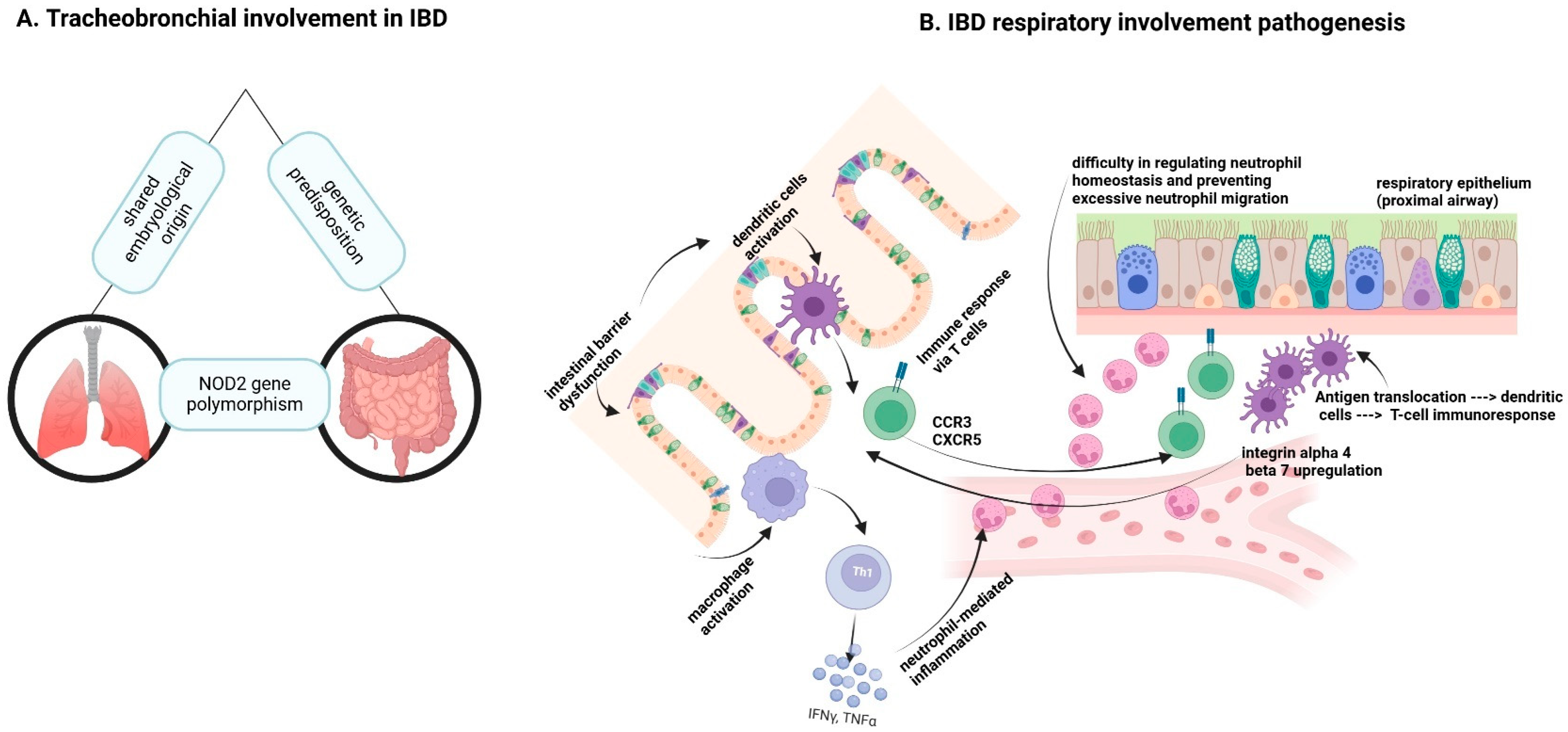
1.3. Pathophysiological Mechanisms
1.4. Rationale for the Review
2. Materials and Methods
2.1. Study Design and Strategy
2.2. Inclusion and Exclusion Criteria
- -
- Reported pulmonary manifestations, specifically ILD, in IBD patients.
- -
- Were original research articles, including cohort studies, case-control studies, case series, and case reports.
- -
- Were published in English.
- -
- Studies focusing solely on pediatric populations.
- -
- Non-human studies.
- -
- Reviews, meta-analyses, and editorials without original patient data.
- -
- Articles lacking sufficient detail on pulmonary involvement in IBD.
2.3. Study Selection, Data Extraction, and Synthesis
2.4. Quality Assessment and Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. [Updated 4 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 7 September 2025).
- Ulcerative Colitis Epidemiology Analysis and Forecast to 2031; (12 March 2024); Market Research Reports & Consulting|GlobalData UK Ltd.: London, UK; Available online: https://www.globaldata.com/store/report/ulcerative-colitis-epidemiology-analysis/ (accessed on 7 September 2025).
- Clinical Trials Arena. (6 December 2023). Exploring Prevalence Patterns of CD and UC in the US, Canada, 5EU, and Japan. Clinical Trials Arena. Available online: https://www.clinicaltrialsarena.com/analyst-comment/exploring-prevalence-patterns-of-cd-and-uc-in-the-us-canada-5eu-and-japan/#:~:text=According%20to%20GlobalData’s%20epidemiology%20forecast,to%201.7%20million%20cases%20by (accessed on 7 September 2025).
- Statista. (14 May 2025). Projected IBD Population in Select Countries in NA and Europe in 2010, 2020, and 2030. Available online: https://www.statista.com/statistics/1206510/projected-ibd-population-in-select-countries-north-america-europe/ (accessed on 7 September 2025).
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, CGast-S12731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scientific Image and Illustration Software|BioRender. (n.d.) Available online: https://www.biorender.com/ (accessed on 11 June 2025).
- Bastón-Rey, I.; Calviño-Suarez, C.; Acosta, M.B. Should we investigate respiratory diseases in inflammatory bowel disease patients? Arch. Bronconeumol. 2020, 56, 481–482. [Google Scholar] [CrossRef]
- Cavalli, C.A.M.; Gabbiadini, R.; Dal Buono, A.; Quadarella, A.; De Marco, A.; Repici, A.; Bezzio, C.; Simonetta, E.; Aliberti, S.; Armuzzi, A. Lung Involvement in Inflammatory Bowel Diseases: Shared Pathways and Unwanted Connections. J. Clin. Med. 2023, 12, 6419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papanikolaou, I.; Kagouridis, K.; Papiris, S.A. Patterns of airway involvement in inflammatory bowel diseases. World J. Gastrointest. Pathophysiol. 2014, 5, 560–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman, E.A.; Lynch, D.A.; Barr, R.G.; van Beek, E.J.; Parraga, G. IWPFI Investigators. Pulmonary CT and MRI phenotypes that help explain chronic pulmonary obstruction disease pathophysiology and outcomes. J. Magn. Reson. Imaging 2016, 43, 544–557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caloian, A.-D.; Cristian, M.; Calin, E.; Pricop, A.-R.; Mociu, S.-I.; Seicaru, L.; Deacu, S.; Ciufu, N.; Suceveanu, A.-I.; Suceveanu, A.-P.; et al. Epigenetic Symphony in Diffuse Large B-Cell Lymphoma: Orchestrating the Tumor Microenvironment. Biomedicines 2025, 13, 853. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Margaritopoulos, G.A.; Tomassetti, S.; Bonella, F.; Costabel, U.; Poletti, V. Interstitial lung disease. Eur. Respir. Rev. 2014, 23, 40–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nonspecific Interstitial Pneumonia (NSIP). Cleveland Clinic. 1 May 2024. Available online: https://my.clevelandclinic.org/health/diseases/14804-nonspecific-interstitial-pneumonia-nsip (accessed on 7 September 2025).
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammer, T.; Langholz, E. The epidemiology of inflammatory bowel disease: Balance between East and West? A narrative review. Dig. Med. Res. 2020, 3, 48. [Google Scholar] [CrossRef]
- Cukic, V.; Lovre, V.; Dragisic, D.; Ustamujic, A. Asthma and Chronic Obstructive Pulmonary Disease (COPD)—Differences and Similarities. Mater. Sociomed. 2012, 24, 100–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, J.M.; Liu, Z.Q.; Eugene, C.Y. Overexpression of pulmonary surfactant protein A like molecules in inflammatory bowel disease tissues. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008, 33, 979–986. [Google Scholar] [PubMed]
- Liu, Y.; Wang, X.Y.; Yang, X.; Jing, S.; Zhu, L.; Gao, S.H. Lung and intestine: A specific link in an ulcerative colitis rat model. Gastroenterol. Res. Pract. 2013, 2013, 124530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kia’i, N.; Bajaj, T. Histology, Respiratory Epithelium. [Updated 1 May 2023]; In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541061/ (accessed on 7 September 2025).
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Canani, R.B.; Caminati, M.; Carucci, L.; Eguiluz-Gracia, I. Skin, gut, and lung barrier: Physiological interface and target of intervention for preventing and treating allergic diseases. Allergy 2024, 79, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 September 2025).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics. (n.d.) Available online: https://www.ibm.com/products/spss-statistics (accessed on 17 November 2024).
- Jussila, A.; Virta, L.J.; Pukkala, E.; Färkkilä, M.A. Mortality and causes of death in patients with inflammatory bowel disease: A nationwide register study in Finland. J. Crohns Colitis 2014, 8, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, R.; Walsh, G.; Flower, C.D.; Shneerson, J.M. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur. Respir. J. 2000, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Tremaine, W.J.; Melton, L.J., 3rd; Munkholm, P.; Sandborn, W.J. Survival and cause specific mortality in patients with inflammatory bowel disease: A long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut 2006, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parry, S.D.; Barbatzas, C.; Peel, E.T.; Barton, J.R. Sulphasalazine and lung toxicity. Eur. Respir. J. 2002, 19, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Hussein, A.A.; Mohamed, N.A.; Ibrahim, M.E. Changes in pulmonary function in patients with ulcerative colitis. Respir. Med. 2007, 101, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: A population-based study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.G.; Frizelle, F.A.; Thornley, P.T.; Beckert, L.; Epton, M.; Lynch, A.C. Inflammatory bowel disease and the lung: Is there a link between surgery and bronchiectasis? Int. J. Color. Dis. 2006, 21, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Yilmaz Demirci, N.; Hoşgün, D.; Uner, E.; Erdoğan, Y.; Gökçek, A.; Cağlar, A. Pulmonary involvement in inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 4952–4957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendoza, J.L.; Lana, R.; Taxonera, C.; Alba, C.; Izquierdo, S.; Díaz-Rubio, M. Manifestaciones extraintestinales en la enfermedad inflamatoria intestinal: Diferencias entre la enfermedad de Crohn y la colitis ulcerosa. Med. Clínica 2005, 125, 297–300. [Google Scholar] [CrossRef]
- Kraft, S.C.; Earle, R.H.; Roesler, M.; Esterly, J.R. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch. Intern. Med. 1976, 136, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.A.; Birring, S.S.; Green, R.; Grant, A.; de Caestecker, J.; Pavord, I.D. Prevalence of inflammatory bowel disease in patients with airways disease. Respir. Med. 2008, 102, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, K.R.; Noftz, M.K.; Dalhoff, K.; Ludwig, D.; Stange, E.F.; Fellermann, K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am. J. Gastroenterol. 2002, 97, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Black, H.; Mendoza, M.; Murin, S. Thoracic manifestations of inflammatory bowel disease. Chest 2007, 131, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Patil, S.; Udwadia, Z.; Maheshwari, S.; Abraham, P.; Joshi, A. Pulmonary manifestations in inflammatory bowel disease: A prospective study. Indian J. Gastroenterol. 2011, 30, 225–228. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.P.; Engels, L.G.; Moers, A.M. Pneumonitis induced by sulphasalazine. Postgrad. Med. J. 1997, 73, 99–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chew, M.T.; Chak, E.; Matsukuma, K. A Rare Cause of Pulmonary Nodules. Case Rep. Gastroenterol. 2016, 10, 633–639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freeman, H.J.; Davis, J.E.; Prest, M.E.; Lawson, E.J. Granulomatous bronchiolitis with necrobiotic pulmonary nodules in Crohn’s disease. Can. J. Gastroenterol. 2004, 18, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Garg, C.; Shrimanker, I.; Goel, S.; Mclaughlin, J.; Nookala, V. Extraintestinal Manifestations of Crohn’s Disease in the Form of Pulmonary Nodules: A Case Report. Cureus 2020, 12, e7161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golpe, R.; Mateos, A.; Pérez-Valcárcel, J.; Lapeña, J.A.; García-Figueiras, R.; Blanco, J. Multiple pulmonary nodules in a patient with Crohn’s disease. Respiration 2003, 70, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Deshwal, H.; Haraf, R.; Raju, S.; Saeedan, M.B.; Sarkar, P.; Gildea, T.; Farver, C.; Mehta, A.C. Pulmonary manifestations of inflammatory bowel disease and treatment strategies. CHEST Pulm. 2023, 1, 100018. [Google Scholar] [CrossRef]
- Haenen, S.; Verstockt, B.; Ferrante, M.; Vermeire, S.; Sabino, J. A curious presentation of Crohn’s disease with pulmonary involvement: A case report. Acta Gastroenterol. Belg. 2023, 86, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Abu Shtaya, A.; Cohen, S.; Kogan, Y.; Shteinberg, M.; Sagool, O. Crohn’s Disease with Atypical Extra-Intestinal Manifestations Developing Under Treatment with Vedolizumab. Eur. J. Case Rep. Intern. Med. 2021, 8, 002265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warwick, G.; Leecy, T.; Silverstone, E.; Rainer, S.; Feller, R.; Yates, D.H. Pulmonary necrobiotic nodules: A rare extraintestinal manifestation of Crohn’s disease. Eur. Respir. Rev. 2009, 18, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Faller, M.; Gasser, B.; Massard, G.; Pauli, G.; Quoix, E. Pulmonary migratory infiltrates and pachypleuritis in a patient with Crohn’s disease. Respiration 2000, 67, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Hotermans, G.; Benard, A.; Guenanen, H.; Demarcq-Delerue, G.; Malart, T.; Wallaert, B. Nongranulomatous interstitial lung disease in Crohn’s disease. Eur. Respir. J. 1996, 9, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Athayde, R.A.B.; Costa, F.M.D.; Nascimento, E.C.T.D.; Sales, R.K.B.; Costa, A.N. Pulmonary involvement in Crohn’s disease. J. Bras. Pneumol. 2018, 44, 519–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Q.; Hu, D.; Li, Y.; Zhou, Z.; Wu, J.; Li, X.; Yu, X. Evaluating the causal association between bronchiectasis and different types of inflammatory bowel disease: A two-sample Mendelian randomization study. Front. Immunol. 2024, 15, 1365108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.S.; Yang, B.; Shin, H.S.; Lee, H.; Chai, H.G.; Choi, H.; Han, J.; Yoon, J.H.; Kim, E.; Lee, H. Increased bronchiectasis risk and related risk factors in inflammatory bowel disease: A 10-year Korean national cohort study. ERJ Open Res. 2024, 10, 00087-2024. [Google Scholar] [CrossRef]
- Andronache, I.T.; Şuţa, V.C.; Şuţa, M.; Ciocodei, S.L.; Vladareanu, L.; Nicoara, A.D.; Arghir, O.C. Better Safe than Sorry: Rheumatoid Arthritis, Interstitial Lung Disease, and Medication-A Narrative Review. Biomedicines 2023, 11, 1755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranasinghe, I.R.; Tian, C.; Hsu, R. Crohn Disease. [Updated 24 February 2024]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK436021/ (accessed on 7 September 2025).
- Belu, A.M.; Nicoara, A.D.; Belu, D.M.; Circo, E. Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis. Medicina 2024, 60, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Q.; Wang, L.X.; Lu, D.G. Pulmonary manifestations of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 13501–13511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


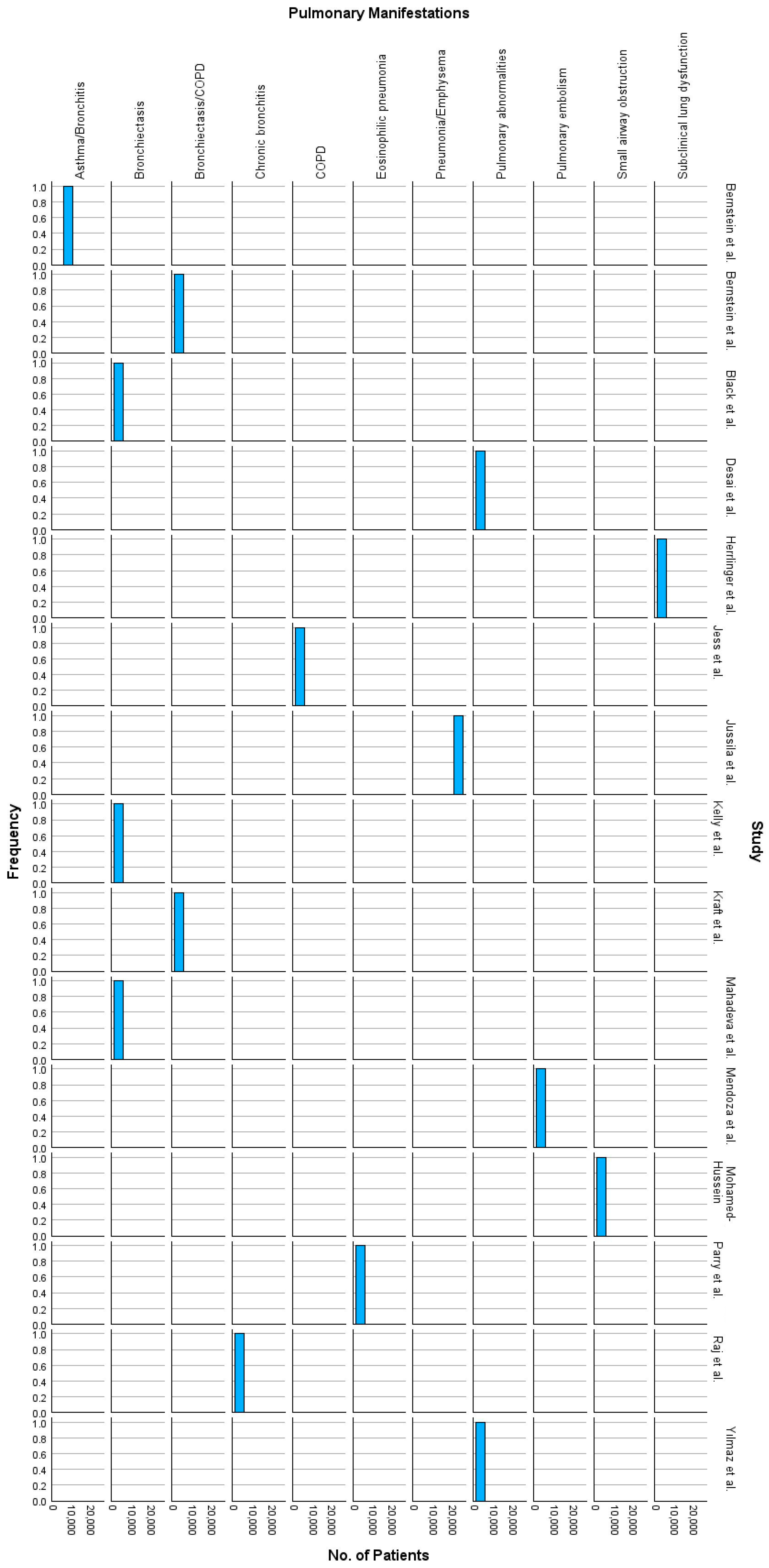

| Study | Patients (n) | Age (Years) | Male (%) | Other Population Characteristics | Smoking (n) | Pulmonary Involvement | Key Findings | Deaths (n) | Conclusion | Years from Onset |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||||||
| Jussila et al. (2014) [27] | 21,964 | 40 | 48% | Nationwide Finnish IBD cohort; 16,649 UC, 5315 CD | Not reported | pulmonary embolism, pneumonia, bronchitis, emphysema | SMR * for respiratory diseases: 2.01 in CD, 1.24 in UC; pneumonia & COPD * common | 2244 | Chronic inflammation contributes to elevated mortality in IBD | 1–25 |
| Mahadeva et al. (1999) [28] | 17 | 38–83 | 58.8% | 14 UC, 3 CD patients | 7 ex-smokers, 3 current smokers, 7 never smoked | 13 patients with bronchiectasis (76%), 9 patients with air trapping, 5 with “tree in bud” pattern, peripheral reticular changes | Pulmonary abnormalities responsive to steroids; concurrent UC and lung exacerbations in some cases | Not reported | Pulmonary involvement in IBD is varied and steroid-responsive in many cases | 1–25 |
| Jess et al. (2006) [29] | 692 | 33.3 | 53.03% | 314 CD, 378 UC; Olmsted County population-based cohort | Smoking prevalent in CD | Increased risk of COPD in CD | CD mortality from GI disease and COPD; UC mortality lower than general population | 56 (CD), 62 (UC) | Long-term outcomes stable; smoking cessation crucial in CD to reduce COPD risks | 14 |
| Parry et al. (2002) [30] | 50 | 48.3 | 66% | 72% UC, 6% CD, various arthritis cases | Data not provided | Eosinophilic pneumonia, interstitial fibrosis | Pulmonary toxicity from sulphasalazine, symptoms improved post drug withdrawal in most cases | 5 | Pulmonary toxicity rare, resolves post drug withdrawal, corticosteroids debatable in treatment | 0.5–10 |
| Mohamed-Hussein et al. (1996) [31] | 26 | 39.5 | 65% | 20 active UC, 6 inactive UC patients | 3 smokers (11.5%) | Small airway obstruction, restrictive and obstructive dysfunction | Pulmonary dysfunction common, more pronounced in active UC, no smoking impact on PFTs | 0 | Early recognition of lung issues is key; PFTs improve with treatment | 1–3 |
| Bernstein et al. (2005) [32] | 8072 | 42.5 | 49% | Manitoba IBD cohort: 3879 UC, 4193 CD | Smoking more prevalent in CD patients | Asthma and bronchitis common as comorbidities | Increased prevalence of asthma and bronchitis among IBD patients compared to controls | Not reported | Pulmonary conditions often underdiagnosed in IBD patients | Not specified |
| Kelly et al. (2006) [33] | 10 | 56 | 5 UC, 5 CD; post-surgical patients | 3 smokers, 4 ex-smokers | Bronchiectasis, small airways disease | Surgery for IBD associated with respiratory symptoms, particularly bronchiectasis | 0 | Pulmonary symptoms often develop post-IBD surgery | 9–35 | |
| Yılmaz et al. (2010) [34] | 39 | 44.3 | 59% | 30 UC, 9 CD, Turkey cohort | 4 smokers, 5 ex-smokers | Peribronchial thickening, bronchiectasis, emphysema | 64.1% had HRCT abnormalities; pulmonary symptoms correlated with active bowel disease | 0 | Early detection and management of respiratory symptoms in IBD is crucial | 0–9 |
| Mendoza et al. (2005) [35] | 566 | 34.5 | 53.5% | Spanish cohort: 295 CD, 271 UC | 12% smokers overall | Rare but documented in both CD and UC | Pulmonary embolism more common in UC; extraintestinal manifestations frequent | Not reported | Comprehensive monitoring needed for systemic complications in IBD | 2–32 |
| Kraft et al. (1976) [36] | 6 | 45–59 | 50% | 5 UC, 1 CD, severe cases | 4 non-smokers, 2 ex-smokers | Chronic bronchitis, bronchiectasis, COPD | Pulmonary symptoms persisted post-colectomy in 2 cases; bronchiectasis linked to IBD | 2 | Pulmonary diseases are a systemic complication in IBD | 3–13 |
| Raj et al. (2008) [37] | 37 | 61 | 45% | Airways disease patients with IBD (22 UC, 13 CD) | 5 current smokers, 16 ex-smokers | Chronic bronchitis, bronchiectasis | IBD prevalence higher in airways disease; non-asthmatic airway disease common in IBD | Not reported | Airways disease frequently coexists with IBD, especially UC | 15–16 |
| Herrlinger et al. (2002) [38] | 66 | 36 | 47% | 35 CD, 31 UC; Germany cohort | 15% smokers overall | Reduced FEV1 *, IVC *, DLCO * in 42% | Pulmonary dysfunction linked to disease activity, persists in remission | Not reported | Subclinical lung dysfunction is a frequent, persistent extraintestinal IBD manifestation | 1–29 |
| Black et al. (2007) [39] | 155 | 45 | 40% | Cases from 55 English-language reports; UC predominates | 19% current smokers | Bronchiectasis most common, BOOP, airway hyperresponsiveness | Pulmonary involvement spans airways to pleura; varied | Not reported | Screening studies reveal high prevalence of respiratory issues in IBD | 2–20 |
| Desai et al. (2011) [40] | 95 | 41.9 | 50.5% | 83 UC, 12 CD; Mumbai cohort | Smoking prevalence low | Bronchiectasis, nodules, emphysema | 28.5% had PFT abnormalities; HRCT detected pulmonary changes in 22% | 0 | Routine HRCT for IBD patients may detect latent pulmonary disease | 1–30 |
| Bernstein et al. (2001) [41] | 4454 | 40 | 48% | Manitoba IBD Database (population-based cohort) | Not reported | COPD, bronchiectasis | Pulmonary conditions more common in IBD patients than in general population | Not reported | Early recognition of extraintestinal manifestations critical in IBD management | 1–20 |
| Case reports | ||||||||||
| Peters et al. (1997) [42] | 1 | 65 | N/A | Patient with rheumatoid arthritis, treated with sulphasalazine | Non-smoker | Eosinophilic pneumonitis | Symptoms (dyspnoea, fever) resolved after sulphasalazine withdrawal | 0 | Sulphasalazine-induced pulmonary toxicity is rare and reversible upon drug cessation | <1 |
| Chew et al. (2016) [43] | 1 | 44 | N/A | Female with Crohn’s Disease | Non-smoker | Pulmonary nodules, necrotizing granulomas | Pulmonary symptoms led to Crohn’s diagnosis; lung disease as EIM * of IBD | 0 | Pulmonary EIM can precede gastrointestinal symptoms in Crohn’s disease | <1 |
| Freeman et al. (2004) [44] | 1 | 37 | N/A | Extensive Crohn’s (stomach, small, large intestine) | Non-smoker | Granulomatous bronchiolitis, necrobiotic nodules | Pulmonary involvement resolved with steroids; necrotizing nodules linked to Crohn’s | 0 | Pulmonary manifestations are rare but manageable with immunosuppression | ~10 |
| Garg et al. (2020) [45] | 1 | 41 | N/A | Female with Crohn’s, post-colectomy | Non-smoker | Ground-glass opacities, pulmonary nodules | Pulmonary symptoms resolved with steroids; nodules linked to Crohn’s as EIM | 0 | Pulmonary involvement in Crohn’s should be considered in unexplained lung diseases | 9 |
| Golpe et al. (2003) [46] | 1 | 68 | N/A | Female with long-standing Crohn’s Disease | Non-smoker | Multiple pulmonary nodules, Nongranulomatous lymphoid infiltration | Pulmonary symptoms resolved with steroids; nodules linked to CD | 0 | Pulmonary nodules in Crohn’s patients may mimic malignancy; respond to steroids | 14 |
| Ghosh et al. (2023) [47] | 1 | 73 | N/A | Female with GERD * and liver haemangiomas; first pulmonary sign of Crohn’s | Non-smoker | Pulmonary nodules, granulomatous inflammation | Pulmonary nodules resolved with infliximab; EIMs preceded GI symptoms | 0 | Pulmonary involvement can precede GI symptoms in CD; thorough work-up is crucial | <1 |
| Haenen et al. (2023) [48] | 1 | 30 | N/A | UC, recent flare, history of immunosuppressive therapy | Non-smoker | Cavitary necrobiotic pulmonary nodules | Nodules resolved with steroids and ustekinumab; lung symptoms mirrored UC flare | 0 | Pulmonary manifestations in UC can mimic malignancy, need biopsy for diagnosis | ~0.5 |
| Shtaya et al. (2021) [49] | 1 | 31 | N/A | Crohn’s disease under vedolizumab therapy | Non-smoker | Pulmonary granulomas, pleural effusion | Lung findings resolved with infliximab; granulomas linked to Crohn’s as EIM | 0 | Vedolizumab may unmask pulmonary EIM; infliximab effectively treats pulmonary symptoms | 13 |
| Warwick et al. (2009) [50] | 1 | 22 | N/A | Female with Crohn’s disease and anterior uveitis | Non-smoker | Necrobiotic pulmonary nodules | Nodules responded to systemic steroids; rare pulmonary manifestation of Crohn’s | 0 | Necrobiotic nodules are rare EIMs of Crohn’s and respond well to steroids | Newly diagnosed |
| Faller et al. (2000) [51] | 1 | 38 | N/A | Female with Crohn’s disease and eosinophilia | Non-smoker | Migratory infiltrates, pachypleuritis, necrotizing nodules | Pulmonary symptoms resolved with corticosteroids; associated with Crohn’s disease or mesalazine use | 0 | Pulmonary manifestations in Crohn’s can mimic infections; steroids are effective | 3 |
| Hotermans et al. (1996) [52] | 1 | 33 | N/A | Female with Crohn’s disease on mesalazine | Non-smoker | Nongranulomatous interstitial lung disease | Significant improvement with corticosteroids and cyclophosphamide; worsened without steroids | 0 | ILD in Crohn’s can mimic IPF *; immunosuppression effective | 3 |
| Athayde et al. (2018) [53] | 1 | 34 | N/A | Male with severe intestinal Crohn’s Disease, treated with infliximab | Non-smoker | Organizing pneumonia with granulomatous inflammation | Symptoms resolved with corticosteroids and immunomodulation with azathioprine and infliximab | 0 | Pulmonary manifestations are rare but treatable with steroids and immunosuppressants | 2 |
| Pulmonary Manifestation | Crohn’s Disease | Ulcerative Colitis |
|---|---|---|
| Bronchiectasis | 76% of patients [28] | 13% of patients [28] |
| Post-surgical CD patients [33] | Post-surgical UC patients (Kelly et al. [33]) | |
| 64.1% showed HRCT abnormalities [34] | Detected in 28.5% via HRCT [40] | |
| Detected in 22% via HRCT [40] | Reported cases in IBD cohort [41] | |
| Pulmonary conditions more common in CD [41] | ||
| COPD | Increased risk of COPD [29] | Less prevalent than CD, but reported [29] |
| Exacerbated by smoking [39] | ||
| Interstitial lung disease | Eosinophilic pneumonia, fibrosis [30,31] | ILD less frequent [30] |
| Pulmonary embolism | More common in CD [27,35] | SMR 1.24 for respiratory diseases [27] |
| Asthma and Bronchitis | Frequently reported [27,32] | Common comorbidities [27,32] |
| Pneumonia, emphysema | SMR 2.01 [27] | SMR 1.24 [27] |
| Pulmonary Manifestation | Crohn’s Disease | Ulcerative Colitis |
|---|---|---|
| Pulmonary nodules | Necrobiotic nodules reported [44,46] | Pulmonary nodules preceding GI symptoms [43] |
| Granulomatous inflammation | Granulomatous bronchiolitis, necrobiotic granulomas (Freeman et al. [44,47,50] | Non-necrotizing granulomas in lung biopsy [48] |
| Organizing pneumonia | Organizing pneumonia with granulomatous inflammation [53] | Pulmonary organizing pneumonia linked to UC flare [48] |
| Eosinophilic pneumonitis | Interstitial pneumonitis, eosinophilic infiltrates [52] | Eosinophilic pneumonitis linked to sulfasalazine therapy [42] |
| Pleural effusion/thickening | Migratory infiltrates, pachypleuritis [51] | Bilateral pleural thickening linked to UC flare [42] |
| Bronchiectasis | Noted in some CD patients post-colectomy [45] | Not explicitly reported in case reports |
| Interstitial lung disease | Nongranulomatous ILD reported; improved with immunosuppression [52] | Rare ILD cases linked to UC [43] |
| Pulmonary embolism | None reported in case studies | Pulmonary embolism rare but recognized (generalized in studies, not specific case) |
| Pulmonary Manifestation | Code | Frequency in CD | Frequency in UC |
|---|---|---|---|
| Pulmonary nodules | 1 | 2 | 1 |
| Granulomatous inflammation | 2 | 3 | 1 |
| Organizing pneumonia | 3 | 1 | 1 |
| Eosinophilic pneumonitis | 4 | 1 | 1 |
| Pleural effusion/thickening | 5 | 1 | 1 |
| Bronchiectasis | 6 | 1 | 0 |
| Interstitial lung disease | 7 | 1 | 1 |
| Pulmonary embolism | 8 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preotesoiu, I.; Alexandrescu, L.; Cimpineanu, B.; Tofolean, I.T.; Stanciu, I.V.; Herlo, A.; Dumitru, E.; Alexandrescu, D.M.; Dina, E.; Aftenie, C.D.; et al. From Gut to Lungs: The Hidden Respiratory Impacts of IBD: A Systematic Review of the Literature. Int. J. Mol. Sci. 2025, 26, 8912. https://doi.org/10.3390/ijms26188912
Preotesoiu I, Alexandrescu L, Cimpineanu B, Tofolean IT, Stanciu IV, Herlo A, Dumitru E, Alexandrescu DM, Dina E, Aftenie CD, et al. From Gut to Lungs: The Hidden Respiratory Impacts of IBD: A Systematic Review of the Literature. International Journal of Molecular Sciences. 2025; 26(18):8912. https://doi.org/10.3390/ijms26188912
Chicago/Turabian StylePreotesoiu, Ionela, Luana Alexandrescu, Bogdan Cimpineanu, Ioan Tiberiu Tofolean, Ionut Valentin Stanciu, Alexandra Herlo, Eugen Dumitru, Daria Maria Alexandrescu, Elena Dina, Cristina Daniela Aftenie, and et al. 2025. "From Gut to Lungs: The Hidden Respiratory Impacts of IBD: A Systematic Review of the Literature" International Journal of Molecular Sciences 26, no. 18: 8912. https://doi.org/10.3390/ijms26188912
APA StylePreotesoiu, I., Alexandrescu, L., Cimpineanu, B., Tofolean, I. T., Stanciu, I. V., Herlo, A., Dumitru, E., Alexandrescu, D. M., Dina, E., Aftenie, C. D., Nelson Twakor, A., & Tofolean, D. E. (2025). From Gut to Lungs: The Hidden Respiratory Impacts of IBD: A Systematic Review of the Literature. International Journal of Molecular Sciences, 26(18), 8912. https://doi.org/10.3390/ijms26188912






