Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges
Abstract
1. Introduction
2. Results
2.1. Platinum-Based Complexes
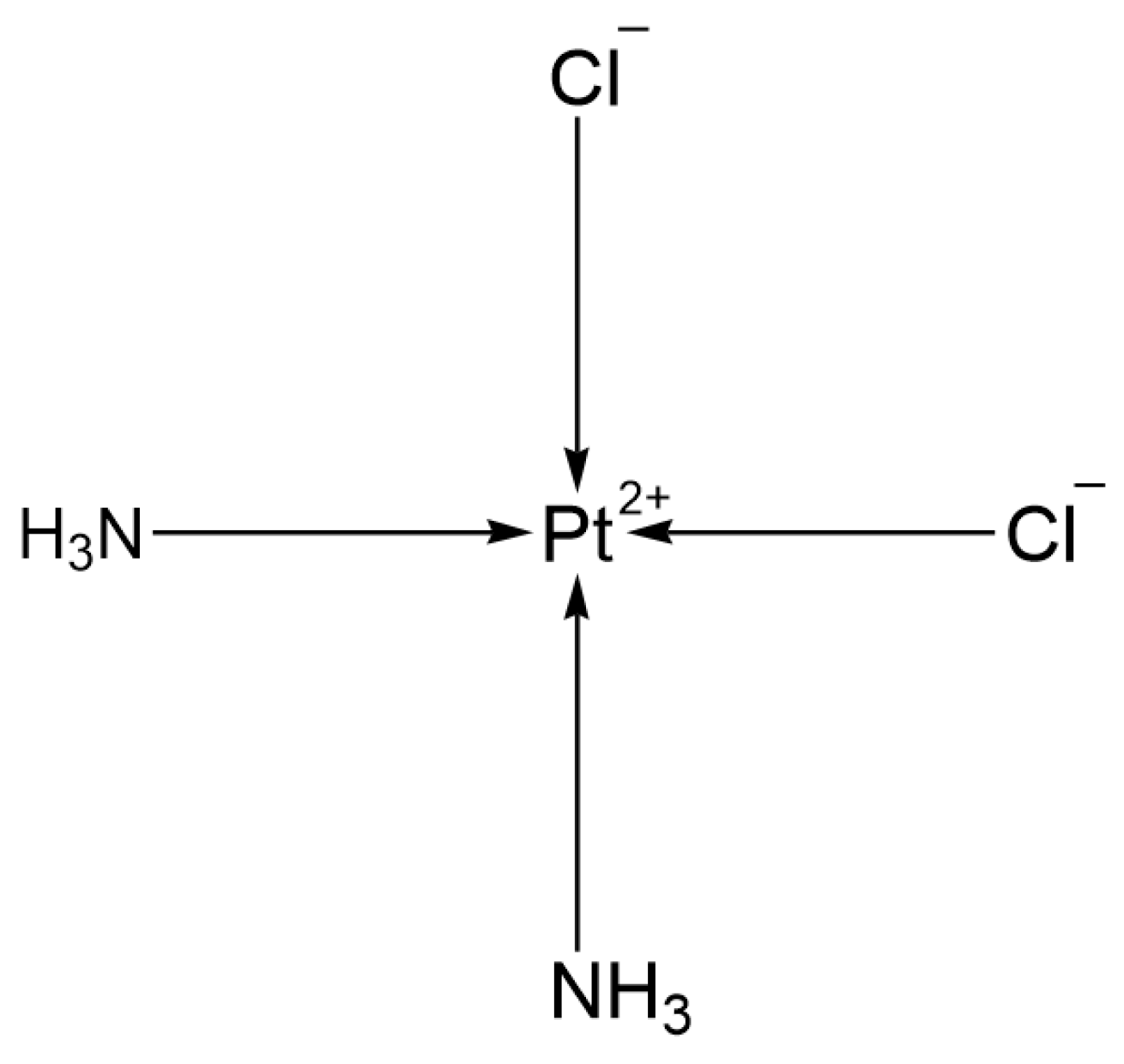
2.1.1. First Generation: Cisplatin
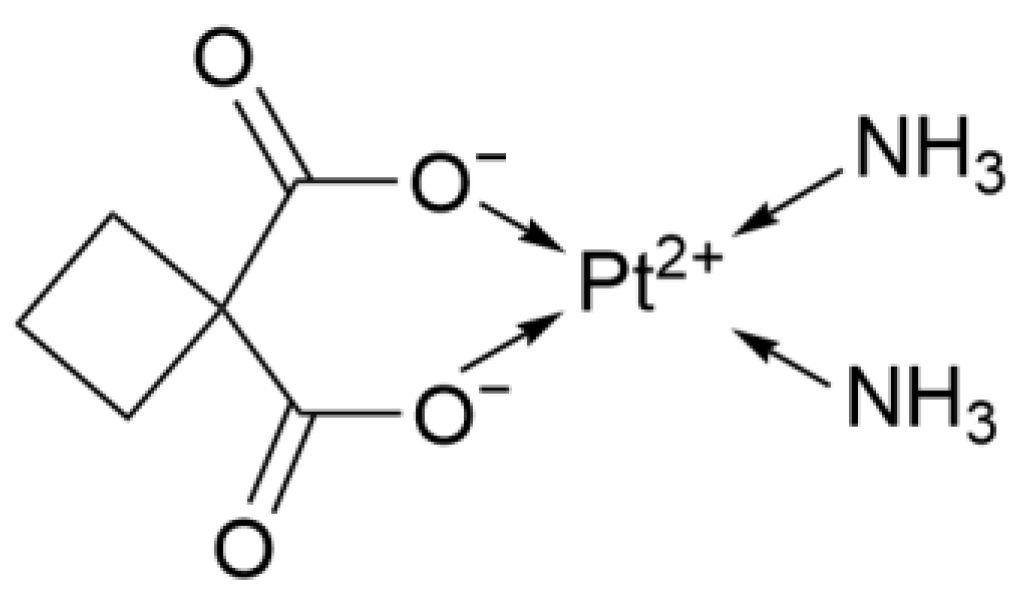
2.1.2. Second Generation: Carboplatin
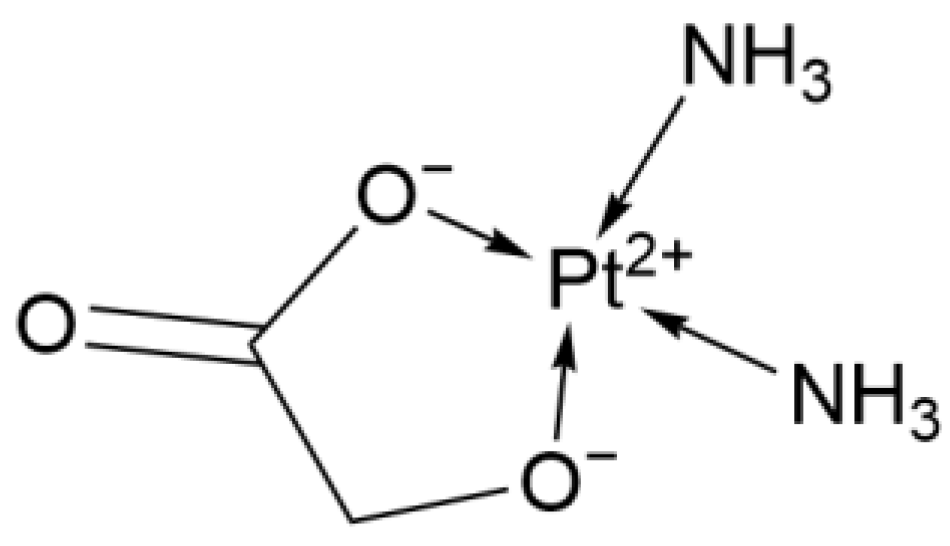
2.1.3. Second-Generation Derivative: Nedaplatin
2.1.4. Third Generation: Oxaliplatin
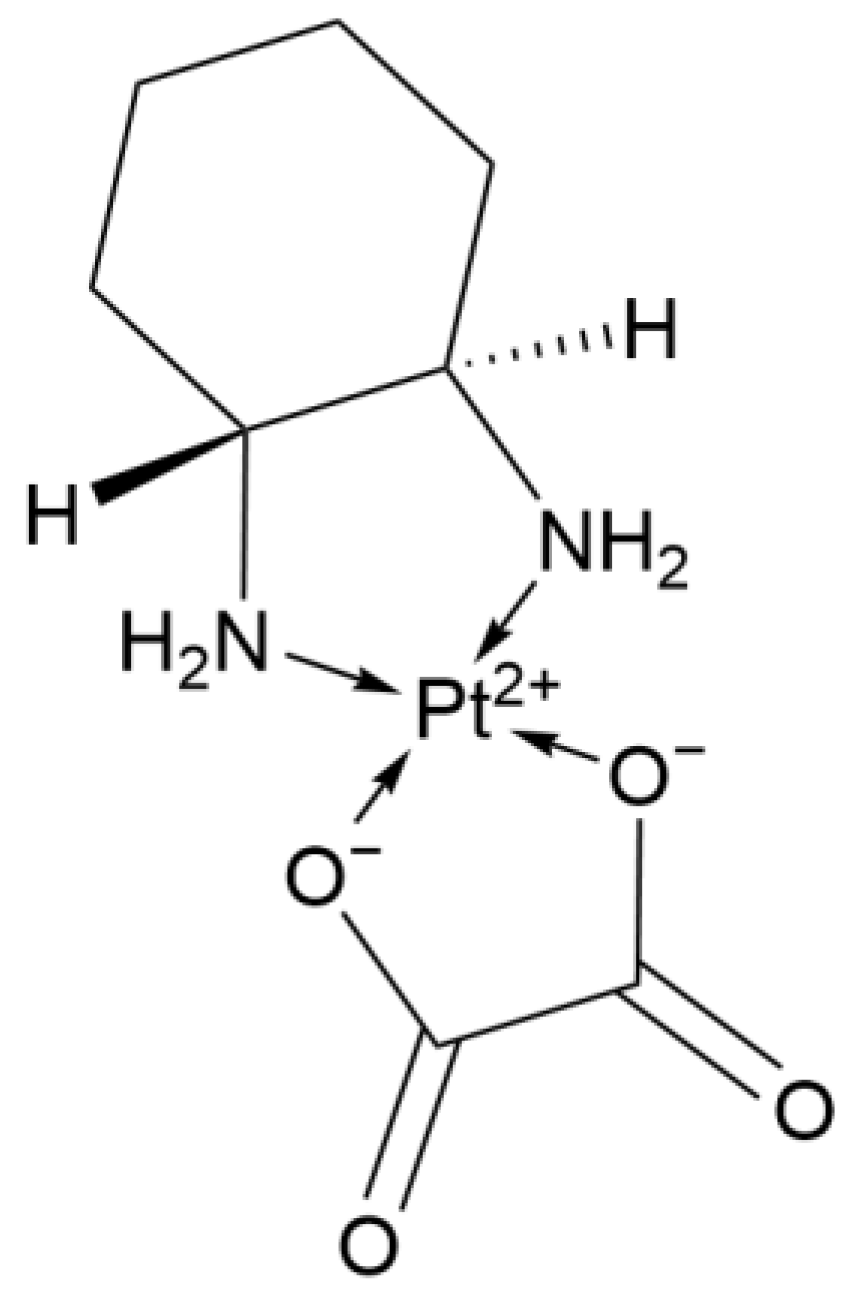
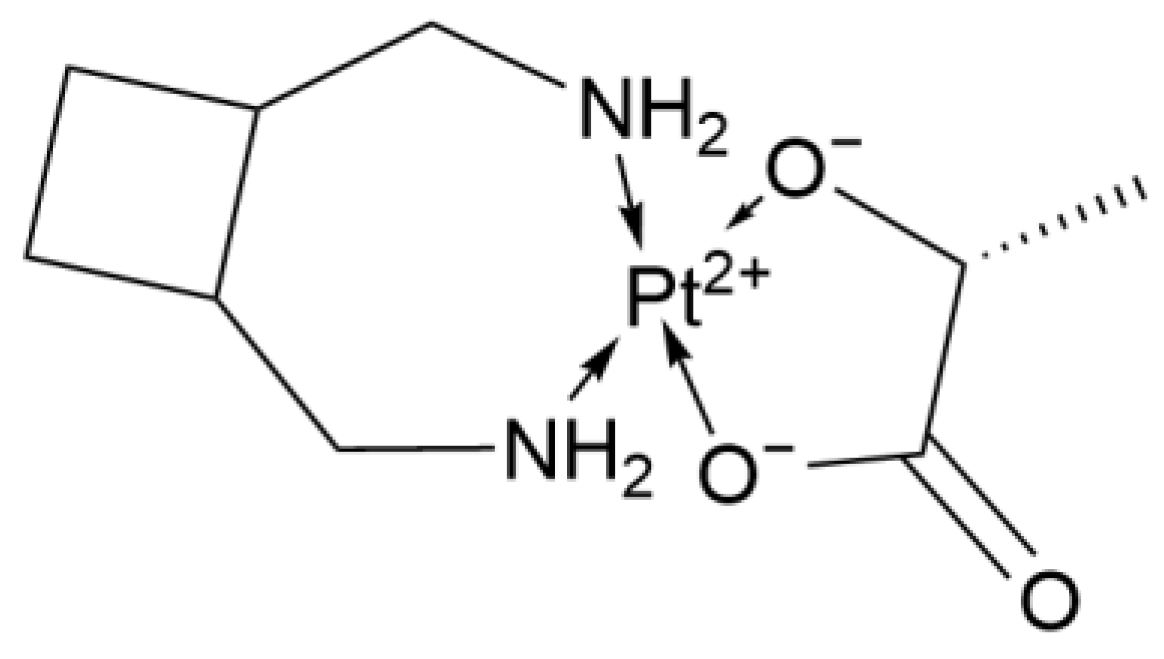
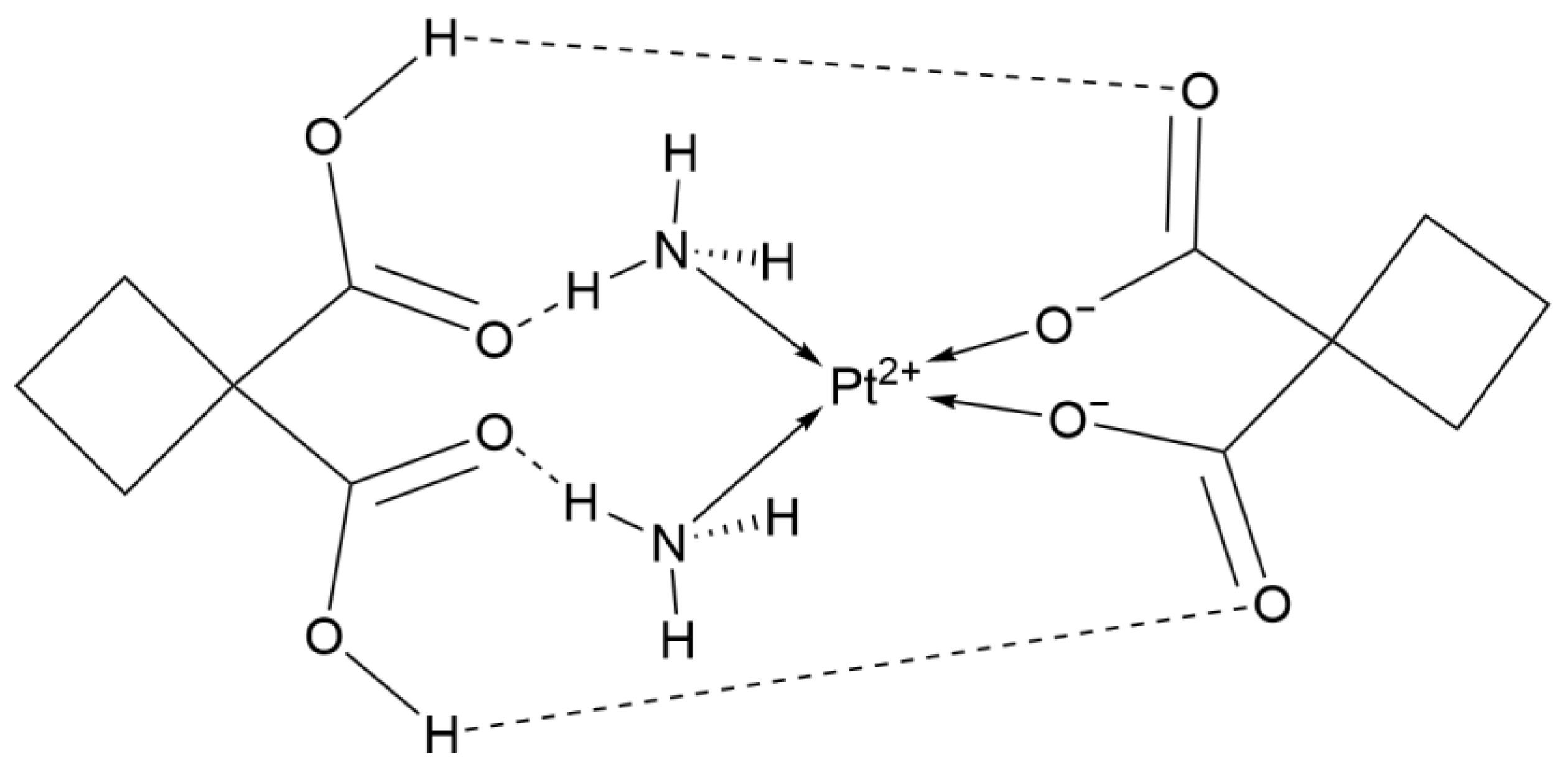
2.1.5. Third-Generation Derivative: Lobaplatin
2.2. Platinum-Based Complexes in the Clinical Trial Stage
2.2.1. Dicycloplatin
2.2.2. Satraplatin
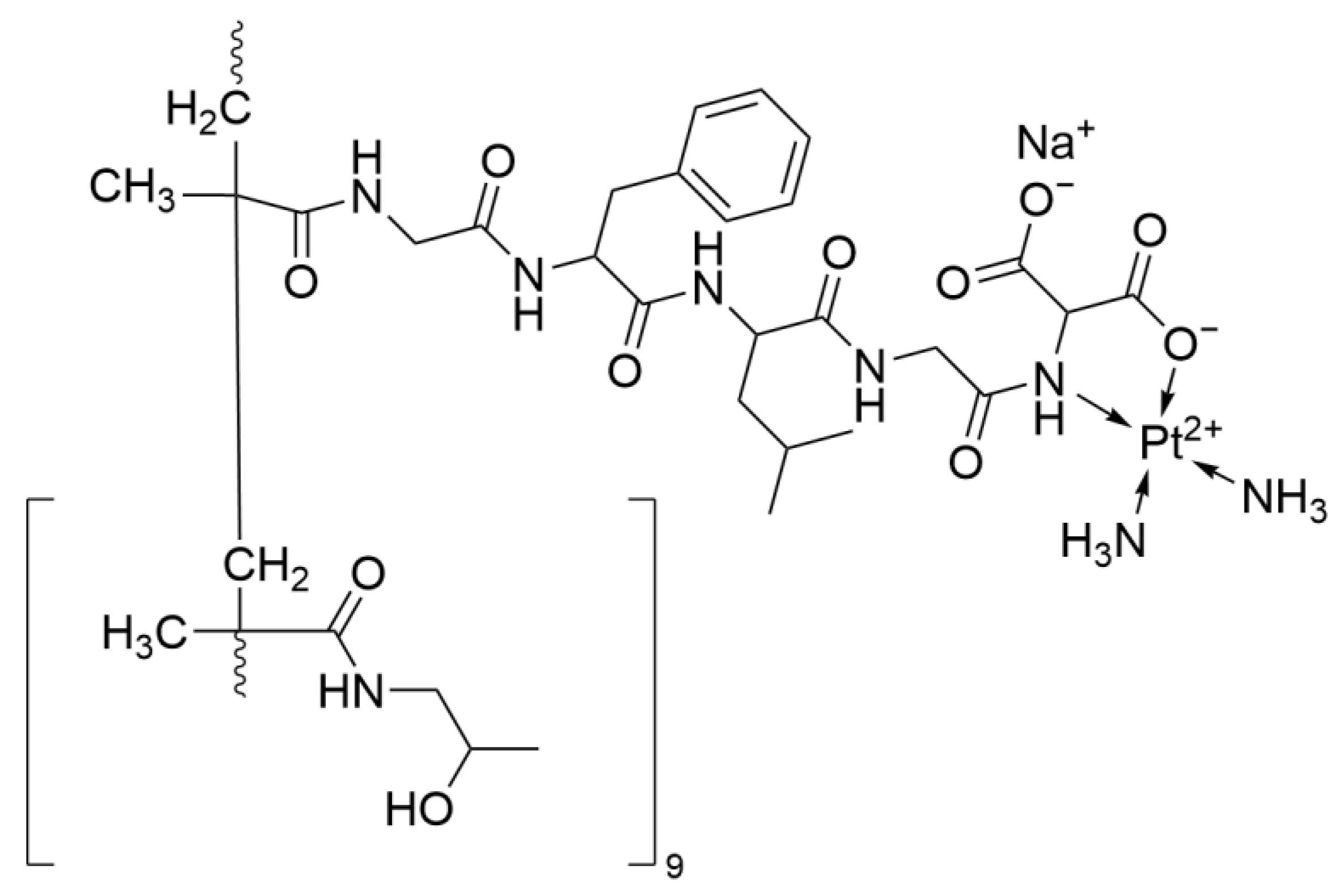
2.2.3. Lipoplatin
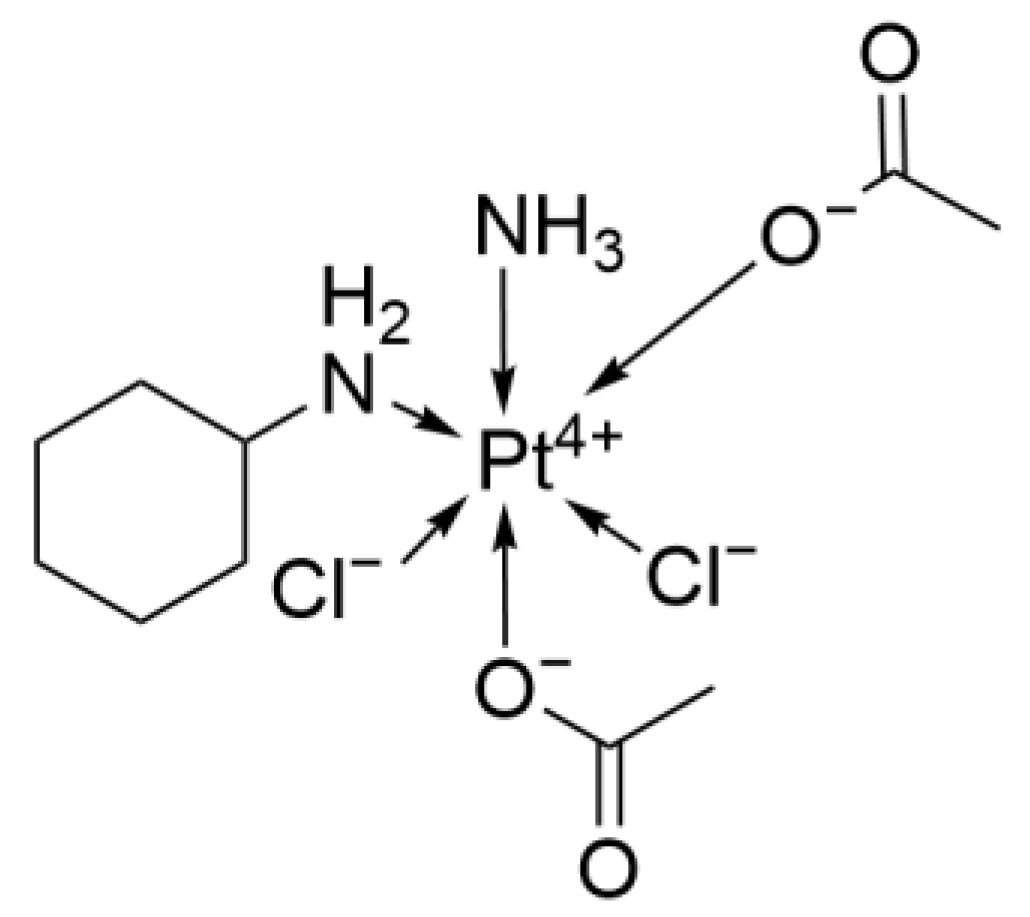
2.2.4. LA-12
2.2.5. Pt-Exo
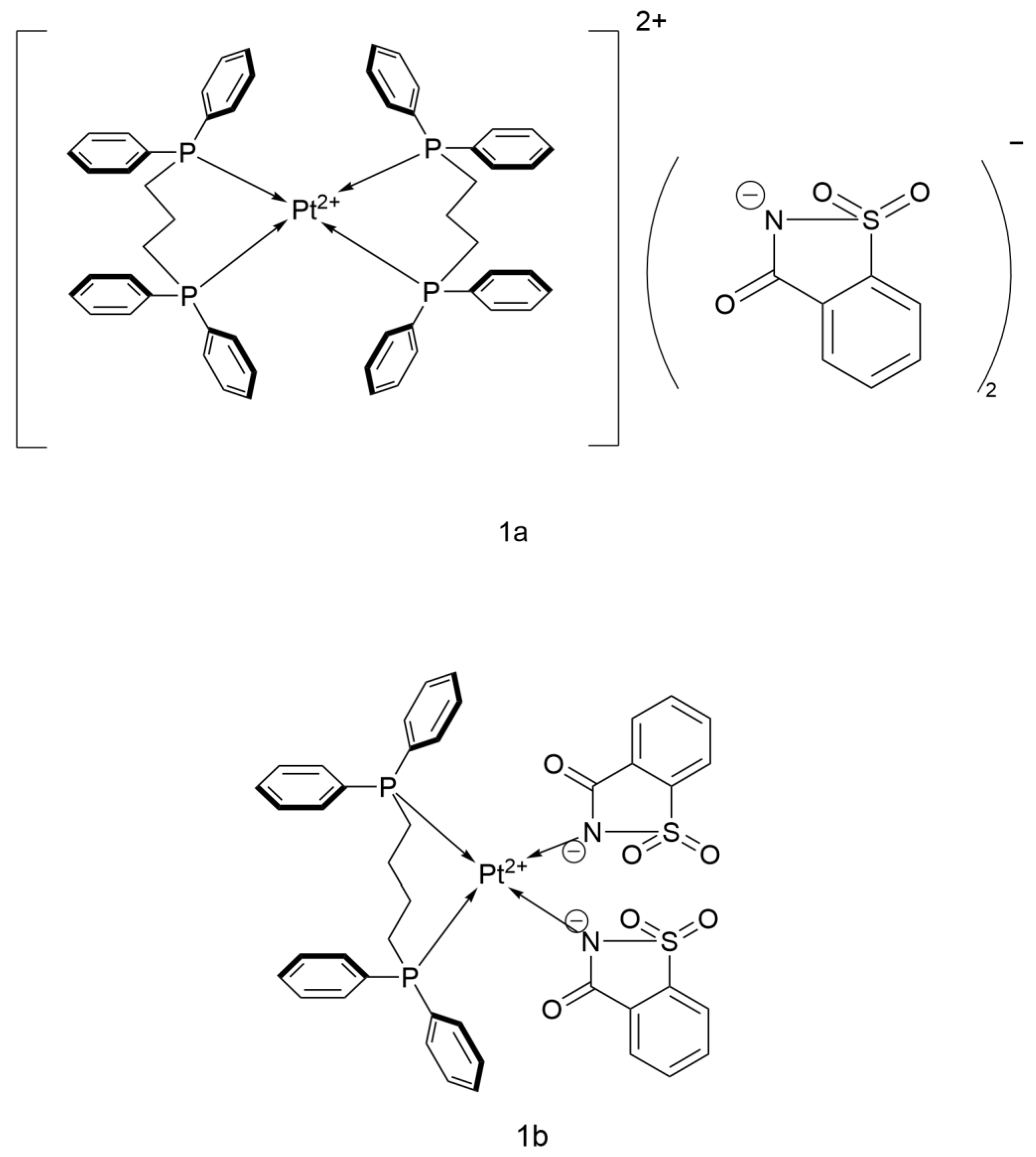
2.2.6. Pt(II) Saccharin Complex
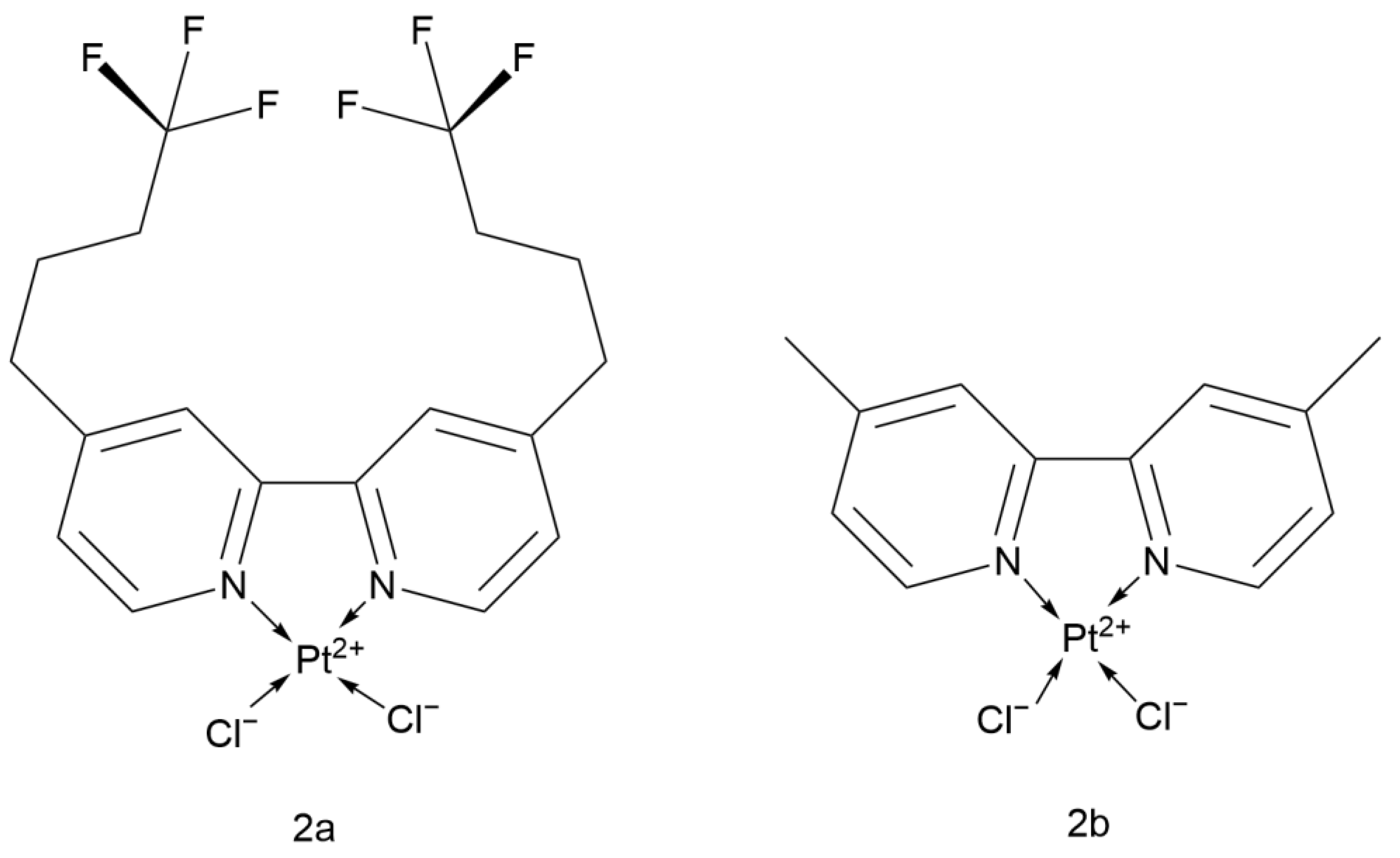
2.2.7. Fluorinated Bipyridine Cisplatin Analogs
2.2.8. Cisplatin Analog with 4,4′-Dialkoxy-2,2′-bipyridine
2.2.9. BBR 3464
2.2.10. Picoplatin
2.2.11. Aerosolized SLIT Cisplatin
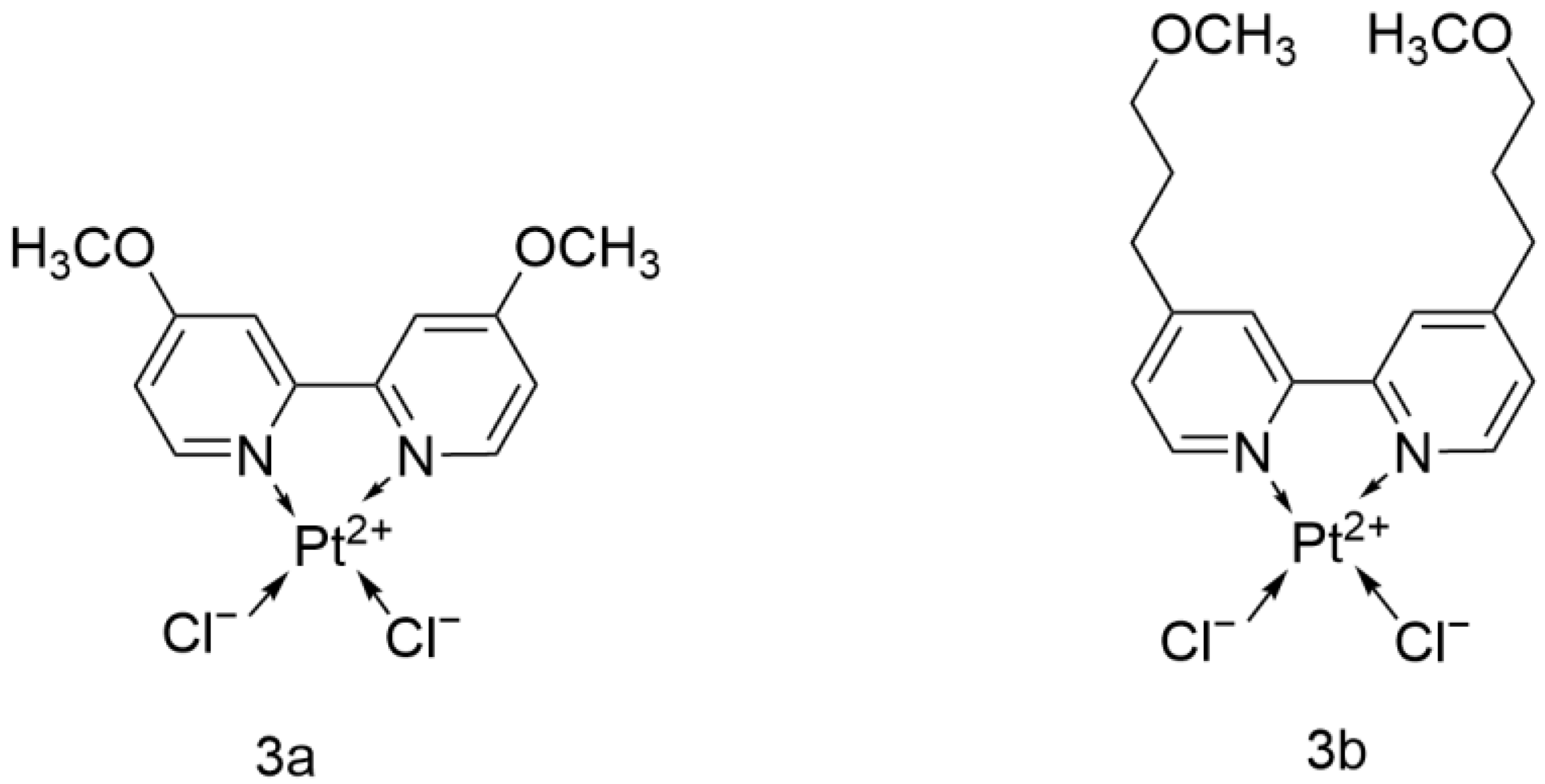
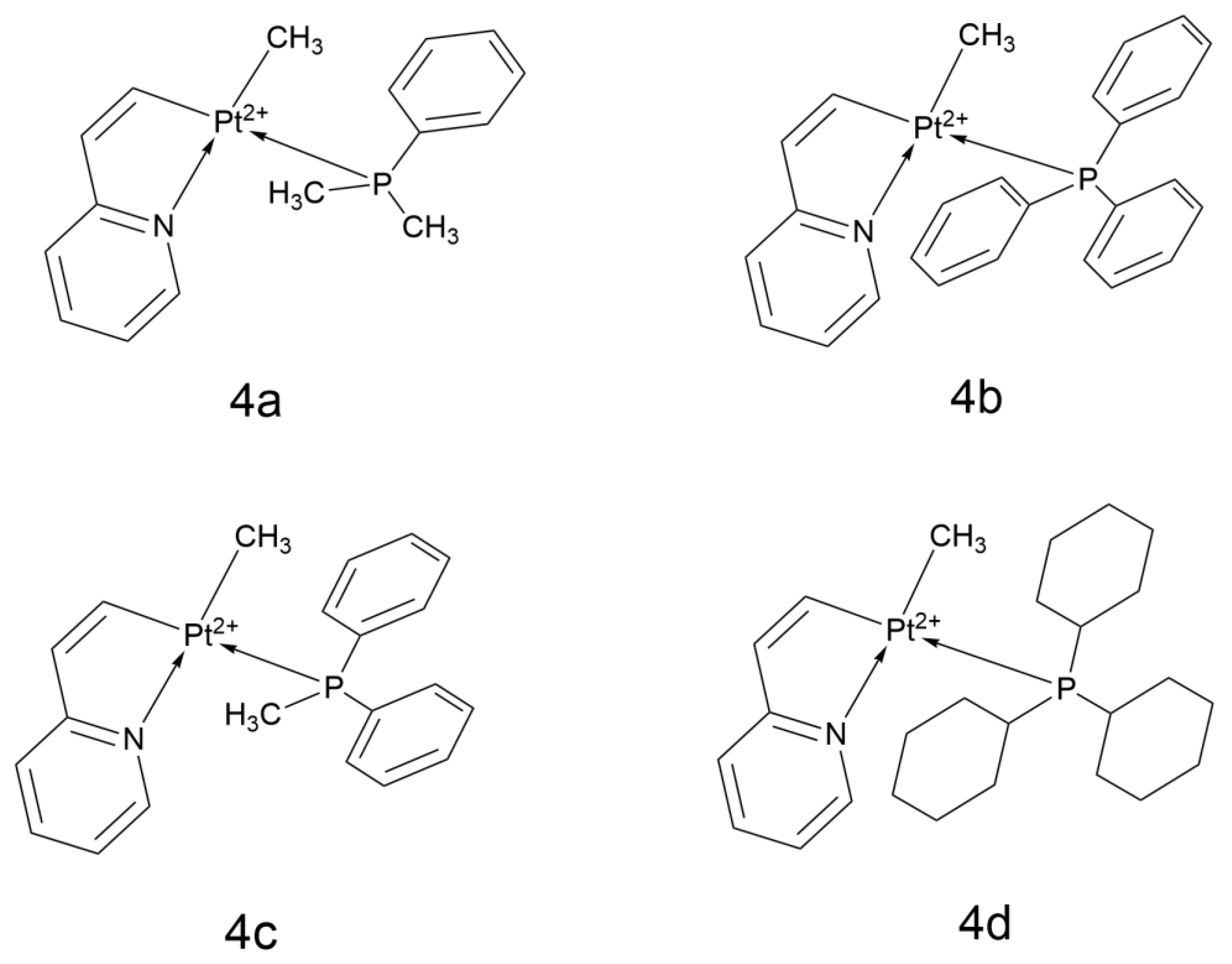
2.2.12. Platinum(II) Cyclometalated Complexes Containing 2-Vinylpyridine
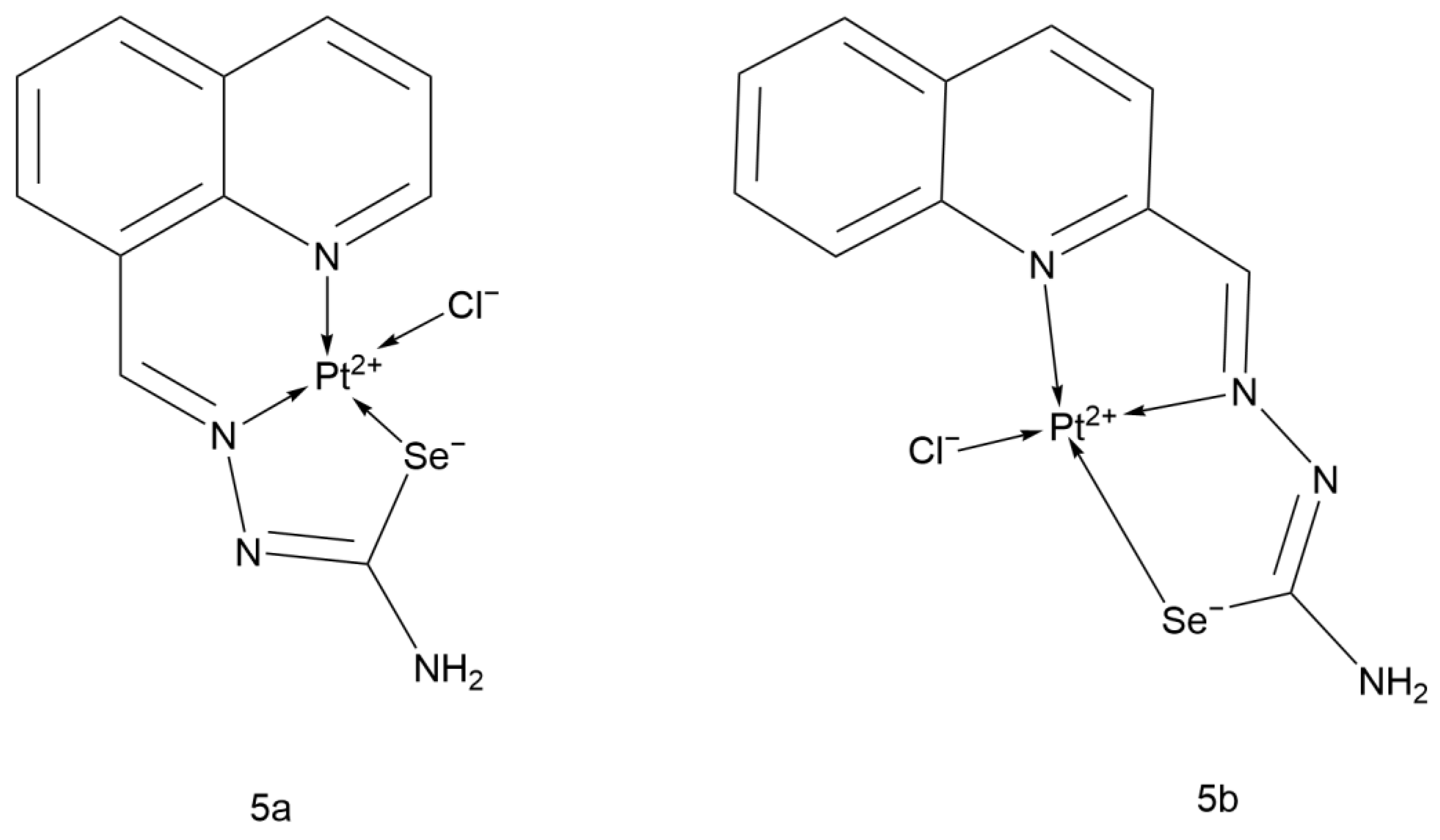
2.2.13. Quinolinecarboxaldehyde Selenosemicarbazone Pt(II) Complex
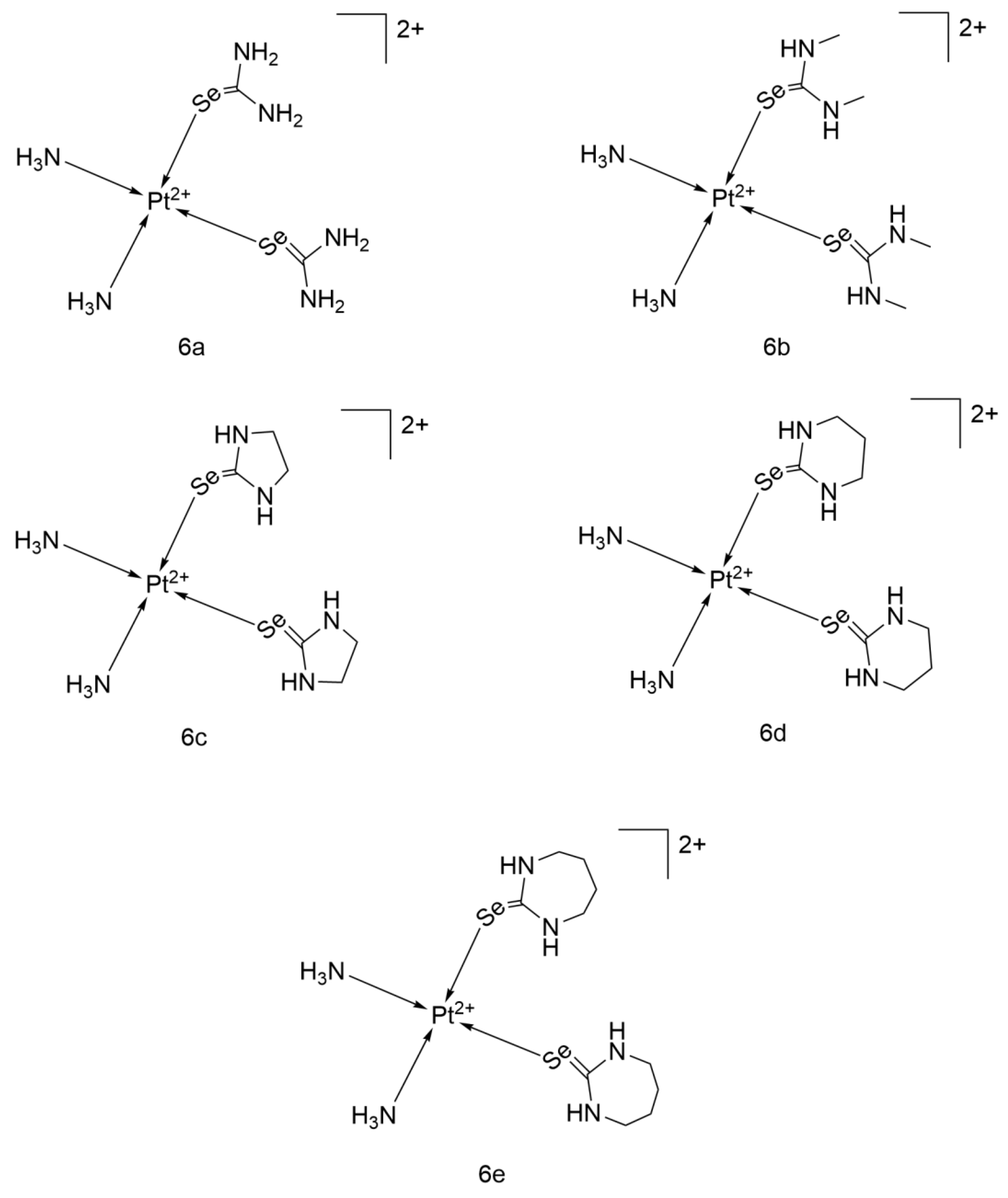
2.2.14. Cisplatin-Derived Complexes of Selenones
2.2.15. Mon-Pt-2
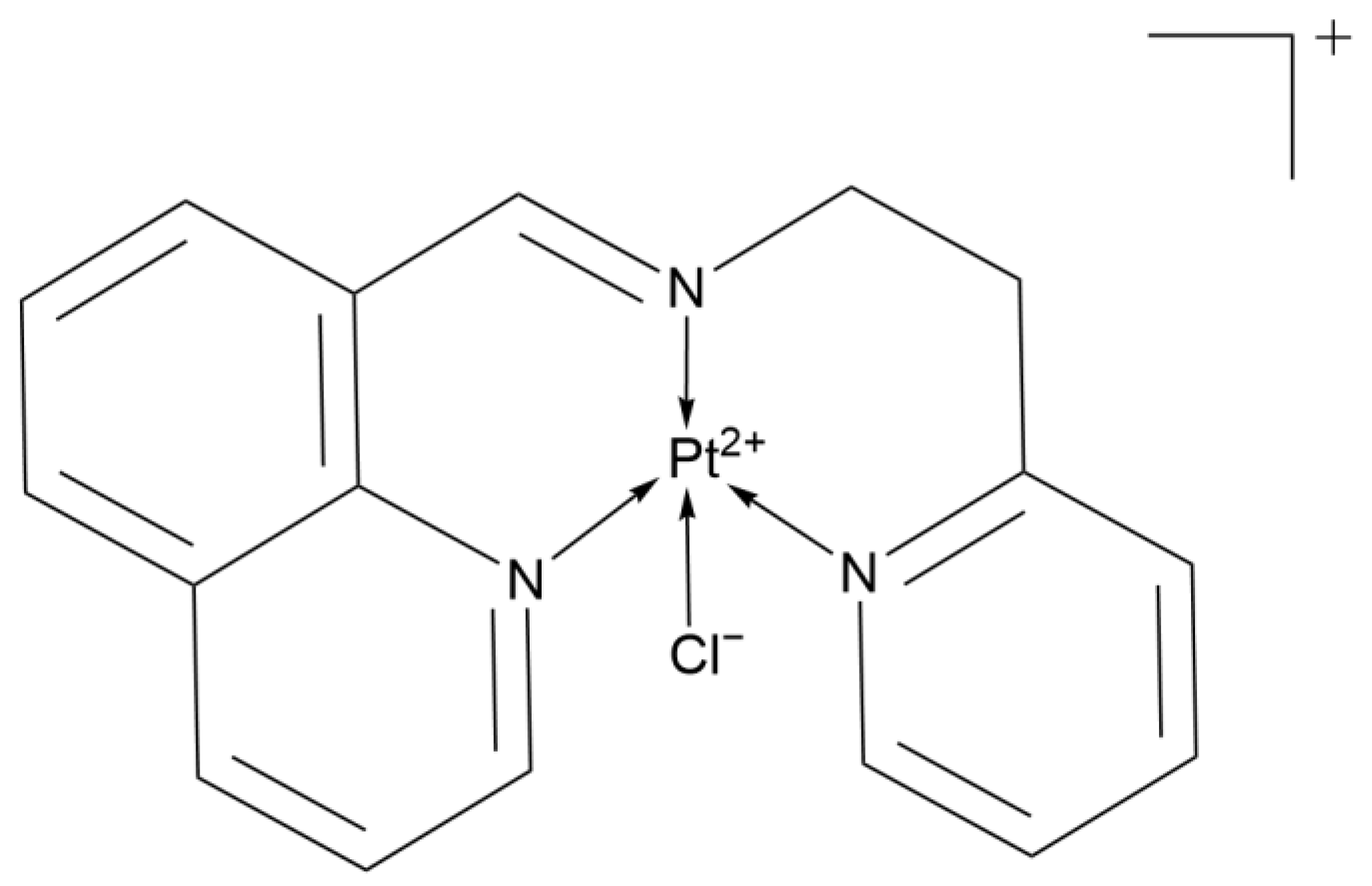
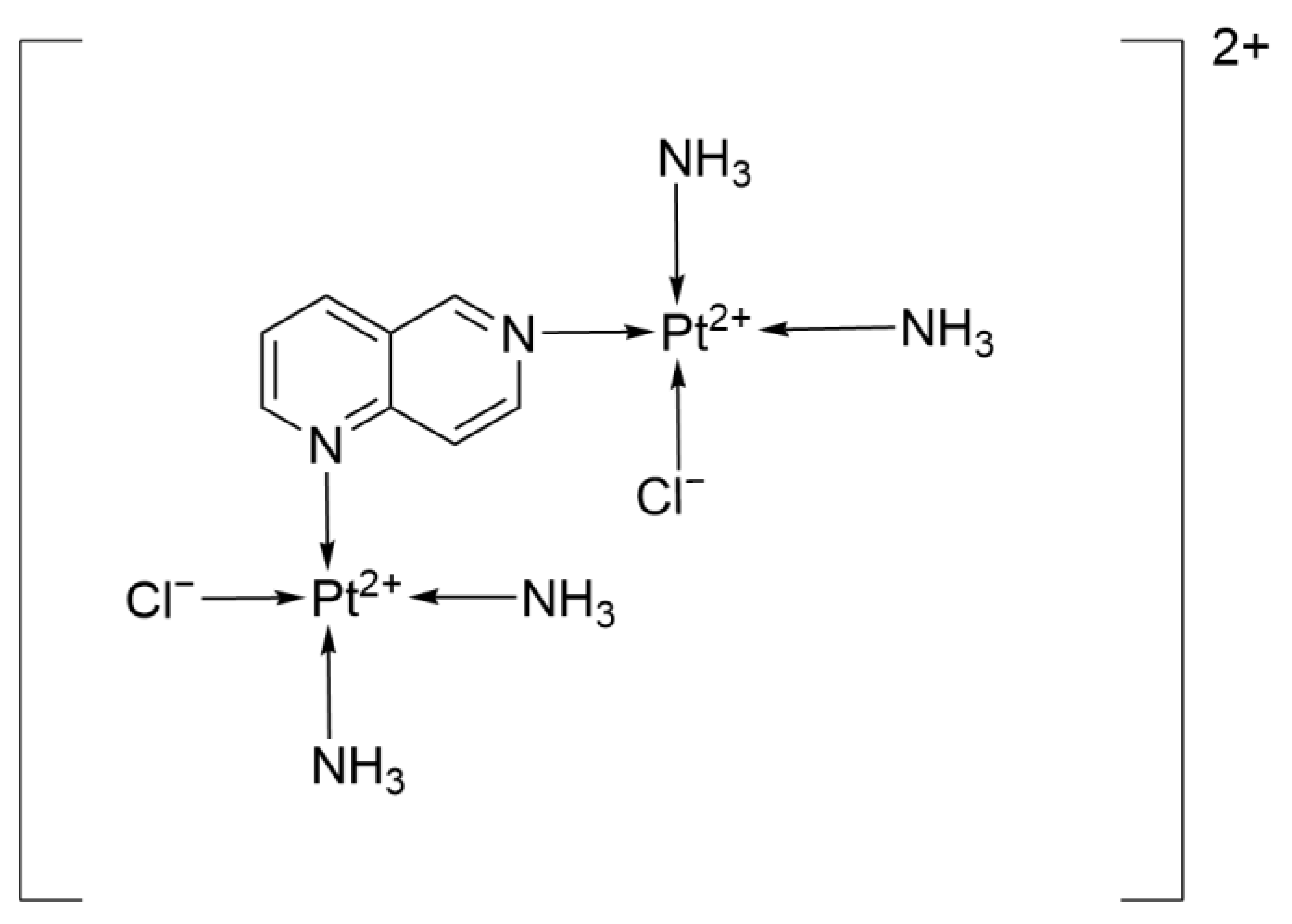
2.2.16. 1,6-Naphthyridine Ligand
2.3. Platinum-Based Complexes in the Laboratory Research Phase
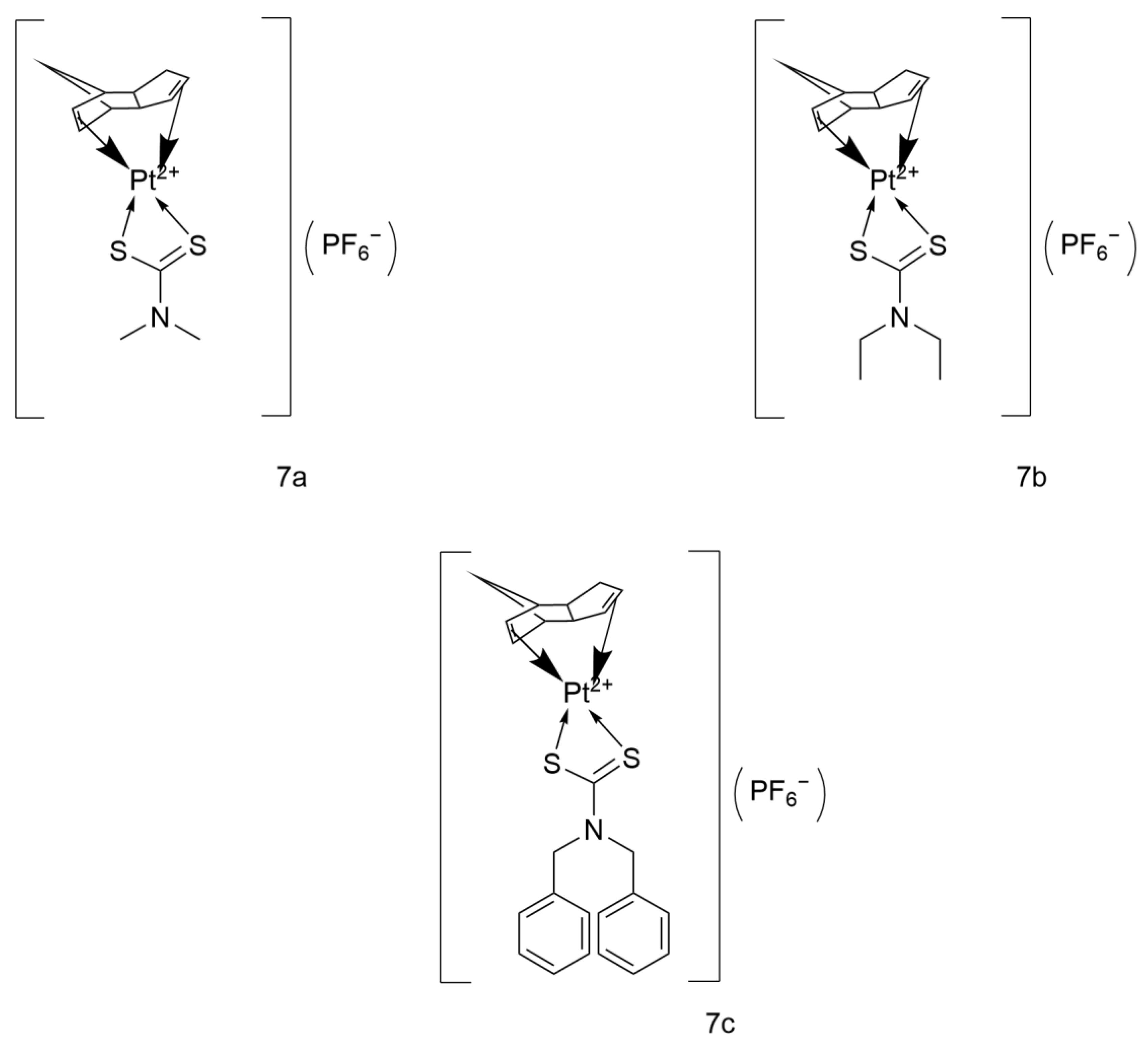
2.3.1. Platinum(II) Cyclopentadienyl-Dithiocarbamate Complexes
2.3.2. Heterometallic Compounds
2.3.3. Cyclometalated Platinum(II) Complexes with O,S-Heterocyclic Ligands
2.4. Innovations in Drug Delivery Systems of Platinum-Based Complexes
2.4.1. AP 5280
2.4.2. SPI-77
2.4.3. Platinum Nanoparticles
2.4.4. EG-Se/Pt
2.4.5. Platinum Coordinated Elenomethionine
2.4.6. Pt-BA
3. Discussion and Summary
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Schaake-Koning, C.; van den Bogaert, W.; Dalesio, O.; Festen, J.; Hoogenhout, J.; van Houtte, P.; Kirkpatrick, A.; Koolen, M.; Maat, B.; Nijs, A. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N. Engl. J. Med. 1992, 326, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Ushijima, S.; Yamamoto, H.; Ito, K.; Araki, J.; Inoue, Y.; Semba, H.; Ichinose, Y. Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: A multi-institutional phase II trial. Br. J. Cancer 2006, 95, 717–721. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, X.; Zhou, X.; Huang, R.; Chu, Z. A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 2007, 57, 348–358. [Google Scholar] [CrossRef]
- Konoshenko, M.; Lansukhay, Y.; Krasilnikov, S.; Laktionov, P. MicroRNAs as Predictors of Lung-Cancer Resistance and Sensitivity to Cisplatin. Int. J. Mol. Sci. 2022, 23, 7594. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef]
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.; Gois, A.F.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 1, CD009256. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Ward, A.; Benfield, P.; Heel, R.C. Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs 1989, 37, 162–190. [Google Scholar] [CrossRef]
- Smit, E.; Moro-Sibilot, D.; de Castro Carpeño, J.; Lesniewski-Kmak, K.; Aerts, J.; Villatoro, R.; Kraaij, K.; Nacerddine, K.; Dyachkova, Y.; Smith, K.T. Cisplatin and carboplatin-based chemotherapy in the first-line treatment of non-small cell lung cancer: Analysis from the European FRAME study. Lung Cancer 2016, 92, 35–40. [Google Scholar] [CrossRef]
- Shukuya, T.; Yamanaka, T.; Seto, T.; Daga, H.; Goto, K.; Saka, H.; Sugawara, S.; Takahashi, T.; Yokota, S.; Kaneda, H. Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): A randomised, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1630–1638. [Google Scholar] [CrossRef]
- Shan, J.; Xiong, Y.; Wang, D.; Xu, M.; Yang, Y.I.; Gong, K.; Yang, Z.; Wang, G.E.; Yang, X. Nedaplatin-versus cisplatin-based chemotherapy in the survival time of patients with non-small cell lung cancer. Mol. Clin. Oncol. 2015, 3, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Feng, F.; Wu, L.; Hu, Y.; Liu, J.; Gao, Y.; Guan, X.; Nan, K.; Suo, A.; Wang, X. Phase II multicenter clinical trial of nedaplatin in the treatment of malignant tumors. Zhonghua Zhong Liu Za Zhi 2006, 28, 230–234. [Google Scholar]
- Jing, C.; Wang, Z.; Lou, R.; Wu, J.Z.; Shi, C.; Chen, D.; Ma, R.; Liu, S.W.; Cao, H.; Feng, J. Nedaplatin reduces multidrug resistance of non-small cell lung cancer by downregulating the expression of long non-coding RNA MVIH. J. Cancer 2020, 11, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Monnet, I.; Brienza, S.; Hugret, F.; Voisin, S.; Gastiaburu, J.; Saltiel, J.C.; Soulié, P.; Armand, J.P.; Cvitkovic, E.; de Cremoux, H. Phase II study of oxaliplatin in poor-prognosis non-small cell lung cancer (NSCLC). ATTIT. Association pour le Traitement des Tumeurs Intra Thoraciques. Eur. J. Cancer 1998, 34, 1124–1127. [Google Scholar] [CrossRef]
- Mir, O.; Alexandre, J.; Ropert, S.; Montheil, V.; Martin, I.; Durand, J.P.; Goldwasser, F. Vinorelbine and oxaliplatin in stage IV nonsmall cell lung cancer patients unfit for cisplatin: A single-center experience. Anticancer Drugs 2009, 20, 105–108. [Google Scholar] [CrossRef]
- Pu, D.; Hou, M.; Li, Z.; Zeng, X. A randomized controlled study of chemotherapy: Etoposide combined with oxaliplatin or cisplatin regimens in the treatment of extensive-stage small cell lung cancer in elderly patients. Zhongguo Fei Ai Za Zhi Chin. J. Lung Cancer 2013, 16, 20–24. [Google Scholar]
- Cheng, Y.; Fan, Y.; Liu, X.; Liu, Y.; Liu, J.; Wang, D.; Yu, Y.; Qin, S.; Liu, W.; Huang, C. Randomized controlled trial of lobaplatin plus etoposide vs. cisplatin plus etoposide as first-line therapy in patients with extensive-stage small cell lung cancer. Oncol. Lett. 2019, 17, 4701–4709. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.; Li, Q.; Yang, W.; Chen, X.; Geng, Y.; Luo, D.; Hu, Y.; Wu, B.; Jiang, W. Preliminary results of randomized phase II study of etoposide plus lobaplatin or etoposide plus cisplatin with concurrent thoracic radiotherapy in the treatment of limited-stage small cell lung cancer. Anticancer Drugs 2023, 34, 1183–1189. [Google Scholar] [CrossRef]
- Zhou, N.N.; Zhao, Y.Y.; Zhai, L.Z.; Ruan, C.M.; Yang, Y.P.; Huang, Y.; Hou, X.; Chen, L.K.; Zhou, T.; Zhang, L. The Efficacy and Toxicity of Lobaplatin-contained Chemotherapy in Extensive-stage Small-cell Lung Cancer. J. Cancer 2018, 9, 2232–2236. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhang, F.; Cui, W.; Zhang, P. Pharmacokinetics and safety of lobaplatin plus etoposide in Chinese men older than 65 years with extensive-stage small cell lung cancer: A phase II clinical trial. Cancer Chemother. Pharmacol. 2019, 84, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, X.; Song, Q.; Tang, K.; Yang, Z.; Zhang, X.; Tang, Y. Structural studies of dicycloplatin, an antitumor supramolecule. Sci. China Chem. 2010, 53, 1346–1351. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Liao, H.; Zhan, J.; Guo, Y.; Zou, B.Y.; Jiang, W.-Q.; Guan, Z.Z.; Yang, X.Q. Phase I clinical trial of the novel platin complex dicycloplatin: Clinical and pharmacokinetic results. Int. J. Clin. Pharmacol. Ther. 2013, 51, 96–105. [Google Scholar] [CrossRef]
- Yu, J.J.; Yang, X.; Song, Q.; Mueller, M.D.; Remick, S.C. Dicycloplatin, a novel platinum analog in chemotherapy: Synthesis of chinese pre-clinical and clinical profile and emerging mechanistic studies. Anticancer Res. 2014, 34, 455–463. [Google Scholar] [PubMed]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A chemical perspective on the clinical use of platinum-based anticancer drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef]
- Bhargava, A.; Vaishampayan, U.N. Satraplatin: Leading the new generation of oral platinum agents. Expert Opin. Investig. Drugs 2009, 18, 1787–1797. [Google Scholar] [CrossRef]
- Grant, S.C.; Gralla, R.J.; Kris, M.G.; Orazem, J.; Kitsis, E.A. Single-agent chemotherapy trials in small-cell lung cancer, 1970 to 1990: The case for studies in previously treated patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1992, 10, 484–498. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Boulikas, T. Lipoplatin Formulation Review Article. J. Drug Deliv. 2012, 2012, 581363. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, M.; Zeng, J.; Feng, J.; Yu, L. Meta-analysis of clinical trials comparing the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN). Medicine 2018, 97, e13169. [Google Scholar] [CrossRef]
- Mylonakis, N.; Athanasiou, A.; Ziras, N.; Angel, J.; Rapti, A.; Lampaki, S.; Politis, N.; Karanikas, C.; Kosmas, C. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer 2010, 68, 240–247. [Google Scholar] [CrossRef]
- Zák, F.; Turánek, J.; Kroutil, A.; Sova, P.; Mistr, A.; Poulová, A.; Mikolin, P.; Zák, Z.; Kasná, A.; Záluská, D. Platinum(IV) complex with adamantylamine as nonleaving amine group: Synthesis, characterization, and in vitro antitumor activity against a panel of cisplatin-resistant cancer cell lines. J. Med. Chem. 2004, 47, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kvardova, V.; Hrstka, R.; Walerych, D.; Muller, P.; Matoulkova, E.; Hruskova, V.; Stelclova, D.; Sova, P.; Vojtesek, B. The new platinum(IV) derivative LA-12 shows stronger inhibitory effect on Hsp90 function compared to cisplatin. Mol. Cancer 2010, 9, 147. [Google Scholar] [CrossRef]
- Roubalová, E.; Kvardová, V.; Hrstka, R.; Borilová, S.; Michalová, E.; Dubská, L.; Müller, P.; Sova, P.; Vojtesek, B. The effect of cellular environment and p53 status on the mode of action of the platinum derivative LA-12. Investig. New Drugs 2010, 28, 445–453. [Google Scholar] [CrossRef]
- Bouchal, P.; Jarkovsky, J.; Hrazdilova, K.; Dvorakova, M.; Struharova, I.; Hernychova, L.; Damborsky, J.; Sova, P.; Vojtesek, B. The new platinum-based anticancer agent LA-12 induces retinol binding protein 4 in vivo. Proteome Sci. 2011, 9, 68. [Google Scholar] [CrossRef]
- Cui, Z.; Ruan, Z.; Zeng, J.; Sun, J.; Ye, W.; Xu, W.; Guo, X.; Zhang, L.; Song, L. Lung-specific exosomes for co-delivery of CD47 blockade and cisplatin for the treatment of non–small cell lung cancer. Thorac. Cancer 2022, 13, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Xie, X.; Zhang, D.; Zhou, Y.; Li, B.; Li, F.; Li, F.; Cheng, Y.; Mei, H.; Meng, H. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Icsel, C.; Aygun, M.; Erkisa, M.; Ulukaya, E. Pd(II) and Pt(II) saccharinate complexes of bis(diphenylphosphino)propane/butane: Synthesis, structure, antiproliferative activity and mechanism of action. Eur. J. Med. Chem. 2018, 158, 534–547. [Google Scholar] [CrossRef]
- Elwell, K.E.; Hall, C.; Tharkar, S.; Giraud, Y.; Bennett, B.; Bae, C.; Carper, S.W. A fluorine containing bipyridine cisplatin analog is more effective than cisplatin at inducing apoptosis in cancer cell lines. Bioorg. Med. Chem. 2006, 14, 8692–8700. [Google Scholar] [CrossRef]
- Vo, V.; Kabuloglu-Karayusuf, Z.G.; Carper, S.W.; Bennett, B.L.; Evilia, C. Novel 4,4′-diether-2,2′-bipyridine cisplatin analogues are more effective than cisplatin at inducing apoptosis in cancer cell lines. Bioorg. Med. Chem. 2010, 18, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C.; Cassidy, J.; Edwards, C.; Samuel, L.; Bisset, D.; Camboni, G.; Young, A.; Boyle, D.; Jodrell, D. A phase I study of the trinuclear platinum compound, BBR 3464, in combination with protracted venous infusional 5-fluorouracil in patients with advanced cancer. Cancer Chemother. Pharmacol. 2004, 53, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hensing, T.A.; Hanna, N.H.; Gillenwater, H.H.; Camboni, M.G.; Allievi, C.; Socinski, M.A. Phase II study of BBR 3464 as treatment in patients with sensitive or refractory small cell lung cancer. Anticancer Drugs 2006, 17, 697–704. [Google Scholar] [CrossRef]
- Lovejoy, K.S.; Serova, M.; Bieche, I.; Emami, S.; D’Incalci, M.; Broggini, M.; Erba, E.; Gespach, C.; Cvitkovic, E.; Faivre, S. Spectrum of cellular responses to pyriplatin, a monofunctional cationic antineoplastic platinum(II) compound, in human cancer cells. Mol. Cancer Ther. 2011, 10, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.R.; Bentsion, D.L.; Lipatov, O.N.; Polyakov, I.S.; MacKintosh, F.R.; Karlin, D.A.; Baker, G.S.; Breitz, H.B. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 2046–2051. [Google Scholar] [CrossRef]
- Hamilton, G. Picoplatin pharmacokinetics and chemotherapy of non-small cell lung cancer. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1381–1390, Erratum in Expert Opin. Drug Metab. Toxicol. 2014, 10, 495. [Google Scholar] [CrossRef]
- Wittgen, B.P.H.; Kunst, P.W.A.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef]
- Mojaddami, A.; Karimi, A.; Mahdavinia, M.; Fereidoonnezhad, M. Antiproliferative activity and DNA binding studies of cyclometalated complexes of platinum(II) containing 2-vinylpyridine. BioMetals 2022, 35, 617–627. [Google Scholar] [CrossRef]
- Filipović, N.; Polović, N.; Rašković, B.; Misirlić-Denčić, S.; Dulović, M.; Savić, M.; Nikšić, M.; Mitić, D.; Anđelković, K.; Todorović, T. Biological activity of two isomeric N-heteroaromatic selenosemicarbazones and their metal complexes. Monatshefte Für Chem. Chem. Mon. 2014, 145, 1089–1099. [Google Scholar] [CrossRef]
- As Sobeai, H.M.; Sulaiman, A.A.A.; Ahmad, S.; Shaikh, A.R.; Sulaimon, R.; Alotiabi, M.R.; AlZoghaibi, F.; Altoum, A.O.; Isab, A.A.; Alhoshani, A.R. Synthesis, characterization, and miRNA-mediated PI3K suppressing activity of novel cisplatin-derived complexes of selenones. Arab. J. Chem. 2021, 14, 103245. [Google Scholar] [CrossRef]
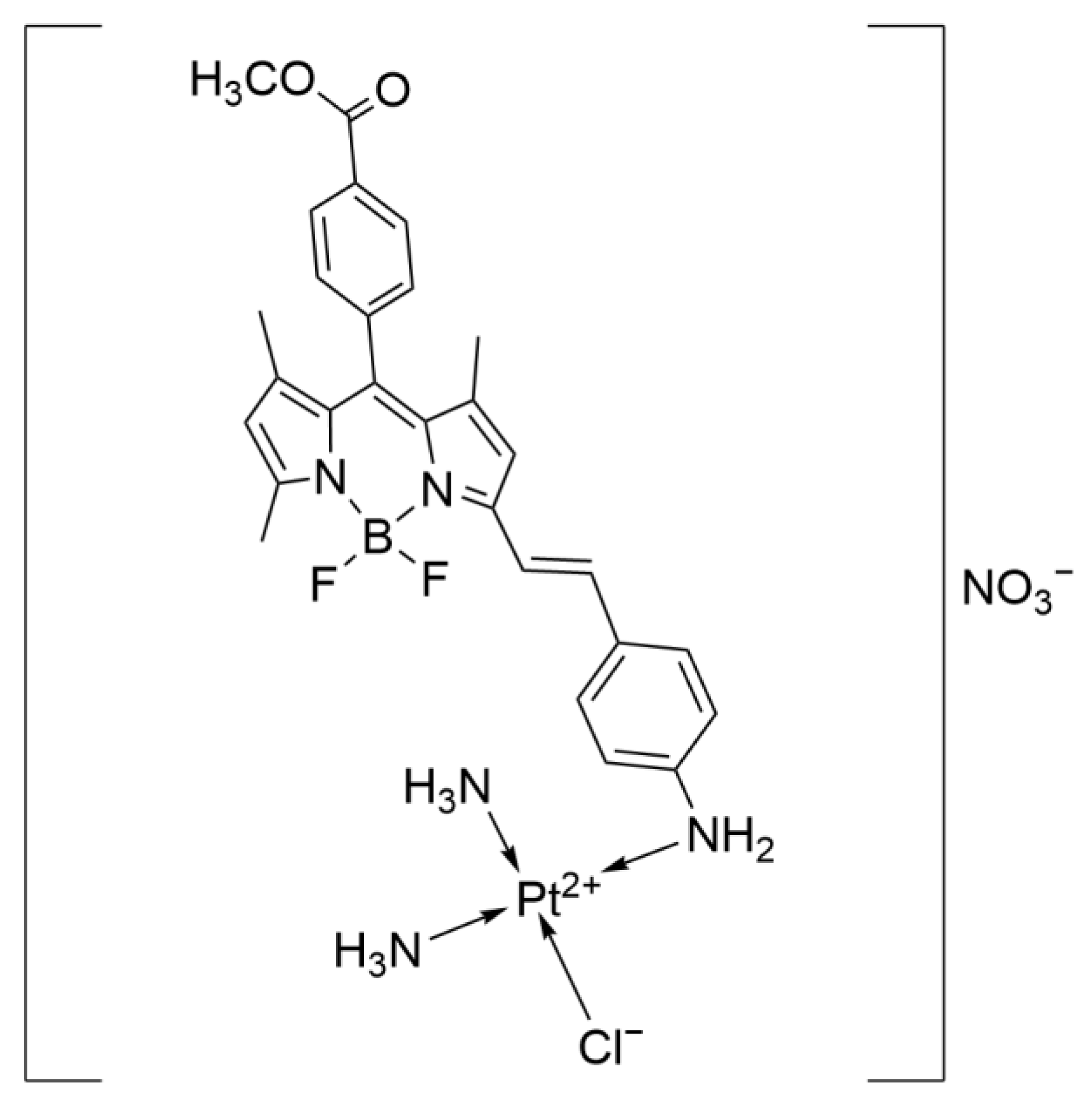
- Wang, F.Y.; Tang, X.M.; Wang, X.; Huang, K.B.; Feng, H.W.; Chen, Z.F.; Liu, Y.N.; Liang, H. Mitochondria-targeted platinum(II) complexes induce apoptosis-dependent autophagic cell death mediated by ER-stress in A549 cancer cells. Eur. J. Med. Chem. 2018, 155, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, B.; Franich, A.A.; Jovanović, M.; Jurisević, M.; Gajović, N.; Jovanović, M.; Arsenijević, N.; Maric, V.; Jovanović, I.; Živković, M.D. Synthesis, DNA-/bovine serum albumin-binding affinity, and cytotoxicity of dinuclear platinum(II) complexes with 1,6-naphthyridine-bridging ligand. Appl. Organomet. Chem. 2021, 35, e6112. [Google Scholar] [CrossRef]
- Sulaiman, A.A.A.; Sobeai, H.M.A.; Aldawood, E.; Abogosh, A.; Alhazzani, K.; Alotaibi, M.R.; Ahmad, S.; Alhoshani, A.; Isab, A.A. In vitro and in vivo antitumor studies of potential anticancer agents of platinum(II) complexes of dicyclopentadiene and dithiocarbamates. Metallomics 2022, 14, mfac054. [Google Scholar] [CrossRef] [PubMed]
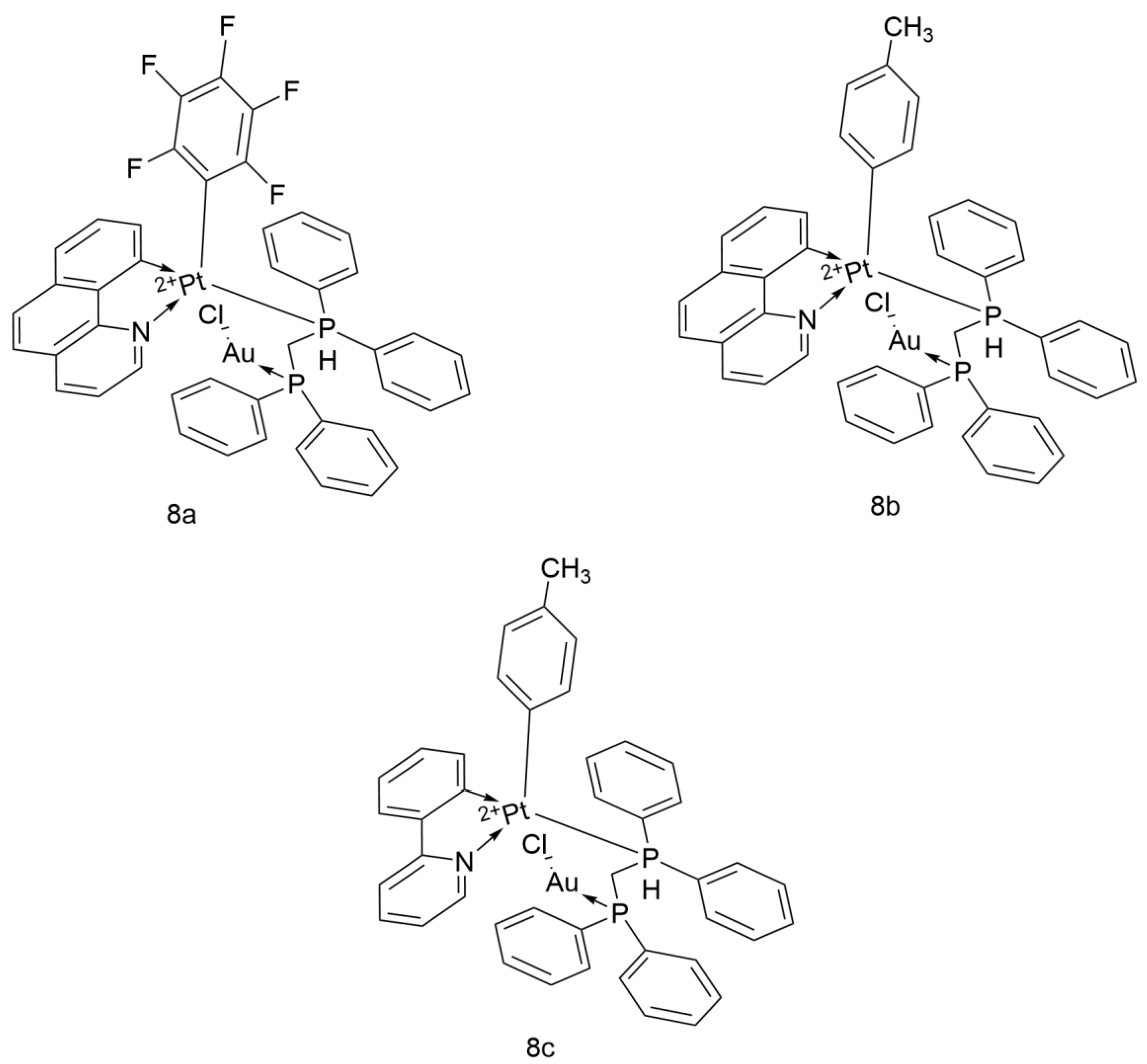
- Shahsavari, H.R.; Giménez, N.; Lalinde, E.; Moreno, M.T.; Fereidoonnezhad, M.; Aghakhanpour, R.B.; Khatami, M.; Kalantari, F.; Jamshidi, Z.; Mohammadpour, M. Heterobimetallic PtII-AuI Complexes Comprising Unsymmetrical 1,1-Bis(diphenylphosphanyl)methane Bridges: Synthesis, Photophysical, and Cytotoxic Studies. Eur. J. Inorg. Chem. 2019, 2019, 1360–1373. [Google Scholar] [CrossRef]
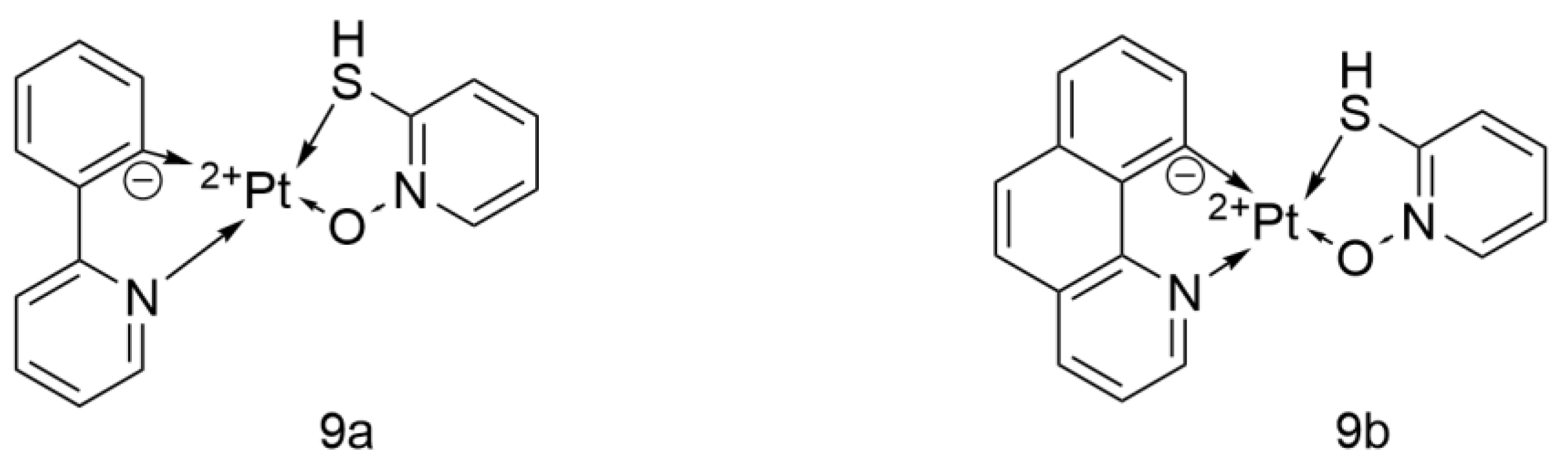
- Fereidoonnezhad, M.; Ramezani, Z.; Nikravesh, M.; Zangeneh, J.; Haghighi, M.G.; Faghih, Z.; Notash, B.; Shahsavari, H.R. Cycloplatinated(II) complexes bearing an O,S-heterocyclic ligand: Search for anticancer drugs. New J. Chem. 2018, 42, 7177–7187. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Terret, C.; Howell, S.B.; Baud, C.M.; Boer, R.F.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M.; Droz, J.P. A Phase I and Pharmacological Study of the Platinum Polymer AP5280 Given as an Intravenous Infusion Once Every 3 Weeks in Patients with Solid Tumors. Clin. Cancer Res. 2004, 10, 3386–3395. [Google Scholar] [CrossRef]
- Terwogt, J.M.; Groenewegen, G.; Pluim, D.; Maliepaard, M.; Tibben, M.; Huisman, A.; Huinink, W.B.; Schot, M.; Welbank, H.; Voest, E. Phase I and pharmacokinetic study of SPI-77, a liposomal encapsulated dosage form of cisplatin. Cancer Chemother. Pharmacol. 2002, 49, 201–210. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Lorigan, P.; Margison, G.P.; Margison, J.M.; Martin, F.; Thatcher, N.; Anderson, H.; Ranson, M. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br. J. Cancer 2006, 95, 822–828. [Google Scholar] [CrossRef]
- Kim, E.S.; Lu, C.; Khuri, F.R.; Tonda, M.; Glisson, B.S.; Liu, D.; Jung, M.; Hong, W.K.; Herbst, R.S. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer 2001, 34, 427–432. [Google Scholar] [CrossRef]
- Ismail, N.A.S.; Lee, J.X.; Yusof, F. Platinum Nanoparticles: The Potential Antioxidant in the Human Lung Cancer Cells. Antioxidants 2022, 11, 986. [Google Scholar] [CrossRef]
- Faderin, E.; Iorkula, T.H.; Aworinde, O.R.; Awoyemi, R.F.; Awoyemi, C.T.; Acheampong, E.; Chukwu, J.U.; Agyemang, P.; Onaiwu, G.E.; Ifijen, I.H. Platinum nanoparticles in cancer therapy: Chemotherapeutic enhancement and ROS generation. Med. Oncol. 2025, 42, 42. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Y.; Li, T.; Cao, W.; Yi, Y.; Geng, W.; Sun, Z.; Xu, H. Selenium–Platinum Coordination Compounds as Novel Anticancer Drugs: Selectively Killing Cancer Cells via a Reactive Oxygen Species (ROS)-Mediated Apoptosis Route. Chem. Asian J. 2014, 9, 2295–2302. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Han, X.; Zhuang, H.; Nie, G.; Xu, H. Nanomedicine Assembled by Coordinated Selenium–Platinum Complexes Can Selectively Induce Cytotoxicity in Cancer Cells by Targeting the Glutathione Antioxidant Defense System. ACS Biomater. Sci. Eng. 2018, 4, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiang, W.; Li, F.; Xu, H. Self-assembly regulated anticancer activity of platinum coordinated selenomethionine. Biomaterials 2018, 157, 17–25. [Google Scholar] [CrossRef]
- Xue, X.; Zhu, C.; Chen, H.; Bai, Y.; Shi, X.; Jiao, Y.; Chen, Z.; Miao, Y.; He, W.; Guo, Z. A New Approach to Sensitize Antitumor Monofunctional Platinum(II) Complexes via Short Time Photo-Irradiation. Inorg. Chem. 2017, 56, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Wang, Y.; Gou, S. Platinum(IV) Prodrugs with Cancer Stem Cell Inhibitory Effects on Lung Cancer for Overcoming Drug Resistance. J. Med. Chem. 2022, 65, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Fronik, P.; Gutmann, M.; Vician, P.; Stojanovic, M.; Kastner, A.; Heffeter, P.; Pirker, C.; Keppler, B.K.; Berger, W.; Kowol, C.R. A platinum(IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance. Commun. Chem. 2022, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Liang, B.B.; Liu, W.; Mao, Z.W. What blocks more anticancer platinum complexes from experiment to clinic: Major problems and potential strategies from drug design perspectives. Coord. Chem. Rev. 2021, 449, 214210. [Google Scholar] [CrossRef]





| Generation | Drug | Molecular Formula | Key Structural Features | Water Solubility Characteristics | First Launched Country | Launch Year |
|---|---|---|---|---|---|---|
| 1st | Cisplatin | Pt(NH3)2Cl2 | Cis-configuration, Cl− ligands | Low (requires hydration therapy) | USA | 1978 |
| 2nd | Carboplatin | Pt(NH3)2(C2O4) | Bidentate cyclobutane dicarboxylate ligand | High (l6× more soluble than Cisplatin, no hydration) | UK | 1986 |
| 2nd Deriv. | Nedaplatin | Pt(NH3)2Cl(CH3COO) | Glycolic acid replaces two Cl− in Cisplatin | High (~10× more soluble than Cisplatin, no hydration) | Japan | 1995 |
| 3rd | Oxaliplatin | C8H14N2O4Pt | Cyclohexane ligand | Moderate (lower toxicity) | France | 1980s |
| 3rd Deriv. | Lobaplatin | C9H18N2O3Pt·3H2O | Cyclohexane ligand | Moderate (lower nephrotoxicity) | China | 2005 |
| Trial Phase | Dicycloplatin | C12H20N2O8Pt | Cyclobutane dicarboxylate ligand | Very high (low toxicity) | / | / |
| Drug | Indication | Major Toxicities | Target Patient Population |
|---|---|---|---|
| Cisplatin | NSCLC, SCLC | Nephrotoxicity, nausea/vomiting, ototoxicity | Patients needing rapid symptom relief in extensive-stage SCLC |
| Carboplatin | NSCLC (patients with renal insufficiency) | Myelosuppression, thrombocytopenia | Patients with renal insufficiency |
| Nedaplatin | SCLC | Myelosuppression, neutropenia, thrombocytopenia | Patients requiring low nephrotoxicity regimens |
| Oxaliplatin | NSCLC (neuropathy-intolerant patients) | Reversible peripheral neuropathy | Patients intolerant to Cisplatin toxicity |
| Lobaplatin | NSCLC (Chinese patients) | Myelosuppression, thrombocytopenia | Patients requiring low gastrointestinal toxicity regimens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Huang, Y.; Wang, Y.; Lu, D.; Sun, Q. Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges. Int. J. Mol. Sci. 2025, 26, 7958. https://doi.org/10.3390/ijms26167958
Liang W, Huang Y, Wang Y, Lu D, Sun Q. Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges. International Journal of Molecular Sciences. 2025; 26(16):7958. https://doi.org/10.3390/ijms26167958
Chicago/Turabian StyleLiang, Wanqi, Yufeng Huang, Yi Wang, Desheng Lu, and Qi Sun. 2025. "Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges" International Journal of Molecular Sciences 26, no. 16: 7958. https://doi.org/10.3390/ijms26167958
APA StyleLiang, W., Huang, Y., Wang, Y., Lu, D., & Sun, Q. (2025). Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges. International Journal of Molecular Sciences, 26(16), 7958. https://doi.org/10.3390/ijms26167958








