Role of Histone Lactylation in Neurological Disorders
Abstract
1. Lactate and Lactylation
2. Addition and Removal of Lactyl Groups
2.1. Lactylation: Enzymatic and Non-Enzymatic Reactions
2.2. Delactylation
3. Pathology Manifestations
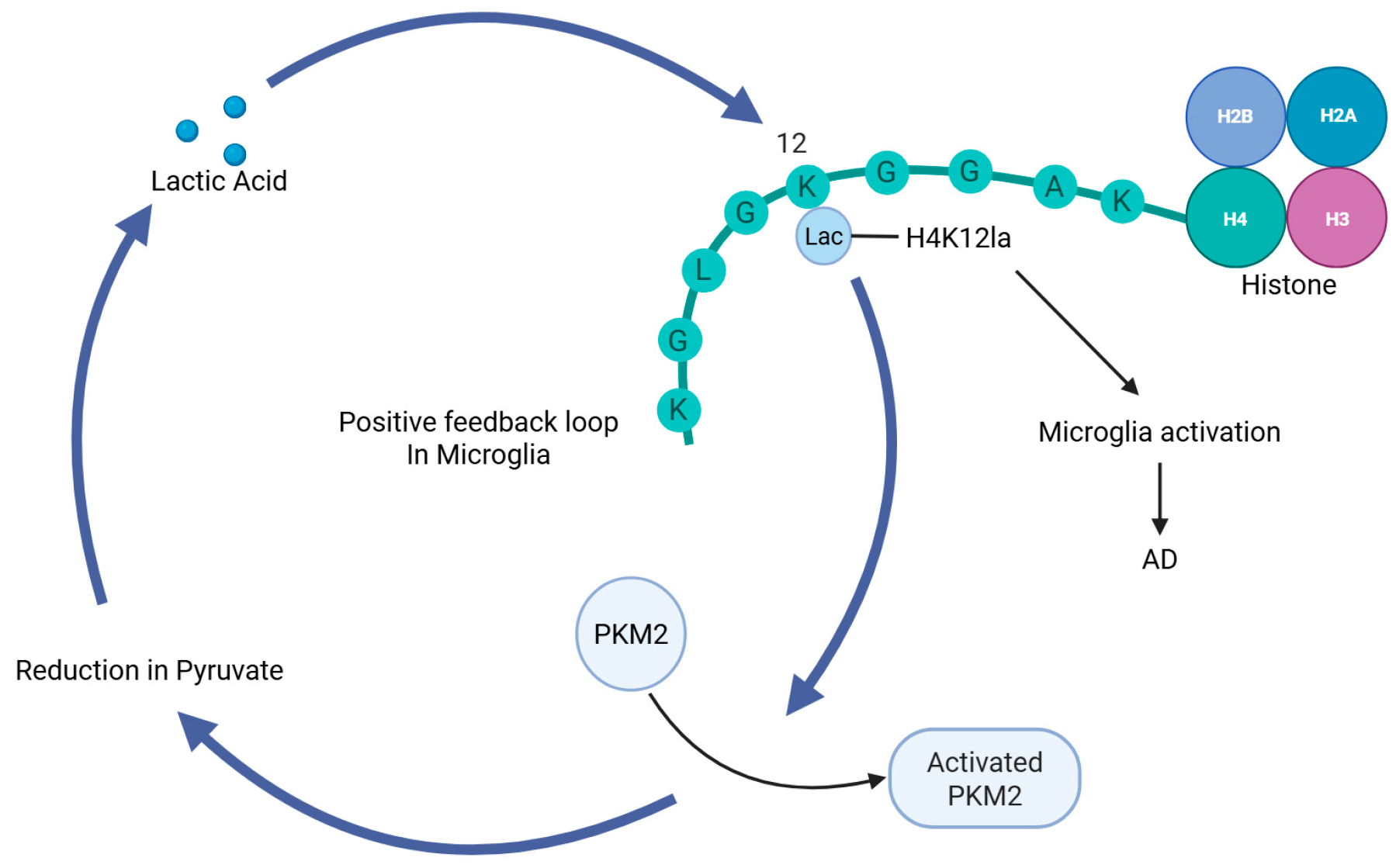
3.1. AD
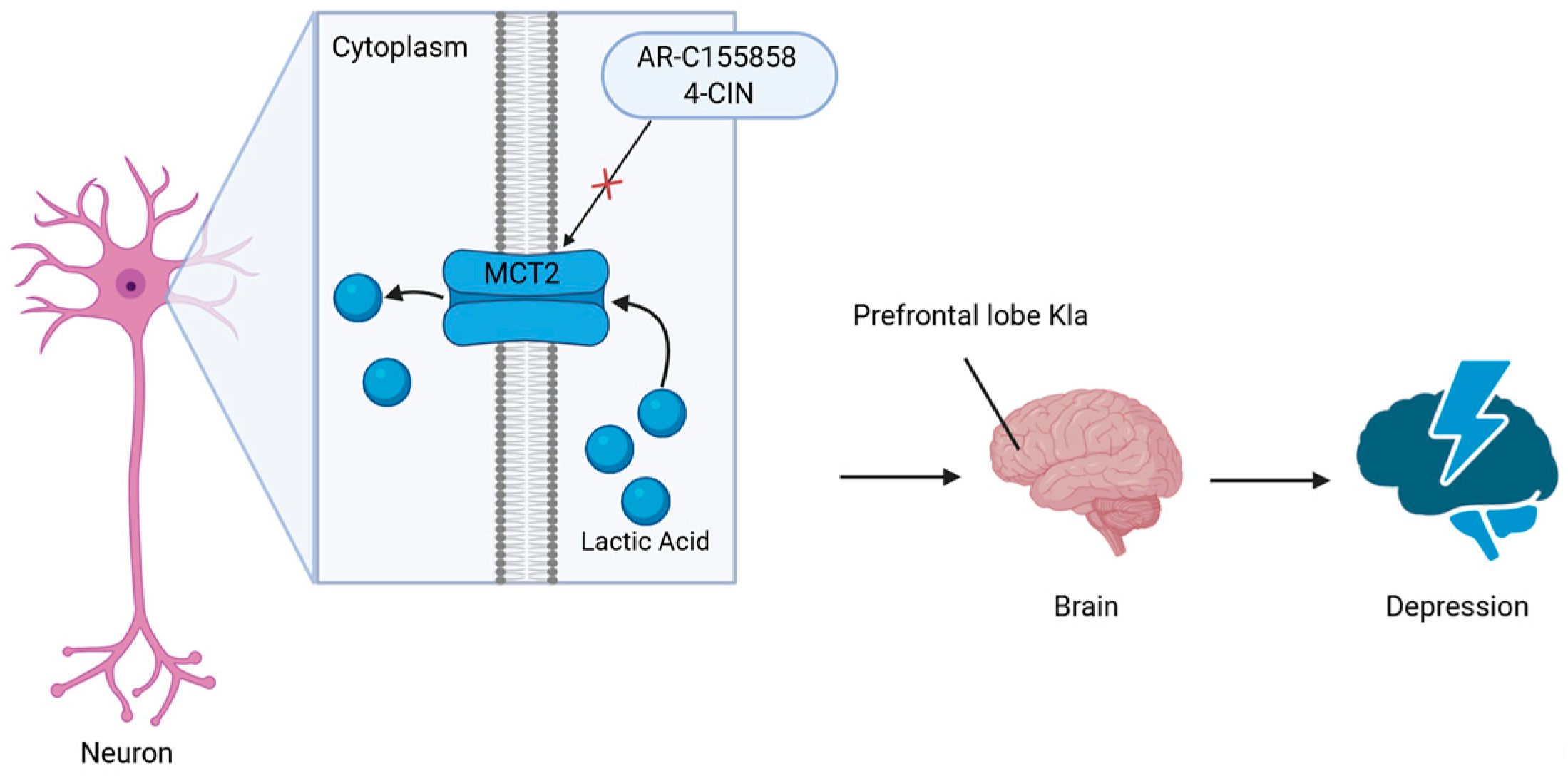
3.2. Depression
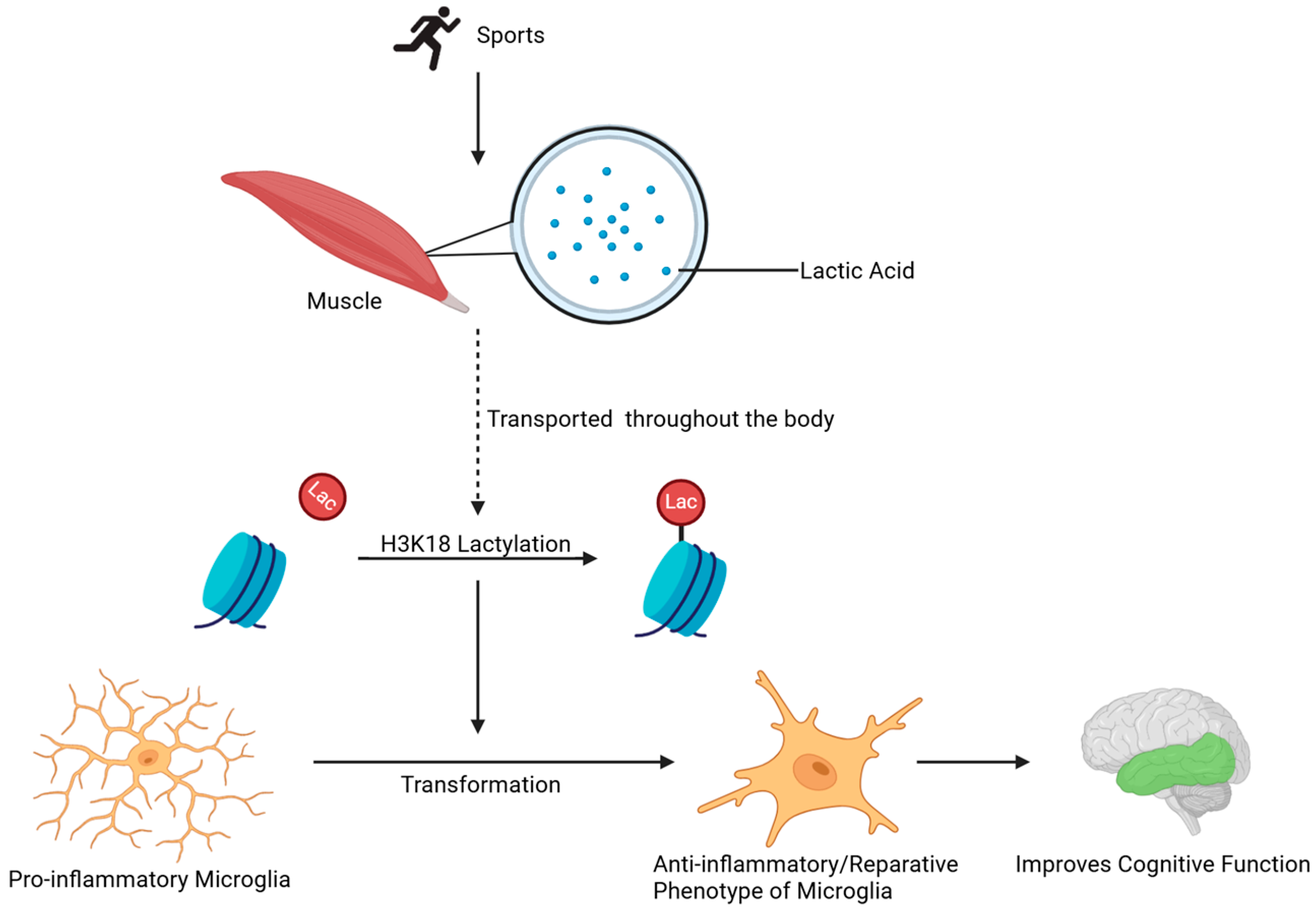
3.3. Neuroinflammation and Aging
3.4. Histone Lactylation in Other Neurological Disorders
3.5. Cell-Type-Specific Regulation of Histone Lactylation in the CNS
4. Drug Development
5. Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AlCl3 | aluminum chloride |
| APP | amyloid precursor protein |
| Arg 1 | arginase 1 |
| Aβ | amyloid-β |
| 4-CIN | α-cyano-4-hydroxycinnamate |
| CNS | central nervous system |
| D-gal | d-galactose |
| ECS | electroconvulsive stimulation |
| ERK | extracellular regulated protein kinases |
| GLO2 | glyoxalase ii |
| HPLC | high-performance liquid chromatography |
| BNDF | brain-derived neurotrophic factor |
| HDAC | histone deacetylase |
| KAT | lysine acetyltransferase |
| Kla | lysine lactylation |
| KL-la | l-lactyl-lysine |
| Kce | n-ε-(carboxyethyl)-lysine |
| KD-la | d-lactyl-lysine |
| LDH | lactate dehydrogenase |
| LGSH | lactic glutathione |
| MCT1 | monocarboxylate transporter 1 |
| MCT2 | monocarboxylate transporter 2 |
| ROS | reactive oxygen species |
| SIRT | sirtuins |
| STAT3 | signal transducer and activator of transcription 3 |
| OX | oxymate |
| PKM2 | pyruvate kinase m2 |
| PSEN1 | presenilin |
| SASP | senescence-associated secretory phenotype |
| SAH | subarachnoid hemorrhage |
| PFC | prefrontal cortex |
| VEGF | vascular endothelial growth factor |
| LDHA | lactate dehydrogenase A |
| BRD4 | bromodomain-containing protein 4 |
References
- Schell, J.C.; Wisidagama, D.R.; Bensard, C.; Zhao, H.; Wei, P.; Tanner, J.; Flores, A.; Mohlman, J.; Sorensen, L.K.; Earl, C.S.; et al. Control of Intestinal Stem Cell Function and Proliferation by Mitochondrial Pyruvate Metabolism. Nat. Cell Biol. 2017, 19, 1027–1036. [Google Scholar] [CrossRef]
- Zoremba, N.; Homola, A.; Rossaint, R.; Syková, E. Interstitial Lactate, Lactate/Pyruvate and Glucose in Rat Muscle Before, During and in the Recovery from Global Hypoxia. Acta Vet. Scand. 2014, 56, 72. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications: Basic Mechanisms and Role in Cardiovascular Disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Sabari, B.R.; Zhang, D.; Allis, C.D.; Zhao, Y. Metabolic Regulation of Gene Expression through Histone Acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, H.; Li, Q.; Liu, S.; Qu, K.; Liu, C.; Zhang, J. Lactylation: A Passing Fad or the Future of Posttranslational Modification. Inflammation 2022, 45, 1419–1429. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhu, Z.; Mao, Q.; Xu, Z.; Singh, P.K.; Rimayi, C.C.; Moreno-Yruela, C.; Xu, S.; Li, G.; et al. Lysine L-Lactylation Is the Dominant Lactylation Isomer Induced by Glycolysis. Nat. Chem. Biol. 2024, 21, 91–99. [Google Scholar] [CrossRef]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y.; et al. Exercise Improves Cognitive Dysfunction and Neuroinflammation in Mice Through Histone H3 Lactylation in Microglia. Immun. Ageing 2023, 20, 63. [Google Scholar] [CrossRef]
- Varner, E.L.; Trefely, S.; Bartee, D.; von Krusenstiern, E.; Izzo, L.; Bekeova, C.; Connor, R.S.O.; Seifert, E.L.; Wellen, K.E.; Meier, J.L.; et al. Quantification of Lactoyl-CoA (Lactyl-CoA) by Liquid Chromatography Mass Spectrometry in Mammalian Cells and Tissues. Open Biol. 2020, 10, 200187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Pal, J.K. Metabolic Ink Lactate Modulates Epigenomic Landscape: A Concerted Role of Pro-Tumor Microenvironment and Macroenvironment During Carcinogenesis. Curr. Mol. Med. 2021, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, R.; Chen, H.Z.; Xiao, Q.; Wang, W.J.; He, J.P.; Li, X.-X.; Yu, X.-W.; Li, L.; Wang, P.; et al. Enhancement of Hypothalamic STAT3 Acetylation by Nuclear Receptor Nur77 Dictates Leptin Sensitivity. Diabetes 2015, 64, 2069–2081. [Google Scholar] [CrossRef]
- Zhu, R.; Ye, X.; Lu, X.; Xiao, L.; Yuan, M.; Zhao, H.; Guo, D.; Meng, Y.; Han, H.; Luo, S.; et al. ACSS2 Acts as a Lactyl-CoA Synthetase and Couples KAT2A to Function as a Lactyltransferase for Histone Lactylation and Tumor Immune Evasion. Cell Metab. 2024, 37, 361–376.e7. [Google Scholar] [CrossRef]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Schou Oxvig, A.M.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-Enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2019, 27, 206–213.e6. [Google Scholar] [CrossRef]
- James, A.M.; Hoogewijs, K.; Logan, A.; Hall, A.R.; Ding, S.; Fearnley, I.M.; Murphy, M.P. Non-Enzymatic N-Acetylation of Lysine Residues by acetylCoA Often Occurs via a Proximal S-Acetylated Thiol Intermediate Sensitive to Glyoxalase II. Cell Rep. 2017, 18, 2105–2112. [Google Scholar] [CrossRef]
- Rajabi, N.; Galleano, I.; Madsen, A.S.; Olsen, C.A. Targeting Sirtuins: Substrate Specificity and Inhibitor Design. Prog. Mol. Biol. Transl. Sci. 2018, 154, 25–69. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. The Rpd3/Hda1 Family of Lysine Deacetylases: From Bacteria and Yeast to Mice and Men. Nat. Rev. Mol. Cell Biol. 2008, 9, 206–218. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I Histone Deacetylases (HDAC1–3) Are Histone Lysine Delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef]
- Madsen, A.S.; Olsen, C.A. Profiling of Substrates for Zinc-Dependent Lysine Deacylase Enzymes: HDAC3 Exhibits Decrotonylase Activity In Vitro. Angew. Chem. Int. Ed. 2012, 124, 9217–9221. [Google Scholar] [CrossRef]
- Millard, C.J.; Watson, P.J.; Fairall, L.; Schwabe, J.W.R. Targeting Class I Histone Deacetylases in a “Complex” Environment. Trends Pharmacol. Sci. 2017, 38, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.G.; Barak, O.; Lazar, M.A. The SMRT and N-CoR Corepressors Are Activating Cofactors for Histone Deacetylase 3. Mol. Cell. Biol. 2001, 21, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.A.; Millard, C.J.; Lin, C.-L.; Gurnett, J.E.; Wu, M.; Lee, K.; Fairall, L.; Schwabe, J.W.; Cole, P.A. Diverse Nucleosome Site-Selectivity Among Histone Deacetylase Complexes. eLife 2020, 9, E57663. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Li, N.; Geng, Q. Lactate and Lactylation in Macrophage Metabolic Reprogramming: Current Progress and Outstanding Issues. Front. Immunol. 2024, 15, 1395786. [Google Scholar] [CrossRef]
- Yu, J.; Chai, P.; Xie, M.; Ge, S.; Ruan, J.; Fan, X.; Jia, R. Histone Lactylation Drives Oncogenesis by Facilitating m6A Reader Protein YTHDF2 Expression in Ocular Melanoma. Genome Biol. 2021, 22, 85. [Google Scholar] [CrossRef]
- Wei, T.; Yi, M.; Gu, W.; Hou, L.; Lu, Q.; Yu, Z.; Chen, H. The Potassium Channel KCa3.1 Represents a Valid Pharmacological Target for Astrogliosis-Induced Neuronal Impairment in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2017, 7, 528. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Miller, M.B.; Huang, A.Y.; Kim, J.; Zhou, Z.; Kirkham, S.L.; Maury, E.A.; Ziegenfuss, J.S.; Reed, H.C.; Neil, J.E.; Rento, L.; et al. Somatic Genomic Changes in Single Alzheimer’s Disease Neurons. Nature 2022, 604, 714–722. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 Lactylation of Senescent Microglia Potentiates Brain Aging and Alzheimer’s Disease Through the NFκB Signaling Pathway. J. NeuroInflamm. 2023, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive Feedback Regulation of Microglial Glucose Metabolism by Histone H4 Lysine 12 Lactylation in Alzheimer’s Disease. Cell Metab. 2022, 34, 634–648.e6. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Guell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Stockmeier, C. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Allaman, I.; Fiumelli, H.; Magistretti, P.J.; Martin, J.-L. Fluoxetine Regulates the Expression of Neurotrophic/Growth Factors and Glucose Metabolism in Astrocytes. Psychopharmacology 2011, 216, 75–84. [Google Scholar] [CrossRef]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnie’re, L.; Mey-Lan, E.M.; Petit, J.-M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.-L. Peripheral Administration of Lactate Produces Antidepressant-like Effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate Is an Antidepressant That Mediates Resilience to Stress by Modulating the Hippocampal Levels and Activity of Histone Deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and Neurobiological Consequences of Prolonged Glucocorticoid Exposure in Rats: Relevance to Depression. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef]
- Carrard, A.; Cassé, F.; Carron, C.; Burlet-Godinot, S.; Toni, N.; Magistretti, P.J.; Martin, J.-L. Role of Adult Hippocampal Neurogenesis in the Antidepressant Actions of Lactate. Mol. Psychiatry 2021, 26, 6723–6735. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Vieira, R.; et al. Dynamic Regulation of Mitochondrial Function by Glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Zafir, A.; Banu, N. Modulation of in Vivo Oxidative Status by Exogenous Corticosterone and Restraint Stress in Rats. Stress 2009, 12, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Covington, H.E.; Berton, O., 3rd; Russo, S.J. A Standardized Protocol for Repeated Social Defeat Stress in Mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, C.M.; Mongeon, R.; Lahmann, C.; Koveal, D.; Zucker, H.; Yellen, G. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 2017, 26, 361–374.e4. [Google Scholar] [CrossRef]
- Busnello, J.V.; Leke, R.; Oses, J.P.; Feier, G.; Bruch, R.; Quevedo, J.; Kapczin-Ski, F.; Souza, D.O.; Cruz Portela, L.V. Acute and Chronic Electro-Convulsive Shock in Rats: Effects on Peripheral Markers of Neuronal Injury and Glial Activity. Life Sci. 2006, 78, 3013–3017. [Google Scholar] [CrossRef]
- Lam, T.K.T.; Gutierrez-Juarez, R.; Pocai, A.; Rossetti, L. Regulation of Blood Glucose by Hypothalamic Pyruvate Metabolism. Science 2005, 309, 943–947. [Google Scholar] [CrossRef]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Epilepsy Treatment. Targeting LDH Enzymes with a Stiripentol Analog to Treat Epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein Lactylation Induced by Neural Excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- Bullitt, E. Expression of c-Fos-like Protein as a Marker for Neuronal Activity Following Noxious Stimulation in the Rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Moreno, B.; Jukes, J.P.; Vergara-Irigaray, N.; Errea, O.; Villoslada, P.; Perry, V.H.; Newman, T.A. Systemic Inflammation Induces Axon Injury During Brain Inflammation. Ann. Neurol. 2011, 70, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Tortuous Path of Lactate Shuttle Discovery: From Cinders and Boards to the Lab and ICU. J. Sport Health Sci. 2020, 9, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Lønbro, S.; Wiggins, J.M.; Wittenborn, T.; Elming, P.B.; Rice, L.; Pampo, C.; Lee, J.A.; Siemann, D.W.; Horsman, M.R.; Sunderland, C. Reliability of Blood Lactate as a Measure of Exercise Intensity in Different Strains of Mice During Forced Treadmill Running. PLoS ONE 2019, 14, e0215584. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamudio, R.I.; Ha, H.C. PARP 1 Enhances Inflammatory Cytokine Expression by Alteration of Promoter Chromatin Structure in Microglia. Brain Behav. 2014, 4, 552–565. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Zhang, W.; Hua, H.; Guo, Y.; Cheng, Y.; Pi, F.; Yao, W.; Xie, Y.; Qian, H. Torularhodin from Sporidiobolus Pararoseus Attenuates d-Galactose/AlCl (3)-Induced Cognitive Impairment, Oxidative Stress, and Neuroinflammation via the Nrf2/NF-κB Pathway. J. Agric. Food Chem. 2020, 68, 6604–6614. [Google Scholar] [CrossRef]
- Gao, L.; Peng, X.M.; Huo, S.X.; Liu, X.M.; Yan, M. Memory Enhancement of Acteoside (Verbascoside) in a Senescent Mice Model Induced by a Combination of D-Gal and AlCl3. Phytother. Res. 2015, 29, 1131–1136. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primers. 2019, 5, 70. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling Pathways Involved in Ischemic Stroke: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Brouns, R.; Sheorajpanday, R.; Wauters, A.; De Surgeloose, D.; Mariën, P.; De Deyn, P.P. Evaluation of Lactate as a Marker of Metabolic Stress and Cause of Secondary Damage in Acute Ischemic Stroke or TIA. Clin. Chim. Acta 2008, 397, 27–31. [Google Scholar] [CrossRef]
- Yao, X.; Li, C. Lactate Dehydrogenase A Mediated Histone Lactylation Induced the Pyroptosis Through Targeting HMGB1. Metab. Brain Dis. 2023, 38, 1543–1553. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Li, N.; Ma, Y.; Yao, M.; Wang, G.; He, S.; Li, W.; Tan, J.; Lu, Q.; et al. YY1 Lactylation in Microglia Promotes Angiogenesis Through Transcription Activation-Mediated Upregulation of FGF2. Genome Biol. 2023, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Deng, H.; Song, P.; Liu, Y.; Zhang, M. Lactylation in Glioblastoma: A Novel Epigenetic Modifier Bridging Epigenetic Plasticity and Metabolic Reprogramming. Int. J. Mol. Sci. 2025, 26, 3368. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Multi-layered Prevention and Treatment of Chronic Inflammation, Organ Fibrosis and Cancer Associated with Canonical WNT/Β-catenin Signaling Activation (Review). Int. J. Mol. Med. 2018, 42, 713–725. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, J.; Lu, P.; Zhang, X.; Yang, L.; Wu, J.; Zhang, L.; Zhang, L.; Pang, J.; Xie, H.; et al. Lactylation of Histone by BRD4 Regulates Astrocyte Polarization After Experimental Subarachnoid Hemorrhage. J. NeuroInflamm. 2024, 21, 186. [Google Scholar] [CrossRef]
- Hancock, R.L.; Dunne, K.; Walport, L.J.; Flashman, E.; Kawamura, A. Epigenetic Regulation by Histone Demethylases in Hypoxia. Epigenomics 2015, 7, 791–811. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM 2, Cancer Metabolism, and the Road Ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1α Activity and IL-1β Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol Metabolism Contributes to Brain Histone Acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef]
- Ovens, M.J.; Davies, A.J.; Wilson, M.C.; Murray, C.M.; Halestrap, A.P. AR-C155858 Is a Potent Inhibitor of Monocarboxylate Transporters MCT1 and MCT2 That Binds to an Intracellular Site Involving Transmembrane Helices 7–10. Biochem. J. 2010, 425, 523–530. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, H.; Lu, W.; Tian, X.; Sun, Y.; Peng, H. Histone Lactylation Is Involved in Mouse Oocyte Maturation and Embryo Development. Int. J. Mol. Sci. 2024, 25, 4821. [Google Scholar] [CrossRef]

| Disorder | Key Lactylation Sites | Core Pathological Mechanism | Disease Phenotypes |
|---|---|---|---|
| Alzheimer’s disease | H4K12la, H3K18la | PKM2/glycolysis positive feedback loop → microglial hyperactivation | Aβ deposition, cognitive decline, neuroinflammation |
| Glioblastoma | H3K9la | BRD4 recruitment to enhancers → oncogene activation | Tumor proliferation, therapy resistance, Warburg effect amplification |
| Ischemic stroke | H3K9la, H3K18la | LDHA-mediated HMGB1 promoter lactylation → NLRP3 inflammasome activation | Infarct volume expansion, blood–brain barrier disruption, neuronal death |
| Depression | H1Kla | MCT2-mediated neuronal lactate uptake → prefrontal cortex protein lactylation | Neuronal hyperexcitability, behavioral despair, social avoidance |
| Aging/Neuro-inflammation | H3K18la | Exercise-induced lactate → microglial phenotype switching (pro- to anti-inflammatory) | Cognitive dysfunction, accelerated neurodegeneration, synaptic impairment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-M.; Yang, F.; Li, Q.; Zhang, J.-N. Role of Histone Lactylation in Neurological Disorders. Int. J. Mol. Sci. 2025, 26, 7949. https://doi.org/10.3390/ijms26167949
Zhang Y-M, Yang F, Li Q, Zhang J-N. Role of Histone Lactylation in Neurological Disorders. International Journal of Molecular Sciences. 2025; 26(16):7949. https://doi.org/10.3390/ijms26167949
Chicago/Turabian StyleZhang, Yu-Mo, Fei Yang, Qian Li, and Jian-Nan Zhang. 2025. "Role of Histone Lactylation in Neurological Disorders" International Journal of Molecular Sciences 26, no. 16: 7949. https://doi.org/10.3390/ijms26167949
APA StyleZhang, Y.-M., Yang, F., Li, Q., & Zhang, J.-N. (2025). Role of Histone Lactylation in Neurological Disorders. International Journal of Molecular Sciences, 26(16), 7949. https://doi.org/10.3390/ijms26167949






